Radiomics-Based Detection of Germ Cell Neoplasia In Situ Using Volumetric ADC and FA Histogram Features: A Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. MRI Protocol
2.3. MRI Data Postprocessing
2.4. Histologic Evaluation
2.5. Statistical Analysis
3. Results
3.1. Patient Population
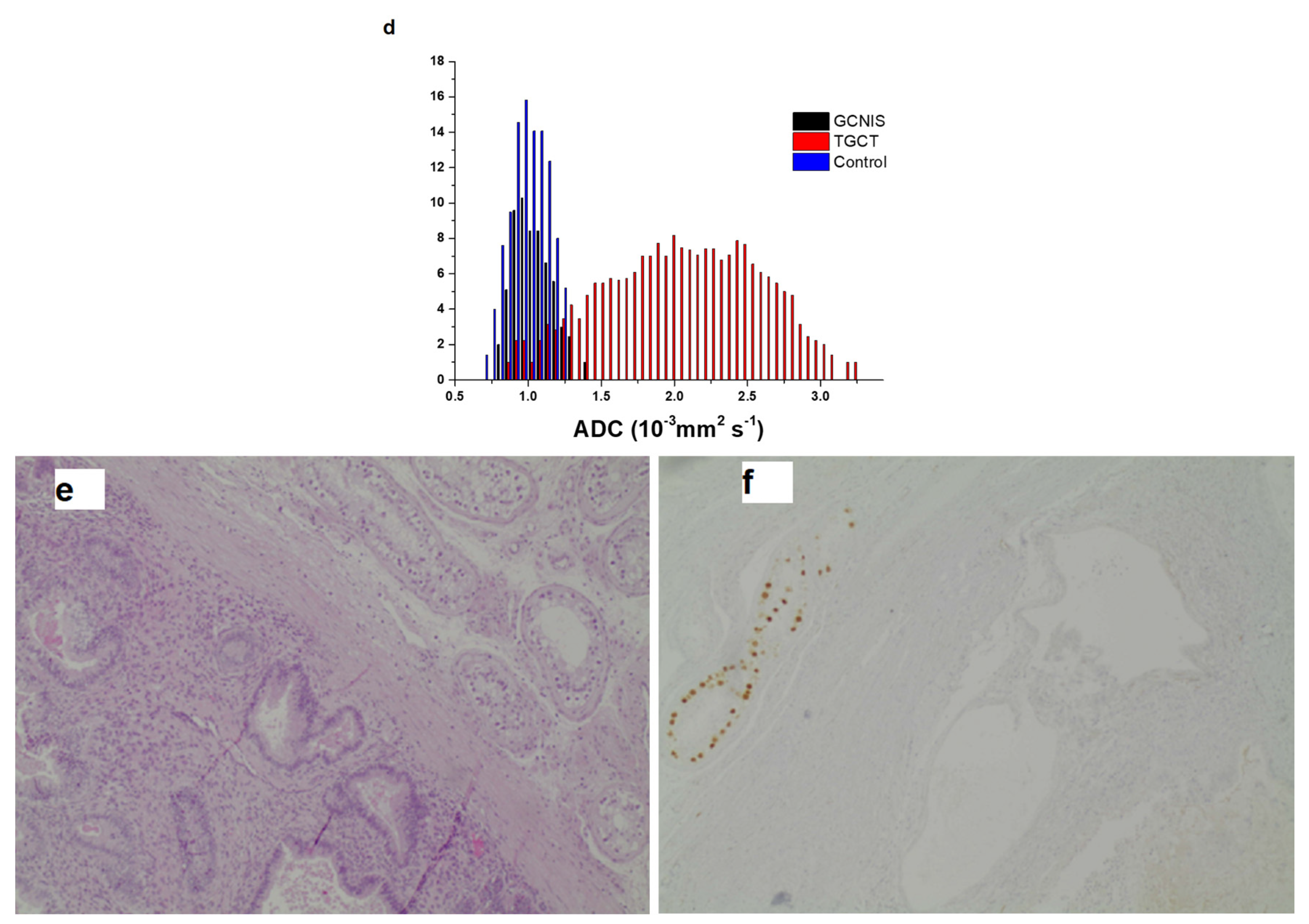
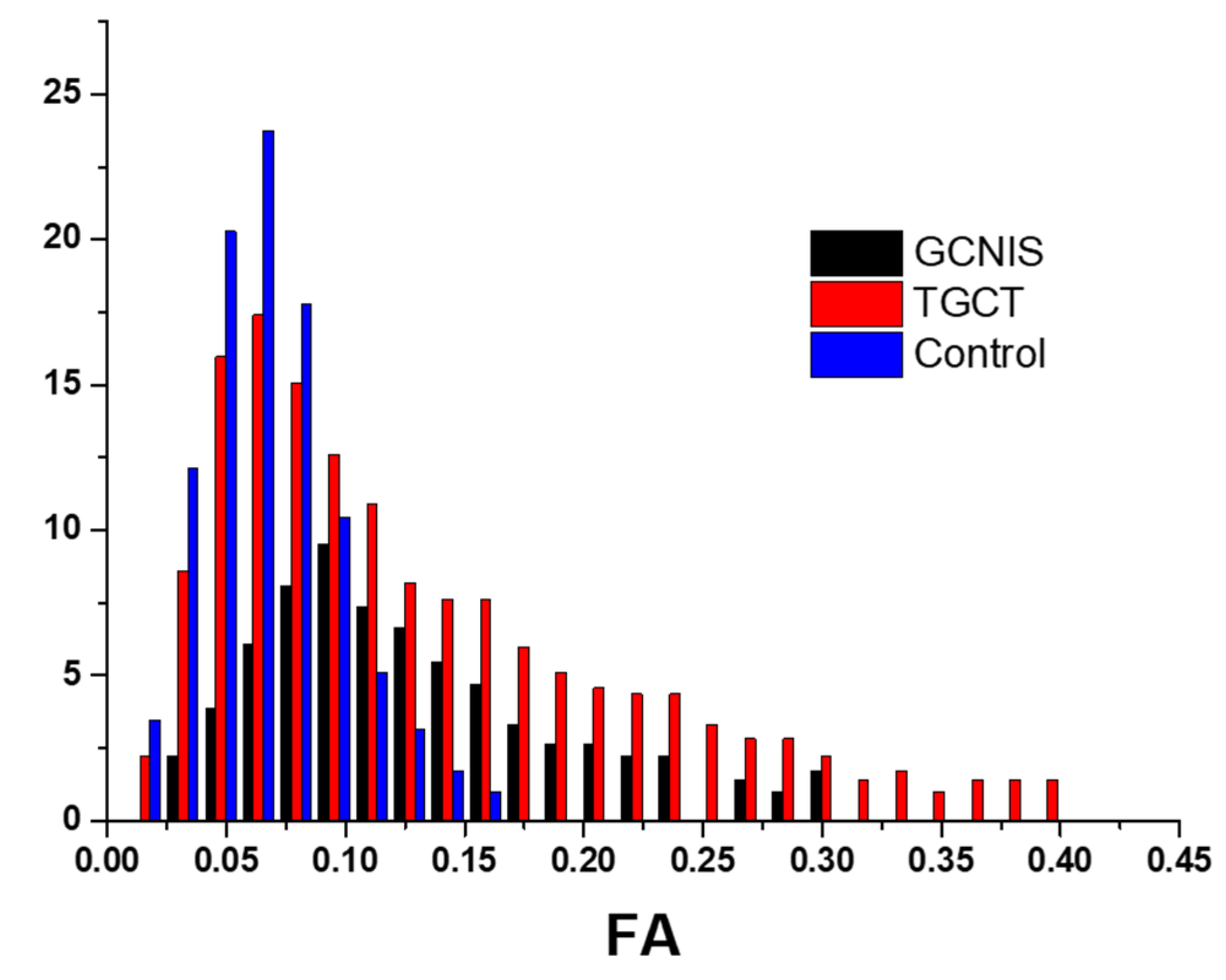
3.2. Histogram Metrics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TGCTS | testicular germ cell tumors |
| GCNIS | Germ Cell Neoplasia In Situ |
| NSGCTS | non-seminomatous germ cell tumors |
| DWI | diffusion-weighted imaging |
| ADC | apparent diffusion coefficient |
| ROI | region of interest |
| 3D | three-dimensional |
| DTI | diffusion tensor imaging |
| FA | fractional anisotropy |
| T2WI | T2-weighted imaging |
| DCE | dynamic contrast-enhanced |
| VOI | volume of interest |
| IQR | interquartile range |
| MAD | mean absolute deviation |
| RMAD | robust mean absolute deviation |
| RMS | root mean square |
| (H&E) | hematoxylin & eosin |
| OCT3/4 | octamer-binding transcription factor 3/4 |
| PLAP | placental alkaline phosphatase |
| ROC | Receiver Operating Characteristic |
References
- Nicol, D.; Berney, D.M.; Boormans, J.L.; Di Nardo, D.; Fankhauser, C.D.; Fischer, S.; Gremmels, H.; Cornes, R.; Heidenreich, A.; Leão, R.; et al. European Association of Urology 2025. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Testicular-Cancer-2025.pdf (accessed on 14 August 2025).
- Park, J.S.; Kim, J.; Elghiaty, A.; Ham, W.S. Recent global trends in testicular cancer incidence and mortality. Medicine 2018, 97, e12390. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Tin, M.S.; Liu, X.; Lok, V.T.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; et al. Worldwide Distribution, Risk Factors, and Temporal Trends of Testicular Cancer Incidence and Mortality: A Global Analysis. Eur. Urol. Oncol. 2022, 5, 566–576. [Google Scholar] [CrossRef]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H.J.; Van der Kwast, T.H.; Grignon, D.; Egevad, L.; Kristiansen, G.; Kao, C.S.; Idrees, M.T. Report from the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers: IV: Current and Future Utilization of Molecular-Genetic Tests for Testicular Germ Cell Tumors. Am. J. Surg. Pathol. 2020, 44, e66–e79. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Cheaib, J.G.; Patel, H.D.; Sharma, R.; Zhang, A.; Bass, E.B.; Pierorazio, P.M. Diagnosis and Management of Intratubular Germ Cell Neoplasia In Situ: A Systematic Review. J. Urol. 2020, 204, 33–41. [Google Scholar] [CrossRef]
- Stephenson, A.; Eggener, S.E.; Bass, E.B.; Chelnick, D.M.; Daneshmand, S.; Feldman, D.; Gilligan, T.; Karam, J.A. Diagnosis and Treatment of Early Stage Testicular Cancer: AUA Guideline. J. Urol. 2019, 202, 272–281. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Loy, V. Prevalence of contralateral testicular intraepithelial neoplasia in patients with testicular germ cell neoplasms. J. Clin. Oncol. 1996, 14, 3126–3132. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Kulejewski, M.; Pichlmeier, U.; Loy, V. Diagnosis of contralateral testicular intraepithelial neoplasia (TIN) in patients with testicular germ cell cancer: Systematic two-site biopsies are more sensitive than a single random biopsy. Eur. Urol. 2007, 51, 175–182. [Google Scholar] [CrossRef]
- Hoei-Hansen, C.E.; Rajpert-De Meyts, E.; Daugaard, G.; Skakkebaek, N.E. Carcinoma in situ testis, the progenitor of testicular germ cell tumours: A clinical review. Ann. Oncol. 2005, 16, 863–868. [Google Scholar] [CrossRef]
- Harland, S.J.; Cook, P.A.; Fossa, S.D.; Horwich, A.; Mead, G.M.; Parkinson, M.C.; Roberts, J.T.; Stenning, S.P. Intratubular germ cell neoplasia of the contralateral testis in testicular cancer: Defining a high risk group. J. Urol. 1998, 160, 1353–1357. [Google Scholar] [CrossRef]
- Elzinga-Tinke, J.E.; Sirre, M.E.; Looijenga, L.H.J.; van Casteren, N.; Wildhagen, M.F.; Dohle, G.R. The predictive value of testicular ultrasound abnormalities for carcinoma in situ of the testis in men at risk for testicular cancer. Int. J. Androl. 2009, 33, 597–603. [Google Scholar] [CrossRef]
- Giwercman, A.; Lenz, S.; Skakkebaek, N.E. Carcinoma in situ in atrophic testis: Biopsy based on abnormal ultrasound pattern. Br. J. Urol. 1993, 72, 118–120. [Google Scholar] [CrossRef]
- Reinges, M.H.; Kaiser, W.A.; Miersch, W.D.; Vogel, J. Dynamic magnetic resonance imaging of the contralateral testis in patients with malignant tumor of the testis. Urology 1994, 44, 540–547. [Google Scholar] [CrossRef]
- Thomsen, C.; Jensen, K.E.; Giwercman, A.; Kjaer, L.; Henriksen, O.; Skakkebaek, N.E. Magnetic resonance: In vivo tissue characterization of the testis in patients with carcinoma-in-situ testis by magnetic resonance imaging. Int. J. Androl. 1987, 10, 191–198. [Google Scholar] [CrossRef]
- Tsili, A.C.; Ntorkou, A.; Baltogiannis, D.; Goussia, A.; Astrakas, L.G.; Malamou-Mitsi, V.; Sofikitis, N.; Argyropoulou, M.I. The role of apparent diffusion coefficient values in detecting testicular intraepithelial neoplasia: Preliminary results. Eur. J. Radiol. 2015, 84, 828–833. [Google Scholar] [CrossRef]
- Le Bihan, D.; Turner, R.; Douek, P.; Patronas, N. Diffusion MR imaging: Clinical applications. AJR Am. J. Roentgenol. 1992, 159, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Iima, M. Diffusion Magnetic Resonance Imaging: What Water Tells Us about Biological Tissues. PLoS Biol. 2015, 13, e1002203. [Google Scholar]
- Just, N. Improving tumour heterogeneity MRI assessment with histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef]
- Gihr, G.; Horvath-Rizea, D.; Kohlhof-Meinecke, P.; Ganslandt, O.; Henkes, H.; Härtig, W.; Donitza, A.; Skalej, M.; Schob, S. Diffusion Weighted Imaging in Gliomas: A Histogram-Based Approach for Tumor Characterization. Cancers 2022, 14, 3393. [Google Scholar] [CrossRef] [PubMed]
- Usuda, K.; Iwai, S.; Yamagata, A.; Iijima, Y.; Motono, N.; Matoba, M.; Doai, M.; Hirata, K.; Uramoto, H. Whole-Lesion Apparent Diffusion Coefficient Histogram Analysis: Significance for Discriminating Lung Cancer from Pulmonary Abscess and Mycobacterial Infection. Cancers 2021, 13, 2720. [Google Scholar] [CrossRef] [PubMed]
- Chong, I.; Ostrom, Q.; Khan, B.; Dandachi, D.; Garg, N.; Kotrotsou, A.; Colen, R.; Morón, F. Whole Tumor Histogram Analysis Using DW MRI in Primary Central Nervous System Lymphoma Correlates with Tumor Biomarkers and Outcome. Cancers 2019, 11, 1506. [Google Scholar] [CrossRef]
- De Robertis, R.; Tomaiuolo, L.; Pasquazzo, F.; Geraci, L.; Malleo, G.; Salvia, R.; D’Onofrio, M. Correlation between ADC Histogram-Derived Metrics and the Time to Metastases in Resectable Pancreatic Adenocarcinoma. Cancers 2022, 14, 6050. [Google Scholar] [CrossRef]
- Donati, O.F.; Mazaheri, Y.; Afaq, A.; Vargas, H.A.; Zheng, J.; Moskowitz, C.S.; Hricak, H.; Akin, O. Prostate cancer aggressiveness: Assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology 2014, 271, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Cho, J.Y.; Kim, S.Y.; Kim, S.H. Histogram analysis of apparent diffusion coefficient map of diffusion-weighted MRI in endometrial cancer: A preliminary correlation study with histological grade. Acta Radiol. 2014, 55, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Min, X.; Feng, Z.; Cai, W.; Li, B.; Zhang, P.; You, H.; Xie, J.; Wang, L. Discrimination between benign and malignant testicular lesions using volumetric apparent diffusion coefficient histogram analysis. Eur. J. Radiol. 2020, 126, 108939. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.D.; Hung, N.D.; Tuan, T.A.; Hien, M.M.; Tuan, H.X.; Duc, N.M. Apparent diffusion coefficient histogram in the differentiation of benign and malignant testicular tumors. Int. J. Med. Sci. 2024, 21, 200–206. [Google Scholar] [CrossRef]
- Min, X.; Feng, Z.; Wang, L.; Cai, J.; Yan, X.; Li, B.; Ke, Z.; Zhang, P.; You, H. Characterization of testicular germ cell tumors: Whole-lesion histogram analysis of the apparent diffusion coefficient at 3T. Eur. J. Radiol. 2018, 98, 25–31. [Google Scholar] [CrossRef]
- Le Bihan, D.; Mangin, J.F.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging 2001, 13, 534–546. [Google Scholar] [CrossRef]
- Dong, Q.; Welsh, R.C.; Chenevert, T.L.; Carlos, R.C.; Maly-Sundgren, P.; Gomez-Hassan, D.M.; Mukherji, S.K. Clinical applications of diffusion tensor imaging. J. Magn. Reson. Imaging 2004, 19, 6–18. [Google Scholar] [CrossRef]
- Manan, A.A.; Yahya, N.A.; Taib, N.H.M.; Idris, Z.; Manan, H.A. The Assessment of White Matter Integrity Alteration Pattern in Patients with Brain Tumor Utilizing Diffusion Tensor Imaging: A Systematic Review. Cancers 2023, 15, 3326. [Google Scholar] [CrossRef]
- Manan, A.A.; Yahya, N.; Idris, Z.; Manan, H.A. The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review. Cancers 2022, 14, 2466. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Randall, J.W.; Elhalawani, H.; Al Feghali, K.A.; Elliott, A.M.; Anderson, B.M.; Lacerda, L.; Tran, B.L.; Mohamed, A.S.; Brock, K.K.; et al. Detection of Glioblastoma Subclinical Recurrence Using Serial Diffusion Tensor Imaging. Cancers 2020, 12, 568. [Google Scholar] [CrossRef]
- Lanzman, R.S.; Wittsack, H.J. Diffusion tensor imaging in abdominal organs. NMR Biomed. 2017, 30, e3434. [Google Scholar] [CrossRef]
- Shenhar, C.; Degani, H.; Ber, Y.; Baniel, J.; Tamir, S.; Benjaminov, O.; Rosen, P.; Furman-Haran, E.; Margel, D. Diffusion Is Directional: Innovative Diffusion Tensor Imaging to Improve Prostate Cancer Detection. Diagnostics 2012, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Tsili, A.C.; Ntorkou, A.; Astrakas, L.G.; Boukali, E.; Giannakis, D.; Maliakas, V.; Sofikitis, N.; Argyropoulou, M.I. Magnetic resonance diffusion tensor imaging of the testis: Preliminary observations. Eur. J. Radiol. 2017, 95, 265–270. [Google Scholar] [CrossRef]
- Tsili, A.C.; Sofikitis, N.; Pappa, O.; Bougia, C.K.; Argyropoulou, M.I. An Overview of the Role of Multiparametric MRI in the Investigation of Testicular Tumors. Cancers 2022, 14, 3912. [Google Scholar] [CrossRef] [PubMed]
- Nissan, N.; Anaby, D.; Tavor, I.; Kleinbaum, Y.; Dotan, Z.; Konen, E.; Portnoy, O. The Diffusion Tensor Imaging Properties of the Normal Testicles at 3 Tesla Magnetic Resonance Imaging. Acad. Radiol. 2019, 26, 1010–1016. [Google Scholar] [CrossRef]
- Tsili, A.C.; Ntorkou, A.; Goussia, A.; Astrakas, L.; Panopoulou, E.; Sofikitis, N.; Argyropoulou, M.I. Diffusion tensor imaging parameters in testes with nonobstructive azoospermia. J. Magn. Reson. Imaging 2018, 48, 1318–1325. [Google Scholar] [CrossRef]
- Gao, S.; Yang, J.; Chen, D.; Min, X.; Fan, C.; Zhang, P.; Wang, Q.; Li, Z.; Cai, W. Noninvasive Prediction of Sperm Retrieval Using Diffusion Tensor Imaging in Patients with Nonobstructive Azoospermia. J. Imaging 2023, 9, 182. [Google Scholar] [CrossRef]
- Pappa, O.; Astrakas, L.; Anagnostou, N.; Bougia, C.Κ.; Maliakas, V.; Sofikitis, N.; Argyropoulou, M.I.; Tsili, A.C. 3.0 T diffusion tensor imaging and fiber tractography of the testes in nonobstructive azoospermia. Abdom. Radiol. 2024, 49, 4543–4555. [Google Scholar] [CrossRef]
- Tsili, A.C.; Sofikitis, N.; Xiropotamou, O.; Astrakas, L.G.; Ntorkou, A.; Argyropoulou, M.I. Diffusion tensor imaging as an adjunct tool for the diagnosis of varicocele. Andrologia 2019, 51, e13210. [Google Scholar] [CrossRef]
- Radiomic Features—Pyradiomics v3.0.1.post11+g03d23f7 Documentation. Available online: https://pyradiomics.readthedocs.io/en/latest/features.html#moduleradiomics.firstorder (accessed on 14 August 2025).
- Tsili, A.C.; Argyropoulou, M.I.; Giannakis, D.; Tsampalas, S.; Sofikitis, N.; Tsampoulas, K. Diffusion-weighted MR imaging of normal and abnormal scrotum: Preliminary results. Asian J. Androl. 2012, 14, 649–654. [Google Scholar] [CrossRef]
- Algebally, A.M.; Tantawy, H.I.; Yousef, R.R.; Szmigielski, W.; Darweesh, A. Advantage of Adding Diffusion Weighted Imaging to Routine MRI Examinations in the Diagnostics of Scrotal Lesions. Pol. J. Radiol. 2015, 80, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Tsili, A.C.; Sylakos, A.; Ntorkou, A.; Stavrou, S.; Astrakas, L.G.; Sofikitis, N.; Argyropoulou, M.I. Apparent diffusion coefficient values and dynamic contrast enhancement patterns in differentiating seminomas from nonseminomatous testicular neoplasms. Eur. J. Radiol. 2015, 84, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, Z.; Chen, Y.; Zhao, F.; Yu, H.; Guo, X.; Shi, K. Testicular tumors: Discriminative value of conventional MRI and diffusion weighted imaging. Medicine 2021, 100, e27799. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, J.; Jiang, Y.; Wu, Z.; Ji, J.; Li, A.; Wang, X.; Li, R. The utility of diffusion-weighted imaging and ADC values in the characterization of mumps orchitis and seminoma. Acta Radiol. 2022, 63, 416–423. [Google Scholar] [CrossRef]
- Berney, D.M.; Looijenga, L.H.; Idrees, M.; Oosterhuis, J.W.; Rajpert-De Meyts, E.; Ulbright, T.M.; Skakkebaek, N.E. Germ cell neoplasia in situ (GCNIS): Evolution of the current nomenclature for testicular pre-invasive germ cell malignancy. Histopathology 2016, 69, 7–10. [Google Scholar] [CrossRef]
- Spiller, C.M.; Bowles, J. Germ cell neoplasia in situ: The precursor cell for invasive germ cell tumors of the testis. Int. J. Biochem. Cell Biol. 2017, 86, 22–25. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Skakkebaek, N.E. Carcinoma in situ of the testis: Review of biological and clinical features. Int. J. Cancer 1999, 83, 815–822. [Google Scholar] [CrossRef]
- Basiri, A.; Movahhed, S.; Parvin, M.; Salimi, M.; Rezaeetalab, G.H. The histologic features of intratubular germ cell neoplasia and its correlation with tumor behavior. Investig. Clin. Urol. 2016, 57, 191–195. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Saito, S.; Koyama, Y.; Ueda, J.; Hashido, T. Relationship between Apparent Diffusion Coefficient Distribution and Cancer Grade in Prostate Cancer and Benign Prostatic Hyperplasia. Diagnostics 2022, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shen, M.; He, Y.; Ma, F.; Liu, J.; Zhang, G.; Qiang, J. The role of volumetric ADC histogram analysis in preoperatively evaluating the tumour subtype and grade of endometrial cancer. Eur. J. Radiol. 2021, 140, 109745. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Polat, A.V.; Selcuk, M.B. Whole-lesion ADC histogram analysis versus single-slice ADC measurement for the differentiation of benign and malignant soft tissue tumors. Eur. J. Radiol. 2021, 143, 109934. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, H.; Chen, Z.; Ni, P.; Zhong, Q.; Huang, H.; Sandrasegaran, K. Correlation of histogram analysis of apparent diffusion coefficient with uterine cervical pathologic finding. AJR Am. J. Roentgenol. 2015, 204, 1125–1131. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.J.; Lee, J.W.; Lee, N.K.; Lee, G.; Kang, T.; Park, H.; Son, Y.H.; Grimm, R. Risk stratification of ductal carcinoma in situ using whole-lesion histogram analysis of the apparent diffusion coefficient. Eur. Radiol. 2019, 29, 485–493. [Google Scholar] [CrossRef]
- Loy, V.; Wigand, I.; Dieckmann, K.P.V. Incidence and distribution of carcinoma in situ in testes removed for germ cell tumour: Possible inadequacy of random testicular biopsy in detecting the condition. Histopathology 1990, 16, 198–200. [Google Scholar] [CrossRef]
- Prym, H.; Lauke, H. Carcinoma in situ of the human testis: Tumour cells are distributed focally in the seminiferous tubules. Andrologia 1994, 26, 231–234. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Zhang, X.; Chen, S.; Song, Y.; Xie, L.; Chen, Y.; Ouyang, H. Whole-lesion apparent diffusion coefficient (ADC) histogram as a quantitative biomarker to preoperatively differentiate stage IA endometrial carcinoma from benign endometrial lesions. BMC Med. Imaging 2022, 22, 139. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Fang, X.; Wang, Z.; Xing, Y.; Ma, L.; Wu, B. Rectal cancer: Can T2WI histogram of the primary tumor help predict the existence of lymph node metastasis? Eur. Radiol. 2019, 29, 6469–6476. [Google Scholar] [CrossRef]
- Li, Z.; Han, C.; Wang, L.; Zhu, J.; Yin, Y.; Li, B. Prognostic value of texture analysis based on pretreatment DWI-weighted MRI for esophageal squamous cell carcinoma patients treated with concurrent chemo-radiotherapy. Front. Oncol. 2019, 9, 1057. [Google Scholar] [CrossRef]
- Davnall, F.; Yip, C.S.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Kersemaekers, A.M.; Jacobsen, G.K.; Timmer, A.; Steyerberg, E.W.; Molier, M.; Van Weeren, P.C.; Stoop, H.; Looijenga, L.H. Morphology of testicular parenchyma adjacent to germ cell tumours. An interim report. Acta Pathol. Microbiol. Immunol. Scand. 2003, 111, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kierans, A.S.; Doshi, A.M.; Dunst, D.; Popiolek, D.; Blank, S.V.; Rosenkrantz, A.B. Retrospective assessment of histogram-based diffusion metrics for differentiating benign and malignant endometrial lesions. J. Comput. Assist. Tomogr. 2016, 40, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; You, C.; Zhou, X.; Li, X.; Zhang, C.; Wu, Y.; Shen, W. The volumetric ADC histogram analysis in differentiating stage IA endometrial carcinoma from endometrial polyp. Br. J. Radiol. 2024, 97, 1139–1145. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, Y.S.; Liu, Z.; Liu, H.F.; Zhai, Y.N.; Mao, X.R.; Lei, J.Q. Whole-liver apparent diffusion coefficient histogram analysis for the diagnosis and staging of liver fibrosis. J. Magn. Reson. Imaging 2020, 51, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E. Possible carcinoma-in-situ of the testis. Lancet 1972, 2, 516–517. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Wilken, S.; Loy, V.; Matthies, C.; Kleinschmidt, K.; Bedke, J.; Martinschek, A.; Souchon, R.; Pichlmeier, U.; Kliesch, S. Treatment of testicular intraepithelial neoplasia (intratubular germ cell neoplasia unspecified) with local radiotherapy or with platinum-based chemotherapy: A survey of the German Testicular Cancer Study Group. Ann. Oncol. 2013, 24, 1332–1337. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Gundgaard, M.G.; Daugaard, G. Treatment options for carcinoma in situ testis. Int. J. Androl. 2011, 34, e32–e36. [Google Scholar] [CrossRef]
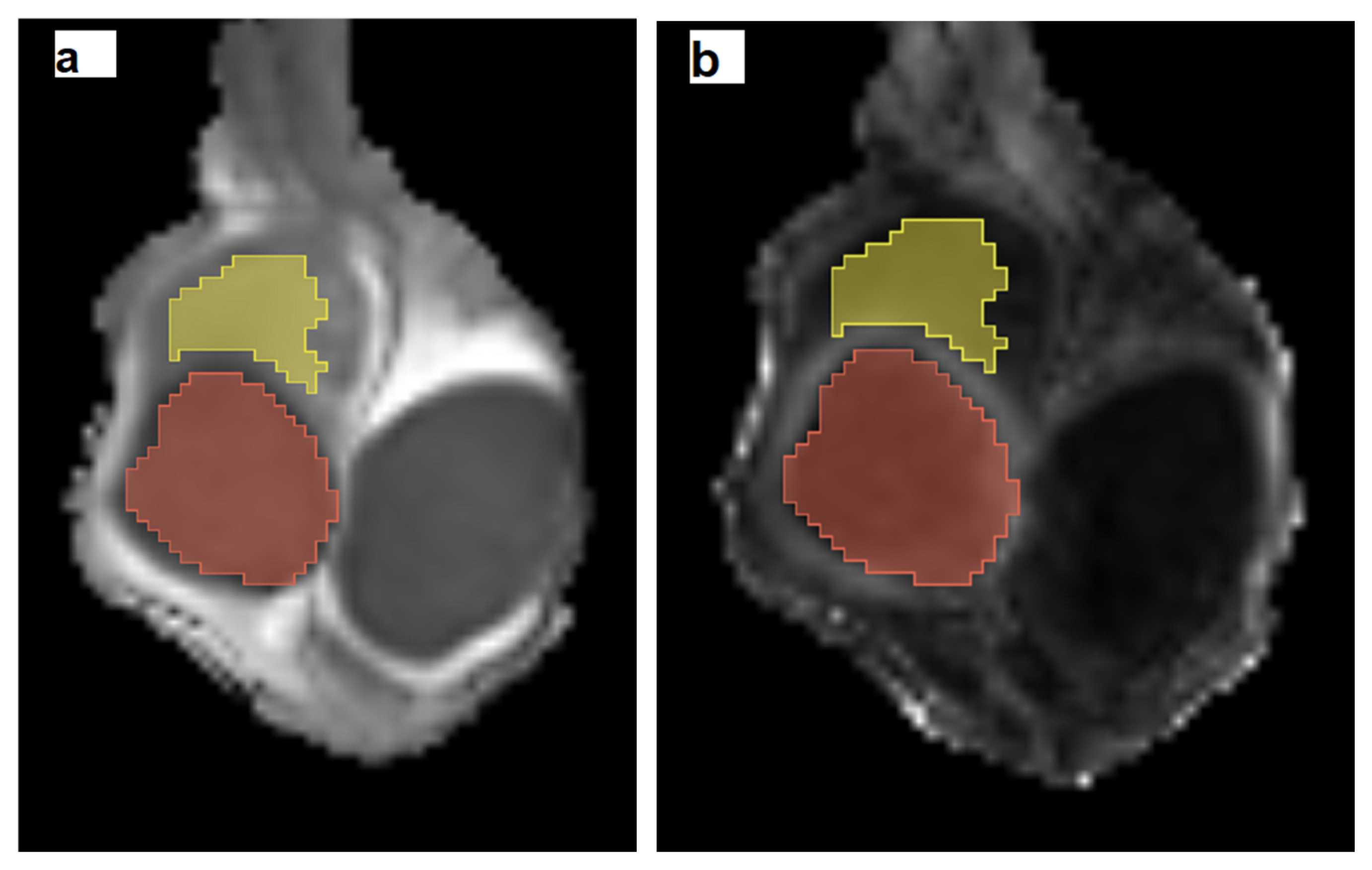
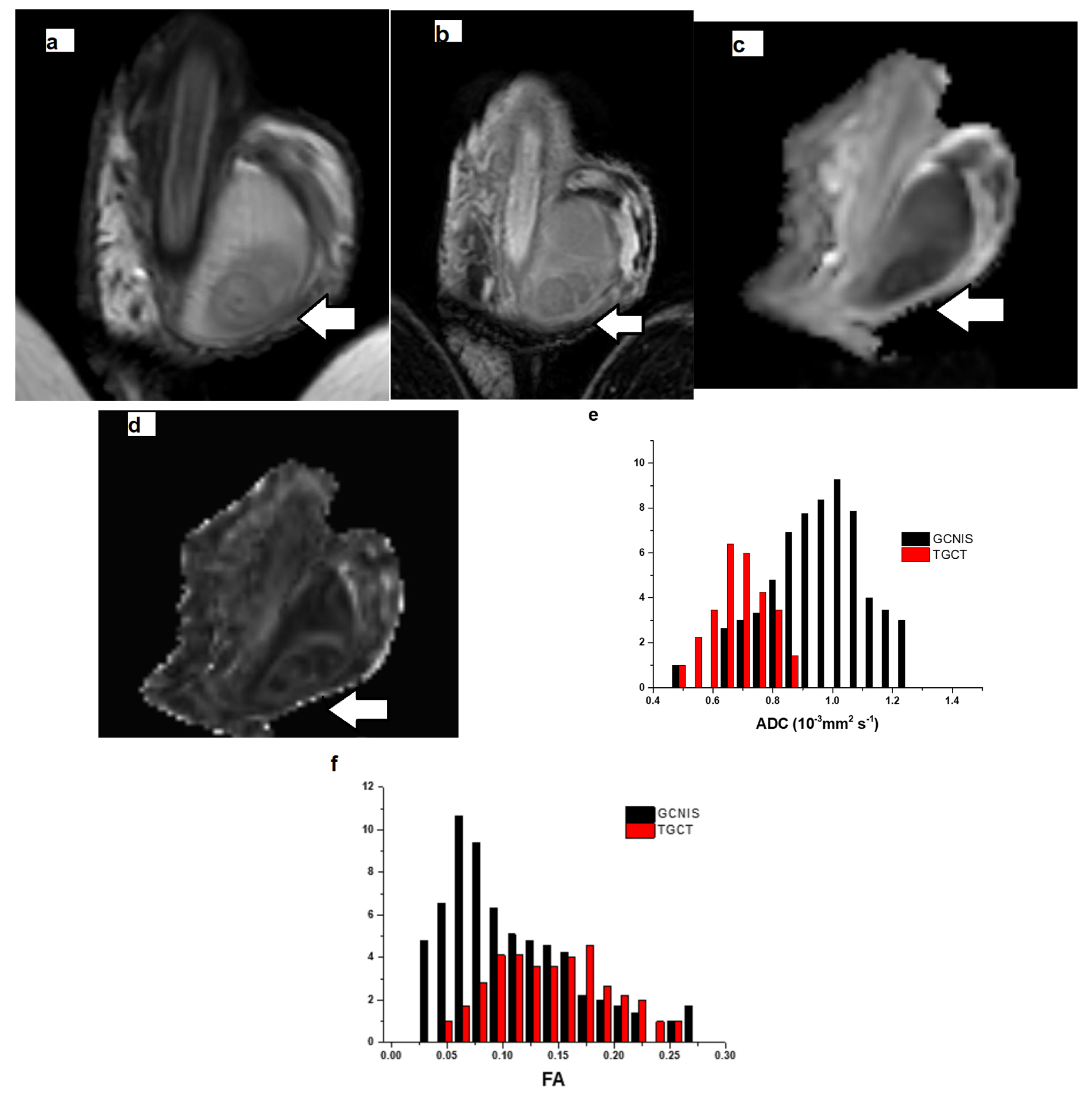
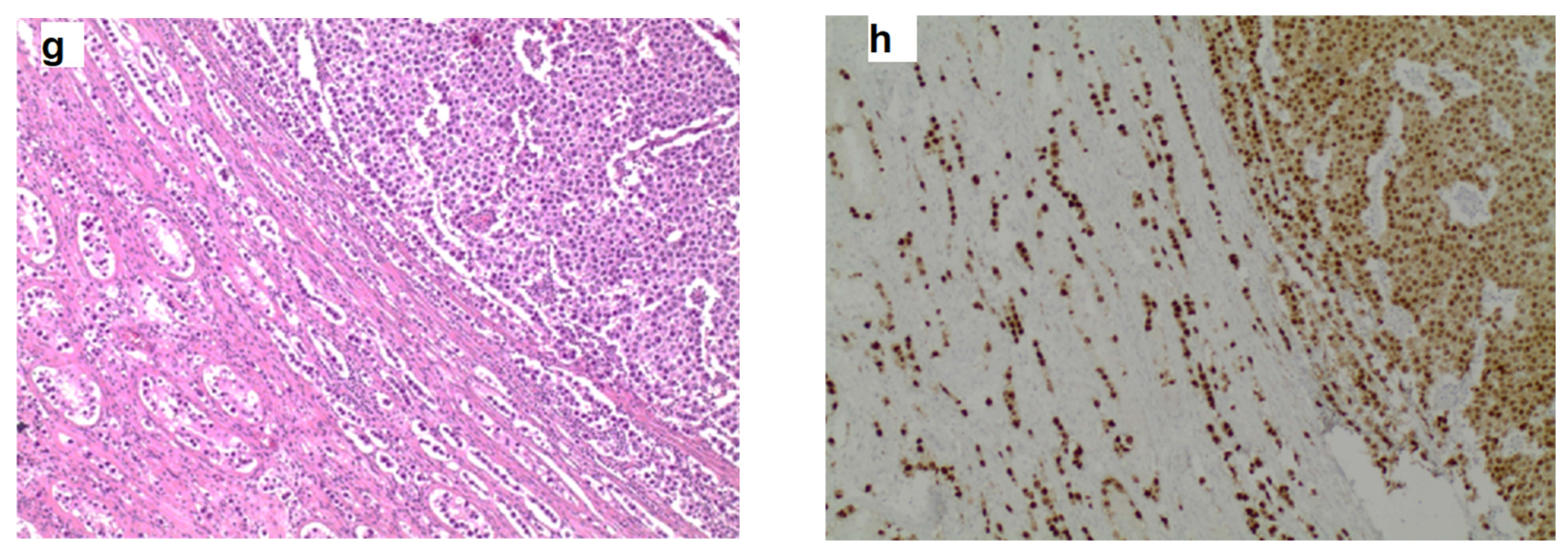


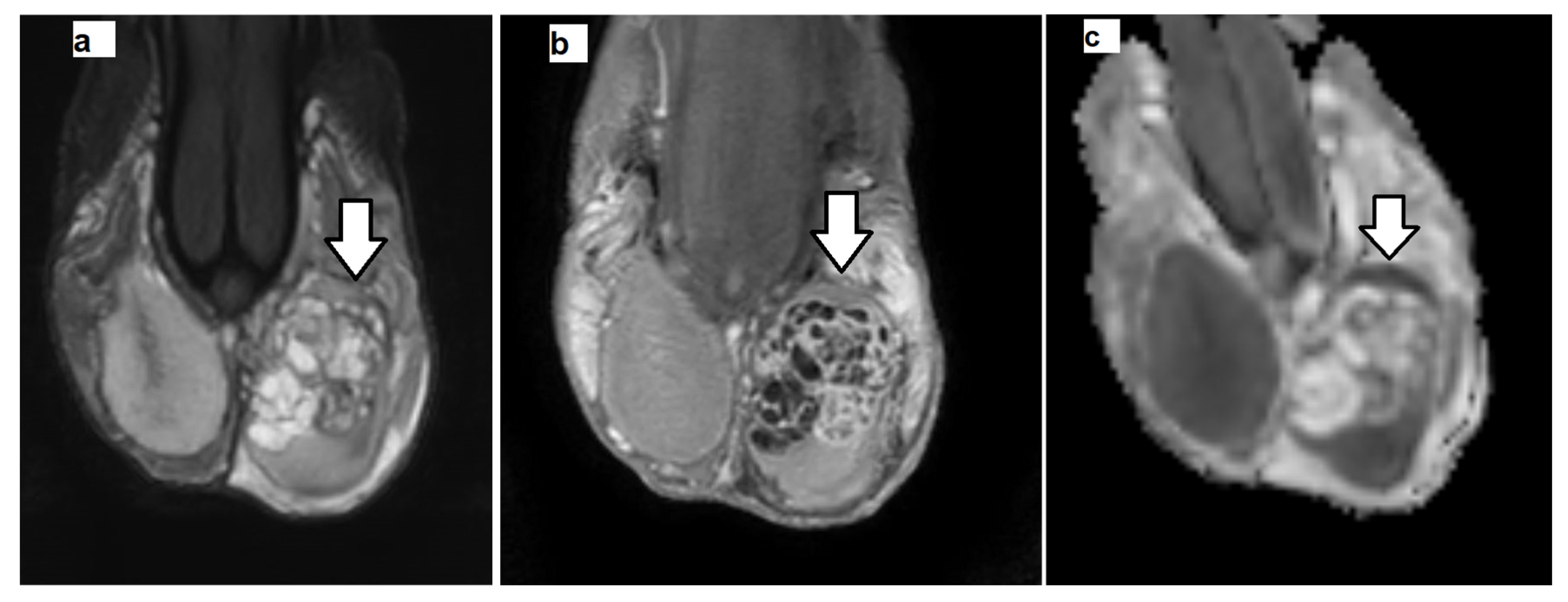

| Protocol | T1WI | T2WI | DTI | DCE-MRI |
|---|---|---|---|---|
| Sequences | Spin-Echo | Turbo Spin-Echo | Fat-Saturated Single-Shot Spin-Echo Planar Imaging | 3D Fast Field Echo |
| plane | axial | axial, sagittal, coronal | coronal | |
| TR (ms) | 485 | 4000 | coronal | 5.5 |
| TE (ms) | 8 | 100–120 | 3.700 | 1.94–3.5 |
| slice thickness (mm) | 3 | 2–3 | 72 | 2 |
| gap (mm) | 0.3 | 0.2–0.3 | 4 | 0 |
| matrix (mm) | 172 × 168 | 180 × 256 | 0.4 | 200 × 200 |
| FOV (mm) | 170 × 170 | 240 × 270 | 128 × 87 | 160 × 177 |
| NSA | 1 | 1–2 | 160 × 80 | 1 |
| b value (s/mm2) | 2 | |||
| flip angle | 0, 900 | 100 | ||
| dynamic scans | 8 | |||
| scan duration (min) | 3 | 2.30–4.30 | 3 | 1 min each |
| ADC/FA Histogram Metrics | Description |
|---|---|
| 10th Percentile | The ADC/FA value below which 10% of all ADC/FA voxel values lie |
| 90th Percentile | The ADC/FA value below which 90% of all ADC/FA voxel values lie |
| Maximum | The maximum ADC/FA value within the VOI |
| Mean | The average ADC/FA value within the VOI |
| Median | The ADC/FA value below which 50% of all ADC/FA voxel values lie |
| Minimum | The minimum ADC/FA value within the VOI |
| Range | Measures the difference between the maximum and minimum ADC/FA values within the VOI |
| IQR | Measures the spread of the distribution of ADC/FA values, defined as the difference between 75th and 25th percentile |
| MAD | Measures the average distance between each ADC/FA intensity value and the mean intensity of a VOI. Quantifies variability, but unlike variance or standard deviation, it does not square the deviations. It is less sensitive to outliers than variance or standard deviation |
| RMAD | Provides a more outlier-resistant measure of variability compared to standard MAD. The lowest and highest 10% of ADC/FA values are excluded |
| RMS | Represents the square root of the average of the squared intensity values in a VOI. Reflects both the mean ADC/FA values and their variability |
| Entropy | Measures the inherent randomness of the ADC/FA values within the VOI |
| Energy | Describes the uniformity of the distribution of ADC/FA voxel values within the VOI |
| Total Energy | Reflects the overall signal strength of ADC/FA voxel values within the VOI |
| Skewness | Measures the asymmetry of the distribution of ADC/FA values around the mean |
| Kurtosis | Measures the peakedness of ADC/FA values within the VOI |
| Uniformity | Quantifies the evenness of the intensity of ADC/FA values in the VOI. It is closely related to energy |
| Variance | Quantifies the dispersion of the intensity of ADC/FA values around the mean within the VOI |
| Histologic Type | n |
|---|---|
| Seminomas | 10 |
| Non-seminomas | 6 |
| embryonal carcinoma | 3 |
| embryonal carcinoma, yolk sac tumor, teratoma | 1 |
| seminoma, embryonal carcinoma, teratoma | 1 |
| yolk scac tumor, teratoma | 1 |
| Groups | 1 Median [Min, Max] | 2 Median [Min, Max] | 3 Median [Min, Max] | p |
|---|---|---|---|---|
| ADC-Derived Metrics (10−3 mm2/s) | ||||
| 10th Percentile | 0.71 [0.05, 1.48] | 1.26 [0.77, 1.91] | 0.93 [0.87, 1.2] | 0.001 |
| 90th Percentile | 0.94 [0.15, 2.64] | 1.62 [1.02, 2.16] | 1.12 [1.06, 1.46] | 0.016 |
| Maximum | 1.36 [0.28, 3.39] | 1.71 [1.1, 2.21] | 1.32 [1.2, 1.9] | 0.217 |
| Mean | 0.81 [0.09, 2.08] | 1.42 [0.88, 2.02] | 1.03 [0.97, 1.31] | 0.007 |
| Median | 0.8 [0.07, 2.1] | 1.42 [0.87, 2.02] | 1.03 [0.97, 1.31] | 0.007 |
| Minimum | 0.52 [0, 1.11] | 1.1 [0.51, 1.79] | 0.8 [0.66, 0.94] | <0.001 |
| Range | 0.6 [0.18, 2.88] | 0.45 [0.29, 0.75] | 0.57 [0.38, 1.01] | 0.240 |
| IQR | 0.14 [0.04, 0.87] | 0.14 [0.09, 0.27] | 0.11 [0.07, 0.22] | 0.065 |
| MAD | 0.08 [0.03, 0.46] | 0.08 [0.05, 0.16] | 0.06 [0.05, 0.15] | 0.039 |
| RMAD | 0.06 [0.02, 0.34] | 0.06 [0.04, 0.12] | 0.04 [0.03, 0.1] | 0.056 |
| RMS | 0.81 [0.1, 2.12] | 1.42 [0.88, 2.02] | 1.03 [0.97, 1.31] | 0.011 |
| Entropy | 7.07 [3, 11.12] | 6.5 [4.89, 8.14] | 4.3 [4, 5.35] | <0.001 |
| Energy | 87.49 [3.87, 45685.44] | 276.59 [81.82, 1150.08] | 1.58 [1.22, 2.81] | <0.001 |
| Total Energy | 785.62 [34.72, 410236.65] | 2483.66 [734.74, 10327.23] | 14183.41 [10985.3, 25261.8] | <0.001 |
| Skewness | 0.46 [−1.01, 2.45] | 0.03 [−0.52, 0.56] | 0.22 [−0.54, 1.2] | 0.114 |
| Kurtosis | 3.34 [1.35, 11.62] | 2.55 [1.98, 3.59] | 3.27 [2.51, 5.4] | 0.006 |
| Uniformity | 0.01 [0, 0.17] | 0.01 [0, 0.03] | 0.06 [0.03, 0.08] | <0.001 |
| Variance | 0.01 [0, 0.31] | 0.01 [0, 0.04] | 0.01 [0, 0.04] | 0.034 |
| FA-derived metrics | ||||
| 10th Percentile | 0.071 [0.024, 0.117] | 0.073 [0.030, 0.606] | 0.036 [0.029, 0.049] | <0.001 |
| 90th Percentile | 0.171 [0.139, 0.282] | 0.152 [0.081, 0.788] | 0.091 [0.065, 0.128] | <0.001 |
| Maximum | 0.327 [0.154, 0.907] | 0.239 [0.112, 0.888] | 0.185 [0.100, 0.453] | 0.002 |
| Mean | 0.117 [0.069, 0.183] | 0.112 [0.061, 0.686] | 0.062 [0.048, 0.083] | <0.001 |
| Median | 0.111 [0.051, 0.179] | 0.107 [0.053, 0.679] | 0.056 [0.047, 0.081] | <0.001 |
| Minimum | 0.042 [0.000, 0.087] | 0.042 [0.009, 0.478] | 0.013 [0.006, 0.021] | <0.001 |
| Range | 0.265 [0.093, 0.907] | 0.199 [0.091, 0.410] | 0.170 [0.093, 0.441] | 0.03 |
| IQR | 0.059 [0.024, 0.107] | 0.043 [0.025, 0.106] | 0.026 [0.018, 0.047] | <0.001 |
| MAD | 0.038 [0.016, 0.069] | 0.024 [0.014, 0.056] | 0.017 [0.011, 0.031] | <0.001 |
| RMAD | 0.026 [0.010, 0.046] | 0.018 [0.010, 0.043] | 0.011 [0.007, 0.020] | <0.001 |
| RMS | 0.126 [0.086, 0.193] | 0.117 [0.064, 0.690] | 0.066 [0.050, 0.089] | <0.001 |
| Entropy | 3.431 [2.156, 4.370] | 2.928 [2.182, 6.593] | 2.452 [1.893, 3.051] | <0.001 |
| Energy | 3.353 [0.091, 368.614] | 2.484 [0.458, 60.479] | 6.068 [3.172, 11.431] | 0.008 |
| Total Energy | 30.109 [0.818, 3310.003] | 22.307 [4.115, 543.076] | 54.485 [28.483, 102.642] | 0.010 |
| Skewness | 0.959 [−0.243, 2.079] | 0.513 [−0.139, 1.448] | 1.135 [0.180, 3.864] | 0.041 |
| Kurtosis | 3.751 [1.984, 10.922] | 4.052 [1.962, 6.322] | 5.722 [2.651, 31.354] | 0.02 |
| Uniformity | 0.115 [0.058, 0.250] | 0.156 [0.011, 0.259] | 0.227 [0.134, 0.314] | <0.001 |
| Variance | 0.003 [0.000, 0.008] | 0.001 [0.000, 0.005] | 0.000 [0.000, 0.002] | <0.001 |
| Variable | β [95% CI] | p |
|---|---|---|
| ADC-derived metrics | ||
| 10th Percentile | 16.23 [0.55, 31.92] | 0.042 |
| Range | 56.41 [7.78, 105.05] | 0.023 |
| IQR | −276.99 [−511.81, −42.17] | 0.021 |
| Entropy | −3.62 [−6.83,−0.41] | 0.027 |
| Total energy | 3.52 × 10−4 [2.8 × 10−5, 0.001] | 0.033 |
| Skewness | 6.25 [0.23, 12.27] | 0.042 |
| Kurtosis | −8.96 [−16.92, −1] | 0.033 |
| Uniformity | 19.27 [−82.03, 120.58] | 0.709 |
| FA-derived metrics | ||
| Median | 39.17 [−5.11, 83.46] | 0.083 |
| Range | 55.93 [−34.8, 146.67] | 0.227 |
| Energy | −0.29 [−0.57, −0.01] | 0.039 |
| Skewness | 1.31 [−4.19, 6.82] | 0.640 |
| Kurtosis | 0.01 [−2.29, 2.31] | 0.995 |
| Uniformity | 73.64 [−38.77, 186.04] | 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, M.-V.; Pappa, O.; Astrakas, L.; Lampri, E.; Paliouras, T.; Sofikitis, N.; Argyropoulou, M.I.; Tsili, A.C. Radiomics-Based Detection of Germ Cell Neoplasia In Situ Using Volumetric ADC and FA Histogram Features: A Retrospective Study. Cancers 2025, 17, 3220. https://doi.org/10.3390/cancers17193220
Christodoulou M-V, Pappa O, Astrakas L, Lampri E, Paliouras T, Sofikitis N, Argyropoulou MI, Tsili AC. Radiomics-Based Detection of Germ Cell Neoplasia In Situ Using Volumetric ADC and FA Histogram Features: A Retrospective Study. Cancers. 2025; 17(19):3220. https://doi.org/10.3390/cancers17193220
Chicago/Turabian StyleChristodoulou, Maria-Veatriki, Ourania Pappa, Loukas Astrakas, Evangeli Lampri, Thanos Paliouras, Nikolaos Sofikitis, Maria I. Argyropoulou, and Athina C. Tsili. 2025. "Radiomics-Based Detection of Germ Cell Neoplasia In Situ Using Volumetric ADC and FA Histogram Features: A Retrospective Study" Cancers 17, no. 19: 3220. https://doi.org/10.3390/cancers17193220
APA StyleChristodoulou, M.-V., Pappa, O., Astrakas, L., Lampri, E., Paliouras, T., Sofikitis, N., Argyropoulou, M. I., & Tsili, A. C. (2025). Radiomics-Based Detection of Germ Cell Neoplasia In Situ Using Volumetric ADC and FA Histogram Features: A Retrospective Study. Cancers, 17(19), 3220. https://doi.org/10.3390/cancers17193220






