Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: A Review of Pathogenesis, Diagnosis and Management
Simple Summary
Abstract
1. Introduction
2. Subtypes of Epidermolysis Bullosa
2.1. EB Simplex
2.2. Junctional EB
2.3. Dystrophic EB
2.4. Kindler Syndrome
2.5. Acquired EB
3. Cutaneous Squamous Cell Carcinoma
3.1. Pathophysiology of cSCC
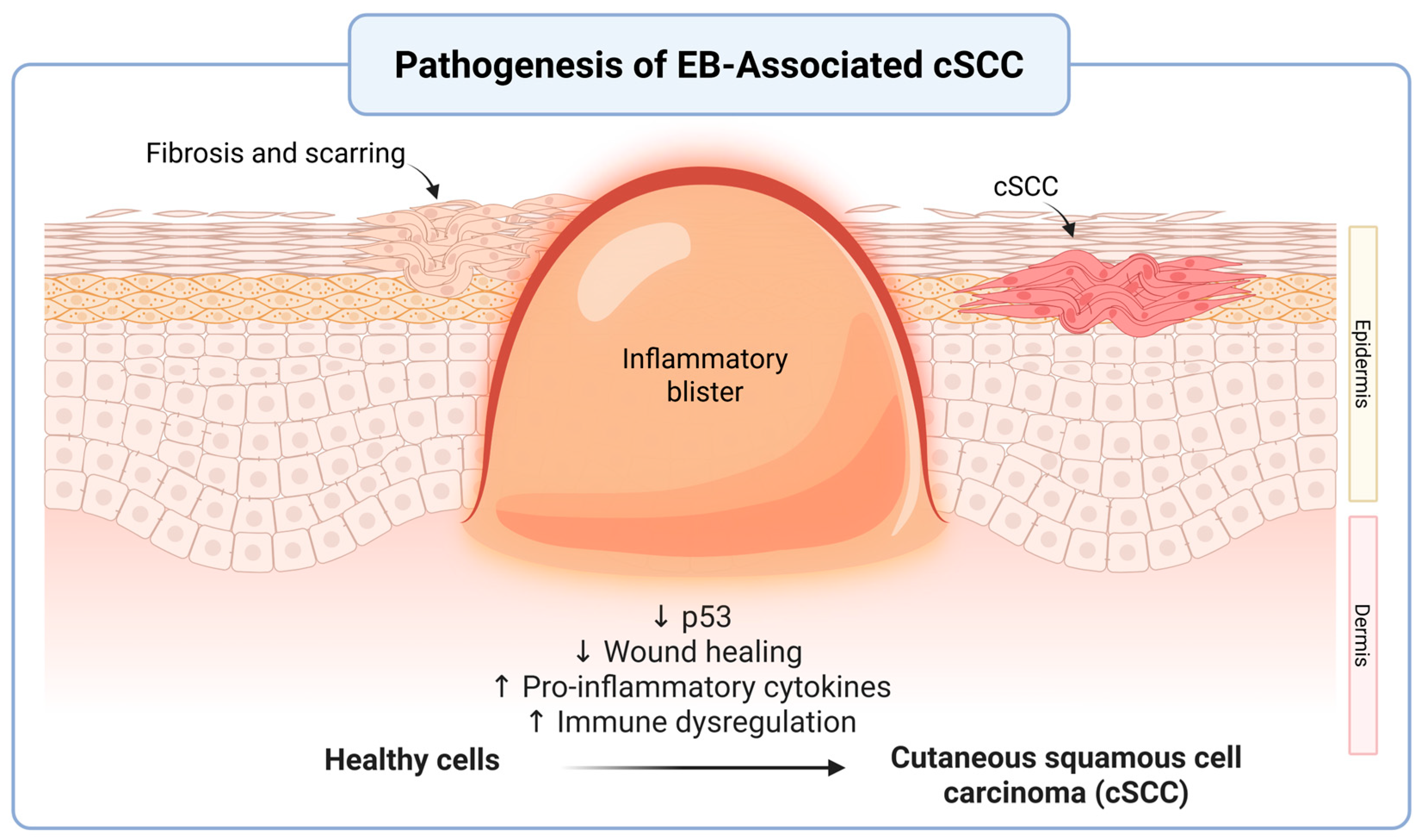
3.2. Pathophysiology of cSCC in EB
3.3. Clinical Presentation of cSCC in EB
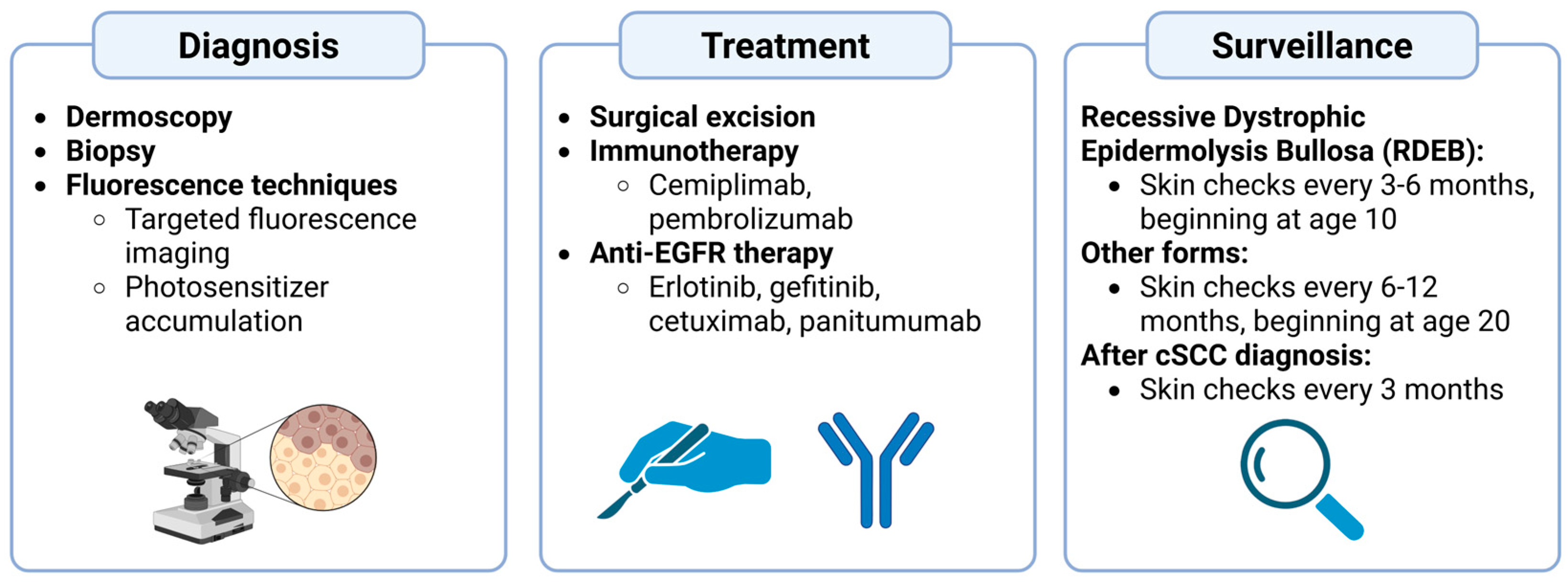
4. Diagnosis of EB-Associated cSCC
5. Management and Treatment
5.1. Surgical Excision
5.2. Immunotherapy
5.3. Anti-EGFR Therapy
5.4. Electrochemotherapy
5.5. Palliative Care
6. Surveillance and Prevention
7. Future Directions and Research Needs
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Khanna, D.; Bardhan, A. Epidermolysis Bullosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- So, J.Y.; Teng, J. Epidermolysis Bullosa Simplex. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Evtushenko, N.A.; Beilin, A.K.; Kosykh, A.V.; Vorotelyak, E.A.; Gurskaya, N.G. Keratins as an Inflammation Trigger Point in Epidermolysis Bullosa Simplex. Int. J. Mol. Sci. 2021, 22, 12446. [Google Scholar] [CrossRef]
- Mariath, L.M.; Santin, J.T.; Schuler-Faccini, L.; Kiszewski, A.E. Inherited Epidermolysis Bullosa: Update on the Clinical and Genetic Aspects. An. Bras. Dermatol. 2020, 95, 551–569. [Google Scholar] [CrossRef]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.R.; et al. A Simplified Laminin Nomenclature. Matrix Biol. J. Int. Soc. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kiritsi, D.; Has, C.; Bruckner-Tuderman, L. Laminin 332 in Junctional Epidermolysis Bullosa. Cell Adhes. Migr. 2013, 7, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mallipeddi, R.; Keane, F.M.; McGrath, J.A.; Mayou, B.J.; Eady, R.A.J. Increased Risk of Squamous Cell Carcinoma in Junctional Epidermolysis Bullosa. J. Eur. Acad. Dermatol. Venereol. JEADV 2004, 18, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Mellerio, J.E.; Kiritsi, D.; Marinkovich, M.P.; Haro, N.R.; Badger, K.; Arora, M.; Dziasko, M.A.; Vithlani, M.; Martinez, A.E. Mapping the Burden of Severe Forms of Epidermolysis Bullosa—Implications for Patient Management. JAAD Int. 2023, 11, 224–232. [Google Scholar] [CrossRef]
- Condorelli, A.G.; Dellambra, E.; Logli, E.; Zambruno, G.; Castiglia, D. Epidermolysis Bullosa-Associated Squamous Cell Carcinoma: From Pathogenesis to Therapeutic Perspectives. Int. J. Mol. Sci. 2019, 20, 5707. [Google Scholar] [CrossRef]
- Alexeev, V.; Huitema, L.; Phillips, T.; Cepeda, R.; de Los Cobos, D.; Perez, R.I.M.; Salas-Garza, M.; Fajardo-Ramirez, O.R.; Ringpfeil, F.; Uitto, J.; et al. T-Cell Activation and Bacterial Infection in Skin Wounds of Recessive Dystrophic Epidermolysis Bullosa Patients. Exp. Dermatol. 2022, 31, 1431–1442. [Google Scholar] [CrossRef]
- Kim, M.; Li, M.; Intong-Wheeler, L.R.A.; Tran, K.; Marucci, D.; Murrell, D.F. Epidemiology and Outcome of Squamous Cell Carcinoma in Epidermolysis Bullosa in Australia and New Zealand. Acta Derm. Venereol. 2018, 98, 70–76. [Google Scholar] [CrossRef]
- Cianfarani, F.; Zambruno, G.; Castiglia, D.; Odorisio, T. Pathomechanisms of Altered Wound Healing in Recessive Dystrophic Epidermolysis Bullosa. Am. J. Pathol. 2017, 187, 1445–1453. [Google Scholar] [CrossRef]
- Martins, V.L.; Vyas, J.J.; Chen, M.; Purdie, K.; Mein, C.A.; South, A.P.; Storey, A.; McGrath, J.A.; O’Toole, E.A. Increased Invasive Behaviour in Cutaneous Squamous Cell Carcinoma with Loss of Basement-Membrane Type VII Collagen. J. Cell Sci. 2009, 122, 1788–1799. [Google Scholar] [CrossRef]
- Shinkuma, S. Dystrophic Epidermolysis Bullosa: A Review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 275–284. [Google Scholar] [CrossRef]
- Michael, M.; Begum, R.; Chan, G.K.; Whitewood, A.J.; Matthews, D.R.; Goult, B.T.; McGrath, J.A.; Parsons, M. Kindlin-1 Regulates Epidermal Growth Factor Receptor Signaling. J. Investig. Dermatol. 2019, 139, 369–379. [Google Scholar] [CrossRef]
- Herz, C.; Aumailley, M.; Schulte, C.; Schlötzer-Schrehardt, U.; Bruckner-Tuderman, L.; Has, C. Kindlin-1 Is a Phosphoprotein Involved in Regulation of Polarity, Proliferation, and Motility of Epidermal Keratinocytes. J. Biol. Chem. 2006, 281, 36082–36090. [Google Scholar] [CrossRef] [PubMed]
- Youssefian, L.; Vahidnezhad, H.; Uitto, J. Kindler Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Kasperkiewicz, M.; Sadik, C.D.; Bieber, K.; Ibrahim, S.M.; Manz, R.A.; Schmidt, E.; Zillikens, D.; Ludwig, R.J. Epidermolysis Bullosa Acquisita: From Pathophysiology to Novel Therapeutic Options. J. Investig. Dermatol. 2016, 136, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Karia, P.S.; Han, J.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma: Estimated Incidence of Disease, Nodal Metastasis, and Deaths from Disease in the United States, 2012. J. Am. Acad. Dermatol. 2013, 68, 957–966. [Google Scholar] [CrossRef]
- Jiang, R.; Fritz, M.; Que, S.K.T. Cutaneous Squamous Cell Carcinoma: An Updated Review. Cancers 2024, 16, 1800. [Google Scholar] [CrossRef] [PubMed]
- Wikonkal, N.M.; Brash, D.E. Ultraviolet Radiation Induced Signature Mutations in Photocarcinogenesis. J. Investig. Dermatol. Symp. Proc. 1999, 4, 6–10. [Google Scholar] [CrossRef]
- Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J.; et al. Guidelines of Care for the Management of Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef]
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of Immunity and Tissue Repair or Regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527. [Google Scholar] [CrossRef]
- Barman, P.K.; Koh, T.J. Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes. Front. Cell Dev. Biol. 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Alexandru, M.; Chen, X.; Mellerio, J.E.; Karagiannis, S.N.; Jacków-Malinowska, J. Unravelling Drivers of Cutaneous Squamous Cell Carcinoma in Recessive Dystrophic Epidermolysis Bullosa. Hum. Immunol. 2024, 85, 110805. [Google Scholar] [CrossRef] [PubMed]
- Odorisio, T.; Di Salvio, M.; Orecchia, A.; Di Zenzo, G.; Piccinni, E.; Cianfarani, F.; Travaglione, A.; Uva, P.; Bellei, B.; Conti, A.; et al. Monozygotic Twins Discordant for Recessive Dystrophic Epidermolysis Bullosa Phenotype Highlight the Role of TGF-β Signalling in Modifying Disease Severity. Hum. Mol. Genet. 2014, 23, 3907–3922. [Google Scholar] [CrossRef] [PubMed]
- Twaroski, K.; Chen, W.; Pickett-Leonard, M.; Tolar, J. Role of Transforming Growth Factor-Β1 in Recessive Dystrophic Epidermolysis Bullosa Squamous Cell Carcinoma. Exp. Dermatol. 2021, 30, 664–675. [Google Scholar] [CrossRef]
- South, A.P.; Laimer, M.; Gueye, M.; Sui, J.Y.; Eichenfield, L.F.; Mellerio, J.E.; Nyström, A. Type VII Collagen Deficiency in the Oncogenesis of Cutaneous Squamous Cell Carcinoma in Dystrophic Epidermolysis Bullosa. J. Investig. Dermatol. 2023, 143, 2108–2119. [Google Scholar] [CrossRef]
- Ng, Y.-Z.; Pourreyron, C.; Salas-Alanis, J.C.; Dayal, J.H.S.; Cepeda-Valdes, R.; Yan, W.; Wright, S.; Chen, M.; Fine, J.-D.; Hogg, F.J.; et al. Fibroblast-Derived Dermal Matrix Drives Development of Aggressive Cutaneous Squamous Cell Carcinoma in Patients with Recessive Dystrophic Epidermolysis Bullosa. Cancer Res. 2012, 72, 3522–3534. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; Moore, K.; Lashari, M.; Nissinen, L.; Kähäri, V.-M.; Alexander, S.; Jones, E.; Harwood, C.A.; Jones, J.; et al. Loss of the Laminin Subunit Alpha-3 Induces Cell Invasion and Macrophage Infiltration in Cutaneous Squamous Cell Carcinoma. Br. J. Dermatol. 2021, 184, 923–934. [Google Scholar] [CrossRef]
- Yuen, W.Y.; Jonkman, M.F. Risk of Squamous Cell Carcinoma in Junctional Epidermolysis Bullosa, Non-Herlitz Type: Report of 7 Cases and a Review of the Literature. J. Am. Acad. Dermatol. 2011, 65, 780–789. [Google Scholar] [CrossRef]
- Nishie, W. Collagen XVII Processing and Blistering Skin Diseases. Acta Derm. Venereol. 2020, 100, adv00054. [Google Scholar] [CrossRef]
- Fine, J.-D.; Johnson, L.B.; Weiner, M.; Li, K.-P.; Suchindran, C. Epidermolysis Bullosa and the Risk of Life-Threatening Cancers: The National EB Registry Experience, 1986-2006. J. Am. Acad. Dermatol. 2009, 60, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Castelo, B.; Viñal, D.; Maseda, R.; Ostios, L.; Sánchez, D.; García-Salvatierra, B.; Escámez, M.J.; Martínez-Santamaría, L.; Del Río, M.; Mora-Rillo, M.; et al. Epidemiology and Natural History of Cutaneous Squamous Cell Carcinoma in Recessive Dystrophic Epidermolysis Bullosa Patients: 20 Years’ Experience of a Reference Centre in Spain. Clin. Transl. Oncol. 2019, 21, 1573–1577. [Google Scholar] [CrossRef]
- Robertson, S.J.; Orrin, E.; Lakhan, M.K.; O’Sullivan, G.; Felton, J.; Robson, A.; Greenblatt, D.T.; Bernardis, C.; McGrath, J.A.; Martinez, A.E.; et al. Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: A 28-Year Retrospective Study. Acta Derm. Venereol. 2021, 101, adv00523. [Google Scholar] [CrossRef]
- Montaudié, H.; Chiaverini, C.; Sbidian, E.; Charlesworth, A.; Lacour, J.-P. Inherited Epidermolysis Bullosa and Squamous Cell Carcinoma: A Systematic Review of 117 Cases. Orphanet J. Rare Dis. 2016, 11, 117. [Google Scholar] [CrossRef]
- Bonamonte, D.; Filoni, A.; De Marco, A.; Lospalluti, L.; Nacchiero, E.; Ronghi, V.; Colagrande, A.; Giudice, G.; Cazzato, G. Squamous Cell Carcinoma in Patients with Inherited Epidermolysis Bullosa: Review of Current Literature. Cells 2022, 11, 1365. [Google Scholar] [CrossRef]
- Mellerio, J.E.; Robertson, S.J.; Bernardis, C.; Diem, A.; Fine, J.D.; George, R.; Goldberg, D.; Halmos, G.B.; Harries, M.; Jonkman, M.F.; et al. Management of Cutaneous Squamous Cell Carcinoma in Patients with Epidermolysis Bullosa: Best Clinical Practice Guidelines. Br. J. Dermatol. 2016, 174, 56–67. [Google Scholar] [CrossRef]
- Tartaglia, G.; Cao, Q.; Padron, Z.M.; South, A.P. Impaired Wound Healing, Fibrosis, and Cancer: The Paradigm of Recessive Dystrophic Epidermolysis Bullosa. Int. J. Mol. Sci. 2021, 22, 5104. [Google Scholar] [CrossRef] [PubMed]
- Larisch, P.; Verwanger, T.; Onder, K.; Krammer, B. In Vitro Analysis of Photosensitizer Accumulation for Assessment of Applicability of Fluorescence Diagnosis of Squamous Cell Carcinoma of Epidermolysis Bullosa Patients. BioMed Res. Int. 2013, 2013, 521281. [Google Scholar] [CrossRef][Green Version]
- Paganelli, A.; Reggiani, C.; Fiorentini, C.; Lando, M.; Cesinaro, A.M.; Magnoni, C. Surgical Management of Squamous Cell Carcinoma Arising in Patients Affected by Epidermolysis Bullosa: A Comparative Study. Int. Wound, J. 2020, 17, 519–521. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.D.R.; Komine, M.; Ohtsuki, M. Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges. Int. J. Mol. Sci. 2022, 23, 8530. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.; Wong, D.; Barrett, A.; Price, H. Successful Use of Immunotherapy to Treat Advanced Cutaneous Squamous Cell Carcinoma in Recessive Dystrophic Epidermolysis Bullosa. BMJ Case Rep. 2021, 14, e238966. [Google Scholar] [CrossRef] [PubMed]
- Rafei-Shamsabadi, D.; Scholten, L.; Lu, S.; Castiglia, D.; Zambruno, G.; Volz, A.; Arnold, A.; Saleva, M.; Martin, L.; Technau-Hafsi, K.; et al. Epidermolysis-Bullosa-Associated Squamous Cell Carcinomas Support an Immunosuppressive Tumor Microenvironment: Prospects for Immunotherapy. Cancers 2024, 16, 471. [Google Scholar] [CrossRef]
- Foote, M.C.; McGrath, M.; Guminski, A.; Hughes, B.G.M.; Meakin, J.; Thomson, D.; Zarate, D.; Simpson, F.; Porceddu, S.V. Phase II Study of Single-Agent Panitumumab in Patients with Incurable Cutaneous Squamous Cell Carcinoma. Ann. Oncol. 2014, 25, 2047–2052. [Google Scholar] [CrossRef]
- Burns, C.; Kubicki, S.; Nguyen, Q.-B.; Aboul-Fettouh, N.; Wilmas, K.M.; Chen, O.M.; Doan, H.Q.; Silapunt, S.; Migden, M.R. Advances in Cutaneous Squamous Cell Carcinoma Management. Cancers 2022, 14, 3653. [Google Scholar] [CrossRef]
- Diociaiuti, A.; Steinke, H.; Nyström, A.; Schwieger-Briel, A.; Meiss, F.; Pfannenberg, C.; Bruckner-Tuderman, L.; Ruf, J.; De Vito, R.; El Hachem, M.; et al. EGFR Inhibition for Metastasized Cutaneous Squamous Cell Carcinoma in Dystrophic Epidermolysis Bullosa. Orphanet J. Rare Dis. 2019, 14, 278. [Google Scholar] [CrossRef]
- Hwang, A.; Kwon, A.; Miller, C.H.; Reimer-Taschenbrecker, A.; Paller, A.S. Therapies for Cutaneous Squamous Cell Carcinoma in Recessive Dystrophic Epidermolysis Bullosa: A Systematic Review of 157 Cases. Orphanet J. Rare Dis. 2024, 19, 206. [Google Scholar] [CrossRef]
- Gothelf, A.; Mir, L.M.; Gehl, J. Electrochemotherapy: Results of Cancer Treatment Using Enhanced Delivery of Bleomycin by Electroporation. Cancer Treat. Rev. 2003, 29, 371–387. [Google Scholar] [CrossRef]
- Denyer, J.; Pillay, E. Best Practice Guidelines for Skin and Wound Care in Epidermolysis Bullosa. International Consensus. DEBRA; Wounds International: London, UK, 2012. [Google Scholar]
- Onoufriadis, A.; Proudfoot, L.E.; Ainali, C.; Torre, D.; Papanikolaou, M.; Rayinda, T.; Rashidghamat, E.; Danarti, R.; Mellerio, J.E.; Ma’ayan, A.; et al. Transcriptomic Profiling of Recessive Dystrophic Epidermolysis Bullosa Wounded Skin Highlights Drug Repurposing Opportunities to Improve Wound Healing. Exp. Dermatol. 2022, 31, 420–426. [Google Scholar] [CrossRef]
- Guide, S.V.; Gonzalez, M.E.; Bağcı, I.S.; Agostini, B.; Chen, H.; Feeney, G.; Steimer, M.; Kapadia, B.; Sridhar, K.; Quesada Sanchez, L.; et al. Trial of Beremagene Geperpavec (B-VEC) for Dystrophic Epidermolysis Bullosa. N. Engl. J. Med. 2022, 387, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Guttmann-Gruber, C.; Tockner, B.; Scharler, C.; Hüttner, C.; Common, J.E.; Tay, A.S.L.; Denil, S.L.I.J.; Klausegger, A.; Trost, A.; Breitenbach, J.; et al. Low-Dose Calcipotriol Can Elicit Wound Closure, Anti-Microbial, and Anti-Neoplastic Effects in Epidermolysis Bullosa Keratinocytes. Sci. Rep. 2018, 8, 13430. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M. The Role of Cathelicidins in the Innate Host Defenses of Mammals. Curr. Issues Mol. Biol. 2005, 7, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Prodinger, C.; Reichelt, J.; Bauer, J.W.; Laimer, M. Epidermolysis Bullosa: Advances in Research and Treatment. Exp. Dermatol. 2019, 28, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Wally, V.; Hovnanian, A.; Ly, J.; Buckova, H.; Brunner, V.; Lettner, T.; Ablinger, M.; Felder, T.K.; Hofbauer, P.; Wolkersdorfer, M.; et al. Diacerein Orphan Drug Development for Epidermolysis Bullosa Simplex: A Phase 2/3 Randomized, Placebo-Controlled, Double-Blind Clinical Trial. J. Am. Acad. Dermatol. 2018, 78, 892–901.e7. [Google Scholar] [CrossRef]
- Retrosi, C.; Diociaiuti, A.; De Ranieri, C.; Corbeddu, M.; Carnevale, C.; Giancristoforo, S.; Marchili, M.R.; Salvatori, G.; Atti, M.L.C.D.; El Hachem, M.; et al. Multidisciplinary Care for Patients with Epidermolysis Bullosa from Birth to Adolescence: Experience of One Italian Reference Center. Ital. J. Pediatr. 2022, 48, 58. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, A.; Moser, J.C. Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: A Review of Pathogenesis, Diagnosis and Management. Cancers 2025, 17, 3211. https://doi.org/10.3390/cancers17193211
Chandrasekaran A, Moser JC. Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: A Review of Pathogenesis, Diagnosis and Management. Cancers. 2025; 17(19):3211. https://doi.org/10.3390/cancers17193211
Chicago/Turabian StyleChandrasekaran, Abarajithan, and Justin C. Moser. 2025. "Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: A Review of Pathogenesis, Diagnosis and Management" Cancers 17, no. 19: 3211. https://doi.org/10.3390/cancers17193211
APA StyleChandrasekaran, A., & Moser, J. C. (2025). Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: A Review of Pathogenesis, Diagnosis and Management. Cancers, 17(19), 3211. https://doi.org/10.3390/cancers17193211





