VSIG4 Is Dispensable for Tumor Growth and Metastasis in Murine Colorectal and Breast Cancer Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Cell Culture and Tumor Models
2.2. Ex Vivo Preparation of Single-Cell Suspensions
2.3. Flow Cytometry
2.4. Sandwich ELISA
2.5. SDS-PAGE
2.6. Western Blot
2.7. In Silico Analysis Human CRC and TNBC: scRNA-Sequencing Dataset and Correlation Analysis
2.8. Statistics
3. Results
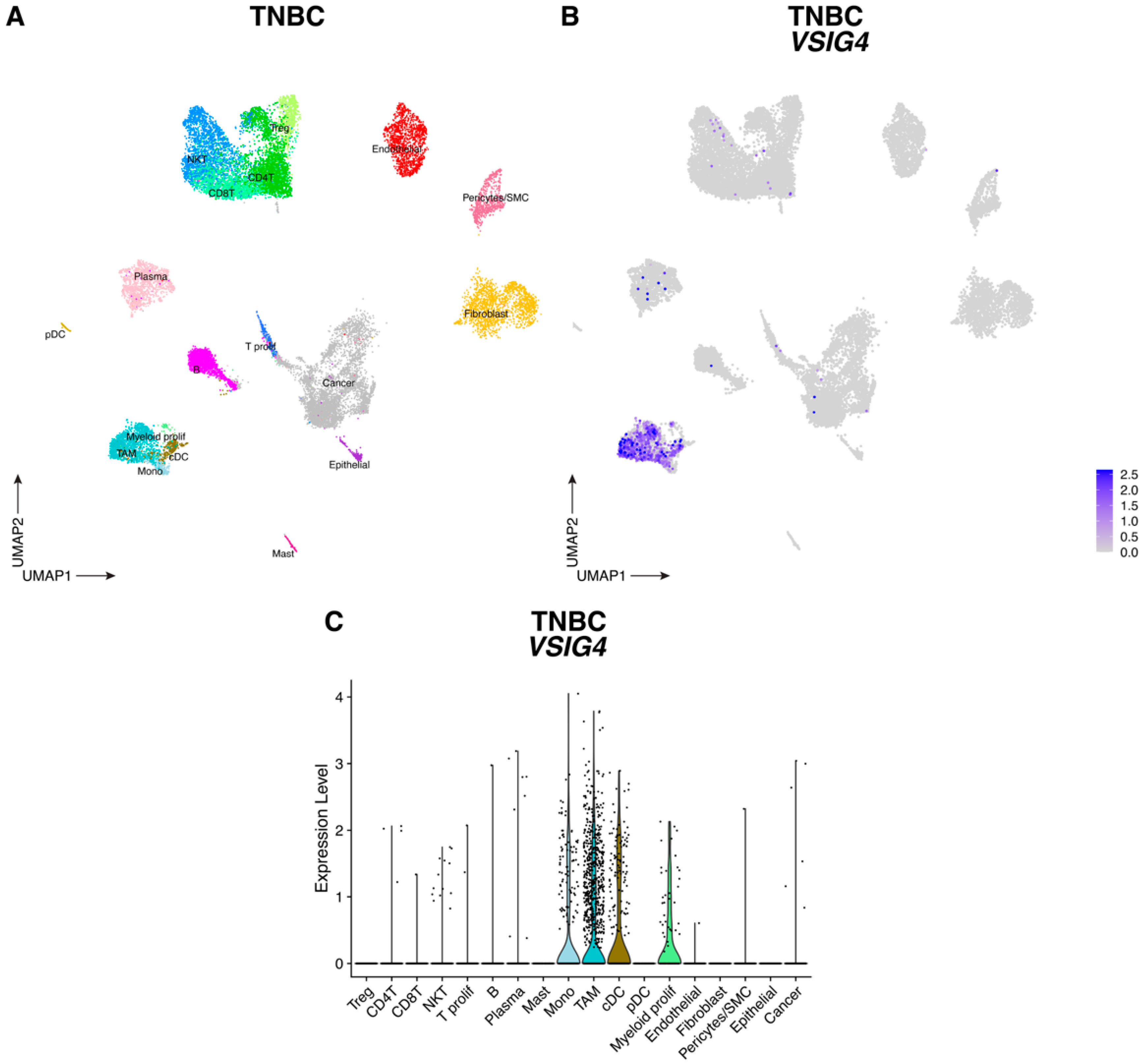
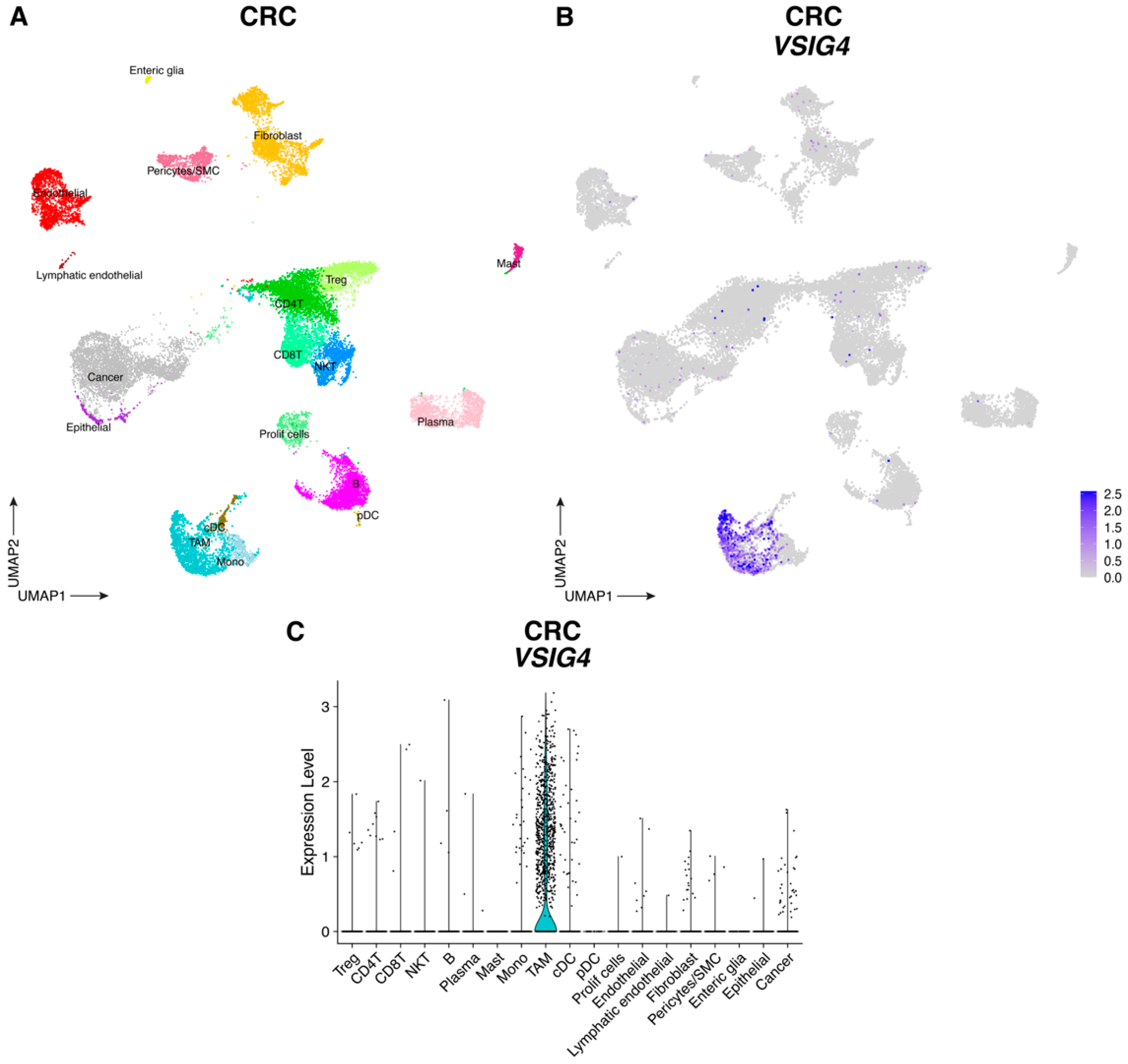
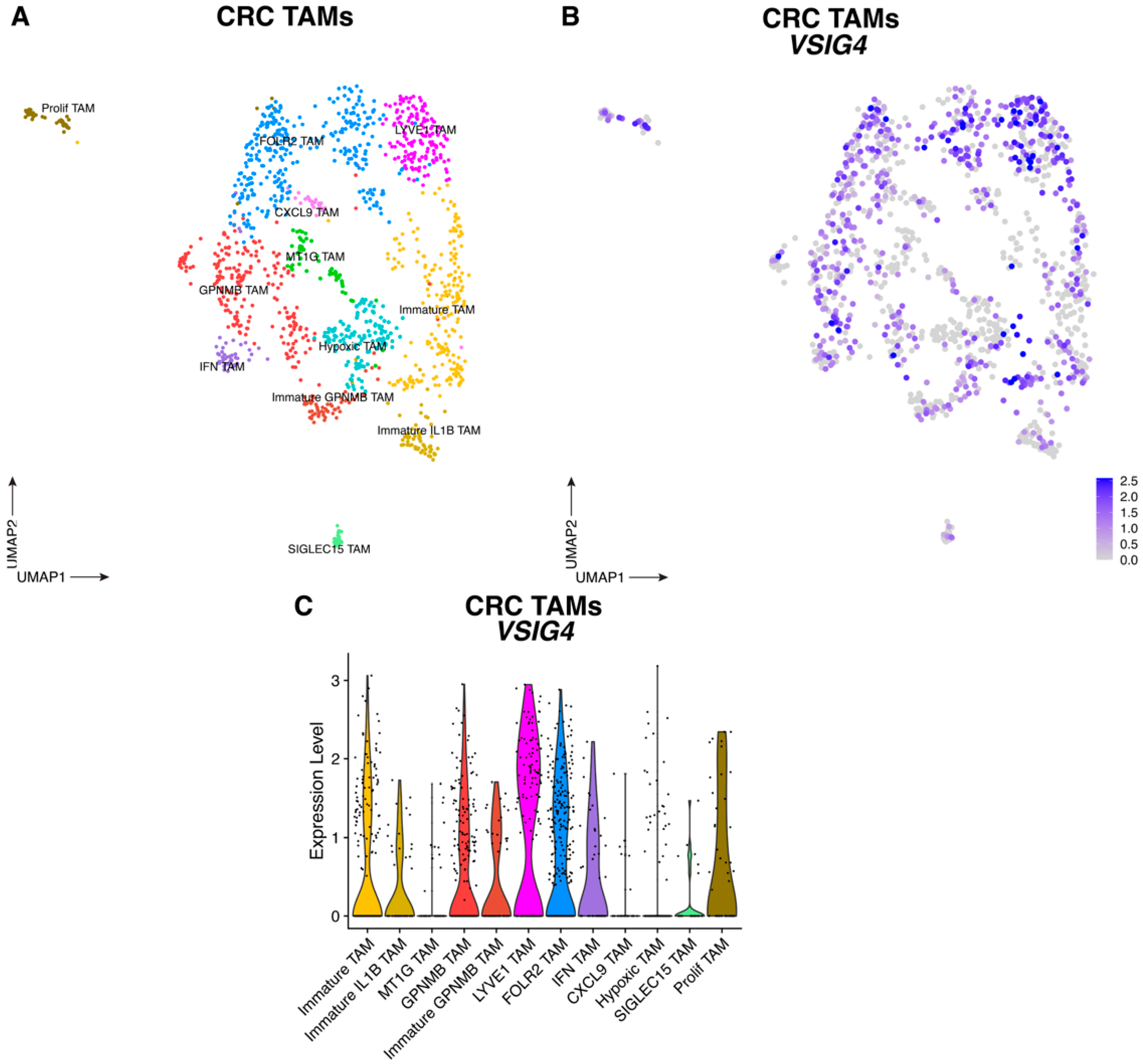
3.1. VSIG4 Is Expressed by Human TNBC and CRC TAM Subsets and Correlates with a Worse Prognosis
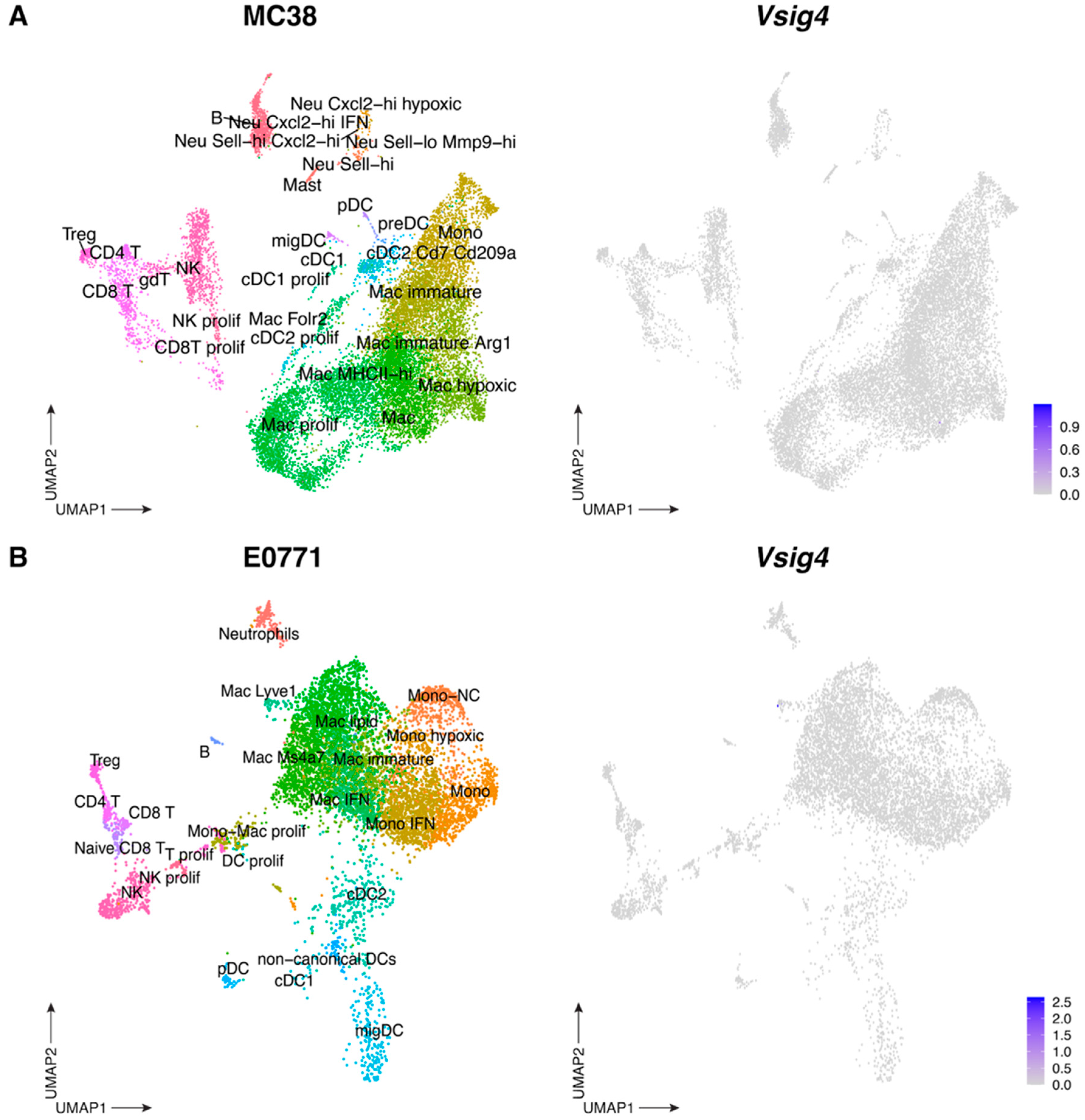
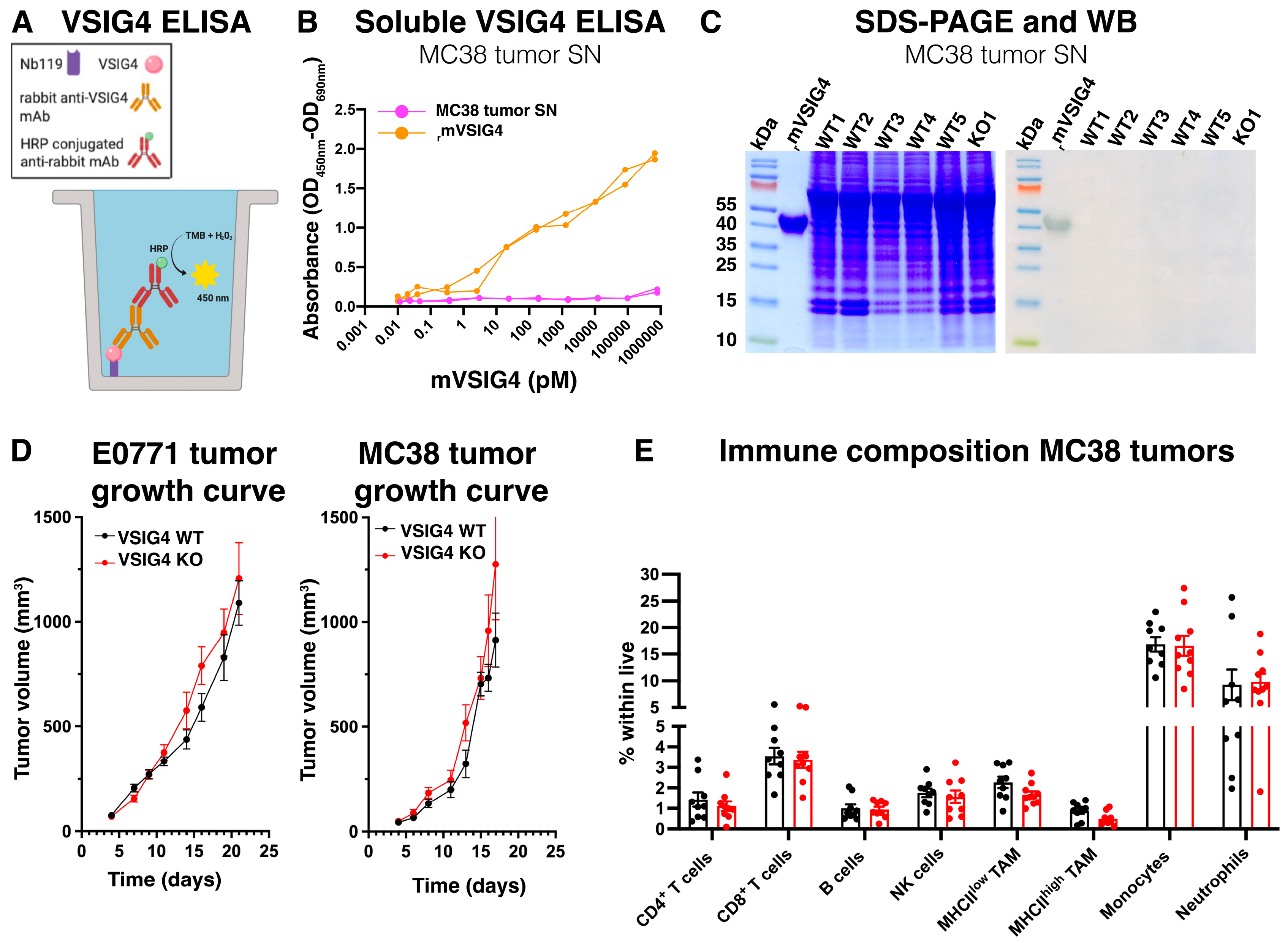
3.2. The VSIG4 Protein Is Absent from Mouse Primary Tumors and, Consequently, Does Not Influence Tumor Growth
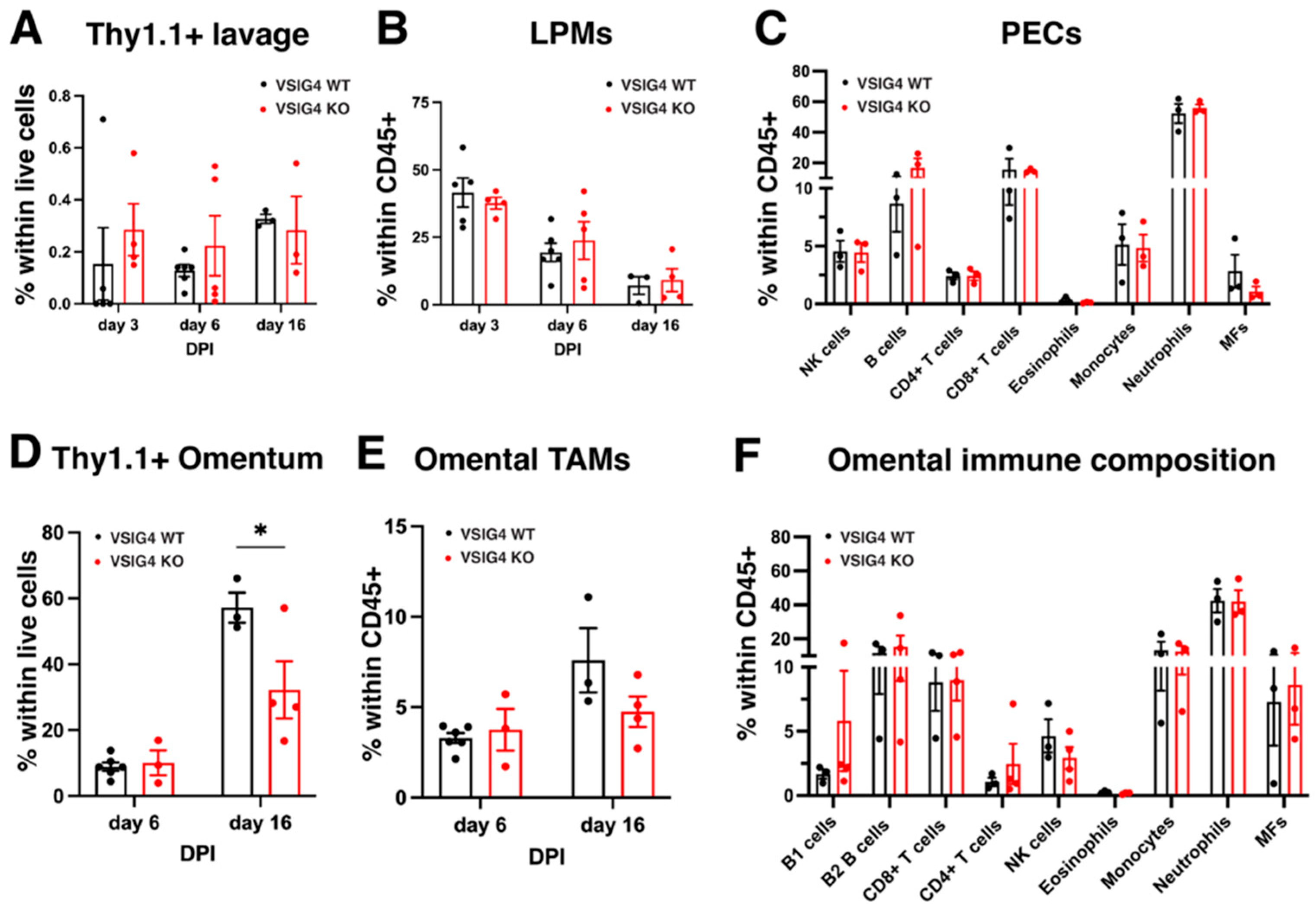
3.3. The VSIG4 Receptor Does Not Play a Role in Metastatic Growth of Cancer Cells in the Peritoneal Cavity and Liver but Could Play a Role in the Omentum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagasaki, J.; Ishino, T.; Togashi, Y. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Cancer Sci. 2022, 113, 3303–3312. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Wang, J.; Si, T.; Xing, W. Tumor-Associated Macrophage-Derived Transforming Growth Factor-β Promotes Colorectal Cancer Progression through HIF1-TRIB3 Signaling. Cancer Sci. 2021, 112, 4198–4207. [Google Scholar] [CrossRef]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between Cancer Cells and Tumor Associated Macrophages Is Required for Mesenchymal Circulating Tumor Cell-Mediated Colorectal Cancer Metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Yuan, Z.-Y.; Luo, R.-Z.; Peng, R.-J.; Wang, S.-S.; Xue, C. High Infiltration of Tumor-Associated Macrophages in Triple-Negative Breast Cancer Is Associated with a Higher Risk of Distant Metastasis. OncoTargets Ther. 2014, 7, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-Cell Analyses Reveal Key Immune Cell Subsets Associated with Response to PD-L1 Blockade in Triple-Negative Breast Cancer. Cancer Cell 2021, 39, 1578–1593.e8. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, H.; Zhang, X.; Zhao, H.; Xiao, Y.; Li, N.; Yao, Y.; Xing, X. Disruption of GPSM1/CSF1 Signaling Reprograms Tumor-Associated Macrophages to Overcome Anti-PD-1 Resistance in Colorectal Cancer. J. Immunother. Cancer 2025, 13, e010826. [Google Scholar] [CrossRef]
- Vogt, L.; Schmitz, N.; Kurrer, M.O.; Bauer, M.; Hinton, H.I.; Behnke, S.; Gatto, D.; Sebbel, P.; Beerli, R.R.; Sonderegger, I.; et al. VSIG4, a B7 Family-Related Protein, Is a Negative Regulator of T Cell Activation. J. Clin. Investig. 2006, 116, 2817–2826. [Google Scholar] [CrossRef]
- Helmy, K.Y.; Katschke, K.J.; Gorgani, N.N.; Kljavin, N.M.; Elliott, J.M.; Diehl, L.; Scales, S.J.; Ghilardi, N.; Van Lookeren Campagne, M. CRIg: A Macrophage Complement Receptor Required for Phagocytosis of Circulating Pathogens. Cell 2006, 124, 915–927. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, L.; Gao, H.; Zhang, J.; Dong, Y.; Gao, W.; Yuan, S.; Gong, T.; Huang, W. The Biology of VSIG4: Implications for the Treatment of Immune-Mediated Inflammatory Diseases and Cancer. Cancer Lett. 2023, 553, 215996. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fu, Y.; Yosri, M.; Chen, Y.; Sun, P.; Xu, J.; Zhang, M.; Sun, D.; Strickland, A.B.; Mackey, Z.B.; et al. CRIg Plays an Essential Role in Intravascular Clearance of Bloodborne Parasites by Interacting with Complement. Proc. Natl. Acad. Sci. USA 2019, 116, 24214–24220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Surewaard, B.G.J.; Wong, C.H.Y.; Geoghegan, J.A.; Jenne, C.N.; Kubes, P. CRIg Functions as a Macrophage Pattern Recognition Receptor to Directly Bind and Capture Blood-Borne Gram-Positive Bacteria. Cell Host Microbe 2016, 20, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, C.; Katschke, K.J.; Yin, J.; Helmy, K.Y.; Steffek, M.; Fairbrother, W.J.; McCallum, S.A.; Embuscado, L.; DeForge, L.; Hass, P.E.; et al. Structure of C3b in Complex with CRIg Gives Insights into Regulation of Complement Activation. Nature 2006, 444, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Diao, B.; Guo, S.; Huang, X.; Yang, C.; Feng, Z.; Yan, W.; Ning, Q.; Zheng, L.; Chen, Y.; et al. VSIG4 Inhibits Proinflammatory Macrophage Activation by Reprogramming Mitochondrial Pyruvate Metabolism. Nat. Commun. 2017, 8, 1322. [Google Scholar] [CrossRef]
- Widyagarini, A.; Nishii, N.; Kawano, Y.; Zhang, C.; Azuma, M. VSIG4/CRIg Directly Regulates Early CD8+ T Cell Activation through Its Counter-Receptor in a Narrow Window. Biochem. Biophys. Res. Commun. 2022, 614, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Yao, W.R.; Zhou, Z.Y.; Liu, L. The Immunosuppressive Role of VSIG4 in Colorectal Cancer and Its Interaction with the Tumor Microenvironment. Discov. Oncol. 2025, 16, 664. [Google Scholar] [CrossRef]
- Kim, S.-W.; Roh, J.; Lee, H.S.; Ryu, M.-H.; Park, Y.-S.; Park, C.-S. Expression of the Immune Checkpoint Molecule V-Set Immunoglobulin Domain-Containing 4 Is Associated with Poor Prognosis in Patients with Advanced Gastric Cancer. Gastric Cancer 2021, 24, 327–340. [Google Scholar] [CrossRef]
- Roh, J.; Jeon, Y.; Lee, A.-N.; Lee, S.M.; Kim, Y.; Sung, C.O.; Park, C.-J.; Hong, J.Y.; Yoon, D.H.; Suh, C.; et al. The Immune Checkpoint Molecule V-Set Ig Domain-Containing 4 Is an Independent Prognostic Factor for Multiple Myeloma. Oncotarget 2017, 8, 58122–58132. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Y.; Luo, H.; Jiang, Z.; Qiao, B.; Jiang, Y.; Hu, Y.; Cheng, Y.; Chen, X.; Gong, W.; et al. Serum Soluble VSIG4 as a Surrogate Marker for the Diagnosis of Lymphoma-Associated Hemophagocytic Lymphohistiocytosis. Br. J. Haematol. 2020, 189, 72–83. [Google Scholar] [CrossRef]
- Zhu, S.; Tan, W.; Li, W.; Zhou, R.; Wu, X.; Chen, X.; Li, W.; Shang, C.; Chen, Y. Low Expression of VSIG4 Is Associated with Poor Prognosis in Hepatocellular Carcinoma Patients with Hepatitis B Infection. Cancer Manag. Res. 2018, 10, 3697–3705. [Google Scholar] [CrossRef]
- Byun, J.M.; Jeong, D.H.; Choi, I.H.; Lee, D.S.; Kang, M.S.; Jung, K.O.; Jeon, Y.K.; Kim, Y.N.; Jung, E.J.; Lee, K.B.; et al. The Significance of VSIG4 Expression in Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 872–878. [Google Scholar] [CrossRef]
- Lebegge, E.; Kancheva, D.; Van Craenenbroeck, J.; Ernst, S.; Bardet, P.M.R.; Caro, A.A.; Kiss, M.; Jumapili, N.A.; Barthelmess, R.M.; Zivalj, M.; et al. VSIG4-Expressing Macrophages Contribute to Antiparasitic and Antimetastatic Responses in the Peritoneal Cavity. Eur. J. Immunol. 2025, 55, e202551804. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Ernst, M.; Poh, A.R. An Intrasplenic Injection Model of Pancreatic Cancer Metastasis to the Liver in Mice. STAR Protoc. 2023, 4, 102021. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, X.; Fernández-Garcia, J.; Hartmann, F.J.; de Goede, K.E.; Martens, L.; Elkrim, Y.; Debraekeleer, A.; Stijlemans, B.; Vandekeere, A.; Rinaldi, G.; et al. Macrophages Are Metabolically Heterogeneous within the Tumor Microenvironment. Cell Rep. 2021, 37, 110171. [Google Scholar] [CrossRef]
- Musrati, M.A.; Stijlemans, B.; Azouz, A.; Kancheva, D.; Mesbahi, S.; Hadadi, E.; Lebegge, E.; Ali, L.; De Vlaminck, K.; Scheyltjens, I.; et al. Infection History Imprints Prolonged Changes to the Epigenome, Transcriptome and Function of Kupffer Cells. J. Hepatol. 2024, 81, 1023–1039. [Google Scholar] [CrossRef]
- Zheng, F.; Sparkes, A.; De Baetselier, P.; Schoonooghe, S.; Stijlemans, B.; Muyldermans, S.; Flamand, V.; Van Ginderachter, J.A.; Devoogdt, N.; Raes, G.; et al. Molecular Imaging with Kupffer Cell-Targeting Nanobodies for Diagnosis and Prognosis in Mouse Models of Liver Pathogenesis. Mol. Imaging Biol. 2017, 19, 49–58. [Google Scholar] [CrossRef]
- Vincke, C.; Gutiérrez, C.; Wernery, U.; Devoogdt, N.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S. Generation of Single Domain Antibody Fragments Derived from Camelids and Generation of Manifold Constructs. Methods Mol. Biol. 2012, 907, 145–176. [Google Scholar] [CrossRef]
- Qian, J.; Olbrecht, S.; Boeckx, B.; Vos, H.; Laoui, D.; Etlioglu, E.; Wauters, E.; Pomella, V.; Verbandt, S.; Busschaert, P.; et al. A Pan-Cancer Blueprint of the Heterogeneous Tumor Microenvironment Revealed by Single-Cell Profiling. Cell Res. 2020, 30, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Essalihi, A.; Bouchra, O.; Khadiri, K.; Khadrouf, Z.; Karkouri, M. Immunotherapy for Triple-Negative Breast Cancer: Current Trends and Future Prospects. J. Egypt. Natl. Cancer Inst. 2025, 37, 51. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, X.; Chen, G.; Du, J. How to Overcome Resistance to Immune Checkpoint Inhibitors in Colorectal Cancer: From Mechanisms to Translation. Int. J. Cancer 2023, 153, 709–722. [Google Scholar] [CrossRef]
- Hamouda, A.E.I.; Filtjens, J.; Brabants, E.; Kancheva, D.; Debraekeleer, A.; Brughmans, J.; Jacobs, L.; Bardet, P.M.R.; Knetemann, E.; Lefesvre, P.; et al. Intratumoral Delivery of Lipid Nanoparticle-Formulated mRNA Encoding IL-21, IL-7, and 4-1BBL Induces Systemic Anti-Tumor Immunity. Nat. Commun. 2024, 15, 10635. [Google Scholar] [CrossRef]
- Clappaert, E.J.; Kancheva, D.; Brughmans, J.; Debraekeleer, A.; Bardet, P.M.R.; Elkrim, Y.; Lacroix, D.; Živalj, M.; Hamouda, A.E.I.; Van Ginderachter, J.A.; et al. Flt3L Therapy Increases the Abundance of Treg-Promoting CCR7+ cDCs in Preclinical Cancer Models. Front. Immunol. 2023, 14, 1166180. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.M.; Banh, X.; Gadd, V.L.; Wojcik, K.K.; Ariffin, J.K.; Jose, S.; Lukowski, S.; Baillie, G.J.; Sweet, M.J.; Powell, E.E. CRIg-Expressing Peritoneal Macrophages Are Associated with Disease Severity in Patients with Cirrhosis and Ascites. JCI Insight 2016, 1, e86914. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Yang, Y.G.; Jeon, Y.; Lee, C.-K.; Choi, I.; Lee, S.-W. VSIG4(+) Peritoneal Macrophages Induce Apoptosis of Double-Positive Thymocyte via the Secretion of TNF-α in a CLP-Induced Sepsis Model Resulting in Thymic Atrophy. Cell Death Dis. 2021, 12, 526. [Google Scholar] [CrossRef]
- Jeon, G.; Lee, D.S.; Byun, J.M.; Kim, K.T.; Jeong, D.H. Immunoregulatory Protein V-set and Immunoglobulin Domain-containing 4 Is Overexpressed in Patients with Endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 119–127. [Google Scholar] [CrossRef]
- Reißing, J.; Lutz, P.; Frissen, M.; Ibidapo-Obe, O.; Reuken, P.A.; Wirtz, T.H.; Stengel, S.; Quickert, S.; Rooney, M.; Große, K.; et al. Immunomodulatory Receptor VSIG4 Is Released during Spontaneous Bacterial Peritonitis and Predicts Short-Term Mortality. JHEP Rep. Innov. Hepatol. 2022, 4, 100391. [Google Scholar] [CrossRef]
- Zheng, F.; Put, S.; Bouwens, L.; Lahoutte, T.; Matthys, P.; Muyldermans, S.; De Baetselier, P.; Devoogdt, N.; Raes, G.; Schoonooghe, S. Molecular Imaging with Macrophage CRIg-Targeting Nanobodies for Early and Preclinical Diagnosis in a Mouse Model of Rheumatoid Arthritis. J. Nucl. Med. 2014, 55, 824–829. [Google Scholar] [CrossRef]
- Bayón, L.G.; Izquierdo, M.A.; Sirovich, I.; van Rooijen, N.; Beelen, R.H.; Meijer, S. Role of Kupffer Cells in Arresting Circulating Tumor Cells and Controlling Metastatic Growth in the Liver. Hepatology 1996, 23, 1224–1231. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Yao, Q.; Chen, C.; Zhang, Q.; Ding, K.; Li, L.; Zeng, Z. In Situ Expansion and Reprogramming of Kupffer Cells Elicit Potent Tumoricidal Immunity against Liver Metastasis. J. Clin. Investig. 2023, 133, e157937. [Google Scholar] [CrossRef]
- Song, H.; Wang, T.; Tian, L.; Bai, S.; Chen, L.; Zuo, Y.; Xue, Y. Macrophages on the Peritoneum Are Involved in Gastric Cancer Peritoneal Metastasis. J. Cancer 2019, 10, 5377–5387. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Guiral, D.; Hübner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and Metastatic Peritoneal Surface Malignancies. Nat. Rev. Dis. Primer 2021, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Moulin, M.; Doktor, T.K.; Delfini, M.; Mossadegh-Keller, N.; Bajenoff, M.; Sieweke, M.H.; Moestrup, S.K.; Auphan-Anezin, N.; Lawrence, T. Tissue-Resident Macrophages in Omentum Promote Metastatic Spread of Ovarian Cancer. J. Exp. Med. 2020, 217, e20191869. [Google Scholar] [CrossRef]
- Wijffels, J.F.A.M.; Hendrickx, R.J.B.M.; Steenbergen, J.J.E.; Eestermans, I.L.; Beelen, R.H.J. Milky Spots in the Mouse Omentum May Play an Important Role in the Origin of Peritoneal Macrophages. Res. Immunol. 1992, 143, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Meza-Perez, S.; Randall, T.D. Immunological Functions of the Omentum. Trends Immunol. 2017, 38, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Langnaese, K.; Colleaux, L.; Kloos, D.U.; Fontes, M.; Wieacker, P. Cloning of Z39Ig, a Novel Gene with Immunoglobulin-like Domains Located on Human Chromosome X. Biochim. Biophys. Acta 2000, 1492, 522–525. [Google Scholar] [CrossRef]
- Sazinsky, S.; Zafari, M.; Klebanov, B.; Ritter, J.; Nguyen, P.A.; Phennicie, R.T.; Wahle, J.; Kauffman, K.J.; Razlog, M.; Manfra, D.; et al. Antibodies Targeting Human or Mouse VSIG4 Repolarize Tumor-Associated Macrophages Providing the Potential of Potent and Specific Clinical Anti-Tumor Response Induced across Multiple Cancer Types. Int. J. Mol. Sci. 2024, 25, 6160. [Google Scholar] [CrossRef] [PubMed]
- Louwe, P.A.; Badiola Gomez, L.; Webster, H.; Perona-Wright, G.; Bain, C.C.; Forbes, S.J.; Jenkins, S.J. Recruited Macrophages That Colonize the Post-Inflammatory Peritoneal Niche Convert into Functionally Divergent Resident Cells. Nat. Commun. 2021, 12, 1770. [Google Scholar] [CrossRef]
- Gorgani, N.N.; Thathaisong, U.; Mukaro, V.R.S.; Poungpair, O.; Tirimacco, A.; Hii, C.S.T.; Ferrante, A. Regulation of CRIg Expression and Phagocytosis in Human Macrophages by Arachidonate, Dexamethasone, and Cytokines. Am. J. Pathol. 2011, 179, 1310–1318. [Google Scholar] [CrossRef]
- Van Lookeren Campagne, M.; Wiesmann, C.; Brown, E.J. Macrophage Complement Receptors and Pathogen Clearance. Cell. Microbiol. 2007, 9, 2095–2102. [Google Scholar] [CrossRef]
- Zhao, D.; Yue, P.; Wang, T.; Wang, P.; Song, Q.; Wang, J.; Jiao, Y. Personalized Analysis of Minimal Residual Cancer Cells in Peritoneal Lavage Fluid Predicts Peritoneal Dissemination of Gastric Cancer. J. Hematol. Oncol. 2021, 14, 164. [Google Scholar] [CrossRef]
- Iwagoi, Y.; Motohara, T.; Hwang, S.; Fujimoto, K.; Ikeda, T.; Katabuchi, H. Omental Metastasis as a Predictive Risk Factor for Unfavorable Prognosis in Patients with Stage III–IV Epithelial Ovarian Cancer. Int. J. Clin. Oncol. 2021, 26, 995–1004. [Google Scholar] [CrossRef]
- Chong, Y.Y.; Thiagarajan, S.; Tan, Q.X.; Lim, H.J.; Tan, J.W.-S.; Hendrikson, J.; Ng, G.; Liu, Y.; Chong, C.Y.L.; Guo, W.; et al. The Immunomodulatory Role of Paracrine Signalling Factor VSIG4 in Peritoneal Metastases. Sci. Rep. 2024, 14, 17522. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebegge, E.; Jumapili, N.A.; Van Craenenbroeck, J.; Kancheva, D.; Kiss, M.; Mora Barthelmess, R.; Hamouda, A.E.I.; Elkrim, Y.; Raes, G.; Hadadi, É.; et al. VSIG4 Is Dispensable for Tumor Growth and Metastasis in Murine Colorectal and Breast Cancer Models. Cancers 2025, 17, 3207. https://doi.org/10.3390/cancers17193207
Lebegge E, Jumapili NA, Van Craenenbroeck J, Kancheva D, Kiss M, Mora Barthelmess R, Hamouda AEI, Elkrim Y, Raes G, Hadadi É, et al. VSIG4 Is Dispensable for Tumor Growth and Metastasis in Murine Colorectal and Breast Cancer Models. Cancers. 2025; 17(19):3207. https://doi.org/10.3390/cancers17193207
Chicago/Turabian StyleLebegge, Els, Neema Ahishakiye Jumapili, Jolien Van Craenenbroeck, Daliya Kancheva, Máté Kiss, Romina Mora Barthelmess, Ahmed E. I. Hamouda, Yvon Elkrim, Geert Raes, Éva Hadadi, and et al. 2025. "VSIG4 Is Dispensable for Tumor Growth and Metastasis in Murine Colorectal and Breast Cancer Models" Cancers 17, no. 19: 3207. https://doi.org/10.3390/cancers17193207
APA StyleLebegge, E., Jumapili, N. A., Van Craenenbroeck, J., Kancheva, D., Kiss, M., Mora Barthelmess, R., Hamouda, A. E. I., Elkrim, Y., Raes, G., Hadadi, É., Laoui, D., Van Ginderachter, J. A., & Arnouk, S. M. (2025). VSIG4 Is Dispensable for Tumor Growth and Metastasis in Murine Colorectal and Breast Cancer Models. Cancers, 17(19), 3207. https://doi.org/10.3390/cancers17193207




