Quercetin Increases Expression of Membrane-TRAIL in Glioblastoma Cells Resulting in Apoptosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Cell Culture
2.3. Western Blot Analysis for Markers of Apoptosis
2.4. FITC-Annexin V Flow Cytometry Apoptosis Assay
2.5. Flow Cytometry Analysis of Membrane DR Expression
2.6. Western Blot Analysis for Membrane-TRAIL Expression
2.7. Flow Cytometry Analysis of Membrane-TRAIL Expression
2.8. Double-Label Immunofluorescence
2.9. Statistical Analysis
3. Results
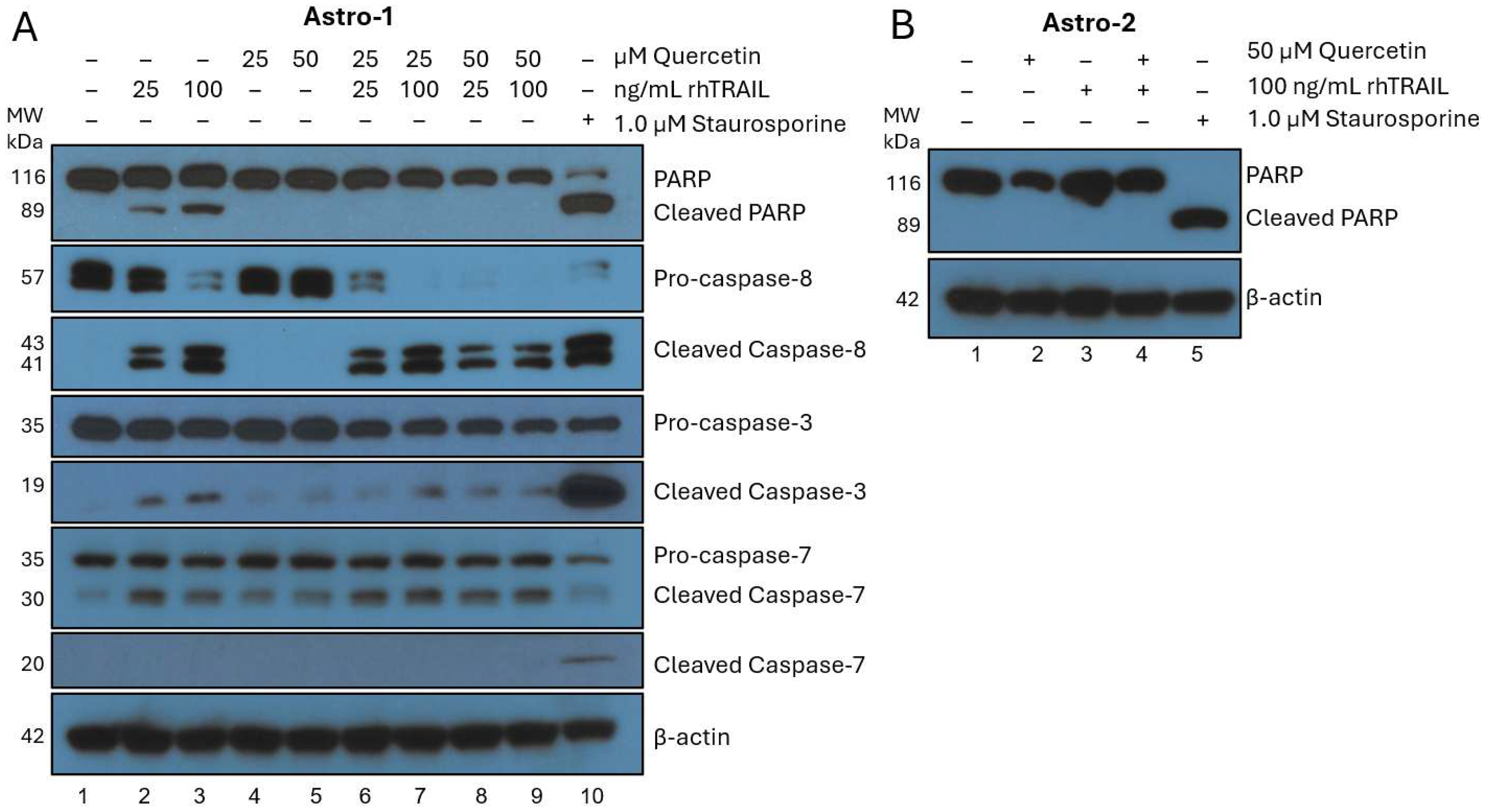
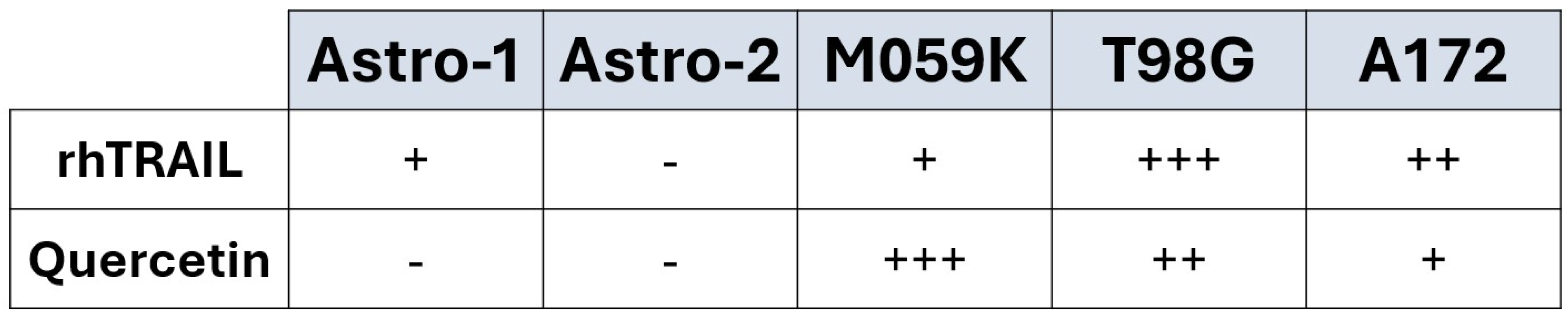
3.1. Quercetin Alone Does Not Induce Apoptosis in Normal Astrocytes
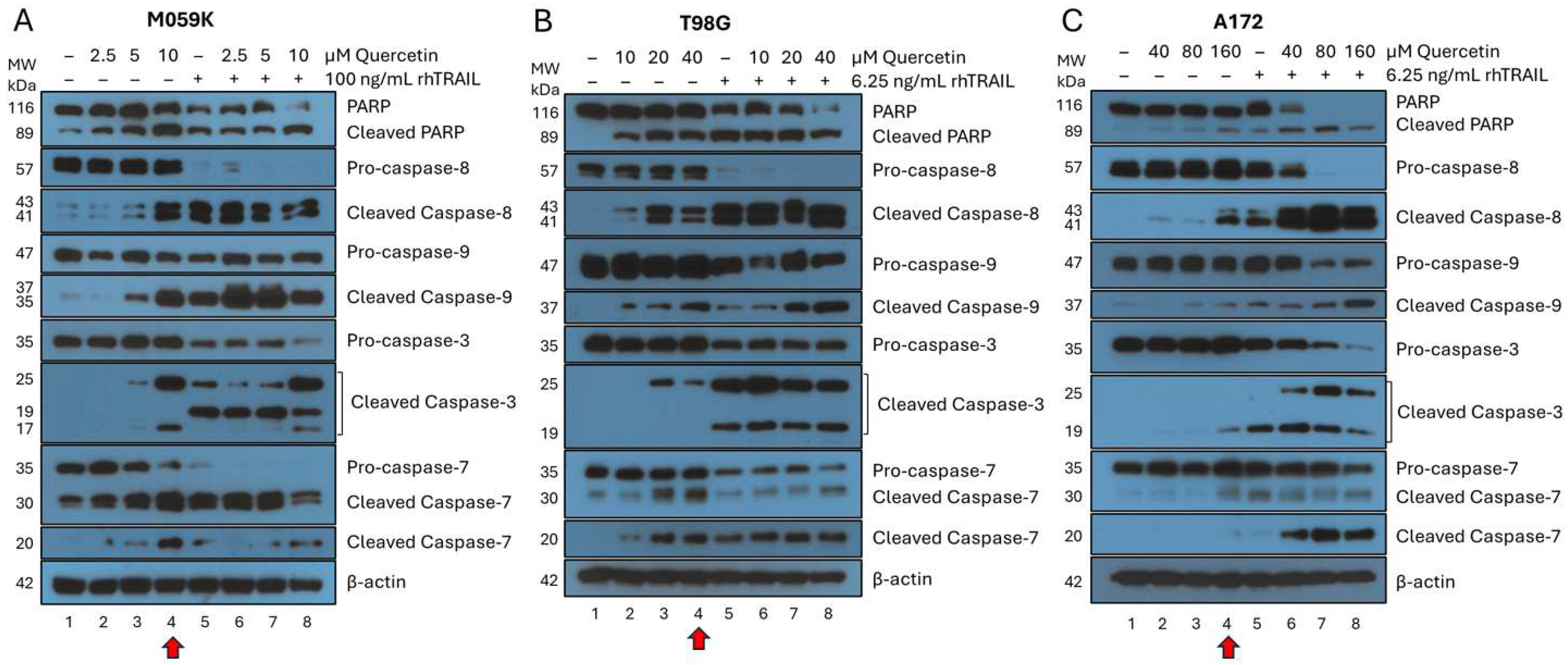
3.2. Treatment of Cancer Cells with Quercetin Alone Induces Cell Death Through Both the Intrinsic and Extrinsic Pathways of Apoptosis
3.3. Cotreatment Substantially Increases the Percentage of Cells Undergoing Apoptosis in All GBM Cell Lines
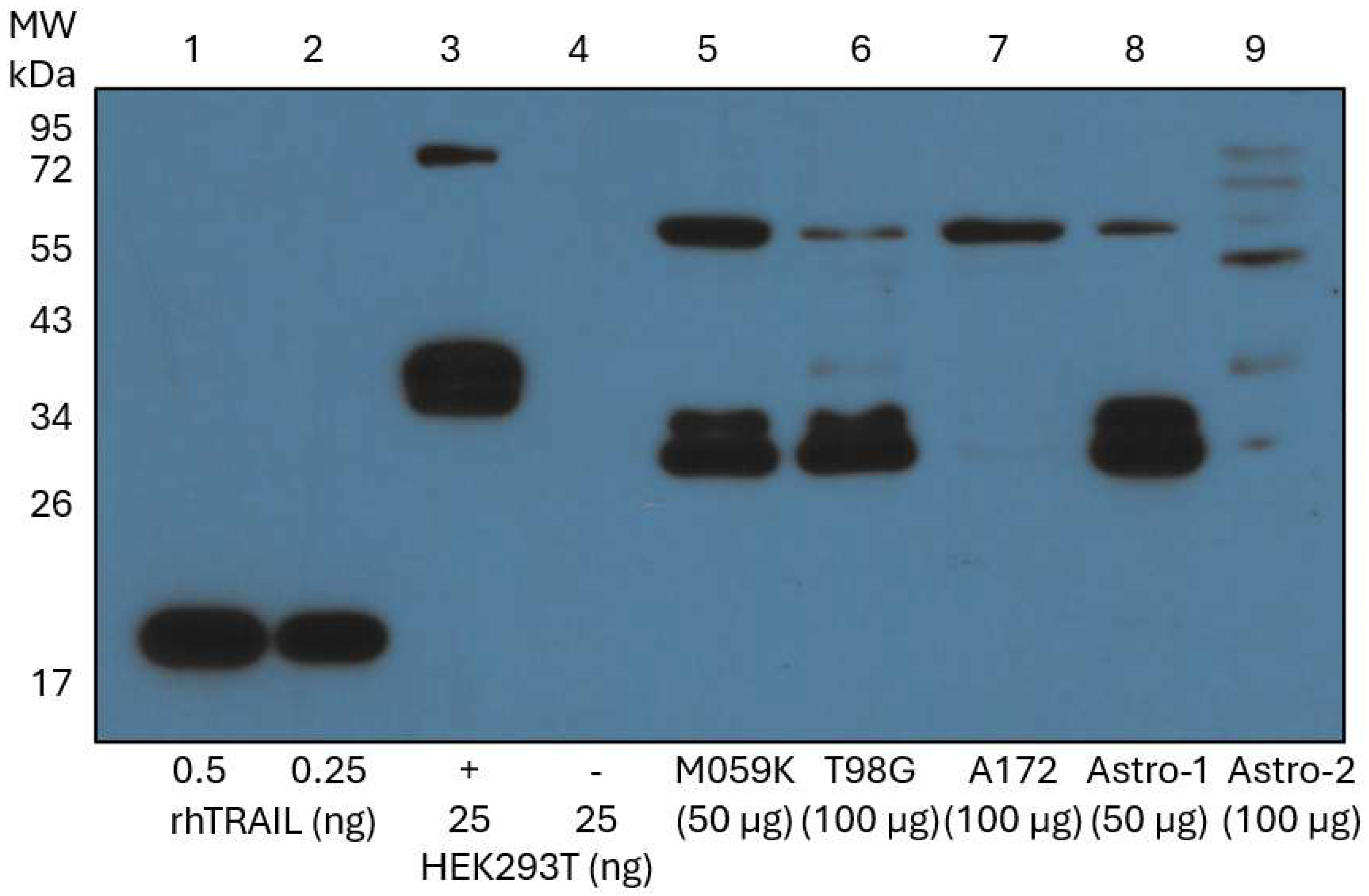
3.4. Endogenous Membrane-TRAIL Protein Is Detected in GBM Cells and Astrocytes
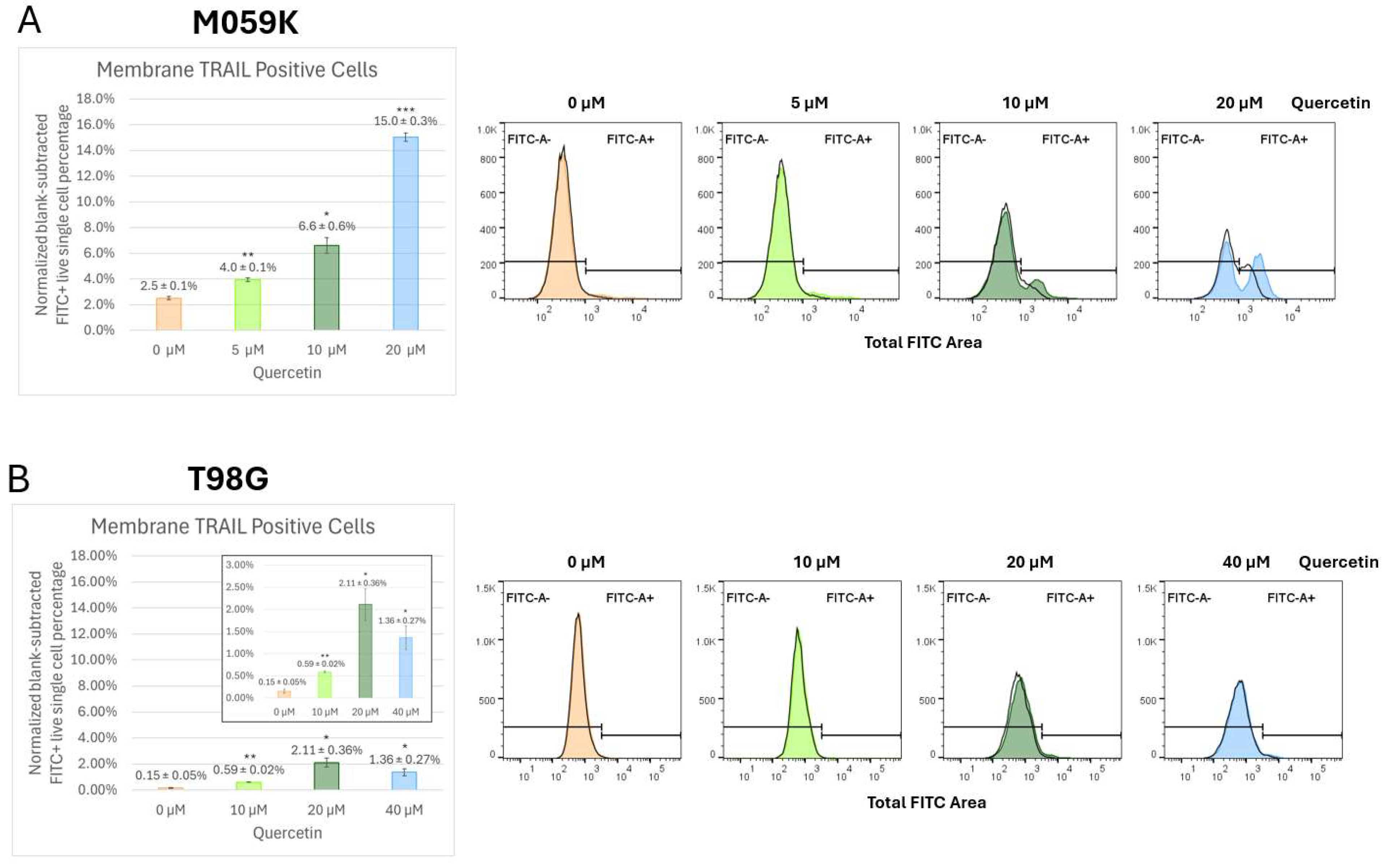
3.5. Quercetin Enhances Endogenous Cell Surface Membrane-TRAIL Expression in Cancer Cells but Not Astrocytes
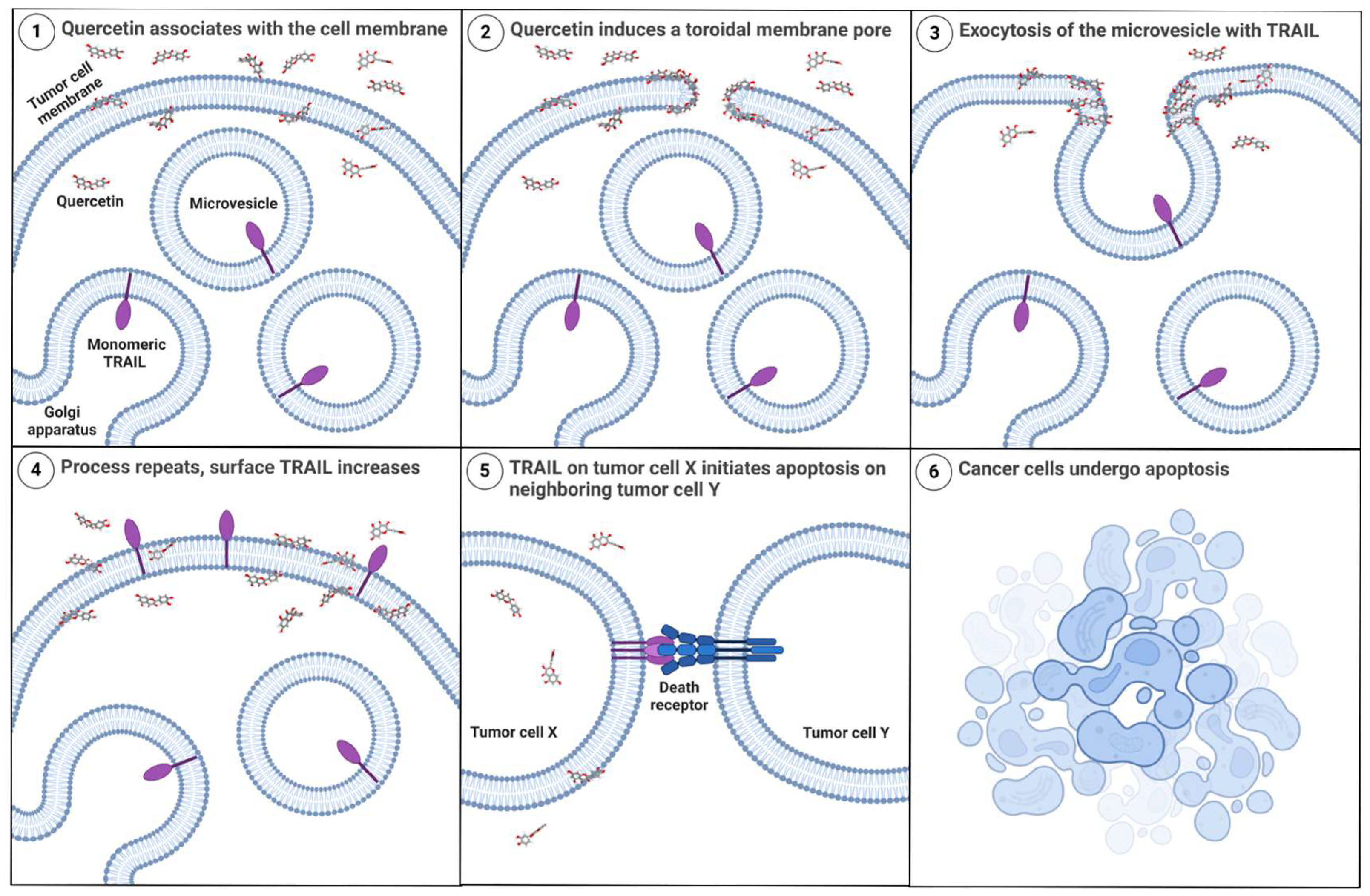
3.6. Quercetin Increases Trafficking of Endogenous Membrane-TRAIL to the Cell Surface of Cancer Cells but Not Normal Astrocytes
3.7. Quercetin Effects Membrane DR4 and DR5 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCG2 | ATP-binding cassette subfamily G member 2 |
| ACC1 | acetyl-CoA Carboxylase 1 |
| APAF1 | apoptotic protease-activating factor 1 |
| BBB | blood–brain barrier |
| BCA | bicinchoninic acid |
| BID | BH3-interacting domain death agonist |
| cFLIPS | cellular FLICE-inhibitory protein short isoform |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| DR | death receptor |
| FADD | Fas-associated death domain |
| FITC | fluorescein isothiocyanate |
| FPLC | fast protein liquid chromatography |
| GBM | glioblastoma isocitrate dehydrogenase (IDH)-wild type |
| HMGCR | HMG-CoA reductase |
| HPLC | high-performance liquid chromatography |
| HRP | horseradish peroxidase |
| kDa | kilodalton |
| MGMT | O6-methylguanine-DNA methyltransferase |
| MW | molecular weight |
| p53 | tumor protein 53 |
| PAGE | polyacrylamide gel electrophoresis |
| PARP | poly (ADP-ribose) polymerase |
| PBS | phosphate-buffered saline |
| PE | phycoerythrin |
| PI | propidium iodide |
| PTEN | phosphatase and tensin homolog gene |
| rhTRAIL | recombinant human TRAIL |
| RIPA | radioimmunoprecipitation assay |
| SDS | sodium dodecyl sulfate |
| SEM | standard error of the mean |
| SREBP2 | sterol regulatory element-binding protein 2 |
| TMZ | temozolomide |
| TP53 | tumor protein 53 gene |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| WGA | wheat germ agglutinin |
References
- Kuijlen, J.M.A.; Bremer, E.; Mooij, J.J.A.; den Dunnen, W.F.A.; Helfrich, W. Review: On TRAIL for Malignant Glioma Therapy? Neuropathol. Appl. Neurobiol. 2010, 36, 168–182. [Google Scholar] [CrossRef]
- Stritzelberger, J.; Distel, L.; Buslei, R.; Fietkau, R.; Putz, F. Acquired Temozolomide Resistance in Human Glioblastoma Cell Line U251 Is Caused by Mismatch Repair Deficiency and Can Be Overcome by Lomustine. Clin. Transl. Oncol. 2018, 20, 508–516. [Google Scholar] [CrossRef]
- Chamberlain, M.C. Salvage Therapy with Lomustine for Temozolomide Refractory Recurrent Anaplastic Astrocytoma: A Retrospective Study. J. Neurooncol. 2015, 122, 329–338. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs Less Travelled: TRAIL in Cancer Biology and Therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Duiker, E.W.; Mom, C.H.; de Jong, S.; Willemse, P.H.B.; Gietema, J.A.; van der Zee, A.G.J.; de Vries, E.G.E. The Clinical Trail of TRAIL. Eur. J. Cancer 2006, 42, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Gores, G.J. Life and Death by Death Receptors. FASEB J. 2009, 23, 1625–1637. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Kalafatis, M.A.; Lindner, D.J.; Kalafatis, M. Recombinant Human Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Selectively Induces Apoptosis in Malignant Melanoma. Int. J. Cancer Oncol. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, B. Mechanisms of Resistance to TRAIL-Induced Apoptosis in Cancer. Cancer Gene Ther. 2005, 12, 228–237. [Google Scholar] [CrossRef]
- Manouchehri, J.M.; Turner, K.A.; Kalafatis, M. TRAIL-Induced Apoptosis in TRAIL-Resistant Breast Carcinoma through Quercetin Cotreatment. Breast Cancer 2018, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, J.M.; Kalafatis, M. Sensitization of RhTRAIL-Resistant Triple-Negative Breast Carcinoma through Silibinin Co-Treatment. Anticancer Res. 2017, 37, 6593–6599. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, J.M.; Kalafatis, M. Ursolic Acid Promotes the Sensitization of RhTRAIL-Resistant Triple-Negative Breast Cancer. Anticancer Res. 2018, 38, 6789–6795. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.A.; Manouchehri, J.M.; Kalafatis, M. Sensitization of Recombinant Human Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Resistant Malignant Melanomas by Quercetin. Melanoma Res. 2018, 28, 277–285. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Rami, A.; Von Deimling, A. Quercetin Promotes Degradation of Survivin and Thereby Enhances Death-Receptor- Mediated Apoptosis in Glioma Cells. Neuro-Oncology 2009, 11, 122–131. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Herold-Mende, C.; Von Deimling, A. The Flavonoid Kaempferol Sensitizes Human Glioma Cells to TRAIL-Mediated Apoptosis by Proteasomal Degradation of Survivin. Mol. Cancer Ther. 2008, 7, 3566–3574. [Google Scholar] [CrossRef]
- Bobak, A.; Steves, M.; McMahon, E.; Spahiu, M.; Rahman, A.S.W.; Casarcia, N.; Banerjee, S.; Bishayee, A. Potential of Phytochemicals for Pharmacotherapy of Glioblastoma Multiforme: Progress, Pitfalls, and Promise. Phytomedicine 2025, 144, 156850. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A Critical Review of the Data Related to the Safety of Quercetin and Lack of Evidence of In Vivo Toxicity, Including Lack of Genotoxic/Carcinogenic Properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer Potential of Quercetin: A Comprehensive Review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Shen, Y.L.; Zhang, Y.; Sun, A.Y.; Xia, X.X.; Wei, D.Z.; Yang, S.L. High-Level Production of Soluble Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (Apo2L/TRAIL) in High-Density Cultivation of Recombinant Escherichia Coli Using a Combined Feeding Strategy. Biotechnol. Lett. 2004, 26, 981–984. [Google Scholar] [CrossRef]
- Luo, Q.; Shen, Y.L.; Wei, D.Z.; Cao, W. Optimization of Culture on the Overproduction of TRAIL in High-Cell-Density Culture by Recombinant Escherichia Coli. Appl. Microbiol. Biotechnol. 2006, 71, 184–191. [Google Scholar] [CrossRef]
- Wang, D.; Shi, L. High-Level Expression, Purification, and in Vitro Refolding of Soluble Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). Appl. Microbiol. Biotechnol. 2009, 157, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pitti, R.M.; Marsters, S.A.; Ruppert, S.; Donahue, C.J.; Moore, A.; Ashkenazi, A. Induction of Apoptosis by Apo-2 Ligand, a New Member of the Tumor Necrosis Factor Cytokine Family. J. Biol. Chem. 1996, 271, 12687–12690. [Google Scholar] [CrossRef] [PubMed]
- Müller-Greven, G.; Carlin, C.R.; Burgett, M.E.; Ahluwalia, M.S.; Lauko, A.; Nowacki, A.S.; Herting, C.J.; Qadan, M.A.; Bredel, M.; Toms, S.A.; et al. Macropinocytosis of Bevacizumab by Glioblastoma Cells in the Perivascular Niche Affects Their Survival. Clin. Cancer Res. 2017, 23, 7059–7071. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Huang, P.; Müller-Greven, G.; Hambardzumyan, D.; Sloan, A.E.; Nowacki, A.S.; Marko, N.; Carlin, C.R.; Gladson, C.L. Integrin A3β1 Promotes Vessel Formation of Glioblastoma-Associated Endothelial Cells through Calcium-Mediated Macropinocytosis and Lysosomal Exocytosis. Nat. Commun. 2022, 13, 4268. [Google Scholar] [CrossRef]
- D’Alimonte, I.; Ballerini, P.; Nargi, E.; Buccella, S.; Giuliani, P.; Di Iorio, P.; Caciagli, F.; Ciccarelli, R. Staurosporine-Induced Apoptosis in Astrocytes Is Prevented by A1 Adenosine Receptor Activation. Neurosci. Lett. 2007, 418, 66–71. [Google Scholar] [CrossRef]
- Samarasekera, G.; Go, N.E.; Choutka, C.; Xu, J.; Takemon, Y.; Chan, J.; Chan, M.; Perera, S.; Aparicio, S.; Morin, G.B.; et al. Caspase 3 and Caspase 7 Promote Cytoprotective Autophagy and the DNA Damage Response during Non-Lethal Stress Conditions in Human Breast Cancer Cells. PLoS Biol. 2025, 23, e3003034. [Google Scholar] [CrossRef]
- Ishisaka, A.; Ichikawa, S.; Sakakibara, H.; Piskula, M.K.; Nakamura, T.; Kato, Y.; Ito, M.; Miyamoto, K.I.; Tsuji, A.; Kawai, Y.; et al. Accumulation of Orally Administered Quercetin in Brain Tissue and Its Antioxidative Effects in Rats. Free. Radic. Biol. Med. 2011, 51, 1329–1336. [Google Scholar] [CrossRef]
- Daniels, R.A.; Turley, H.; Kimberley, F.C.; Liu, X.S.; Mongkolsapaya, J.; Ch’en, P.; Xu, X.N.; Jin, B.; Pezzella, F.; Screaton, G.R. Expression of TRAIL and TRAIL Receptors in Normal and Malignant Tissues. Cell Res. 2005, 15, 430–438. [Google Scholar] [CrossRef]
- Spitzer, D.; McDunn, J.E.; Plambeck-Suess, S.; Goedegebuure, P.S.; Hotchkiss, R.S.; Hawkins, W.G. A Genetically Encoded Multifunctional Trail Trimer Facilitates Cell-Specific Targeting and Tumor Cell Killing. Mol. Cancer Ther. 2010, 9, 2142–2151. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kong, W.H.; Kim, H.; Hahn, S.K. Targeted Systemic Mesenchymal Stem Cell Delivery Using Hyaluronate—Wheat Germ Agglutinin Conjugate. Biomaterials 2016, 106, 217–227. [Google Scholar] [CrossRef]
- Artykov, A.A.; Yagolovich, A.V.; Dolgikh, D.A.; Kirpichnikov, M.P.; Trushina, D.B.; Gasparian, M.E. Death Receptors DR4 and DR5 Undergo Spontaneous and Ligand-Mediated Endocytosis and Recycling Regardless of the Sensitivity of Cancer Cells to TRAIL. Front. Cell Dev. Biol. 2021, 9, 733688. [Google Scholar] [CrossRef] [PubMed]
- Pestana, F.; Edwards-Faret, G.; Belgard, T.G.; Martirosyan, A.; Holt, M.G. No Longer Underappreciated: The Emerging Concept of Astrocyte Heterogeneity in Neuroscience. Brain Sci. 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Bellail, A.; Tse, M.C.L.; Yong, V.W.; Hao, C. Human Astrocytes Are Resistant to Fas Ligand and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis. J. Neurosci. 2006, 26, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Feng, L.; Hu, B.G.; Lu, Y.F.; Wang, W.M.; Guo, W.; Suen, C.W.; Jiao, B.H.; Pang, J.X.; Fu, W.M.; et al. MiR-133a Promotes TRAIL Resistance in Glioblastoma via Suppressing Death Receptor 5 and Activating NF-ΚB Signaling. Mol. Ther. Nucleic Acids 2017, 8, 482–492. [Google Scholar] [CrossRef]
- Sherman, J.H.; Bobak, A.; Arsiwala, T.; Lockman, P.; Aulakh, S. Targeting Drug Resistance in Glioblastoma (Review). Int. J. Oncol. 2024, 65, 80. [Google Scholar] [CrossRef]
- Bobustuc, G.C.; Baker, C.H.; Limaye, A.; Jenkins, W.D.; Pearl, G.; Avgeropoulos, N.G.; Konduri, S.D. Levetiracetam Enhances P53-Mediated MGMT Inhibition and Sensitizes Glioblastoma Cells to Temozolomide. Neuro-Oncology 2010, 12, 917–927. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide Resistance in Glioblastoma Multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Dai, B.; Pieper, R.O.; Li, D.; Wei, P.; Liu, M.; Woo, S.Y.; Aldape, K.D.; Sawaya, R.; Xie, K.; Huang, S. FoxM1B Regulates NEDD4-1 Expression, Leading to Cellular Transformation and Full Malignant Phenotype in Immortalized Human Astrocytes. Cancer Res. 2010, 70, 2951–2961. [Google Scholar] [CrossRef]
- Anderson, C.W.; Allalunis-Turner, M.J. Human TP53 from the Malignant Glioma-Derived Cell Lines M059J and M059K Has a Cancer-Associated Mutation in Exon 8. Radiat. Res. 2000, 154, 473–476. [Google Scholar] [CrossRef]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R.; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-Generation Characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef]
- Ishii, N.; Maier, D.; Merlo, A.; Tada, M.; Sawamura, Y.; Diserens, A.; Van Meir, E.G. Frequent Co-Alterations of TP53, P16/CDKN2A, P14ARF, PTEN Tumor Suppressor Genes in Human Glioma Cell Lines. Brain Pathol. 1999, 9, 469–479. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Misiorek, J.O.; Kruhlenia, N.; Przybyl, L.; Kleszcz, R.; Rolle, K.; Krajka-Kuźniak, V. COXIBs and 2,5-Dimethylcelecoxib Counteract the Hyperactivated Wnt/β-Catenin Pathway and COX-2/PGE2/EP4 Signaling in Glioblastoma Cells. BMC Cancer 2021, 21, 493. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Zhang, L.; Zhang, J. Targeting Mutant P53 Stabilization for Cancer Therapy. Front. Pharmacol. 2023, 14, 1215995. [Google Scholar] [CrossRef] [PubMed]
- Pedrote, M.M.; Motta, M.F.; Ferretti, G.D.S.; Norberto, D.R.; Spohr, T.C.L.S.; Lima, F.R.S.; Gratton, E.; Silva, J.L.; de Oliveira, G.A.P. Oncogenic Gain of Function in Glioblastoma Is Linked to Mutant P53 Amyloid Oligomers. iScience 2020, 23, 100820. [Google Scholar] [CrossRef] [PubMed]
- Panner, A.; Crane, C.A.; Weng, C.; Feletti, A.; Fang, S.; Parsa, A.T.; Pieper, R.O. Ubiquitin-Specific Protease 8 Links the PTEN-Akt-AIP4 Pathway to the Control of FLIPS Stability and TRAIL Sensitivity in Glioblastoma Multiforme. Cancer Res. 2010, 70, 5046–5053. [Google Scholar] [CrossRef] [PubMed]
- Bleau, A.M.; Hambardzumyan, D.; Ozawa, T.; Fomchenko, E.I.; Huse, J.T.; Brennan, C.W.; Holland, E.C. PTEN/PI3K/Akt Pathway Regulates the Side Population Phenotype and ABCG2 Activity in Glioma Tumor Stem-like Cells. Cell Stem Cell 2009, 4, 226–235. [Google Scholar] [CrossRef]
- Sesink, A.L.A.; Arts, I.C.W.; De Boer, V.C.J.; Breedveld, P.; Schellens, J.H.M.; Hollman, P.C.H.; Russel, F.G.M. Breast Cancer Resistance Protein (Bcrp1/Abcg2) Limits Net Intestinal Uptake of Quercetin in Rats by Facilitating Apical Efflux of Glucuronides. Mol. Pharmacol. 2005, 67, 1999–2006. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Morris, M.E. Flavonoids Are Inhibitors of Breast Cancer Resistance Protein (ABCG2)-Mediated Transport. Mol. Pharmacol. 2004, 65, 1208–1216. [Google Scholar] [CrossRef]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and Characterization of a New Member of the TNF Family That Induces Apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Mariani, S.M.; Krammer, P.H. Differential Regulation of TRAIL and CD95 Ligand in Transformed Cells of the T and B Lymphocyte Lineage. Eur. J. Immunol. 1998, 28, 973–982. [Google Scholar] [CrossRef]
- Frank, S.; Köhler, U.; Schackert, G.; Schackert, H.K. Expression of TRAIL and Its Receptors in Human Brain Tumors. Biochem. Biophys. Res. Commun. 1999, 257, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, J.S.; Choi, I.H. Human Brain Astrocytes Mediate TRAIL-Mediated Apoptosis after Treatment with IFN-γ. Yonsei Med. J. 2006, 47, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.; Ohgaki, H.; Kleihues, P.; Weller, M. Human Astrocytic Brain Tumors Express APO2L/TRAIL. Acta Neuropathol. 1999, 97, 1–4. [Google Scholar] [CrossRef]
- Kichev, A.; Rousset, C.I.; Baburamani, A.A.; Levison, S.W.; Wood, T.L.; Gressens, P.; Thornton, C.; Hagberg, H. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Signaling and Cell Death in the Immature Central Nervous System after Hypoxia-Ischemia and Inflammation. J. Biol. Chem. 2014, 289, 9430–9439. [Google Scholar] [CrossRef]
- Sanmarco, L.M.; Wheeler, M.A.; Gutiérrez-Vázquez, C.; Polonio, C.M.; Linnerbauer, M.; Pinho-Ribeiro, F.A.; Li, Z.; Giovannoni, F.; Batterman, K.V.; Scalisi, G.; et al. Gut-Licensed IFNγ+ NK Cells Drive LAMP1+TRAIL+ Anti-Inflammatory Astrocytes. Nature 2021, 590, 473–479. [Google Scholar] [CrossRef]
- Johann, L.; Waisman, A. The Astrocyte LAMP Lights a T Cell TRAIL of Death. Neuron 2021, 109, 1423–1425. [Google Scholar] [CrossRef]
- Wajant, H.; Moosmayer, D.; Wüest, T.; Bartke, T.; Gerlach, E.; Schönherr, U.; Peters, N.; Scheurich, P.; Pfizenmaier, K. Differential Activation of TRAIL-R1 and-2 by Soluble and Membrane TRAIL Allows Selective Surface Antigen-Directed Activation of TRAIL-R2 by a Soluble TRAIL Derivative. Oncogene 2001, 20, 4101–4106. [Google Scholar] [CrossRef]
- Berg, D.; Lehne, M.; Müller, N.; Siegmund, D.; Münkel, S.; Sebald, W.; Pfizenmaier, K.; Wajant, H. Enforced Covalent Trimerization Increases the Activity of the TNF Ligand Family Members TRAIL and CD95L. Cell Death Differ. 2007, 14, 2021–2034. [Google Scholar] [CrossRef]
- Naval, J.; De Miguel, D.; Lleyda, A.G.; Anel, A.; Lostao, L.M. Importance of Trail Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy. Cancers 2019, 11, 444. [Google Scholar] [CrossRef]
- Martínez-Lorenzo, M.J.; Anel, A.; Gamen, S.; Monleón, I.; Lasierra, P.; Larrad, L.; Piñeiro, A.; Alava, M.A.; Naval, J. Activated Human T Cells Release Bioactive Fas Ligand and APO2 Ligand in Microvesicles. J. Immunol. 1999, 163, 1274–1281. [Google Scholar] [CrossRef]
- Monleón, I.; Martínez-Lorenzo, M.J.; Monteagudo, L.; Lasierra, P.; Taulés, M.; Iturralde, M.; Piñeiro, A.; Larrad, L.; Alava, M.A.; Naval, J.; et al. Differential Secretion of Fas Ligand- or APO2 Ligand/TNF-Related Apoptosis-Inducing Ligand-Carrying Microvesicles During Activation-Induced Death of Human T Cells. J. Immunol. 2001, 167, 6736–6744. [Google Scholar] [CrossRef] [PubMed]
- Sträter, J.; Hinz, U.; Walczak, H.; Mechtersheimer, G.; Koretz, K.; Herfarth, C.; Möller, P.; Lehnert, T. Expression of TRAIL and TRAIL Receptors in Colon Carcinoma: TRAIL-R1 Is an Independent Prognostic Parameter. Clin. Cancer Res. 2002, 8, 3734–3740. [Google Scholar] [PubMed]
- Liu, S.; Polsdofer, E.V.; Zhou, L.; Ruan, S.; Lyu, H.; Hou, D.; Liu, H.; Thor, A.D.; He, Z.; Liu, B. Upregulation of Endogenous TRAIL-Elicited Apoptosis Is Essential for Metformin-Mediated Antitumor Activity against TNBC and NSCLC. Mol. Ther. Oncolytics 2021, 21, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of Liposomal Membrane Fluidity by Flavonoids and Isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef]
- Movileanu, L.; Neagoe, I.; Flonta, M.L. Interaction of the Antioxidant Flavonoid Quercetin with Planar Lipid Bilayers. Int. J. Pharm. 2000, 205, 135–146. [Google Scholar] [CrossRef]
- Ollila, F.; Halling, K.; Vuorela, P.; Vuorela, H.; Slotte, J.P. Characterization of Flavonoid-Biomembrane Interactions. Arch. Biochem. Biophys. 2002, 399, 103–108. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlega, B.; Ignacy Gruszecki, W.; Misiak, L.; Paduch, R.; Piersiak, T.; Zarzyka, B.; Pawelec, J.; Gawron, A. Modification of Membranes by Quercetin, a Naturally Occurring Flavonoid, via Its Incorporation in the Polar Head Group. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2195–2204. [Google Scholar] [CrossRef]
- Drǎguşin, M.; Ţugulea, L.; Ganea, C. The Effects of the Natural Antioxidant Quercetin and Anions of the Hofmeister Series on Liposomes Marked with Chlorophyll a. Gen. Physiol. Biophys. 2010, 29, 41–49. [Google Scholar] [CrossRef]
- Sinha, R.; Gadhwal, M.K.; Joshi, U.J.; Srivastava, S.; Govil, G. Interaction of Quercetin with DPPC Model Membrane: Molecular Dynamic Simulation, DSC and Multinuclear NMR Studies. J. Indian Chem. Soc. 2011, 88, 1203–1210. [Google Scholar]
- Košinová, P.; Berka, K.; Wykes, M.; Otyepka, M.; Trouillas, P. Positioning of Antioxidant Quercetin and Its Metabolites in Lipid Bilayer Membranes: Implication for Their Lipid-Peroxidation Inhibition. J. Phys. Chem. B 2012, 116, 1309–1318. [Google Scholar] [CrossRef]
- De Granada-Flor, A.; Sousa, C.; Filipe, H.A.L.; Santos, M.S.C.S.; De Almeida, R.F.M. Quercetin Dual Interaction at the Membrane Level. Chem. Commun. 2019, 55, 1750–1753. [Google Scholar] [CrossRef]
- Eid, J.; Jraij, A.; Greige-Gerges, H.; Monticelli, L. Effect of Quercetin on Lipid Membrane Rigidity: Assessment by Atomic Force Microscopy and Molecular Dynamics Simulations. BBA Adv. 2021, 1, 100018. [Google Scholar] [CrossRef]
- Leite, N.B.; Martins, D.B.; Alvares, D.S.; Cabrera, M.P. dos S. Quercetin Induces Lipid Domain-Dependent Permeability. Chem. Phys. Lipids 2022, 242, 105160. [Google Scholar] [CrossRef]
- Sanver, D.; Sadeghpour, A.; Rappolt, M.; Di Meo, F.; Trouillas, P. Structure and Dynamics of Dioleoyl-Phosphatidylcholine Bilayers under the Influence of Quercetin and Rutin. Langmuir 2020, 36, 11776–11786. [Google Scholar] [CrossRef]
- Meleleo, D.; Avato, P.; Conforti, F.; Argentieri, M.P.; Messina, G.; Cibelli, G.; Mallamaci, R. Interaction of Quercetin, Cyanidin, and Their O-Glucosides with Planar Lipid Models: Implications for Their Biological Effects. Membranes 2023, 13, 600. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Arismendi-Morillo, G.; Zuccoli, G.; Lee, D.C.; Duraj, T.; Elsakka, A.M.; Maroon, J.C.; Mukherjee, P.; Ta, L.; Shelton, L.; et al. Metabolic Management of Microenvironment Acidity in Glioblastoma. Front. Oncol. 2022, 12, 968351. [Google Scholar] [CrossRef] [PubMed]
- Theparambil, S.M.; Hosford, P.S.; Ruminot, I.; Kopach, O.; Reynolds, J.R.; Sandoval, P.Y.; Rusakov, D.A.; Barros, L.F.; Gourine, A.V. Astrocytes Regulate Brain Extracellular PH via a Neuronal Activity-Dependent Bicarbonate Shuttle. Nat. Commun. 2020, 11, 5073. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, M.; Kusaczuk, M.; Kotyńska, J.; Gál, M.; Krętowski, R.; Cechowska-Pasko, M.; Naumowicz, M. The Effect of Quercetin on the Electrical Properties of Model Lipid Membranes and Human Glioblastoma Cells. Bioelectrochemistry 2018, 124, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Garduño-Juárez, R.; Munoz-Garay, C. Estimation of Pore Dimensions in Lipid Membranes Induced by Peptides and Other Biomolecules: A Review. Biochim. Biophys. Acta (BBA)—Biomembr. 2021, 1863, 183551. [Google Scholar] [CrossRef]
- Ren, M.X.; Deng, X.H.; Ai, F.; Yuan, G.Y.; Song, H.Y. Effect of Quercetin on the Proliferation of the Human Ovarian Cancer Cell Line SKOV-3 in Vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef]
- Chang, J.H.; Lai, S.L.; Chen, W.S.; Hung, W.Y.; Chow, J.M.; Hsiao, M.; Lee, W.J.; Chien, M.H. Quercetin Suppresses the Metastatic Ability of Lung Cancer through Inhibiting Snail-Dependent Akt Activation and Snail-Independent ADAM9 Expression Pathways. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 1746–1758. [Google Scholar] [CrossRef]
- Tsai, T.F.; Hwang, T.I.S.; Lin, J.F.; Chen, H.E.; Yang, S.C.; Lin, Y.C.; Chou, K.Y. Suppression of Quercetin-Induced Autophagy Enhances Cytotoxicity through Elevating Apoptotic Cell Death in Human Bladder Cancer Cells. Urol. Sci. 2019, 30, 58–66. [Google Scholar] [CrossRef]
- Deng, X.H.; Song, H.Y.; Zhou, Y.F.; Yuan, G.Y.; Zheng, F.J. Effects of Quercetin on the Proliferation of Breast Cancer Cells and Expression of Survivin in Vitro. Exp. Ther. Med. 2013, 6, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Lee, Y.H.; Sharma, A.R.; Park, J.B.; Jagga, S.; Sharma, G.; Lee, S.S.; Nam, J.S. Quercetin Induces Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells through Modulation of Foxo3a Activity. Korean J. Physiol. Pharmacol. 2017, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; De Freitas, V.; Mateus, N.; Calhau, C. Flavonoid Transport across RBE4 Cells: A Blood-Brain Barrier Model. Cell. Mol. Biol. Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef]
- Schaffer, S.; Halliwell, B. Do Polyphenols Enter the Brain and Does It Matter? Some Theoretical and Practical Considerations. Genes Nutr. 2012, 7, 99–109. [Google Scholar] [CrossRef]
- De Boer, V.C.J.; Dihal, A.A.; Van Der Woude, H.; Arts, I.C.W.; Wolffram, S.; Alink, G.M.; Rietjens, I.M.C.M.; Keijer, J.; Hollman, P.C.H. Tissue Distribution of Quercetin in Rats and Pigs. J. Nutr. 2005, 135, 1718–1725. [Google Scholar] [CrossRef]
- Huebbe, P.; Wagner, A.E.; Boesch-Saadatmandi, C.; Sellmer, F.; Wolffram, S.; Rimbach, G. Effect of Dietary Quercetin on Brain Quercetin Levels and the Expression of Antioxidant and Alzheimer’s Disease Relevant Genes in Mice. Pharmacol. Res. 2010, 61, 242–246. [Google Scholar] [CrossRef]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; Del Grande, P.; Ninfali, P. Enhancement of Flavonoid Ability to Cross the Blood-Brain Barrier of Rats by Co-Administration with α-Tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [PubMed]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with Quercetin Markedly Increases Plasma Quercetin Concentration without Effect on Selected Risk Factors for Heart Disease in Healthy Subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily Quercetin Supplementation Dose-Dependently Increases Plasma Quercetin Concentrations in Healthy Humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [CrossRef]
- Jin, F.; Nieman, D.C.; Shanely, R.A.; Knab, A.M.; Austin, M.D.; Sha, W. The Variable Plasma Quercetin Response to 12-Week Quercetin Supplementation in Humans. Eur. J. Clin. Nutr. 2010, 64, 692–697. [Google Scholar] [CrossRef]
- Damiano, F.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Gnoni, A. Quercetin Inhibition of SREBPs and ChREBP Expression Results in Reduced Cholesterol and Fatty Acid Synthesis in C6 Glioma Cells. Int. J. Biochem. Cell Biol. 2019, 117, 105618. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, S.; Yang, Z.; Li, Z.A.; Hu, W.; Dai, L.; Liang, W.; Wang, X. Cholesterol Metabolism and Its Implication in Glioblastoma Therapy. J. Cancer 2022, 13, 1745–1757. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorpe, E.M.; Muller-Greven, G.; Hirbawi, J.; Gladson, C.L.; Kalafatis, M. Quercetin Increases Expression of Membrane-TRAIL in Glioblastoma Cells Resulting in Apoptosis. Cancers 2025, 17, 3197. https://doi.org/10.3390/cancers17193197
Thorpe EM, Muller-Greven G, Hirbawi J, Gladson CL, Kalafatis M. Quercetin Increases Expression of Membrane-TRAIL in Glioblastoma Cells Resulting in Apoptosis. Cancers. 2025; 17(19):3197. https://doi.org/10.3390/cancers17193197
Chicago/Turabian StyleThorpe, Erin M., Gaëlle Muller-Greven, Jamila Hirbawi, Candece L. Gladson, and Michael Kalafatis. 2025. "Quercetin Increases Expression of Membrane-TRAIL in Glioblastoma Cells Resulting in Apoptosis" Cancers 17, no. 19: 3197. https://doi.org/10.3390/cancers17193197
APA StyleThorpe, E. M., Muller-Greven, G., Hirbawi, J., Gladson, C. L., & Kalafatis, M. (2025). Quercetin Increases Expression of Membrane-TRAIL in Glioblastoma Cells Resulting in Apoptosis. Cancers, 17(19), 3197. https://doi.org/10.3390/cancers17193197





