Predictors of Distant Metastasis in Patients with Medullary Thyroid Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Variables Analyzed
- Epidemiological: sex and age.
- Hereditary: sporadic/familial.
- ▪
- Phenotype (MCT with/without hyperparathyroidism (HP), pheochromocytoma or paraganglioma).
- ▪
- Mutation (exon, genotype, affected protein).
- ▪
- Mutation risk level according to ATA 2009 [28].
- Complementary examinations:
- (1)
- Laboratory:
- -
- Calcitonin (Ct, pg/mL).
- -
- CEA (ng/mL).
- (2)
- Imaging studies
- -
- Thyroid ultrasound.
- ◦
- Findings (unilateral nodule/unilateral nodules/bilateral nodules/normal/not available).
- ◦
- Nodule size
- ◦
- Suspicious lymphadenopathy (yes/no).
- Surgery-related variables
- (1)
- Age at surgery.
- (2)
- Suspicious lymphadenopathy (yes/no).
- -
- Prophylactic surgery: In asymptomatic patients carrying the RET mutation with no CMT according to clinical and radiological criteria (no nodules or nodules < 5 mm identified by ultrasound; and no suspicious lymphadenopathy) [30,31], prophylactic surgery was considered if performed in the first year of life in the case of MEN2B, before the age of 5 in the case of high-risk MEN2A.
- -
- Early surgery: This is surgery performed on asymptomatic RET mutation carriers with no CMT according to clinical and radiological criteria (no nodules or nodules < 5 mm identified by ultrasound; and no suspicious lymphadenopathy) [30,31]. This surgery is performed after the first year of life in MEN 2b syndrome and after 5 years of age in high-risk MEN2A syndrome.
- -
- Curative surgery: this occurs when the disease has been identified, and the aim of the intervention is to achieve a cure.
- -
- Palliative surgery: performed when the disease is not curable, and the aim is to relieve symptoms.
- (3)
- Surgical technique (total thyroidectomy with/without central lymph node dissection, with/without unilateral/bilateral lateral lymph node dissection), according to ATA recommendations.
- (4)
- Complications (yes/no).
- -
- Postoperative hypoparathyroidism: inappropriate PTH production.
- ◦
- Transient: resolved within 6 months.
- ◦
- Permanent: persistent beyond 6 months.
- -
- Recurrent laryngeal nerve paralysis (transitory/permanent).
- -
- Others: hematoma, infection, chylous fistula, jugular vein thrombosis, hemidiaphragm paralysis, respiratory failure.
- Histological variables
- (1)
- Tumor size (mm).
- (2)
- Focality (unifocal/multifocal).
- (3)
- Capsular invasion.
- (4)
- Vascular invasion.
- (5)
- Lymphatic invasion.
- (6)
- Tumor necrosis.
- (7)
- Number of mitoses per 10 high power field (HPF).
- (8)
- Ki67.
- (9)
- High-risk histology: ≥1 of the following: tumor necrosis, >5 mitoses/2 mm2, Ki67 > 5% [32].
- (10)
- Desmoplasia: fibrotic tissue surrounding tumor tissue, absent in normal thyroid parenchyma [33].
- Staging
- (1)
- Nodal involvement (yes/no).
- (2)
- Central node involvement.
- (3)
- Lateral nodal involvement.
- (4)
- Ipsilateral lateral nodal involvement.
- (5)
- Contralateral lateral nodal involvement.
- (6)
- Number of pathological nodes (0/1/>1).
- (7)
- Number of pathological lymph nodes.
- (8)
- Number of lymph nodes removed.
- (9)
- LNR (lymph node ratio): proportion of metastatic lymph nodes to the total number of lymph nodes removed.
- (10)
- TNM stage (AJCC, 8º Edition) [34].
- (11)
- Stage (I/II/III/IVa/IVb/IVc).
- Evolution
- (1)
- Ct at 6–12 months (pg/mL).
- (2)
- BFS (biochemical-free survival) (months): time from initial surgery to biochemical recurrence.
- (3)
- OS (overall survival, months).
- (4)
- Death and cause.
2.2. Statistical Analysis
3. Results
3.1. Univariate Analysis
3.2. Multivariate Analysis of Predictors of Distant Metastases in Patients with MTC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCT | Medullary Thyroid Cancer |
| HP | Hyperparathyroidism |
| HPF | High-power field |
| Ct | Calcitonin |
| CEA | Carcinoembryonic antigen |
| CI | Confidence interval |
| TAC | Computed axial tomography |
| TT | Total thyroidectomy |
| LCB | Central bilateral lymphadenectomy |
| LLB | Bilateral lateral lymphadenectomy |
| LLU | Unilateral lateral lymphadenectomy |
| LNR | Lymph node ratio |
| BFS | Biochemical-free survival |
| OS | Overall survival |
References
- Prinzi, A.; Frasca, F.; Russo, M.; Le Moli, R.; Belfiore, A.; Malandrino, P. Lymph Node Ratio as a Predictive Factor of Persistent/Recurrent Disease in Patients With Medullary Thyroid Cancer: A Single-Center Retrospective Study. Endocr. Pr. 2023, 30, 194–199. [Google Scholar] [CrossRef]
- Siironen, P.; Hagström, J.; Mäenpää, H.O.; Louhimo, J.; Arola, J.; Haglund, C. Lymph node metastases and elevated postoperative calcitonin: Predictors of poor survival in medullary thyroid carcinoma. Acta Oncol. 2016, 55, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Papachristos, A.J.; ENicholls, L.; Mechera, R.; Aniss, A.M.; Robinson, B.; Clifton-Bligh, R.; Gill, A.J.; Learoyd, D.; Sidhu, S.B.; Glover, A.; et al. Management of Medullary Thyroid Cancer: Patterns of Recurrence and Outcomes of Reoperative Surgery. Oncologist 2023, 28, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mao, Y.; You, T.; Chen, G. Establishment and validation of a nomogram model for predicting distant metastasis in medullary thyroid carcinoma: An analysis of the SEER database based on the AJCC 8th TNM staging system. Front. Endocrinol. 2023, 14, 1119656. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Siddique, M.; Riaz, S.; Khan, A.I.; Nawaz, M.K.; Bashir, H. Medullary Thyroid Carcinoma: Prognostic Variables And Tumour Markers Affecting Survival. J. Ayub Med. Coll. Abbottabad 2018, 30 (Suppl. S1), S627–S632. [Google Scholar]
- Schlumberger, M.; Bastholt, L.; Dralle, H.; Jarzab, B.; Pacini, F.S.J. 2012 European Thyroid Association Guidelines for Metastatic Medullary Thyroid Cancer. Endocr. Surg. 2012, 1, 5–14. [Google Scholar] [CrossRef]
- Galofré, J.C.; Sandi, J.S.; Capdevila, J.; González, E.N.; Llopis, C.Z.; Asensio, T.R.Y.C.; Sáez, J.M.G.; Jiménez-Fonseca, P.; Eizaguirre, G.R.; Grande, E. Consensus on the management of advanced medullary thyroid carcinoma on behalf of the Working Group of Thyroid Cancer of the Spanish Society of Endocrinology (SEEN) and the Spanish Task Force Group for Orphan and Infrequent Tumors (GETHI). Clin. Transl. Oncol. 2015, 62, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Metastatic Risk Profile of Microscopic Lymphatic and Venous Invasion in Medullary Thyroid Cancer. Horm. Metab. Res. 2021, 53, 588–593. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhao, K.; Wang, Y.; He, X. Postoperative nomogram for predicting cancer-specific and overall survival among patients with medullary thyroid cancer. Int. J. Endocrinol. 2020, 2020, 8888677. [Google Scholar] [CrossRef]
- Hyer, S.; Vini, L.; A’Hern, R.; Harmer, C. Medullary thyroid cancer: Multivariate analysis of prognostic factors influencing survival. Eur. J. Surg. Oncol. 2000, 26, 686–690. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Higashiiyama, T.; Fukushima, M.; Miya, A. Static Prognostic Factors and Appropriate Surgical Designs for Patients with Medullary Thyroid Carcinoma: The Second Report from a Single—Institution Study in Japan. World J. Surg. 2018, 42, 3954–3966. [Google Scholar] [CrossRef]
- Rozenblat, T.; Hirsch, D.; Robenshtok, E.; Grozinsky-Glasberg, S.; Gross, D.J.; Mazeh, H.; Benbassat, C.; Twito, O.; Levy, S.; Mizrachi, A.; et al. The prognostic value of lymph node ratio in Medullary thyroid carcinoma: A multi-center study. Eur. J. Surg. Oncol. 2020, 46, 2023–2028. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, S.; Son, H.; Ban, E.; Kang, S.-W.; Lee, J.; Jeong, J.J.; Nam, K.-H.; Chung, W.Y.; Park, C.S. Medullary thyroid carcinoma: A 30-year experience at one institution in Korea. Ann. Surg. Treat. Res. 2016, 91, 278–287. [Google Scholar] [CrossRef]
- Kotwal, A.; Erickson, D.; Geske, J.R.; Hay, I.D.; Castro, M.R. Predicting Outcomes in Sporadic and Hereditary Medullary Thyroid Carcinoma over Two Decades. Thyroid 2021, 31, 616–626. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Kroustrup, J.P.; Vestergaard, P.; Stochholm, K.; Poulsen, P.L.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Schytte, S.; Londero, S.C.; Pedersen, H.B.; et al. Survival and Long-Term Biochemical Cure in Medullary Thyroid Carcinoma in Denmark 1997–2014: A Nationwide Study. Thyroid 2019, 29, 368–377. [Google Scholar] [CrossRef]
- Qu, N.; Shi, R.-L.; Luo, T.-X.; Wang, Y.-L.; Li, D.-S.; Wang, Y.; Huang, C.-P.; Ji, Q.-H. Prognostic significance and optimal cutoff of age in medullary thyroid cancer. Oncotarget 2016, 7, 15937–15947. [Google Scholar] [CrossRef] [PubMed]
- Sahli, Z.T.; Canner, J.K.; Zeiger, M.A.; Mathur, A. Association between age and disease specific mortality in medullary thyroid cancer. Am. J. Surg. 2021, 221, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jiang, S.; Gao, L.; Xi, X.; Zhao, R.; Lai, X.; Zhang, B.; Jiang, Y. Construction and Validation of a Nomogram Based on the Log Odds of Positive Lymph Nodes to Predict the Prognosis of Medullary Thyroid Carcinoma After Surgery. Ann. Surg. Oncol. 2021, 28, 4360–4370. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.H.; Hughes, D.T.; Yin, H.; Banerjee, M.; Haymart, M.R. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, 448–454. [Google Scholar] [CrossRef]
- Kandil, E.; Gilson, M.M.; Alabbas, H.H.; Tufaro, A.P.; Dackiw, A.; Tufano, R.P. Survival implications of cervical lymphadenectomy in patients with medullary thyroid cancer. Ann. Surg. Oncol. 2011, 18, 1028–1034. [Google Scholar] [CrossRef]
- Prassas, D.; Kounnamas, A.; Cupisti, K.; Schott, M.; Knoefel, W.T.; Krieg, A. Prognostic Performance of Alternative Lymph Node Classification Systems for Patients with Medullary Thyroid Cancer: A Single Center Cohort Study. Ann. Surg. Oncol. 2022, 29, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, G.; Wei, T.; Gong, R.; Li, Z.; Zhu, J.; Lei, J. Distant metastasis in medullary thyroid carcinoma: Clinical outcomes and implications of T stage. Clin. Endocrinol. 2022, 97, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Gülben, K.; Berberoğlu, U.; Boyabatlı, M. Prognostic factors for sporadic medullary thyroid carcinoma. World J. Surg. 2006, 30, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Le, M.-K.; Kawai, M.; Odate, T.; Vuong, H.G.; Oishi, N.; Kondo, T. Metastatic Risk Stratification of 2526 Medullary Thyroid Carcinoma Patients: A Study Based on Surveillance, Epidemiology, and End Results Database. Endocr. Pathol. 2022, 33, 348–358. [Google Scholar] [CrossRef]
- Pazaitou-Panayiotou, K.; Chrisoulidou, A.; Mandanas, S.; Tziomalos, K.; Doumala, E.; Patakiouta, F. Predictive factors that influence the course of medullary thyroid carcinoma. Int. J. Clin. Oncol. 2014, 19, 445–451. [Google Scholar] [CrossRef]
- Su, H.; Men, Q.; Hao, J.; Zhang, F. Risk factor analysis of distant metastases in patients with primary medullary thyroid cancer: A population-based study. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 1525–1530. [Google Scholar] [CrossRef]
- Xu, B.; Fuchs, T.L.; Ahmadi, S.; Alghamdi, M.; Alzumaili, B.; Bani, M.-A.; Baudin, E.; Chou, A.; De Leo, A.; Fagin, J.A.; et al. International Medullary Thyroid Carcinoma Grading System: A Validated Grading System for Medullary Thyroid Carcinoma. J. Clin. Oncol. 2022, 40, 96–104. [Google Scholar] [CrossRef]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Schiettecatte, J.; Strasser, O.; Anckaert, E.; Smitz, J. Performance evaluation of an automated electrochemiluminescent calcitonin (CT) immunoassay in diagnosis of medullary thyroid carcinoma. Clin. Biochem. 2016, 49, 929–931. [Google Scholar] [CrossRef]
- Febrero, B.; Rodríguez, J.M.; Ríos, A.; Segura, P.; Pérez-Sánchez, B.; Torregrosa, N.; Hernández, A.M.; Parrilla, P. Prophylactic thyroidectomy in multiple endocrine neoplasia 2 (MEN2) patients with the C634Y mutation: A long-term follow-up in a large single-center cohort. Eur. J. Surg. Oncol. 2019, 45, 625–630. [Google Scholar] [CrossRef]
- Lifante, J.; Blanchard, C.; Mirallié, E.; David, A.; Peix, J. Role of preoperative basal calcitonin levels in the timing of prophylactic thyroidectomy in patients with germline RET mutations. World J. Surg. 2014, 38, 576–581. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Koperek, O.; Scheuba, C.; Cherenko, M.; Neuhold, N.; De Micco, C.; Schmid, K.W.; Niederle, B.; Kaserer, K. Desmoplasia in medullary thyroid carcinoma: A reliable indicator of metastatic potential. Histopathology 2008, 52, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.; Gospodarowicz, M.K.; Witterkind, C.H.E. TNM Classification of Malignant Tumors, 8th ed.; John Wiley & Sons: Oxford, UK; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Machens, A.; Dralle, H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J. Clin. Endocrinol. Metab. 2010, 95, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.; Park, H.; Choi, M.S.; Jang, H.W.; Kim, T.H.; Kim, S.W.; Chung, J.H. Metastatic lymph node ratio for predicting recurrence in medullary thyroid cancer. Cancers 2021, 13, 5842. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhao, J.; Guo, F.; Gu, P.; Zhang, J.; Huang, D.; Ruan, X.; Zeng, Y.; Zheng, X.; Gao, M. Value of lymph node ratio as a prognostic factor of recurrence in medullary thyroid cancer. PeerJ 2023, 11, e15025. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, J.; Xu, H.; Dong, C.; Cao, X.; He, Q.; Zhang, G.; Wang, W.; Wang, L.; Yang, X.; et al. Prognostic factors for progression free survival in patients with medullary thyroid cancer: A multicenter cohort study. Updat. Surg. 2025, 77, 1153–1160. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, C.; Xu, Y.; Su, Y.; Zhang, G.; Xie, L.; Huang, L.; You, S.; Zha, J. Ratio of positive lymph nodes: The prognostic value in stage IV thyroid cancer. Oncotarget 2017, 8, 79462–79468. [Google Scholar] [CrossRef]
- Niederle, M.B.; Riss, P.; Selberherr, A.; Koperek, O.; Kaserer, K.; Niederle, B.; Scheuba, C. Omission of lateral lymph node dissection in medullary thyroid cancer without a desmoplastic stromal reaction. Br. J. Surg. 2021, 108, 174–181. [Google Scholar] [CrossRef]
- Machens, A.; Kaatzsch, P.; Lorenz, K.; Horn, L.-C.; Wickenhauser, C.; Schmid, K.W.; Dralle, H.; Siebolts, U. Abandoning node dissection for desmoplasia-negative encapsulated unifocal sporadic medullary thyroid cancer. Surgery 2022, 171, 360–367. [Google Scholar] [CrossRef]
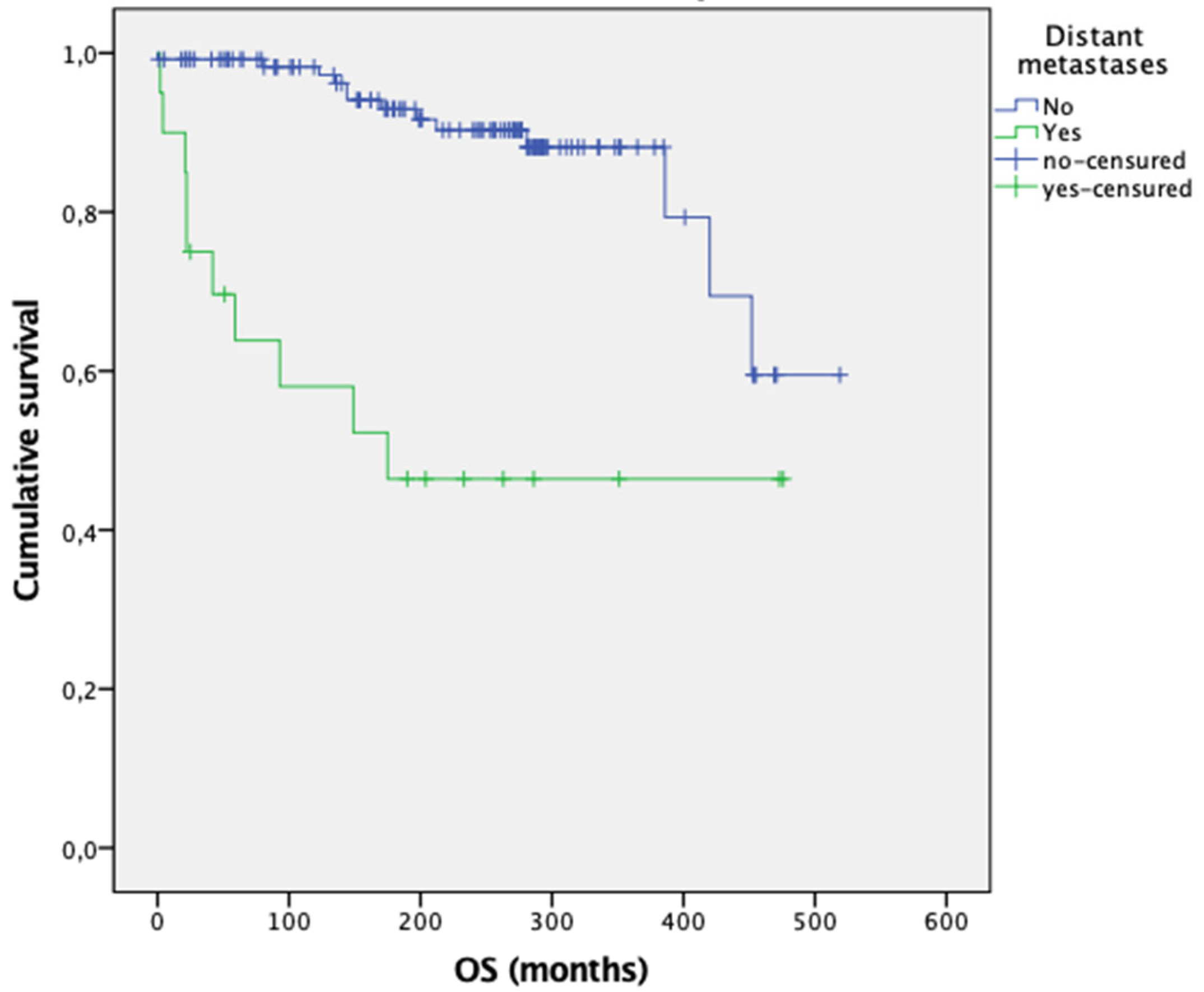
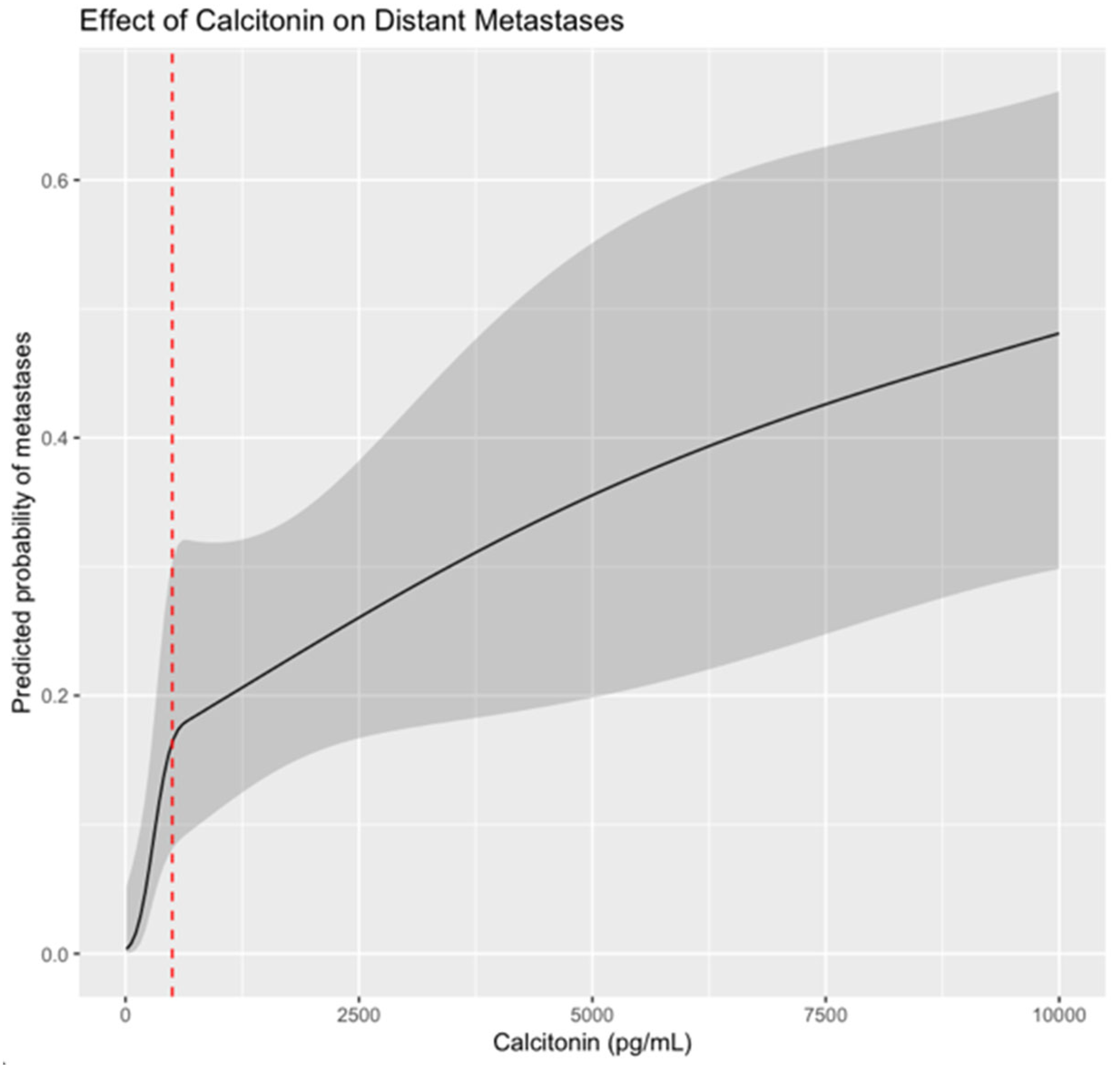
| Patients with MTC | Metastases 14% (n = 21) | No Metastases 86% (n = 125) | p | |
|---|---|---|---|---|
| Sex 1 | Woman (51%, n = 75) | 10 (48%) | 65 (52%) | 0.710 |
| Man (49%, n = 71) | 11 (52%) | 60 (48%) | ||
| Age at diagnosis 2 | Ages (median) | 44 (n = 21) | 33 (n = 125) | 0.006 |
| Heritability 1 | Sporadic (25%, n = 37) | 12 (32%) | 25 (68%) | 0.001 |
| Familial (75%, n = 109) | 9 (8%) | 100 (92%) | ||
| Ct 2 | pg/mL (median) | 1500 (n = 17) | 116 (n = 119) | <0.001 |
| CEA 2 | ng/mL (median) | 51.5 (n = 9) | 5.8 (n = 79) | 0.005 |
| Thyroid ultrasound 1 | Unilateral nodule (42%, n = 52) | 12 (63%) | 40 (38%) | 0.090 |
| Bilateral nodules (30%, n = 37) | 5 (26%) | 32 (31%) | ||
| Normal (28%, n = 35) | 2 (11%) | 33 (31%) | ||
| Suspicious adenopathy (19%, n = 28) | 11 (52%) | 17 (14%) | <0.001 | |
| No (66%, n = 96) | 8 (38%) | 88 (70%) | ||
| Unknown (15%, n = 22) | 2 (10%) | 20 (16%) | ||
| Thyroid nodule size in the ultrasound 1 | Millimeters (median) | 17 (n = 15) | 15 (n = 66) | 0.398 |
| Patients with Familial MTC | Metastases (n = 9) | No Metastases (n = 100) | p | |
|---|---|---|---|---|
| Phenotype—Pheochromocytoma/ paraganglioma 1 | Yes (58%, n = 63) | 4 (44%) | 59 (59%) | 0.489 |
| No (42%, n = 46) | 5 (56%) | 41 (41%) | ||
| Phenotype—HP 1 | Yes (12%, n = 13) | 0 (0%) | 13 (13%) | 0.596 |
| No (88%, n = 96) | 9 (100%) | 87 (87%) | ||
| Genetic mutation 1 | Very high risk (3%, n = 3) | 0 (0) | 3 (3%) | - |
| High risk (92%, n = 98) | 5 (71%) | 93 (93%) | ||
| Moderate risk (5%, n = 6) | 2 (29%) | 4 (4%) | ||
| Patients with MTC | Metastases 14% (n = 21) | No Metastases 86% (n = 125) | p | |
|---|---|---|---|---|
| Treatment 1 | Early/prophylactic (25%, n = 36) | 0 (0%) | 36 (29%) | - |
| Curative (72%, n = 105) | 16 (76%) | 89 (71%) | ||
| Palliative (3%, n = 5) | 5 (24%) | 0 (0) | ||
| Surgical technique 1 | Total thyroidectomy (TT) (33%, n = 48) | 4 (19%) | 44 (35%) | - |
| TT + CLND (22%, n = 33) | 3 (14%) | 30 (24%) | ||
| TT + CLND + ULLND (8%, n = 12) | 5 (24%) | 7 (6%) | ||
| TT + CLND + BLLND (36%, n = 52) | 8 (38%) | 44 (35%) | ||
| Somatostatin analogues (1%, n = 1) | 1 (5%) | 0 (0) | ||
| Complications 1 | Yes (33%, n = 48) | 9 (43%) | 39 (31%) | 0.293 |
| No (67%, n = 98) | 12 (57%) | 86 (69%) | ||
| Hypoparathyroidism 1 | Transitory (21%, n = 30) | 7 (35%) | 23 (18%) | 0.187 |
| Permanent (8%, n = 13) | 1 (5%) | 10 (10%) | ||
| No (71%, n = 103) | 12 (60%) | 91 (72%) | ||
| Recurrent laryngeal nerve paralysis 1 | Transient (3.5%, n = 5) | 2 (10%) | 3 (2%) | 0.205 |
| Permanent (3.5%, n = 5) | 1 (5%) | 4 (3%) | ||
| No (93%, n = 134) | 17 (85%) | 117 (95%) | ||
| Patients with MTC | Metastases 14% (n = 21) | No Metastases 86% (n = 125) | p | |
|---|---|---|---|---|
| T 1 | T1–2 (88%, n = 128) | 13 (65%) | 115 (92%) | <0.001 |
| T3–4 (12%, n = 17) | 7 (35%) | 10 (8%) | ||
| N 1 | N0 (28%, n = 41) | 2 (9%) | 39 (31%) | 0.004 |
| N1a (9%, n = 13) | 2 (9%) | 11 (9%) | ||
| N1b (26%, n = 38) | 12 (58%) | 26 (21%) | ||
| Nx (37%, n = 54) | 5 (24%) | 49 (39%) | ||
| Nodal involvement 1 | Yes (35%, n = 51) | 14 (67%) | 37 (30%) | 0.002 |
| No/no lymphadenectomy (65%, n = 95) | 7 (33%) | 88 (70%) | ||
| Central nodal involvement 1 | Yes (15%, n = 15) | 2 (12%) | 13 (16%) | 1 |
| No (85%, n = 82) | 14 (88%) | 68 (84%) | ||
| Ipsilateral lateral nodal involvement 1 | Yes (36%, n = 35) | 12 (75%) | 23 (28%) | <0.001 |
| No (64%, n = 63) | 4 (25%) | 59 (72%) | ||
| Contralateral lateral nodal involvement 1 | Yes (35%, n = 18) | 6 (75%) | 12 (27%) | 0.015 |
| No (65%, n = 34) | 2 (25%) | 32 (73%) | ||
| Stage 1 | Stage I and II (63%, n = 92) | 5 (24%) | 87 (70%) | <0.001 |
| Stage III and IV (37%, n = 54) | 16 (76%) | 38 (30%) | ||
| Number of lymph nodes removed 2 | (median) | 13.5 (n = 14) | 18 (n = 68) | 0.739 |
| Number of pathological lymph nodes 2 | (median) | 7.5 (n = 16) | 0 (n = 81) | <0.001 |
| LNR 2 | (median) | 0.62 (n = 14) | 0.04 (n = 68) | <0.001 |
| Patients with MTC | Metastases 14% (n = 21) | No Metastases 86% (n = 125) | p | |
|---|---|---|---|---|
| Size 2 | Millimeters (median) | 17 (n = 20) | 9 (125) | 0.001 |
| Focality 1 | Unifocal (31%, n = 46) | 8 (39%) | 38 (30%) | 0.402 |
| Multifocal (66%, n = 95) | 12 (57%) | 83 (67%) | ||
| Unknown (3%, n = 5) | 1 (4%) | 4 (3%) | ||
| Capsular invasion 1 | Yes (8%, n = 12) | 7 (33%) | 5 (4%) | - |
| No (7%, n = 10) | 1 (5%) | 9 (7%) | ||
| Unknown (85%, n = 124) | 13 (62%) | 111 (89%) | ||
| Vascular invasion 1 | Yes (13%, n = 19) | 7 (33%) | 12 (9%) | - |
| No (32%, n = 47) | 1 (5%) | 46 (37%) | ||
| Unknown (55%, n = 80) | 13 (62%) | 67 (54%) | ||
| Lymphatic invasion 1 | Yes (16%, n = 24) | 6 (29%) | 18 (14%) | - |
| No (28%, n = 41) | 0 (0) | 41 (33%) | ||
| Unknown (56%, n = 81) | 15 (71%) | 66 (53%) | ||
| Tumor necrosis 1 | Yes (1%, n = 1) | 1(5%) | 0 | - |
| No (31%, n = 46) | 7 (33%) | 39 (27%) | ||
| Unknown (68%, n = 99) | 13 (62%) | 86 (69%) | ||
| Number of mitoses 2 | (median) | 1 (n = 8) | 0 (n = 33) | 0.035 |
| Ki67 2 | % (median) | 1 (n = 8) | 1 (n = 33) | 0.330 |
| Desmoplasia 1 | Yes (24%, n = 35) | 9 (43%) | 31 (25%) | - |
| No (6%, n = 9) | 0 (0) | 17 (14%) | ||
| Unknown (70%, n = 102) | 12 (57%) | 77 (61%) | ||
| High risk 1 | Yes (27%, n = 40) | 4 (19%) | 4 (3%) | - |
| No (12%, n = 17) | 5 (24%) | 27 (22%) | ||
| Unknown (61%, n = 89) | 12 (57%) | 94 (75%) | ||
| Patients with MTC | Metastases 14% (n = 21) | No Metastases 86% (n = 125) | p | |
|---|---|---|---|---|
| Ct at 6–12 months 2 | pg/mL (median) | 246 (n = 16) | 26 (n = 115) | <0.001 |
| BFS 2 | Months (median) | 0 (n = 21) | 341 (n = 124) | <0.001 |
| Death 1 | Yes (16%, n = 23) | 10 (50%) | 13 (10%) | <0.001 |
| No (84%, n = 122) | 10 (50%) | 112 (90%) | ||
| Cause of death 1 | Cancer progression (48%, n = 11) | 9 (90%) | 2 (15%) | 0.001 |
| Others (52%, n = 12) | 1 (10%) | 11 (85%) | ||
| Variables | Odds Ratio [CI] | p-Valor | R Squared | −2log of Likelihood |
|---|---|---|---|---|
| Constant | 0.018 | <0.001 | 38.9 | 64.28 |
| LNR | 16.460 [2.879; 94.093] | <0.001 | ||
| Ct ≧ 500 pg/mL | 7.985 [1.571; 40.594] | 0.062 |
| Variables | Odds Ratio [CI] | p-Valor | R Squared | −2log of Likelihood |
|---|---|---|---|---|
| Constant | 0.014 | <0.001 | 20.6 | 37.959 |
| Ct ≧ 500 pg/mL | 13.846 [1.544; 124.135] | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ros-Madrid, I.; Febrero, B.; Cano-Mármol, R.P.; Ferrer-Gómez, M.; Rodríguez, J.M. Predictors of Distant Metastasis in Patients with Medullary Thyroid Carcinoma. Cancers 2025, 17, 3193. https://doi.org/10.3390/cancers17193193
Ros-Madrid I, Febrero B, Cano-Mármol RP, Ferrer-Gómez M, Rodríguez JM. Predictors of Distant Metastasis in Patients with Medullary Thyroid Carcinoma. Cancers. 2025; 17(19):3193. https://doi.org/10.3390/cancers17193193
Chicago/Turabian StyleRos-Madrid, Inmaculada, Beatriz Febrero, Rosario Paloma Cano-Mármol, Mercedes Ferrer-Gómez, and José M. Rodríguez. 2025. "Predictors of Distant Metastasis in Patients with Medullary Thyroid Carcinoma" Cancers 17, no. 19: 3193. https://doi.org/10.3390/cancers17193193
APA StyleRos-Madrid, I., Febrero, B., Cano-Mármol, R. P., Ferrer-Gómez, M., & Rodríguez, J. M. (2025). Predictors of Distant Metastasis in Patients with Medullary Thyroid Carcinoma. Cancers, 17(19), 3193. https://doi.org/10.3390/cancers17193193







