A Novel Early Memory-Enriched Allogeneic NKG2D CAR-T Cell Therapy Based on CRISPR/Cas9 Technology for Solid Tumors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Buffy Coat Samples
2.2. Peripheral Blood Mononuclear Cell (PBMC) Isolation and T-Cell Purification
2.3. Cell Lines and Culture
2.4. Flow Cytometry
2.5. CRISPR/Cas9-Mediated Double-Gene Knockout in T Cells and TCR-Negative Enrichment
2.6. Lentiviral Production and Transduction
2.7. In Vitro Cytotoxicity Assay
2.8. ELISA Assay
2.9. Karyotype Analysis
2.10. Statistics
3. Results
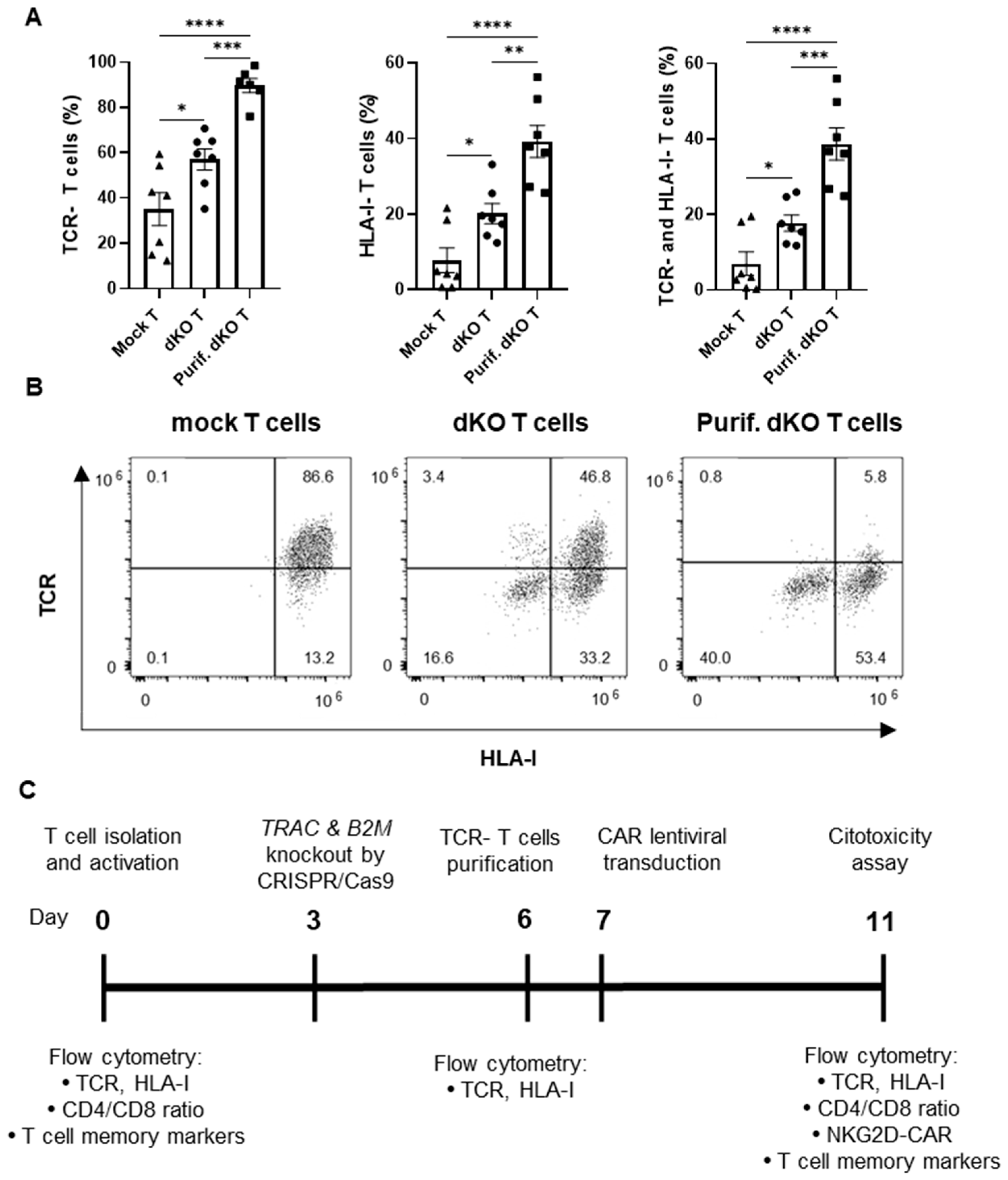
3.1. Simultaneous Disruption of TCR and HLA Class I Expression Using CRISPR/Cas9 Technology
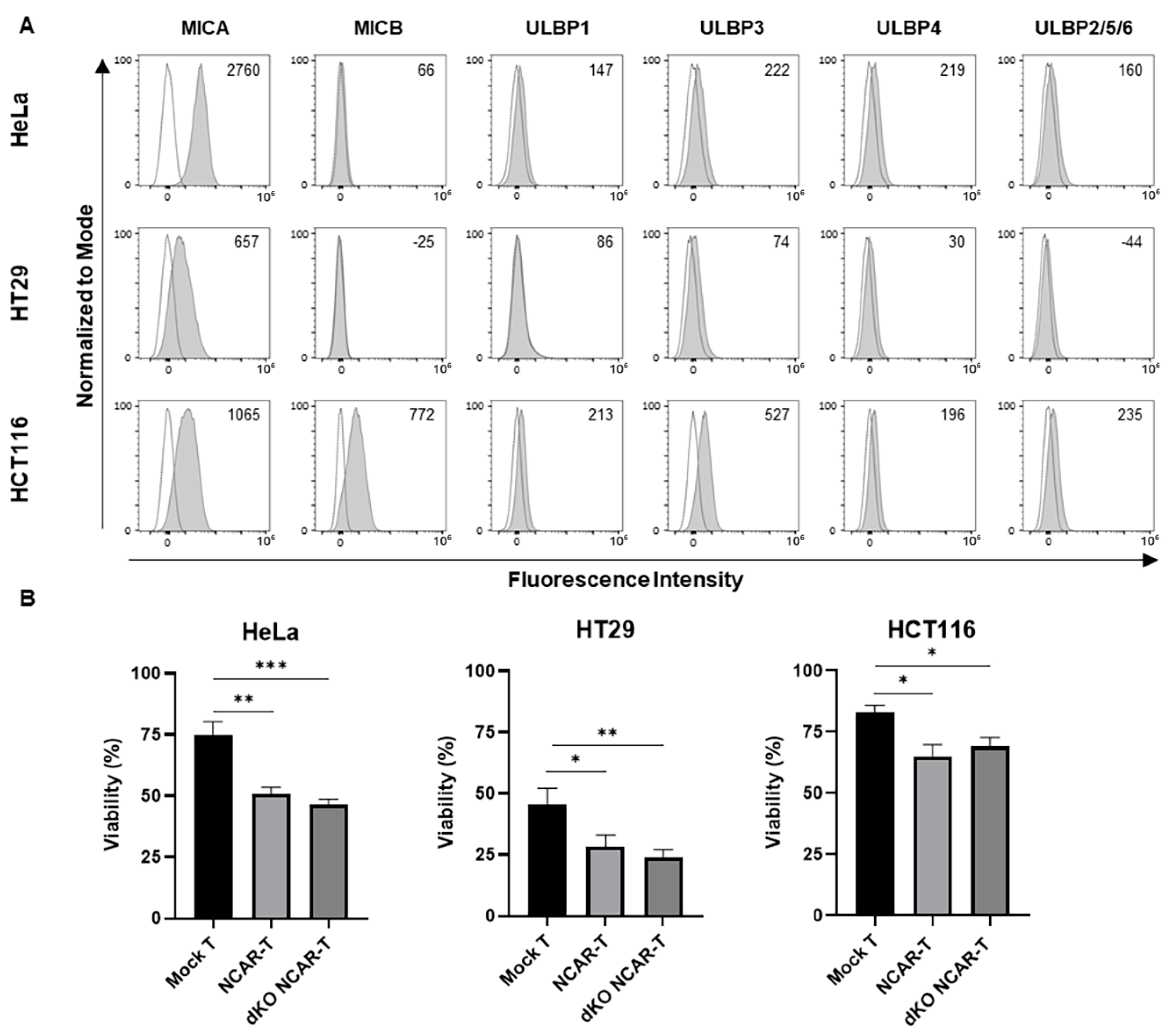
3.2. Generation and Characterization of Allogeneic CAR-T Cells Against NKG2D Ligands
3.3. In Vitro Antitumor Activity of the Allogeneic NKG2D CAR-T Cells Against Human Cervical and Colorectal Tumor Cell Lines
3.4. Effect of Cytokine Supplementation on TRAC and B2M Gene Knockouts Using CRISPR/Cas9 Technology
3.5. Comparison of the Characterization of Allogeneic NKG2D CAR-T Cells Produced with Different Cytokine Supplementations
3.6. Effect of the Different Cytokine Supplementations on the In Vitro Antitumor Activity of the Allogeneic NKG2D CAR-T Cells Against Solid Cancers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B2M | β-2 microglobulin |
| CAR | Chimeric antigen receptor |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CRISPR/Cas9 | CRISPR/Cas9 protein |
| CRS | Cytokine release syndrome |
| E:T | Effector-to-target |
| FACS | Fluorescence-Activated Cell Sorting |
| FBS | Fetal bovine serum |
| GvHD | Graft-versus-host disease |
| gRNAs | Guide RNAs |
| HLA | Human leukocyte antigen |
| HLA-I | HLA class I |
| IL | Interleukin |
| iPSC | Induced pluripotent stem cells |
| MFI | Median Fluorescence Intensity |
| MLR | Mixed lymphocyte reaction |
| NK | Natural killer |
| NKG2D | Natural-killer group 2 member D |
| NKG2D-L | NKG2D ligands |
| PBMC | Peripheral blood mononuclear cells |
| RNP | Ribonucleoprotein |
| TALEN | Transcription activator-like effector nucleases |
| Tcm | Central memory T cells |
| TCR | T-cell receptor |
| Teff | Effector T cells |
| Tem | Effector memory T cells |
| Tscm | Stem cell memory T cells |
| TU | Transducing units |
| ZFN | Zinc finger nucleases |
References
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [PubMed]
- Chen, Y.J.; Abila, B.; Mostafa Kamel, Y. CAR-T: What Is Next? Cancers 2023, 15, 663. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Gruber, I.; Arber, C. Off-the-Shelf Allogeneic T Cell Therapies for Cancer: Opportunities and Challenges Using Naturally Occurring “Universal” Donor T Cells. Front. Immunol. 2020, 11, 583716. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Jozwik, A.; Pepper, A.; Benjamin, R. Allogeneic Car-t Cells: More than Ease of Access? Cells 2018, 7, 155. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. ‘Off-the-Shelf’ Allogeneic CAR T Cells: Development and Challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Baguet, C.; Larghero, J.; Mebarki, M. Early Predictive Factors of Failure in Autologous CAR T-Cell Manufacturing and/or Efficacy in Hematologic Malignancies. Blood Adv. 2024, 8, 337–342. [Google Scholar] [CrossRef]
- Lee, J.; Sheen, J.H.; Lim, O.; Lee, Y.; Ryu, J.; Shin, D.; Kim, Y.Y.; Kim, M. Abrogation of HLA Surface Expression Using CRISPR/Cas9 Genome Editing: A Step toward Universal T Cell Therapy. Sci. Rep. 2020, 10, 17753. [Google Scholar] [CrossRef]
- Tipanee, J.; Samara-Kuko, E.; Gevaert, T.; Chuah, M.K.; VandenDriessche, T. Universal Allogeneic CAR T Cells Engineered with Sleeping Beauty Transposons and CRISPR-CAS9 for Cancer Immunotherapy. Mol. Ther. 2022, 30, 3155–3175. [Google Scholar] [CrossRef]
- Martínez Bedoya, D.; Dutoit, V.; Migliorini, D. Allogeneic CAR T Cells: An Alternative to Overcome Challenges of CAR T Cell Therapy in Glioblastoma. Front. Immunol. 2021, 12, 640082. [Google Scholar] [CrossRef]
- Aparicio, C.; Acebal, C.; González-Vallinas, M. Current Approaches to Develop “off-the-Shelf” Chimeric Antigen Receptor (CAR)-T Cells for Cancer Treatment: A Systematic Review. Exp. Hemato Oncol. 2023, 12, 73. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Fang, C.; Jiang, S.; June, C.H.; Zhao, Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin. Cancer Res. 2017, 23, 2255–2266. [Google Scholar] [CrossRef]
- Razeghian, E.; Nasution, M.K.M.; Rahman, H.S.; Gardanova, Z.R.; Abdelbasset, W.K.; Aravindhan, S.; Bokov, D.O.; Suksatan, W.; Nakhaei, P.; Shariatzadeh, S.; et al. A Deep Insight into CRISPR/Cas9 Application in CAR-T Cell-Based Tumor Immunotherapies. Stem Cell Res. Ther. 2021, 12, 428. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: First Regulatory Approvals for CRISPR-Cas9 Therapeutic Gene Editing for Sickle Cell Disease and Transfusion-Dependent β-Thalassemia. Med. Sci. Monit. 2024, 30, e944204-1. [Google Scholar]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Rodriguez, L.; Attia, N.; Gallego, I.; Mashal, M.; Maldonado, I.; Puras, G.; Pedraz, J.L. Expanding the Horizon of Transient CAR T Therapeutics Using Virus-Free Technology. Biotechnol. Adv. 2024, 72, 108350. [Google Scholar] [CrossRef] [PubMed]
- Murad, J.M.; Baumeister, S.H.; Werner, L.; Daley, H.; Trébéden-Negre, H.; Reder, J.; Sentman, C.L.; Gilham, D.; Lehmann, F.; Snykers, S.; et al. Manufacturing Development and Clinical Production of NKG2D Chimeric Antigen Receptor–Expressing T Cells for Autologous Adoptive Cell Therapy. Cytotherapy 2018, 20, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Liu, B.; Chen, X.; Huang, C.; Yang, L.; Zhang, W.; Weng, J.; Du, X.; Wu, K.; Lai, P. Optimizing CAR-T Cell Therapy for Solid Tumors: Current Challenges and Potential Strategies. J. Hematol. Oncol. 2024, 17, 105. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, L.; Chen, J. Current Advances and Challenges in CAR T-Cell Therapy for Solid Tumors: Tumor-Associated Antigens and the Tumor Microenvironment. Exp. Hematol. Oncol. 2023, 12, 14. [Google Scholar]
- Wensveen, F.M.; Jelenčić, V.; Polić, B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef]
- Sun, B.; Yang, D.; Dai, H.; Liu, X.; Jia, R.; Cui, X.; Li, W.; Cai, C.; Xu, J.; Zhao, X. Eradication of Hepatocellular Carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol. Res. 2019, 7, 1813–1823. [Google Scholar]
- Demoulin, B.; Cook, W.J.; Murad, J.; Graber, D.J.; Sentman, M.L.; Lonez, C.; Gilham, D.E.; Sentman, C.L.; Agaugue, S. Exploiting Natural Killer Group 2D Receptors for CAR T-Cell Therapy. Future Oncol. 2017, 13, 1593–1605. [Google Scholar]
- Leivas, A.; Valeri, A.; Córdoba, L.; García-Ortiz, A.; Ortiz, A.; Sánchez-Vega, L.; Graña-Castro, O.; Fernández, L.; Carreño-Tarragona, G.; Pérez, M.; et al. NKG2D-CAR-Transduced Natural Killer Cells Efficiently Target Multiple Myeloma. Blood Cancer J. 2021, 11, 146. [Google Scholar]
- Obajdin, J.; Davies, D.M.; Maher, J. Engineering of Chimeric Natural Killer Cell Receptors to Develop Precision Adoptive Immunotherapies for Cancer. Clin. Exp. Immunol. 2020, 202, 11–27. [Google Scholar] [CrossRef]
- Curio, S.; Jonsson, G.; Marinović, S. A Summary of Current NKG2D-Based CAR Clinical Trials. Immunother. Adv. 2021, 1, ltab018. [Google Scholar] [CrossRef]
- Ando, M.; Ito, M.; Srirat, T.; Kondo, T.; Yoshimura, A. Memory T Cell, Exhaustion, and Tumor Immunity. Immunol. Med. 2020, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Marton, C.; Mercier-Letondal, P.; Galaine, J.; Godet, Y. An Unmet Need: Harmonization of IL-7 and IL-15 Combination for the Ex Vivo Generation of Minimally Differentiated T Cells. Cell Immunol. 2021, 363, 104314. [Google Scholar] [PubMed]
- Kaartinen, T.; Luostarinen, A.; Maliniemi, P.; Keto, J.; Arvas, M.; Belt, H.; Koponen, J.; Loskog, A.; Mustjoki, S.; Porkka, K.; et al. Low Interleukin-2 Concentration Favors Generation of Early Memory T Cells over Effector Phenotypes during Chimeric Antigen Receptor T-Cell Expansion. Cytotherapy 2017, 19, 689–702. [Google Scholar] [CrossRef]
- Du, L.; Nai, Y.; Shen, M.; Li, T.; Huang, J.; Han, X.; Wang, W.; Pang, D.; Jin, A. IL-21 Optimizes the CAR-T Cell Preparation Through Improving Lentivirus Mediated Transfection Efficiency of T Cells and Enhancing CAR-T Cell Cytotoxic Activities. Front. Mol. Biosci. 2021, 8, 675179. [Google Scholar]
- Arcangeli, S.; Falcone, L.; Camisa, B.; De Girardi, F.; Biondi, M.; Giglio, F.; Ciceri, F.; Bonini, C.; Bondanza, A.; Casucci, M. Next-Generation Manufacturing Protocols Enriching TSCM CAR T Cells Can Overcome Disease-Specific T Cell Defects in Cancer Patients. Front. Immunol. 2020, 11, 1217. [Google Scholar]
- Oliveros, J.C.; Franch, M.; Tabas-Madrid, D.; San-León, D.; Montoliu, L.; Cubas, P.; Pazos, F. Breaking-Cas—Interactive Design of Guide RNAs for CRISPR-Cas Experiments for ENSEMBL Genomes. Nucleic Acids Res. 2016, 44, 267–271. [Google Scholar] [CrossRef]
- Song, D.G.; Ye, Q.; Santoro, S.; Fang, C.; Best, A.; Powell, D.J. Chimeric NKG2D CAR-Expressing T Cell-Mediated Attack of Human Ovarian Cancer Is Enhanced by Histone Deacetylase Inhibition. Hum. Gene Ther. 2013, 24, 295. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Rodríguez-Paredes, M.; Albrecht, M.; Sticht, C.; Stichel, D.; Gutekunst, J.; Pitea, A.; Sass, S.; Sánchez-Rivera, F.J.; Lorenzo-Bermejo, J.; et al. Epigenetically Regulated Chromosome 14q32 MiRNA Cluster Induces Metastasis and Predicts Poor Prognosis in Lung Adenocarcinoma Patients. Mol. Cancer Res. 2018, 16, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Le Gouill, S.; Di Blasi, R.; Sesques, P.; Manson, G.; Cartron, G.; Beauvais, D.; Roulin, L.; Gros, F.X.; Rubio, M.T.; et al. A Real-World Comparison of Tisagenlecleucel and Axicabtagene Ciloleucel CAR T Cells in Relapsed or Refractory Diffuse Large B Cell Lymphoma. Nat. Med. 2022, 28, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, X.; Liu, X.; Fang, C.; Jiang, S.; June, C.H.; Zhao, Y. A Versatile System for Rapid Multiplex Genome-Edited CAR T Cell Generation. Oncotarget 2017, 8, 17002–17011. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, X.; Xu, Y.; Chen, J.; Zhang, H.; Yang, Z.; Qi, Y.; Hong, J.; Li, Y.; Wang, G.; et al. Simultaneous Editing of TCR, HLA-I/II and HLA-E Resulted in Enhanced Universal CAR-T Resistance to Allo-Rejection. Front. Immunol. 2022, 13, 1052717. [Google Scholar]
- Kagoya, Y.; Guo, T.; Yeung, B.; Saso, K.; Anczurowski, M.; Wang, C.H.; Murata, K.; Sugata, K.; Saijo, H.; Matsunaga, Y.; et al. Genetic Ablation of HLA Class I, Class II, and the T-Cell Receptor Enables Allogeneic T Cells to Be Used for Adoptive T-Cell Therapy. Cancer Immunol. Res. 2020, 8, 926–936. [Google Scholar]
- Wei, W.; Chen, Z.N.; Wang, K. CRISPR/Cas9: A Powerful Strategy to Improve CAR-T Cell Persistence. Int. J. Mol. Sci. 2023, 24, 12317. [Google Scholar] [CrossRef]
- Tsuchida, C.A.; Brandes, N.; Bueno, R.; Trinidad, M.; Mazumder, T.; Yu, B.; Hwang, B.; Chang, C.; Liu, J.; Sun, Y.; et al. Mitigation of Chromosome Loss in Clinical CRISPR-Cas9-Engineered T Cells. Cell 2023, 186, 4567–4582.e20. [Google Scholar]
- Hu, Y.; Zhou, Y.; Zhang, M.; Zhao, H.; Wei, G.; Ge, W.; Cui, Q.; Mu, Q.; Chen, G.; Han, L.; et al. Genetically Modified CD7-Targeting Allogeneic CAR-T Cell Therapy with Enhanced Efficacy for Relapsed/Refractory CD7-Positive Hematological Malignancies: A Phase I Clinical Study. Cell Res. 2022, 32, 995–1007. [Google Scholar]
- Ottaviano, G.; Georgiadis, C.; Gkazi, S.A.; Syed, F.; Zhan, H.; Etuk, A.; Preece, R.; Chu, J.; Kubat, A.; Adams, S.; et al. Phase 1 Clinical Trial of CRISPR-Engineered CAR19 Universal T Cells for Treatment of Children with Refractory B Cell Leukemia. Sci. Transl. Med. 2022, 14, eabq3010. [Google Scholar] [CrossRef]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-Target Effects in CRISPR/Cas9 Gene Editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar]
- Maldonado-Pérez, N.; Tristán-Manzano, M.; Justicia-Lirio, P.; Martínez-Planes, E.; Muñoz, P.; Pavlovic, K.; Cortijo-Gutiérrez, M.; Blanco-Benítez, C.; Castella, M.; Juan, M.; et al. Efficacy and Safety of Universal (TCRKO) ARI-0001 CAR-T Cells for the Treatment of B-Cell Lymphoma. Front. Immunol. 2022, 13, 1011858. [Google Scholar] [CrossRef] [PubMed]
- Abou-el-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Till, B.G.; Bauer, G.; Savoldo, B. Scalable Manufacturing of CAR T Cells for Cancer Immunotherapy. Blood Cancer Discov. 2021, 2, 408–430. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Garcia, J.; Agliardi, G.; Roddie, C. CAR-T Cell Manufacturing Landscape-Lessons from the Past Decade and Considerations for Early Clinical Development. Mol. Ther. Methods Clin. Cevelopment 2024, 32, 101250. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Li, F.; Huang, S.; Chen, F.; Li, Y. Bright Future or Blind Alley? CAR-T Cell Therapy for Solid Tumors. Front. Immunol. 2023, 14, 1045024. [Google Scholar] [CrossRef]
- Kankeu Fonkoua, L.A.; Sirpilla, O.; Sakemura, R.; Siegler, E.L.; Kenderian, S.S. CAR T Cell Therapy and the Tumor Microenvironment: Current Challenges and Opportunities. Mol. Ther. Oncolytics 2022, 25, 69–77. [Google Scholar] [CrossRef]
- Mai, Q.; He, B.; Deng, S.; Zeng, Q.; Xu, Y.; Wang, C.; Pang, Y.; Zhang, S.; Li, J.; Zeng, J.; et al. Efficacy of NKG2D CAR-T Cells with IL-15/IL-15Rα Signaling for Treating Epstein-Barr Virus-Associated Lymphoproliferative Disorder. Exp. Hematol. Oncol. 2024, 13, 85. [Google Scholar]
- Jones, A.B.; Rocco, A.; Lamb, L.S.; Friedman, G.K.; Hjelmeland, A.B. Regulation of NKG2D Stress Ligands and Its Relevance in Cancer Progression. Cancers 2022, 14, 2339. [Google Scholar] [CrossRef]
- Prenen, H.; Dekervel, J.; Hendlisz, A.; Anguille, S.; Awada, A.; Cerf, E.; Lonez, C.; Breman, E.; Dheur, M.-S.; Alcantar-Orozco, E.; et al. Updated Data from AlloSHRINK Phase I First-in-Human Study Evaluating CYAD-101, an Innovative Non-Gene Edited Allogeneic CAR-T in MCRC. J. Clin. Oncol. 2021, 39, 74. [Google Scholar]
- Hansen, K.; Cho, C.; Kothari, N.; Shook, D.; Trager, J. Abstract 3604: NKX101, an Allogeneic off-the-Shelf NKG2D CAR-NK Cell Therapy, Has Potent in Vitro Cytotoxicity against Patient-Derived AML Leukemic Stem Cells and Non-Leukemic Stem Cell Blasts. Cancer Res. 2024, 84, 3604. [Google Scholar] [CrossRef]
- Ibáñez-Navarro, M.; Fernández, A.; Escudero, A.; Esteso, G.; Campos-Silva, C.; Navarro-Aguadero, M.Á.; Leivas, A.; Caracuel, B.R.; Rodríguez-Antolín, C.; Ortiz, A.; et al. NKG2D-CAR Memory T Cells Target Pediatric T-Cell Acute Lymphoblastic Leukemia in Vitro and in Vivo but Fail to Eliminate Leukemia Initiating Cells. Front. Immunol. 2023, 14, 1187665. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Hong, J.; Chen, S.; Chen, M.; Wang, L.; Lin, W.; Ye, Y. Interleukin-15 and Chemokine Ligand 19 Enhance Cytotoxic Effects of Chimeric Antigen Receptor T Cells Using Zebrafish Xenograft Model of Gastric Cancer. Front. Immunol. 2022, 13, 1002361. [Google Scholar] [CrossRef]
- Wei, C.; Xia, K.; Xie, Y.; Ye, S.; Ding, Y.; Liu, Z.; Zheng, R.; Long, J.; Wei, Q.; Li, Y.; et al. Combination of 4-1BB and DAP10 Promotes Proliferation and Persistence of NKG2D(Bbz) CAR-T Cells. Front. Oncol. 2022, 12, 893124. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Leise, J.; Priesner, C.; Aktas, M.; Apel, M.; Assenmacher, M.; Bürger, I.; Richter, A.; Altefrohne, P.; Schubert, C.; et al. Automated Manufacturing and Characterization of Clinical Grade Autologous CD20 CAR T Cells for the Treatment of Patients with Stage III/IV Melanoma. Front. Immunol. 2024, 15, 1328368. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Lam, N.; Vanasse, D.; Shen, Y.; Rose, J.J.; Rossi, J.; Xue, A.; Bot, A.; Scholler, N.; Mikkilineni, L.; et al. Safety and Feasibility of Anti-CD19 CAR T Cells with Fully-Human Binding Domains in Patients with B-Cell Lymphoma. Nat. Med. 2020, 26, 270. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fernández, C.; Escribà-Garcia, L.; Caballero, A.C.; Escudero-López, E.; Ujaldón-Miró, C.; Montserrat-Torres, R.; Pujol-Fernández, P.; Sierra, J.; Briones, J. Memory Stem T Cells Modified with a Redesigned CD30-Chimeric Antigen Receptor Show an Enhanced Antitumor Effect in Hodgkin Lymphoma. Clin. Transl. Immunol. 2021, 10, e1268. [Google Scholar] [CrossRef]
- Arcangeli, S.; Bove, C.; Mezzanotte, C.; Camisa, B.; Falcone, L.; Manfredi, F.; Bezzecchi, E.; El Khoury, R.; Norata, R.; Sanvito, F.; et al. CAR T Cell Manufacturing from Naive/Stem Memory T Lymphocytes Enhances Antitumor Responses While Curtailing Cytokine Release Syndrome. J. Clin. Invest. 2022, 132, e150807. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Ramos, C.A.; Durett, A.; Liu, E.; Dakhova, O.; Liu, H.; Creighton, C.J.; Gee, A.P.; Heslop, H.E.; et al. Closely Related T-Memory Stem Cells Correlate with in Vivo Expansion of CAR.CD19-T Cells and Are Preserved by IL-7 and IL-15. Blood 2014, 123, 3750–3759. [Google Scholar] [CrossRef]
- Hoffmann, J.M.; Schubert, M.L.; Wang, L.; Hückelhoven, A.; Sellner, L.; Stock, S.; Schmitt, A.; Kleist, C.; Gern, U.; Loskog, A.; et al. Differences in Expansion Potential of Naive Chimeric Antigen Receptor T Cells from Healthy Donors and Untreated Chronic Lymphocytic Leukemia Patients. Front. Immunol. 2018, 8, 1956. [Google Scholar] [CrossRef]
- Alvarez-Fernández, C.; Escribà-Garcia, L.; Vidal, S.; Sierra, J.; Briones, J. A Short CD3/CD28 Costimulation Combined with IL-21 Enhance the Generation of Human Memory Stem T Cells for Adoptive Immunotherapy. J. Transl. Med. 2016, 14, 214. [Google Scholar] [CrossRef]
- Chi, X.; Luo, S.; Ye, P.; Hwang, W.L.; Cha, J.H.; Yan, X.; Yang, W.H. T-Cell Exhaustion and Stemness in Antitumor Immunity: Characteristics, Mechanisms, and Implications. Front. Immunol. 2023, 14, 1104771. [Google Scholar] [CrossRef]
- Boulch, M.; Cazaux, M.; Loe-Mie, Y.; Thibaut, R.; Corre, B.; Lemaître, F.; Grandjean, C.L.; Garcia, Z.; Bousso, P. A Cross-Talk between CAR T Cell Subsets and the Tumor Microenvironment Is Essential for Sustained Cytotoxic Activity. Sci. Immunol. 2021, 6, eabd434. [Google Scholar] [CrossRef]
- Boulch, M.; Cazaux, M.; Cuffel, A.; Ruggiu, M.; Allain, V.; Corre, B.; Loe-Mie, Y.; Hosten, B.; Cisternino, S.; Auvity, S.; et al. A Major Role for CD4+ T Cells in Driving Cytokine Release Syndrome during CAR T Cell Therapy. Cell Rep. Med. 2023, 4, 101161. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.; van Steenbergen, S.C.; Sleijfer, S.; Debets, R.; Lamers, C.H. T Cell Maturation Stage Prior to and During GMP Processing Informs on CAR T Cell Expansion in Patients. Front. Immunol. 2016, 7, 648. [Google Scholar] [CrossRef] [PubMed]
- Obajdin, J.; Larcombe-Young, D.; Glover, M.; Kausar, F.; Hull, C.M.; Flaherty, K.R.; Tan, G.; Beatson, R.E.; Dunbar, P.; Mazza, R.; et al. Solid Tumor Immunotherapy Using NKG2D-Based Adaptor CAR T Cells. Cell Rep. Med. 2024, 5, 101827. [Google Scholar] [CrossRef] [PubMed]
- Breman, E.; Demoulin, B.; Agaugué, S.; Mauën, S.; Michaux, A.; Springuel, L.; Houssa, J.; Huberty, F.; Jacques-Hespel, C.; Marchand, C.; et al. Overcoming Target Driven Fratricide for T Cell Therapy. Front. Immunol. 2018, 9, 2940. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y.; Chan, G.C.; Chan, W.K. Designs of NKG2D-based immunotherapeutics for cancer. Front. Immunol. 2025, 16, 1557644. [Google Scholar] [CrossRef]
- Han, Y.; Xie, W.; Song, D.G.; Powell, D.J. Control of Triple-Negative Breast Cancer Using Ex Vivo Self-Enriched, Costimulated NKG2D CAR T Cells. J. Hematol. Oncol. 2018, 11, 92. [Google Scholar] [CrossRef]
- Ieranò, C.; Righelli, D.; D’Alterio, C.; Napolitano, M.; Portella, L.; Rea, G.; Auletta, F.; Santagata, S.; Trotta, A.M.; Guardascione, G.; et al. In PD-1+ human colon cancer cells NIVOLUMAB promotes survival and could protect tumor cells from conventional therapies. J. Immunother. Cancer 2022, 10, e004032. [Google Scholar] [CrossRef]
- McGilvray, R.W.; Eagle, R.A.; Watson, N.F.S.; Al-Attar, A.; Ball, G.; Jafferji, I.; Trowsdale, J.; Durrant, L.G. NKG2D Ligand Expression in Human Colorectal Cancer Reveals Associations with Prognosis and Evidence for Immunoediting. Clin. Cancer Res. 2009, 15, 6993–7002. [Google Scholar] [CrossRef]
- Cho, H.; Chung, J.Y.; Kim, S.; Braunschweig, T.; Kang, T.H.; Kim, J.; Chung, E.J.; Hewitt, S.M.; Kim, J.H. MICA/B and ULBP1 NKG2D Ligands Are Independent Predictors of Good Prognosis in Cervical Cancer. BMC Cancer 2014, 14, 957. [Google Scholar]
- Su, M.; Chen, L.; Xie, L.; Fleurie, A.; Jonquieres, R.; Cao, Q.; Li, B.; Liang, J.; Tang, Y. Identification of early predictive biomarkers for severe cytokine release syndrome in pediatric patients with chimeric antigen receptor T-cell therapy. Front. Immunol. 2024, 15, 1450173. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, Y.; Zhang, M.; Ge, W.; Li, Y.; Yang, L.; Wei, G.; Han, L.; Wang, H.; Yu, S.; et al. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-Targeted CAR-T Cell Therapy for Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2021, 27, 2764–2772. [Google Scholar]
- Mailankody, S.; Matous, J.V.; Chhabra, S.; Liedtke, M.; Sidana, S.; Oluwole, O.O.; Malik, S.; Nath, R.; Anwer, F.; Cruz, J.C.; et al. Allogeneic BCMA-Targeting CAR T Cells in Relapsed/Refractory Multiple Myeloma: Phase 1 UNIVERSAL Trial Interim Results. Nat. Med. 2023, 29, 422–429. [Google Scholar] [PubMed]
- Stenger, D.; Stief, T.A.; Kaeuferle, T.; Willier, S.; Rataj, F.; Schober, K.; Vick, B.; Lotfi, R.; Wagner, B.; Grünewald, T.G.P.; et al. Endogenous TCR Promotes in Vivo Persistence of CD19-CAR-T Cells Compared to a CRISPR/Cas9-Mediated TCR Knockout CAR. Blood 2020, 136, 1407–1418. [Google Scholar] [CrossRef]
- Martin, K.E.; Hammer, Q.; Perica, K.; Sadelain, M.; Malmberg, K.J. Engineering immune-evasive allogeneic cellular immunotherapies. Nat. Rev. Immunol. 2024, 24, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, B.; Wu, Z.; Bo, J.; Tong, C.; Chen, D.; Wang, J.; Wang, H.; Wang, Y.; Han, W. Mutant B2M-HLA-E and B2M-HLA-G Fusion Proteins Protects Universal Chimeric Antigen Receptor-Modified T Cells from Allogeneic NK Cell-Mediated Lysis. Eur. J. Immunol. 2021, 51, 2513–2521. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Agbor-Enoh, S.; Jang, M.K.; Alawi, M.; Saygi, C.; Gravina, A.; Tediashvili, G.; Nguyen, V.Q.; Liu, Y.; et al. The SIRPα–CD47 Immune Checkpoint in NK Cells. J. Exp. Med. 2021, 218, e20200839. [Google Scholar] [CrossRef]
- Jo, S.; Das, S.; Williams, A.; Chretien, A.S.; Pagliardini, T.; Le Roy, A.; Fernandez, J.P.; Le Clerre, D.; Jahangiri, B.; Chion-Sotinel, I.; et al. Endowing Universal CAR T-Cell with Immune-Evasive Properties Using TALEN-Gene Editing. Nat. Commun. 2022, 13, 3453. [Google Scholar]
- Chen, X.; Tan, B.; Xing, H.; Zhao, X.; Ping, Y.; Zhang, Z.; Huang, J.; Shi, X.; Zhang, N.; Lin, B.; et al. Allogeneic CAR-T cells with of HLA-A/B and TRAC disruption exhibit promising antitumor capacity against B cell malignancies. Cancer Immunol. Immunother. 2024, 73, 13. [Google Scholar] [CrossRef]




| Primer | Sequence (5′-3′) |
|---|---|
| TRAC_gRNA_Forward | 5′-TAATACGACTCACTATAGTCAGGGTTCTGGATATCTG-3′ |
| TRAC_gRNA_Reverse | 5′-TTCTAGCTCTAAAACACAGATATCCAGAACCCTGA-3′ |
| B2M_gRNA_Forward | 5′-TAATACGACTCACTATAGGCGAGCACAGCTAAGGCC-3′ |
| B2M_gRNA_Reverse | 5′-TTCTAGCTCTAAAACTGGCCTTAGCTGTGCTCGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio, C.; Queipo, M.; Belver, M.; Espeso, F.; Serna-Pérez, J.; Enríquez-Rodríguez, L.; Acebal, C.; Martín-Muñoz, Á.; Valeri, A.; Leivas, A.; et al. A Novel Early Memory-Enriched Allogeneic NKG2D CAR-T Cell Therapy Based on CRISPR/Cas9 Technology for Solid Tumors. Cancers 2025, 17, 3186. https://doi.org/10.3390/cancers17193186
Aparicio C, Queipo M, Belver M, Espeso F, Serna-Pérez J, Enríquez-Rodríguez L, Acebal C, Martín-Muñoz Á, Valeri A, Leivas A, et al. A Novel Early Memory-Enriched Allogeneic NKG2D CAR-T Cell Therapy Based on CRISPR/Cas9 Technology for Solid Tumors. Cancers. 2025; 17(19):3186. https://doi.org/10.3390/cancers17193186
Chicago/Turabian StyleAparicio, Cristina, Mónica Queipo, Marina Belver, Francisco Espeso, Julia Serna-Pérez, Lucía Enríquez-Rodríguez, Carlos Acebal, Álvaro Martín-Muñoz, Antonio Valeri, Alejandra Leivas, and et al. 2025. "A Novel Early Memory-Enriched Allogeneic NKG2D CAR-T Cell Therapy Based on CRISPR/Cas9 Technology for Solid Tumors" Cancers 17, no. 19: 3186. https://doi.org/10.3390/cancers17193186
APA StyleAparicio, C., Queipo, M., Belver, M., Espeso, F., Serna-Pérez, J., Enríquez-Rodríguez, L., Acebal, C., Martín-Muñoz, Á., Valeri, A., Leivas, A., Río, P., Powell, D. J., Jr., Lobo-Valentín, R., Arrabal, D., Martínez-López, J., Sánchez, A., de la Fuente, M. Á., & González-Vallinas, M. (2025). A Novel Early Memory-Enriched Allogeneic NKG2D CAR-T Cell Therapy Based on CRISPR/Cas9 Technology for Solid Tumors. Cancers, 17(19), 3186. https://doi.org/10.3390/cancers17193186









