Challenges and Opportunities in High-Grade Glioma Management and Imaging-Based Response Monitoring During Novel Immunotherapies
Simple Summary
Abstract
1. Introduction
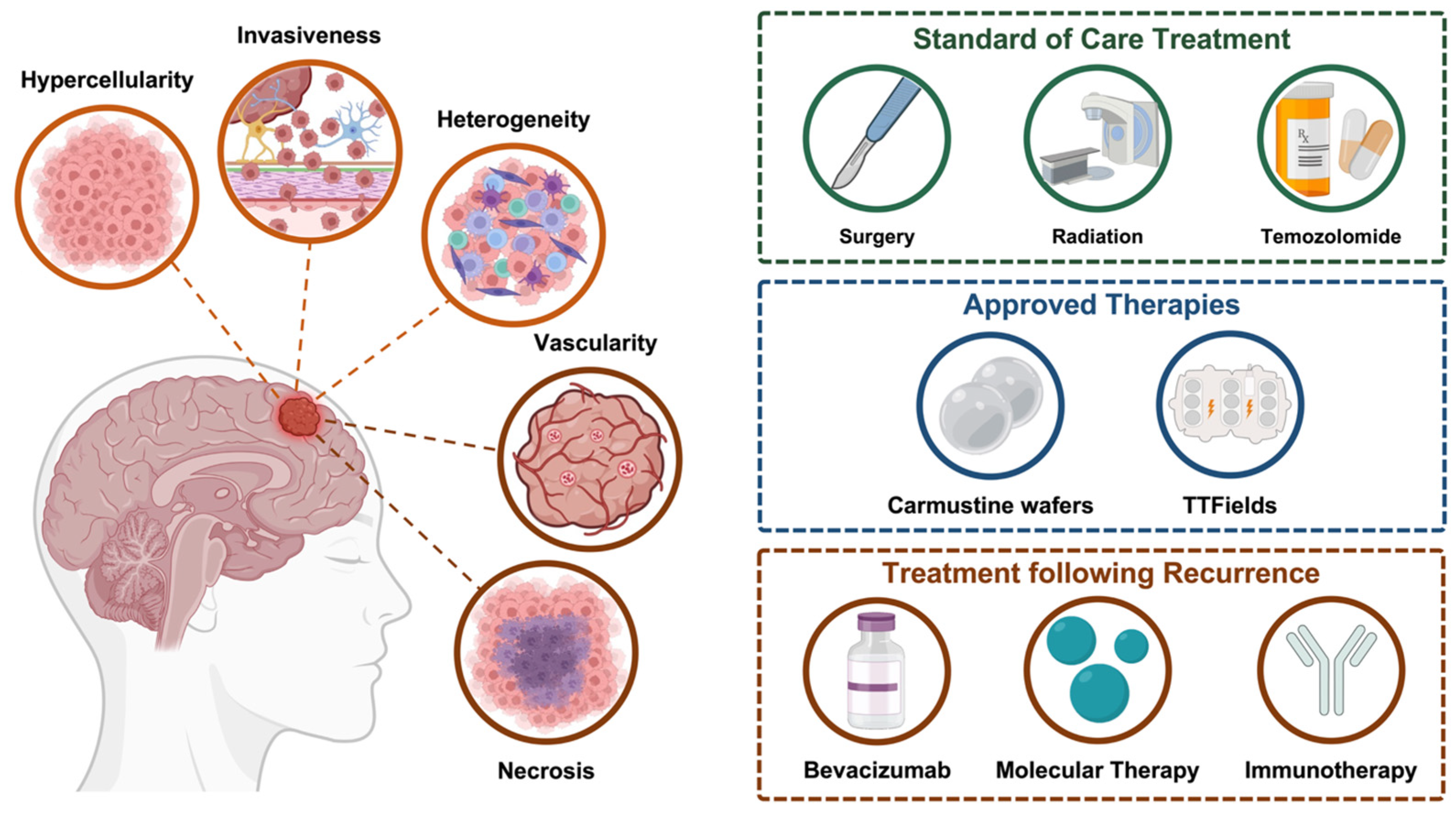
2. High-Grade Glioma Classification and Key Biological Characteristics
3. Current Landscape for High-Grade Glioma Treatment
3.1. Conventional Therapeutic Approaches for High-Grade Glioma and Their Challenges
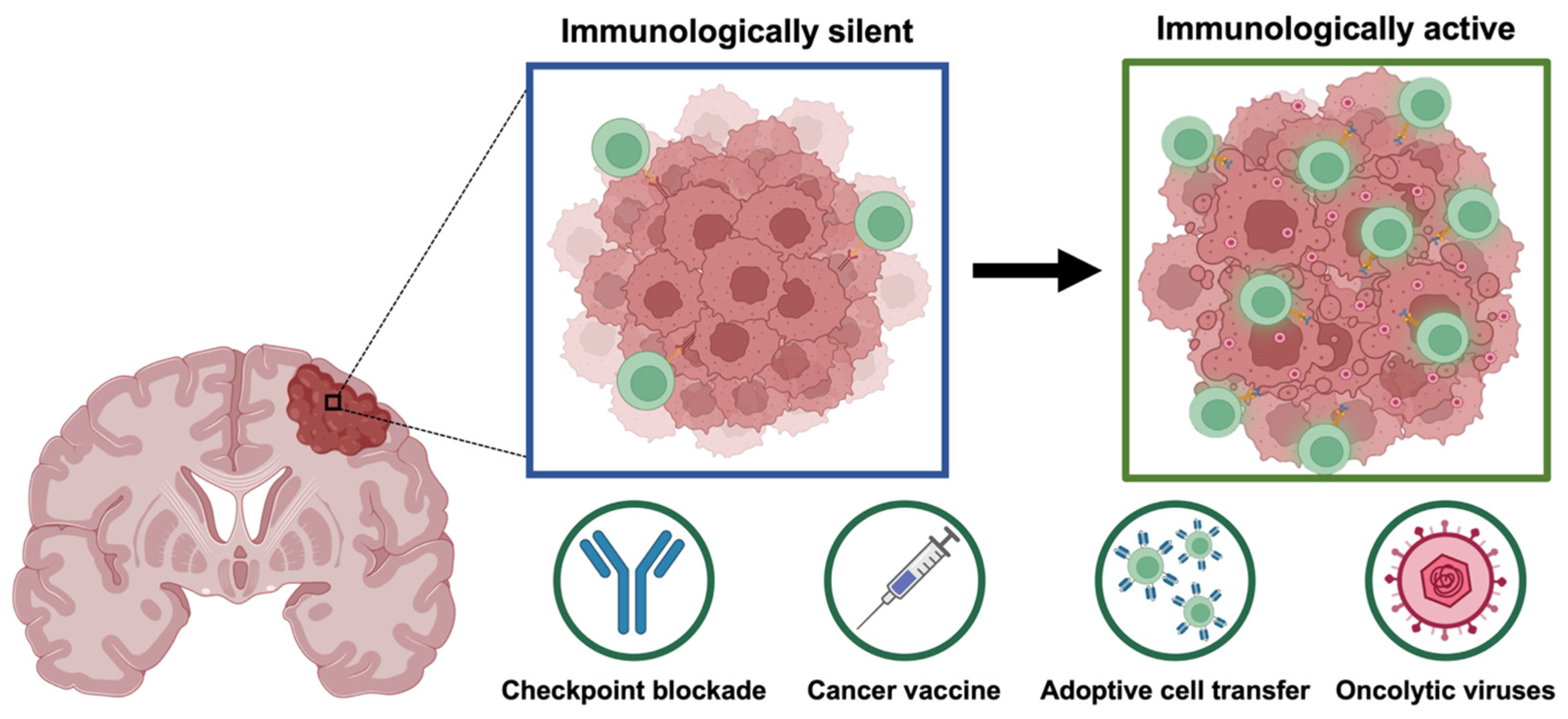
3.2. Novel Immunotherapeutic Approaches for High-Grade Gliomas
3.3. Oncolytic Herpes Simplex Virus and Its Potential for High-Grade Glioma Treatment
4. Conventional Imaging Approaches for High-Grade Glioma Response Assessment
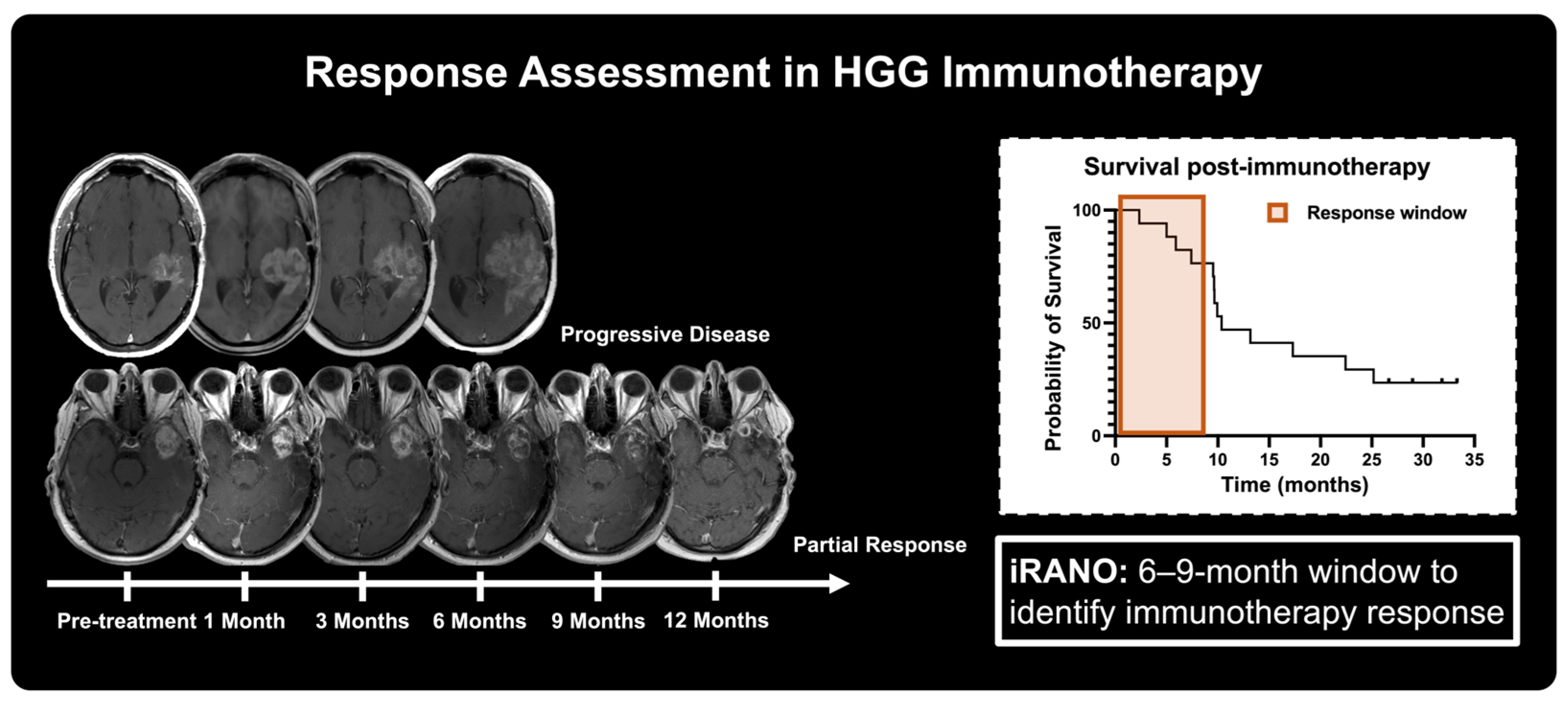
4.1. Standard of Care Magnetic Resonance Imaging in Immunotherapy Response
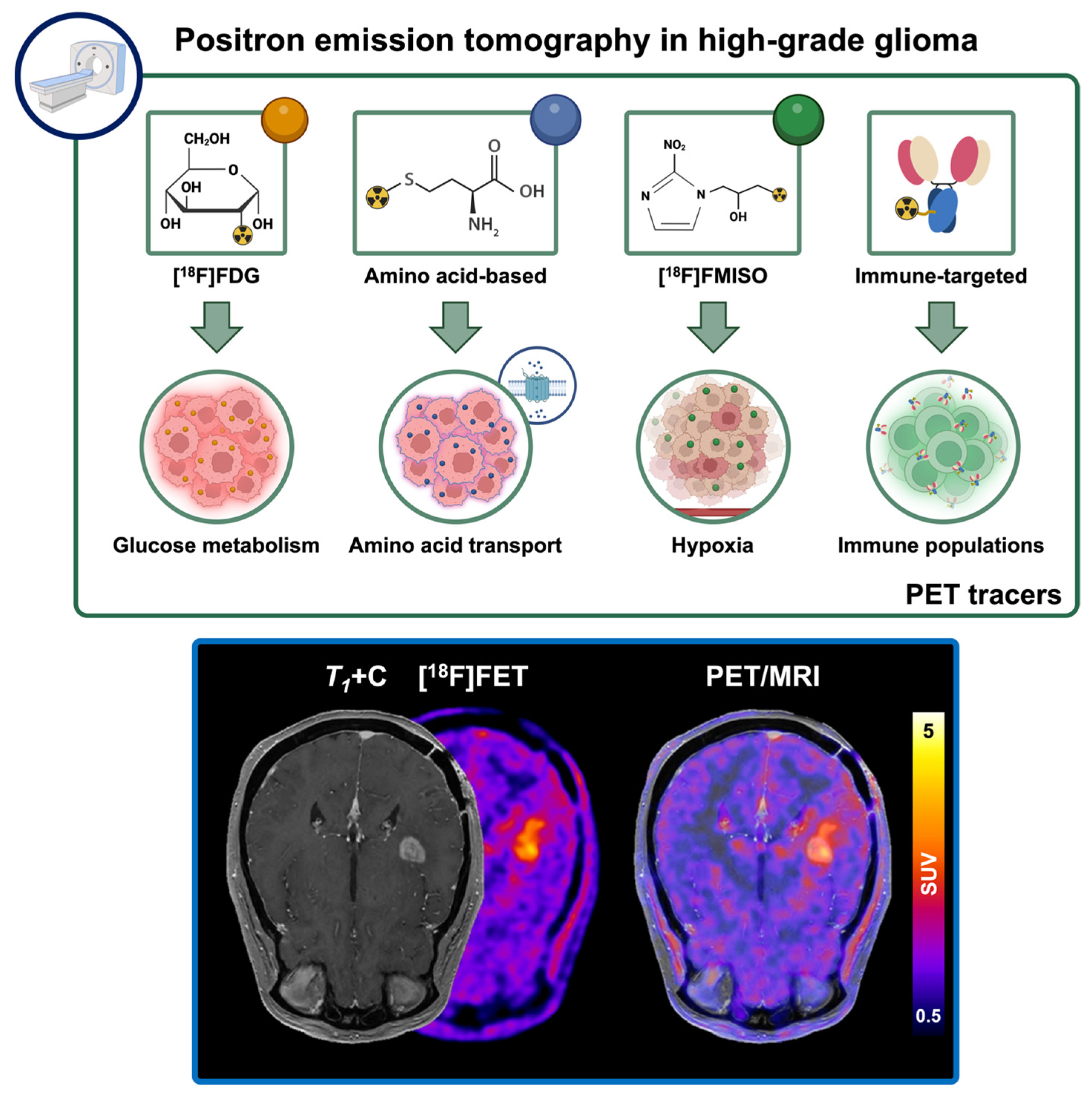
4.2. Conventional Positron Emission Tomography Approaches in High-Grade Glioma Immunotherapy
5. Advanced Imaging Approaches for High-Grade Glioma Response Assessment
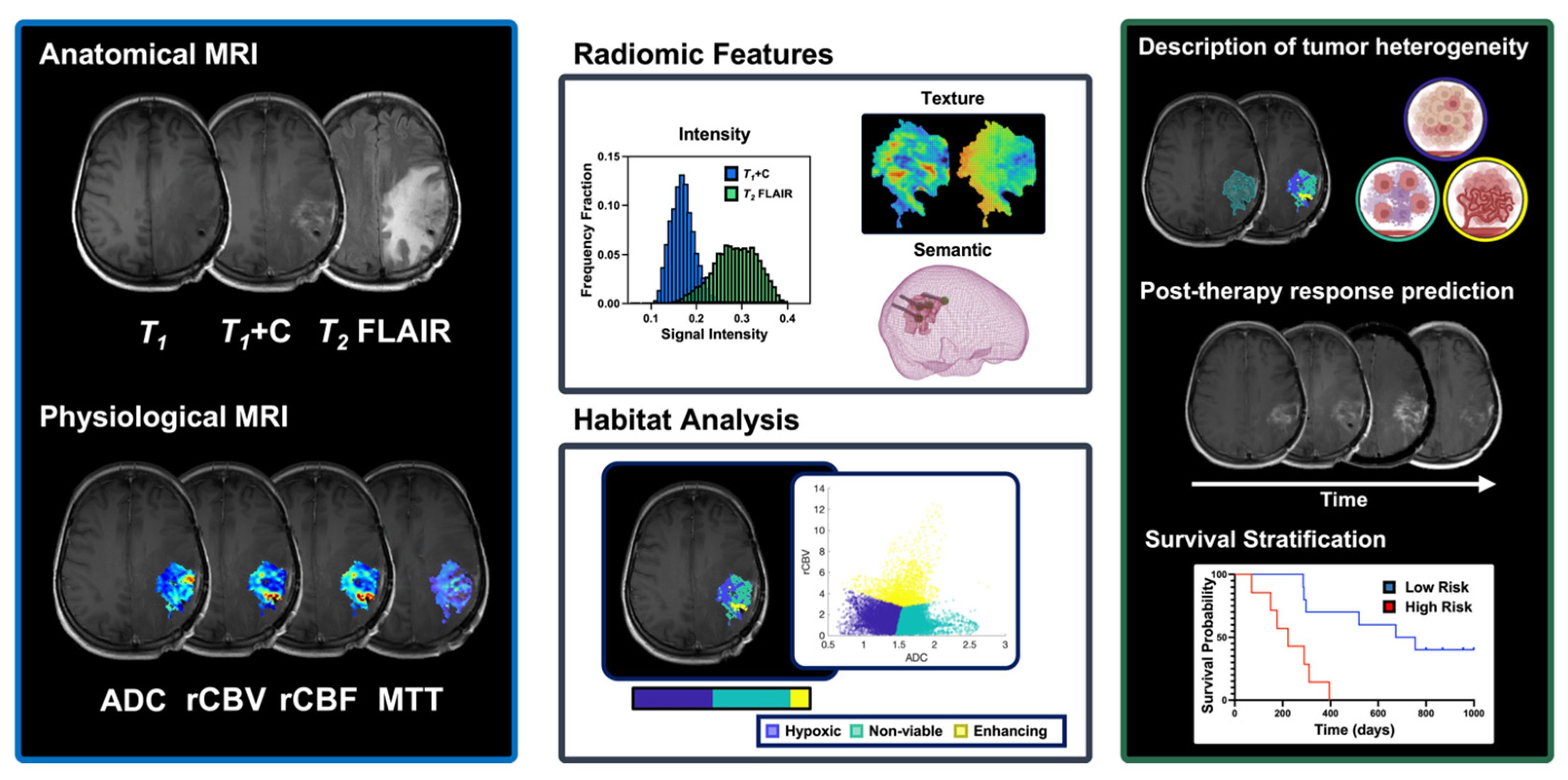
5.1. Biological MRI-Derived Markers and Their Role in Therapeutic Response Assessment
5.2. MRI-Based Multiparametric Assessment of Intratumoral Heterogeneity and Its Potential in Immunotherapy Response
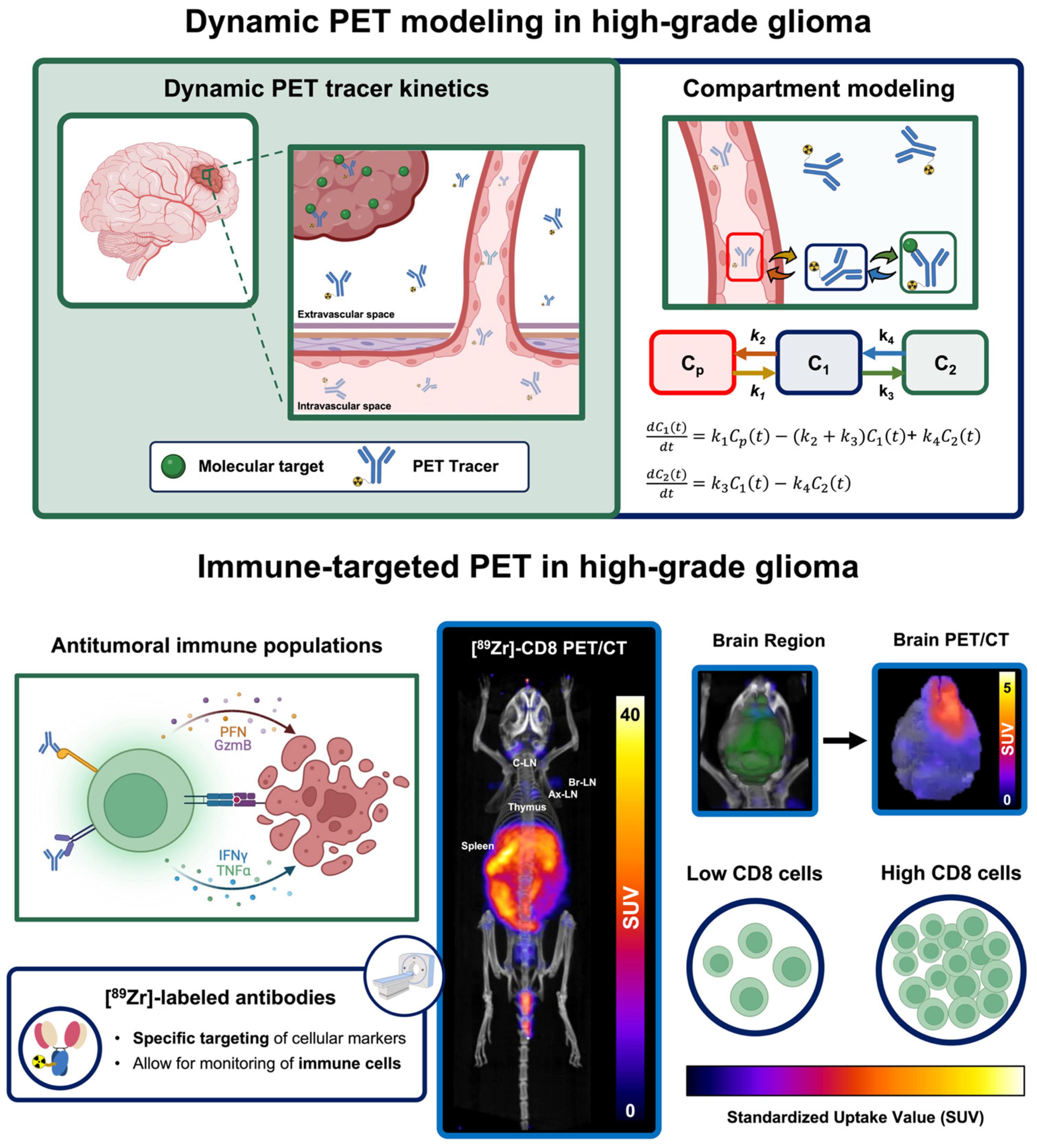
5.3. Dynamic PET Imaging to Enhance Tumor Characterization via Mathematical Modeling
5.4. Immune-Targeted PET Imaging of Populations Driving Immunotherapy Response
6. Current Outlook and Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| [11C]MET | [11C]methionine |
| [18F]FDOPA | [18F]fluorodihydroxyphenylalanine |
| [18F]FDG | [18F]Fluorodeoxyglucose |
| [18F]FET | [18F]fluoroethyl-L-tyrosine |
| [18F]FMISO | [18F]fluoromisonidazole |
| ADC | apparent diffusion coefficient |
| ADT | adoptive cell transfer |
| BBB | blood–brain barrier |
| CAR | chimeric antigen receptor |
| CD3 | cluster of differentiation 3 |
| CNS | Central Nervous System |
| CT | computed tomography |
| DAMP | damage-associated molecular pattern |
| DC | dendritic cell |
| DCE | dynamic contrast enhanced |
| DSC | dynamic susceptibility contrast |
| DWI | diffusion-weighted imaging |
| EGFR | epidermal growth factor receptor |
| EGFRvIII | epidermal growth factor receptor variant III |
| EOR | extend of resection |
| FGFR | fibroblast growth factor receptor |
| FLAIR | fluid attenuation inversion recovery |
| GBM | glioblastoma |
| GSC | glioma stem cell |
| GZP | granzyme B specific positron emission tomography imaging agent |
| HER2 | human epidermal growth factor receptor 2 |
| HGG | high-grade glioma |
| ICB | immune checkpoint blockade |
| IL | interleukin |
| iRANO | immune response assessment in neuro-oncology |
| iRECIST | immune response evaluation criteria in solid tumors |
| kep | efflux transfer constant |
| Ktrans | influx transfer constant |
| MRI | magnetic resonance imaging |
| MTT | mean transit time |
| NK | natural killer |
| oHSV | oncolytic herpes simplex virus |
| OV | oncolytic virus |
| PAMP | pathogen-associated molecular pattern |
| PD-1 | programmed death-1 |
| PDGFR | platelet-derived growth factor receptor |
| PET | positron emission tomography |
| PET-RANO | positron emission tomography-based response assessment criteria in neuro-oncology |
| PSMA | prostate specific membrane antigen |
| PWI | perfusion-weighted imaging |
| rADC | relative apparent diffusion coefficient |
| RANO | response assessment in neuro-oncology |
| rCBF | relative cerebral blood flow |
| rCBV | relative cerebral blood volume |
| RECIST | response evaluation criteria in solid tumors |
| SUV | standardized uptake value |
| T-VEC | tamilogene laherparepvec |
| TAC | time-activity curve |
| TBR | target-to-background ratio |
| TME | tumor microenvironment |
| TMZ | temozolomide |
| Tregs | T regulatory cells |
| TTP | time to peak |
| ve | extracellular-extravascular space |
| VEGF | vascular endothelial growth factor |
| vp | intravascular space |
| WHO | World Health Organization |
References
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro Oncol. 2024, 26, vi1–vi85. [Google Scholar] [CrossRef]
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Guo, X.; Gu, L.; Li, Y.; Zheng, Z.; Chen, W.; Wang, Y.; Wang, Y.; Xing, H.; Shi, Y.; Liu, D.; et al. Histological and Molecular Glioblastoma, IDH-Wildtype: A Real-World Landscape Using the 2021 WHO Classification of Central Nervous System Tumors. Front. Oncol. 2023, 13, 1200815. [Google Scholar] [CrossRef]
- Wang, L.M.; Englander, Z.K.; Miller, M.L.; Bruce, J.N. Malignant Glioma. Adv. Exp. Med. Biol. 2023, 1405, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Comba, A.; Faisal, S.M.; Varela, M.L.; Hollon, T.; Al-Holou, W.N.; Umemura, Y.; Nunez, F.J.; Motsch, S.; Castro, M.G.; Lowenstein, P.R. Uncovering Spatiotemporal Heterogeneity of High-Grade Gliomas: From Disease Biology to Therapeutic Implications. Front. Oncol. 2021, 11, 703764. [Google Scholar] [CrossRef]
- Barthel, L.; Hadamitzky, M.; Dammann, P.; Schedlowski, M.; Sure, U.; Thakur, B.K.; Hetze, S. Glioma: Molecular Signature and Crossroads with Tumor Microenvironment. Cancer Metastasis Rev. 2022, 41, 53–75. [Google Scholar] [CrossRef]
- Rabah, N.; Ait Mohand, F.-E.; Kravchenko-Balasha, N. Understanding Glioblastoma Signaling, Heterogeneity, Invasiveness, and Drug Delivery Barriers. Int. J. Mol. Sci. 2023, 24, 14256. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef]
- Hu, L.S.; D’Angelo, F.; Weiskittel, T.M.; Caruso, F.P.; Fortin Ensign, S.P.; Blomquist, M.R.; Flick, M.J.; Wang, L.; Sereduk, C.P.; Meng-Lin, K.; et al. Integrated Molecular and Multiparametric MRI Mapping of High-Grade Glioma Identifies Regional Biologic Signatures. Nat. Commun. 2023, 14, 6066. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging Therapies for Glioblastoma: Current State and Future Directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A Systematic Review and Meta-Analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Bonosi, L.; Marrone, S.; Benigno, U.E.; Buscemi, F.; Musso, S.; Porzio, M.; Silven, M.P.; Torregrossa, F.; Grasso, G. Maximal Safe Resection in Glioblastoma Surgery: A Systematic Review of Advanced Intraoperative Image-Guided Techniques. Brain Sci. 2023, 13, 216. [Google Scholar] [CrossRef]
- Ndirangu, B.; Bryan, K.; Nduom, E. Extent of Resection and Outcomes of Patients with Primary Malignant Brain Tumors. Curr. Treat. Options Oncol. 2023, 24, 1948–1961. [Google Scholar] [CrossRef]
- Role of Surgical Resection in Low- and High-Grade Gliomas|Current Treatment Options in Neurology. Available online: https://link.springer.com/article/10.1007/s11940-014-0284-7 (accessed on 17 March 2025).
- Bex, A.; Mathon, B. Advances, Technological Innovations, and Future Prospects in Stereotactic Brain Biopsies. Neurosurg. Rev. 2022, 46, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, F.; Jin, L.; Wu, J. Evolution of Neurosurgical Robots: Historical Progress and Future Direction. World Neurosurg. 2024, 191, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Porto Junior, S.; Meira, D.A.; da Cunha, B.L.B.; Fontes, J.H.M.; Pustilnik, H.N.; Medrado Nunes, G.S.; Cerqueira, G.A.; Vassoler, M.E.M.; Monteiro, P.Q.; da Silva da Paz, M.G.; et al. Robot-Assisted Stereotactic Brain Biopsy: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2024, 47, 886. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.C.; Oliveira, F.T.; Carvalho, D.D.F.; Zampirolo, F.B.; Garcia, A.G.P.V.; Larcipretti, A.L.L.; Meneses, A.C.; de Castro, I.C.S.; Ferreira, M.Y.; Oberman, D.Z.; et al. Robot-Assisted versus Manually Guided Stereotactic Biopsy for Intracranial Lesions—A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2024, 47, 880. [Google Scholar] [CrossRef]
- Audette, M.A.; Bordas, S.P.A.; Blatt, J.E. Robotically Steered Needles: A Survey of Neurosurgical Applications and Technical Innovations. Robot. Surg. 2020, 7, 1–23. [Google Scholar] [CrossRef]
- Taha, B.R.; Osswald, C.R.; Rabon, M.; Sandoval-Garcia, C.; Guillaume, D.J.; Wong, X.; Venteicher, A.S.; Darrow, D.P.; Park, M.C.; McGovern, R.A.; et al. Learning Curve Associated with ClearPoint Neuronavigation System: A Case Series. World Neurosurg. X 2022, 13, 100115. [Google Scholar] [CrossRef]
- Schumacher, X.; Hudelist, B.; Paun, L.; Benzakoun, J.; Demasi, M.; Hamza, M.; Roux, A.; Moiraghi, A.; Elia, A.; Parraga, E.; et al. Prevalence and Risk Factors of Nonyield Brain Biopsy: A 21-Year Experience with Robot-Assisted Stereotactic Biopsies. J. Neurosurg. 2025. publish before print. [Google Scholar] [CrossRef] [PubMed]
- Deboeuf, L.; Moiraghi, A.; Debacker, C.; Peeters, S.M.; Simboli, G.A.; Roux, A.; Dezamis, E.; Oppenheim, C.; Chretien, F.; Pallud, J.; et al. Feasibility and Accuracy of Robot-Assisted, Stereotactic Biopsy Using 3-Dimensional Intraoperative Imaging and Frameless Registration Tool. Neurosurgery 2023, 92, 803. [Google Scholar] [CrossRef]
- Gurses, M.E.; Khalafallah, A.M.; Gecici, N.N.; Gökalp, E.; Shah, K.H.; DeLong, C.A.; Susic, N.; Brochu, B.; Lu, V.M.; Shah, A.H.; et al. The Safety, Accuracy, and Feasibility of Robotic Assistance in Neuro-Oncological Surgery. Neurosurg. Focus 2024, 57, E3. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Ricciardi, L.; Manini, I.; Cesselli, D.; Trungu, S.; Piazza, A.; Mangraviti, A.; Miscusi, M.; Raco, A.; Ius, T. Carmustine Wafers Implantation in Patients with Newly Diagnosed High Grade Glioma: Is It Still an Option? Front. Neurol. 2022, 13, 884158. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Dobelbower, M.C.; Burnett III, O.L.; Nordal, R.A.; Nabors, L.B.; Markert, J.M.; Hyatt, M.D.; Fiveash, J.B. Patterns of Failure for Glioblastoma Multiforme Following Concurrent Radiation and Temozolomide. J. Med. Imaging Radiat. Oncol. 2011, 55, 77–81. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, X.; Zhang, B.; He, H.; Shui, Y.; Xu, W.; Jiang, C.; Shen, L.; Wei, Q. Recurrence Patterns in Patients with High-Grade Glioma Following Temozolomide-Based Chemoradiotherapy. Mol. Clin. Oncol. 2016, 5, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Faustino, A.C.; Viani, G.A.; Hamamura, A.C. Patterns of Recurrence and Outcomes of Glioblastoma Multiforme Treated with Chemoradiation and Adjuvant Temozolomide. Clinics 2020, 75, e1553. [Google Scholar] [CrossRef]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarrán, V.; Alía, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martín, M.; Fernandez, E.; et al. Recurrent Glioblastoma: A Review of the Treatment Options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef]
- Oppenlander, M.E.; Wolf, A.B.; Snyder, L.A.; Bina, R.; Wilson, J.R.; Coons, S.W.; Ashby, L.S.; Brachman, D.; Nakaji, P.; Porter, R.W.; et al. An Extent of Resection Threshold for Recurrent Glioblastoma and Its Risk for Neurological Morbidity. J. Neurosurg. 2014, 120, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Furtak, J.; Kwiatkowski, A.; Śledzińska, P.; Bebyn, M.; Krajewski, S.; Szylberg, T.; Birski, M.; Druszcz, A.; Krystkiewicz, K.; Gasiński, P.; et al. Survival after Reoperation for Recurrent Glioblastoma Multiforme: A Prospective Study. Surg. Oncol. 2022, 42, 101771. [Google Scholar] [CrossRef]
- Elshanawany, A.; Waer, M.S.; Algheriany, A.; Farrag, F.M. Surgery in Recurrent Brain Glioma, Does It Improve Clinical Outcome? Egypt. J. Neurosurg. 2024, 39, 71. [Google Scholar] [CrossRef]
- Navarria, P.; Pessina, F.; Clerici, E.; Bellu, L.; Franzese, C.; Franzini, A.; Simonelli, M.; Bello, L.; Santoro, A.; Politi, L.S.; et al. Re-Irradiation for Recurrent High Grade Glioma (HGG) Patients: Results of a Single Arm Prospective Phase 2 Study. Radiother. Oncol. 2022, 167, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Baehr, A.; Trog, D.; Oertel, M.; Welsch, S.; Kröger, K.; Grauer, O.; Haverkamp, U.; Eich, H.T. Re-Irradiation for Recurrent Glioblastoma Multiforme: A Critical Comparison of Different Concepts. Strahlenther. Onkol. 2020, 196, 457–464. [Google Scholar] [CrossRef]
- Tsien, C.I.; Pugh, S.L.; Dicker, A.P.; Raizer, J.J.; Matuszak, M.M.; Lallana, E.C.; Huang, J.; Algan, O.; Deb, N.; Portelance, L.; et al. NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma. J. Clin. Oncol. 2023, 41, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, D.P.; Sheppard, J.P.; Nguyen, T.; Kondajji, A.M.; Unterberger, A.; Duong, C.; Enomoto, A.; Patel, K.; Yang, I. Radiotherapy versus Combination Radiotherapy-Bevacizumab for the Treatment of Recurrent High-Grade Glioma: A Systematic Review. Acta Neurochir. 2021, 163, 1921–1934. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef]
- Lassman, A.B.; Pugh, S.L.; Wang, T.J.C.; Aldape, K.; Gan, H.K.; Preusser, M.; Vogelbaum, M.A.; Sulman, E.P.; Won, M.; Zhang, P.; et al. Depatuxizumab Mafodotin in EGFR-Amplified Newly Diagnosed Glioblastoma: A Phase III Randomized Clinical Trial. Neuro Oncol. 2023, 25, 339–350. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Gerstner, E.R.; Ye, X.; Desideri, S.; Duda, D.G.; Peereboom, D.; Lesser, G.J.; Chowdhary, S.; Wen, P.Y.; Grossman, S.; et al. Feasibility, Phase I, and Phase II Studies of Tandutinib, an Oral Platelet-Derived Growth Factor Receptor-β Tyrosine Kinase Inhibitor, in Patients with Recurrent Glioblastoma. Neuro Oncol. 2017, 19, 567–575. [Google Scholar] [CrossRef]
- Lassman, A.B.; Sepúlveda-Sánchez, J.M.; Cloughesy, T.F.; Gil-Gil, M.J.; Puduvalli, V.K.; Raizer, J.J.; De Vos, F.Y.F.; Wen, P.Y.; Butowski, N.A.; Clement, P.M.J.; et al. Infigratinib in Patients with Recurrent Gliomas and FGFR Alterations: A Multicenter Phase II Study. Clin. Cancer Res. 2022, 28, 2270–2277. [Google Scholar] [CrossRef]
- Davies, M.J. PD-1/PD-L1 Inhibitors for Non–Small Cell Lung Cancer: Incorporating Care Step Pathways for Effective Side-Effect Management. J. Adv. Pract. Oncol. 2019, 10, 21–35. [Google Scholar] [CrossRef]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; de Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 9235638. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, J.W.; Park, J.Y. Nivolumab for Advanced Hepatocellular Carcinoma with Multiple Lung Metastases after Sorafenib Failure. J. Liver Cancer 2020, 20, 72–77. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.; Yazdani, O.; Kahrizi, M.S.; Soltanzadeh, S.; Javididashtbayaz, H.; Mivefroshan, A.; Ilkhani, S.; Esbati, R. Clinical Potential of PD-1/PD-L1 Blockade Therapy for Renal Cell Carcinoma (RCC): A Rapidly Evolving Strategy. Cancer Cell Int. 2022, 22, 401. [Google Scholar] [CrossRef]
- Yang, L.; Ning, Q.; Tang, S. Recent Advances and Next Breakthrough in Immunotherapy for Cancer Treatment. J. Immunol. Res. 2022, 2022, 8052212. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for Relapsed or Refractory Adult B-Cell Acute Lymphoblastic Leukaemia: Phase 2 Results of the Single-Arm, Open-Label, Multicentre ZUMA-3 Study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; et al. Ide-Cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 388, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A Single Dose of Peripherally Infused EGFRvIII-Directed CAR T Cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Vujanovic, L.; Santos, P.M.; Maurer, D.M.; Gambotto, A.; Lohr, J.; Li, C.; Waldman, J.; Chandran, U.; Lin, Y.; et al. Multiple Antigen-Engineered DC Vaccines with or without IFNα to Promote Antitumor Immunity in Melanoma. J. Immunother. Cancer 2019, 7, 113. [Google Scholar] [CrossRef]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.-J.; Glantz, M.; Peereboom, D.M.; et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef]
- Cibula, D.; Rob, L.; Mallmann, P.; Knapp, P.; Klat, J.; Chovanec, J.; Minar, L.; Melichar, B.; Hein, A.; Kieszko, D.; et al. Dendritic Cell-Based Immunotherapy (DCVAC/OvCa) Combined with Second-Line Chemotherapy in Platinum-Sensitive Ovarian Cancer (SOV02): A Randomized, Open-Label, Phase 2 Trial. Gynecol. Oncol. 2021, 162, 652–660. [Google Scholar] [CrossRef]
- Adhikaree, J.; Moreno-Vicente, J.; Kaur, A.P.; Jackson, A.M.; Patel, P.M. Resistance Mechanisms and Barriers to Successful Immunotherapy for Treating Glioblastoma. Cells 2020, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Rocha Pinheiro, S.L.; Lemos, F.F.B.; Marques, H.S.; Silva Luz, M.; de Oliveira Silva, L.G.; Faria Souza Mendes dos Santos, C.; da Costa Evangelista, K.; Calmon, M.S.; Sande Loureiro, M.; Freire de Melo, F. Immunotherapy in Glioblastoma Treatment: Current State and Future Prospects. World J. Clin. Oncol. 2023, 14, 138–159. [Google Scholar] [CrossRef] [PubMed]
- Himes, B.T.; Geiger, P.A.; Ayasoufi, K.; Bhargav, A.G.; Brown, D.A.; Parney, I.F. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front. Oncol. 2021, 11, 770561. [Google Scholar] [CrossRef]
- Yasinjan, F.; Xing, Y.; Geng, H.; Guo, R.; Yang, L.; Liu, Z.; Wang, H. Immunotherapy: A Promising Approach for Glioma Treatment. Front. Immunol. 2023, 14, 1255611. [Google Scholar] [CrossRef]
- Srinivasan, V.M.; Lang, F.F.; Kan, P. Intraarterial Delivery of Virotherapy for Glioblastoma. Neurosurg. Focus 2021, 50, E7. [Google Scholar] [CrossRef] [PubMed]
- Asija, S.; Chatterjee, A.; Goda, J.S.; Yadav, S.; Chekuri, G.; Purwar, R. Oncolytic Immunovirotherapy for High-Grade Gliomas: A Novel and an Evolving Therapeutic Option. Front. Immunol. 2023, 14, 1118246. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Patel, D.M.; Foreman, P.M.; Nabors, L.B.; Riley, K.O.; Gillespie, G.Y.; Markert, J.M. Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered HSV-1 Expressing IL-12, in Patients with Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma. Hum. Gene Ther. Clin. Dev. 2016, 27, 69–78. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Wakimoto, H.; Martuza, R.L.; Kaufman, H.L.; Rabkin, S.D.; Saha, D. Oncolytic Herpes Simplex Virus Expressing IL-2 Controls Glioblastoma Growth and Improves Survival. J. Immunother. Cancer 2024, 12, e008880. [Google Scholar] [CrossRef]
- Aghi, M.K.; Liu, T.-C.; Rabkin, S.; Martuza, R.L. Hypoxia Enhances the Replication of Oncolytic Herpes Simplex Virus. Mol. Ther. 2009, 17, 51–56. [Google Scholar] [CrossRef]
- Sgubin, D.; Wakimoto, H.; Kanai, R.; Rabkin, S.D.; Martuza, R.L. Oncolytic Herpes Simplex Virus Counteracts the Hypoxia-Induced Modulation of Glioblastoma Stem-like Cells. Stem Cells Transl. Med. 2012, 1, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Wakimoto, H. Oncolytic Herpes Simplex Virus-Based Strategies: Toward a Breakthrough in Glioblastoma Therapy. Front. Microbiol. 2014, 5, 303. [Google Scholar] [CrossRef] [PubMed]
- Markert, J.M.; Razdan, S.N.; Kuo, H.-C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A Phase 1 Trial of Oncolytic HSV-1, G207, given in Combination with Radiation for Recurrent GBM Demonstrates Safety and Radiographic Responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.-D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral Oncolytic Herpes Virus G47∆ for Residual or Recurrent Glioblastoma: A Phase 2 Trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Estevez-Ordonez, D.; Stein, J.; Maleknia, P.; Gallegos, C.; Atchley, T.; Laskay, N.; Clements, J.; Lobbous, M.; Leavenworth, J.; Riley, K.; et al. CTIM-13. Phase I Clinical Trial of Oncolytic HSV-1 M032, A Second-Generation Virus Armed to Expressed IL-12, for the Treatment of Adult Patients with Recurrent or Progressive Malignant Glioma. Neuro Oncol. 2023, 25, v64. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Oncolytic Herpes Simplex Virus Immunovirotherapy in Combination with Immune Checkpoint Blockade to Treat Glioblastoma. Immunotherapy 2018, 10, 779–786. [Google Scholar] [CrossRef]
- Hooper, G.W.; Ansari, S.; Johnson, J.M.; Ginat, D.T. Advances in the Radiological Evaluation of and Theranostics for Glioblastoma. Cancers 2023, 15, 4162. [Google Scholar] [CrossRef]
- Kilcoyne, R.F.; Richardson, M.L.; Porter, B.A.; Olson, D.O.; Greenlee, T.K.; Lanzer, W. Magnetic Resonance Imaging of Soft Tissue Masses. Clin. Orthop. Relat. Res. 1988, 228, 13–19. [Google Scholar] [CrossRef]
- Horowitz, A.L. MRI Physics for Physicians; Springer: New York, NY, USA, 1989; ISBN 978-0-387-96904-6. [Google Scholar]
- Eichberg, D.G.; Di, L.; Morell, A.A.; Shah, A.H.; Semonche, A.M.; Chin, C.N.; Bhatia, R.G.; Jamshidi, A.M.; Luther, E.M.; Komotar, R.J.; et al. Incidence of High Grade Gliomas Presenting as Radiographically Non-Enhancing Lesions: Experience in 111 Surgically Treated Non-Enhancing Gliomas with Tissue Diagnosis. J. Neurooncol. 2020, 147, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.; Waldman, A.D. Conventional MRI Evaluation of Gliomas. Br. J. Radiol. 2011, 84, S107–S111. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C.; Cairncross, J.G. Response Criteria for Phase II Studies of Supratentorial Malignant Glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. JNCI J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Hygino da Cruz, L.C.; Rodriguez, I.; Domingues, R.C.; Gasparetto, E.L.; Sorensen, A.G. Pseudoprogression and Pseudoresponse: Imaging Challenges in the Assessment of Posttreatment Glioma. AJNR Am. J. Neuroradiol. 2011, 32, 1978–1985. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; van den Bent, M.; Youssef, G.; Cloughesy, T.F.; Ellingson, B.M.; Weller, M.; Galanis, E.; Barboriak, D.P.; de Groot, J.; Gilbert, M.R.; et al. RANO 2.0: Update to the Response Assessment in Neuro-Oncology Criteria for High- and Low-Grade Gliomas in Adults. J. Clin. Oncol. 2023, 41, 5187–5199. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Wu, C.J. Dynamics and Specificities of T Cells in Cancer Immunotherapy. Nat. Rev. Cancer 2023, 23, 295–316. [Google Scholar] [CrossRef]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. JCO 2015, 33, 3541–3543. [Google Scholar] [CrossRef]
- Jia, W.; Gao, Q.; Han, A.; Zhu, H.; Yu, J. The Potential Mechanism, Recognition and Clinical Significance of Tumor Pseudoprogression after Immunotherapy. Cancer Biol. Med. 2019, 16, 655–670. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy Response Assessment in Neuro-Oncology (iRANO): A Report of the RANO Working Group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef]
- Kasten, B.B.; Udayakumar, N.; Leavenworth, J.W.; Wu, A.M.; Lapi, S.E.; McConathy, J.E.; Sorace, A.G.; Bag, A.K.; Markert, J.M.; Warram, J.M. Current and Future Imaging Methods for Evaluating Response to Immunotherapy in Neuro-Oncology. Theranostics 2019, 9, 5085–5104. [Google Scholar] [CrossRef] [PubMed]
- Almuhaideb, A.; Papathanasiou, N.; Bomanji, J. 18F-FDG PET/CT Imaging In Oncology. Ann. Saudi Med. 2011, 31, 3–13. [Google Scholar] [CrossRef]
- Muthukumar, S.; Darden, J.; Crowley, J.; Witcher, M.; Kiser, J. A Comparison of PET Tracers in Recurrent High-Grade Gliomas: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Galldiks, N.; Ellingson, B.M.; van den Bent, M.J.; Chang, S.M.; Cicone, F.; de Groot, J.; Koh, E.-S.; Law, I.; Le Rhun, E.; et al. PET-Based Response Assessment Criteria for Diffuse Gliomas (PET RANO 1.0): A Report of the RANO Group. Lancet Oncol. 2024, 25, e29–e41. [Google Scholar] [CrossRef] [PubMed]
- Santra, A.; Kumar, R.; Sharma, P.; Bal, C.; Julka, P.K.; Malhotra, A. F-18 FDG PET-CT for Predicting Survival in Patients with Recurrent Glioma: A Prospective Study. Neuroradiology 2011, 53, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Colavolpe, C.; Chinot, O.; Metellus, P.; Mancini, J.; Barrie, M.; Bequet-Boucard, C.; Tabouret, E.; Mundler, O.; Figarella-Branger, D.; Guedj, E. FDG-PET Predicts Survival in Recurrent High-Grade Gliomas Treated with Bevacizumab and Irinotecan. Neuro Oncol. 2012, 14, 649–657. [Google Scholar] [CrossRef]
- Todeschi, J.; Bund, C.; Cebula, H.; Chibbaro, S.; Lhermitte, B.; Pin, Y.; Lefebvre, F.; Namer, I.J.; Proust, F. Diagnostic Value of Fusion of Metabolic and Structural Images for Stereotactic Biopsy of Brain Tumors without Enhancement after Contrast Medium Injection. Neurochirurgie 2019, 65, 357–364. [Google Scholar] [CrossRef]
- Robert, J.A.; Leclerc, A.; Ducloie, M.; Emery, E.; Agostini, D.; Vigne, J. Contribution of [18F]FET PET in the Management of Gliomas, from Diagnosis to Follow-Up: A Review. Pharmaceuticals 2024, 17, 1228. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, S.; Wei, Y.; Xu, G.; An, Y.; Ma, J.; Yang, H.; Qi, Z.; Xiao, X.; Bai, J.; et al. Glioma Imaging by O-(2-18F-Fluoroethyl)-L-Tyrosine PET and Diffusion-Weighted MRI and Correlation with Molecular Phenotypes, Validated by PET/MR-Guided Biopsies. Front. Oncol. 2021, 11, 743655. [Google Scholar] [CrossRef]
- Schwarzenberg, J.; Czernin, J.; Cloughesy, T.F.; Ellingson, B.M.; Pope, W.B.; Grogan, T.; Elashoff, D.; Geist, C.; Silverman, D.H.S.; Phelps, M.E.; et al. Treatment Response Evaluation Using 18F-FDOPA PET in Patients with Recurrent Malignant Glioma on Bevacizumab Therapy. Clin. Cancer Res. 2014, 20, 3550–3559. [Google Scholar] [CrossRef]
- Kebir, S.; Fimmers, R.; Galldiks, N.; Schäfer, N.; Mack, F.; Schaub, C.; Stuplich, M.; Niessen, M.; Tzaridis, T.; Simon, M.; et al. Late Pseudoprogression in Glioblastoma: Diagnostic Value of Dynamic O-(2-[18F]Fluoroethyl)-L-Tyrosine PET. Clin. Cancer Res. 2016, 22, 2190–2196. [Google Scholar] [CrossRef]
- Werner, J.-M.; Weller, J.; Ceccon, G.; Schaub, C.; Tscherpel, C.; Lohmann, P.; Bauer, E.K.; Schäfer, N.; Stoffels, G.; Baues, C.; et al. Diagnosis of Pseudoprogression Following Lomustine–Temozolomide Chemoradiation in Newly Diagnosed Glioblastoma Patients Using FET-PET. Clin. Cancer Res. 2021, 27, 3704–3713. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, H.-G.; Roelcke, U.; Weller, J.; Hundsberger, T.; Hottinger, A.F.; von Moos, R.; Caparrotti, F.; Conen, K.; Remonda, L.; Roth, P.; et al. MRI and 18FET-PET Predict Survival Benefit from Bevacizumab Plus Radiotherapy in Patients with Isocitrate Dehydrogenase Wild-Type Glioblastoma: Results from the Randomized ARTE Trial. Clin. Cancer Res. 2021, 27, 179–188. [Google Scholar] [CrossRef]
- de Zwart, P.L.; van Dijken, B.R.J.; Holtman, G.A.; Stormezand, G.N.; Dierckx, R.A.J.O.; van Laar, P.J.; van der Hoorn, A. Diagnostic Accuracy of PET Tracers for the Differentiation of Tumor Progression from Treatment-Related Changes in High-Grade Glioma: A Systematic Review and Metaanalysis. J. Nucl. Med. 2020, 61, 498–504. [Google Scholar] [CrossRef]
- Chiba, Y.; Kinoshita, M.; Okita, Y.; Tsuboi, A.; Isohashi, K.; Kagawa, N.; Fujimoto, Y.; Oji, Y.; Oka, Y.; Shimosegawa, E.; et al. Use of 11C-Methionine PET Parametric Response Map for Monitoring WT1 Immunotherapy Response in Recurrent Malignant Glioma: Clinical Article. J. Neurosurg. 2012, 116, 835–842. [Google Scholar] [CrossRef]
- Spence, A.M.; Muzi, M.; Swanson, K.R.; O’Sullivan, F.; Rockhill, J.K.; Rajendran, J.G.; Adamsen, T.C.H.; Link, J.M.; Swanson, P.E.; Yagle, K.J.; et al. Regional Hypoxia in Glioblastoma Multiforme Quantified with [18F]Fluoromisonidazole Positron Emission Tomography before Radiotherapy: Correlation with Time to Progression and Survival. Clin. Cancer Res. 2008, 14, 2623–2630. [Google Scholar] [CrossRef]
- Abdo, R.; Lamare, F.; Fernandez, P.; Bentourkia, M. Quantification of Hypoxia in Human Glioblastoma Using PET with 18F-FMISO. Nucl. Med. Mol. Imaging 2021, 55, 107–115. [Google Scholar] [CrossRef]
- Barajas, R.F.; Ambady, P.; Link, J.; Krohn, K.A.; Raslan, A.; Mallak, N.; Woltjer, R.; Muldoon, L.; Neuwelt, E.A. [18F]-Fluoromisonidazole (FMISO) PET/MRI Hypoxic Fraction Distinguishes Neuroinflammatory Pseudoprogression from Recurrent Glioblastoma in Patients Treated with Pembrolizumab. Neuro Oncol. Pract. 2022, 9, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; Heiland, S.; Falini, A.; Jäger, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma Imaging in Europe: A Survey of 220 Centres and Recommendations for Best Clinical Practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.S.; Smits, M.; Kaufmann, T.J.; Knutsson, L.; Rapalino, O.; Galldiks, N.; Sundgren, P.C.; Cha, S. Advanced Imaging in the Diagnosis and Response Assessment of High-Grade Glioma: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2025, 224, e2330612. [Google Scholar] [CrossRef] [PubMed]
- Schmainda, K.M. Diffusion-Weighted MRI as a Biomarker for Treatment Response in Glioma. CNS Oncol. 2012, 1, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Shigematu, Y.; Hirai, T.; Okuda, T.; Liang, L.; Ge, Y.; Komohara, Y.; et al. Usefulness of Diffusion-Weighted MRI with Echo-Planar Technique in the Evaluation of Cellularity in Gliomas. J. Magn. Reason. Imaging 1999, 9, 53–60. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Malkin, M.G.; Rand, S.D.; Connelly, J.M.; Quinsey, C.; LaViolette, P.S.; Bedekar, D.P.; Schmainda, K.M. Validation of Functional Diffusion Maps (fDMs) as a Biomarker for Human Glioma Cellularity. J. Magn. Reason. Imaging 2010, 31, 538–548. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, B.R.J.; van Laar, P.J.; Smits, M.; Dankbaar, J.W.; Enting, R.H.; van der Hoorn, A. Perfusion MRI in Treatment Evaluation of Glioblastomas: Clinical Relevance of Current and Future Techniques. J. Magn. Reson. Imaging 2019, 49, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chen, M.M.; Liu, H.-L.; Ucisik, F.E.; Wintermark, M.; Kumar, V.A. MR Perfusion Imaging for Gliomas. Magn. Reason. Imaging Clin. N. Am. 2024, 32, 73–83. [Google Scholar] [CrossRef]
- Gupta, R.K.; Haris, M.; Husain, N.; Husain, M.; Prasad, K.N.; Pauliah, M.; Srivastava, C.; Tripathi, M.; Rastogi, M.; Behari, S.; et al. Relative Cerebral Blood Volume Is a Measure of Angiogenesis in Brain Tuberculoma. J. Comput. Assist. Tomogr. 2007, 31, 335. [Google Scholar] [CrossRef]
- Hu, L.S.; Eschbacher, J.M.; Dueck, A.C.; Heiserman, J.E.; Liu, S.; Karis, J.P.; Smith, K.A.; Shapiro, W.R.; Pinnaduwage, D.S.; Coons, S.W.; et al. Correlations between Perfusion MR Imaging Cerebral Blood Volume, Microvessel Quantification, and Clinical Outcome Using Stereotactic Analysis in Recurrent High-Grade Glioma. AJNR Am. J. Neuroradiol. 2012, 33, 69–76. [Google Scholar] [CrossRef]
- Jain, R.; Gutierrez, J.; Narang, J.; Scarpace, L.; Schultz, L.R.; Lemke, N.; Patel, S.C.; Mikkelsen, T.; Rock, J.P. In Vivo Correlation of Tumor Blood Volume and Permeability with Histologic and Molecular Angiogenic Markers in Gliomas. AJNR Am. J. Neuroradiol. 2011, 32, 388–394. [Google Scholar] [CrossRef]
- Sourbron, S.P.; Buckley, D.L. Classic Models for Dynamic Contrast-Enhanced MRI. NMR Biomed. 2013, 26, 1004–1027. [Google Scholar] [CrossRef]
- Heye, A.K.; Culling, R.D.; Valdés Hernández, M.d.C.; Thrippleton, M.J.; Wardlaw, J.M. Assessment of Blood–Brain Barrier Disruption Using Dynamic Contrast-Enhanced MRI. A Systematic Review. Neuroimage Clin. 2014, 6, 262–274. [Google Scholar] [CrossRef]
- Keil, V.C.; Gielen, G.H.; Pintea, B.; Baumgarten, P.; Datsi, A.; Hittatiya, K.; Simon, M.; Hattingen, E. DCE-MRI in Glioma, Infiltration Zone and Healthy Brain to Assess Angiogenesis: A Biopsy Study. Clin. Neuroradiol. 2021, 31, 1049–1058. [Google Scholar] [CrossRef]
- Liang, J.; Liu, D.; Gao, P.; Zhang, D.; Chen, H.; Shi, C.; Luo, L. Diagnostic Values of DCE-MRI and DSC-MRI for Differentiation Between High-Grade and Low-Grade Gliomas: A Comprehensive Meta-Analysis. Acad. Radiol. 2018, 25, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lei, D.; Yuan, Y.; Xiong, N. Accuracy of ADC Derived from DWI for Differentiating High-Grade from Low-Grade Gliomas: Systematic Review and Meta-Analysis. Medicine 2020, 99, e19254. [Google Scholar] [CrossRef]
- Shin, K.E.; Ahn, K.J.; Choi, H.S.; Jung, S.L.; Kim, B.S.; Jeon, S.S.; Hong, Y.G. DCE and DSC MR Perfusion Imaging in the Differentiation of Recurrent Tumour from Treatment-Related Changes in Patients with Glioma. Clin. Radiol. 2014, 69, e264–e272. [Google Scholar] [CrossRef]
- Prager, A.J.; Martinez, N.; Beal, K.; Omuro, A.; Zhang, Z.; Young, R.J. Diffusion and Perfusion MRI to Differentiate Treatment-Related Changes Including Pseudoprogression from Recurrent Tumors in High-Grade Gliomas with Histopathologic Evidence. AJNR Am. J. Neuroradiol. 2015, 36, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Ekert, J.O.; Sefcikova, V.; Fersht, N.; Samandouras, G. Discriminators of Pseudoprogression and True Progression in High-Grade Gliomas: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 13258. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Wiestler, B.; Burth, S.; Wick, A.; Nowosielski, M.; Heiland, S.; Schlemmer, H.-P.; Wick, W.; Bendszus, M.; Radbruch, A. Relative Cerebral Blood Volume Is a Potential Predictive Imaging Biomarker of Bevacizumab Efficacy in Recurrent Glioblastoma. Neuro Oncol. 2015, 17, 1139–1147. [Google Scholar] [CrossRef]
- Kickingereder, P.; Radbruch, A.; Burth, S.; Wick, A.; Heiland, S.; Schlemmer, H.-P.; Wick, W.; Bendszus, M.; Bonekamp, D. MR Perfusion–Derived Hemodynamic Parametric Response Mapping of Bevacizumab Efficacy in Recurrent Glioblastoma. Radiology 2016, 279, 542–552. [Google Scholar] [CrossRef]
- Auer, T.A.; Breit, H.-C.; Marini, F.; Renovanz, M.; Ringel, F.; Sommer, C.J.; Brockmann, M.A.; Tanyildizi, Y. Evaluation of the Apparent Diffusion Coefficient in Patients with Recurrent Glioblastoma under Treatment with Bevacizumab with Radiographic Pseudoresponse. J. Neuroradiol. 2019, 46, 36–43. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Wu, Z.; Xie, R.; Zeng, F.; Cai, H.; Lui, S.; Song, B.; Chen, L.; Wu, M. Advanced Imaging Techniques for Differentiating Pseudoprogression and Tumor Recurrence After Immunotherapy for Glioblastoma. Front. Immunol. 2021, 12, 790674. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kadaba, P.; Kravitz, A.; Hormigo, A.; Friedman, J.; Belani, P.; Hadjipanayis, C.; Ellingson, B.M.; Nael, K. Multiparametric MRI for Early Identification of Therapeutic Response in Recurrent Glioblastoma Treated with Immune Checkpoint Inhibitors. Neuro Oncol. 2020, 22, 1658–1666. [Google Scholar] [CrossRef]
- Hagiwara, A.; Oughourlian, T.C.; Cho, N.S.; Schlossman, J.; Wang, C.; Yao, J.; Raymond, C.; Everson, R.; Patel, K.; Mareninov, S.; et al. Diffusion MRI Is an Early Biomarker of Overall Survival Benefit in IDH Wild-Type Recurrent Glioblastoma Treated with Immune Checkpoint Inhibitors. Neuro Oncol. 2021, 24, 1020–1028. [Google Scholar] [CrossRef]
- Cuccarini, V.; Aquino, D.; Gioppo, A.; Anghileri, E.; Pellegatta, S.; Schettino, C.; Mazzi, F.; Finocchiaro, G.; Bruzzone, M.G.; Eoli, M. Advanced MRI Assessment during Dendritic Cell Immunotherapy Added to Standard Treatment Against Glioblastoma. J. Clin. Med. 2019, 8, 2007. [Google Scholar] [CrossRef]
- Vrabec, M.; Van Cauter, S.; Himmelreich, U.; Van Gool, S.W.; Sunaert, S.; De Vleeschouwer, S.; Suput, D.; Demaerel, P. MR Perfusion and Diffusion Imaging in the Follow-up of Recurrent Glioblastoma Treated with Dendritic Cell Immunotherapy: A Pilot Study. Neuroradiology 2011, 53, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Fathi Kazerooni, A.; Bagley, S.J.; Akbari, H.; Saxena, S.; Bagheri, S.; Guo, J.; Chawla, S.; Nabavizadeh, A.; Mohan, S.; Bakas, S.; et al. Applications of Radiomics and Radiogenomics in High-Grade Gliomas in the Era of Precision Medicine. Cancers 2021, 13, 5921. [Google Scholar] [CrossRef]
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and Radiogenomics in Gliomas: A Contemporary Update. Br. J. Cancer 2021, 125, 641–657. [Google Scholar] [CrossRef]
- Molina, D.; Pérez-Beteta, J.; Luque, B.; Arregui, E.; Calvo, M.; Borrás, J.M.; López, C.; Martino, J.; Velasquez, C.; Asenjo, B.; et al. Tumour Heterogeneity in Glioblastoma Assessed by MRI Texture Analysis: A Potential Marker of Survival. Br. J. Radiol. 2016, 89, 20160242. [Google Scholar] [CrossRef]
- Jain, R.; Poisson, L.M.; Gutman, D.; Scarpace, L.; Hwang, S.N.; Holder, C.A.; Wintermark, M.; Rao, A.; Colen, R.R.; Kirby, J.; et al. Outcome Prediction in Patients with Glioblastoma by Using Imaging, Clinical, and Genomic Biomarkers: Focus on the Nonenhancing Component of the Tumor. Radiology 2014, 272, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Hill, V.; Statsevych, V.; Huang, R.; Prasanna, P.; Correa, R.; Singh, G.; Bera, K.; Beig, N.; Thawani, R.; et al. Shape Features of the Lesion Habitat to Differentiate Brain Tumor Progression from Pseudoprogression on Routine Multiparametric MRI: A Multisite Study. Am. J. Neuroradiol. 2018, 39, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, P.; Narayan, V.; Chang, K.; Rahman, R.; Abrey, L.; Reardon, D.A.; Schwartz, L.H.; Wen, P.Y.; Alexander, B.M.; Huang, R.; et al. Quantitative Imaging Biomarkers for Risk Stratification of Patients with Recurrent Glioblastoma Treated with Bevacizumab. Neuro Oncol. 2017, 19, 1688–1697. [Google Scholar] [CrossRef]
- Bahrami, N.; Piccioni, D.; Karunamuni, R.; Chang, Y.-H.; White, N.; Delfanti, R.; Seibert, T.M.; Hattangadi-Gluth, J.A.; Dale, A.; Farid, N.; et al. Edge Contrast of the FLAIR Hyperintense Region Predicts Survival in Patients with High-Grade Gliomas Following Treatment with Bevacizumab. AJNR Am. J. Neuroradiol. 2018, 39, 1017–1024. [Google Scholar] [CrossRef]
- Khalili, N.; Kazerooni, A.F.; Familiar, A.; Haldar, D.; Kraya, A.; Foster, J.; Koptyra, M.; Storm, P.B.; Resnick, A.C.; Nabavizadeh, A. Radiomics for Characterization of the Glioma Immune Microenvironment. npj Precis. Oncol. 2023, 7, 59. [Google Scholar] [CrossRef]
- George, E.; Flagg, E.; Chang, K.; Bai, H.X.; Aerts, H.J.; Vallières, M.; Reardon, D.A.; Huang, R.Y. Radiomics-Based Machine Learning for Outcome Prediction in a Multicenter Phase II Study of Programmed Death-Ligand 1 Inhibition Immunotherapy for Glioblastoma. Am. J. Neuroradiol. 2022, 43, 675–681. [Google Scholar] [CrossRef]
- Molina, D.; Pérez-Beteta, J.; Martínez-González, A.; Martino, J.; Velasquez, C.; Arana, E.; Pérez-García, V.M. Lack of Robustness of Textural Measures Obtained from 3D Brain Tumor MRIs Impose a Need for Standardization. PLoS ONE 2017, 12, e0178843. [Google Scholar] [CrossRef] [PubMed]
- Buch, K.; Kuno, H.; Qureshi, M.M.; Li, B.; Sakai, O. Quantitative Variations in Texture Analysis Features Dependent on MRI Scanning Parameters: A Phantom Model. J. Appl. Clin. Med. Phys. 2018, 19, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Mema, E.; Himoto, Y.; Veeraraghavan, H.; Brenton, J.D.; Snyder, A.; Weigelt, B.; Vargas, H.A. Unravelling Tumour Heterogeneity Using Next-Generation Imaging: Radiomics, Radiogenomics, and Habitat Imaging. Clin. Radiol. 2017, 72, 3–10. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Y.; Liang, C.; Zhao, Y.; Lv, X.; Zou, Y.; Yan, K.; Zheng, H.; Liang, D.; Li, Z.-C. Biologic Pathways Underlying Prognostic Radiomics Phenotypes from Paired MRI and RNA Sequencing in Glioblastoma. Radiology 2021, 301, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.K.; Whisenant, J.G.; Barnes, S.L.; Sorace, A.G.; Yankeelov, T.E. Multiparametric Analysis of Longitudinal Quantitative MRI Data to Identify Distinct Tumor Habitats in Preclinical Models of Breast Cancer. Cancers 2020, 12, 1682. [Google Scholar] [CrossRef]
- Li, S.; Dai, Y.; Chen, J.; Yan, F.; Yang, Y. MRI-Based Habitat Imaging in Cancer Treatment: Current Technology, Applications, and Challenges. Cancer Imaging 2024, 24, 107. [Google Scholar] [CrossRef]
- Jardim-Perassi, B.V.; Huang, S.; Dominguez-Viqueira, W.; Poleszczuk, J.; Budzevich, M.M.; Abdalah, M.A.; Pillai, S.R.; Ruiz, E.; Bui, M.M.; Zuccari, D.A.P.C.; et al. Multiparametric MRI and Coregistered Histology Identify Tumor Habitats in Breast Cancer Mouse Models. Cancer Res. 2019, 79, 3952–3964. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.E.; Kim, N.; Park, S.Y.; Kim, Y.-H.; Cho, Y.H.; Kim, J.H.; Kim, H.S. Tumor Habitat Analysis Using Longitudinal Physiological MRI to Predict Tumor Recurrence After Stereotactic Radiosurgery for Brain Metastasis. Korean J. Radiol. 2023, 24, 235–246. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.E.; Kim, H.S.; Kim, N.; Park, S.Y.; Kim, Y.-H.; Kim, J.H. Spatiotemporal Habitats from Multiparametric Physiologic MRI Distinguish Tumor Progression from Treatment-Related Change in Post-Treatment Glioblastoma. Eur. Radiol. 2021, 31, 6374–6383. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Kim, N.; Park, S.Y.; Kim, Y.-H.; Kim, J.H. Spatiotemporal Heterogeneity in Multiparametric Physiologic MRI Is Associated with Patient Outcomes in IDH-Wildtype Glioblastoma. Clin. Cancer Res. 2021, 27, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Luo, Y.; Gu, F.; Tian, B.; Xiong, Y.; Wu, G.; Nie, X.; Yu, J.; Tong, J.; Liao, X. Artificial Intelligence-Based MRI Radiomics and Radiogenomics in Glioma. Cancer Imaging 2024, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Lung, T.; Muniyappa, S.; Hadley, C.; Templeton, B.; Fritz, J.; Boulter, D.; Shah, K.; Singh, R.; Zhu, S.; et al. Radiomics and Radiogenomics in Differentiating Progression, Pseudoprogression, and Radiation Necrosis in Gliomas. Biomedicines 2025, 13, 1778. [Google Scholar] [CrossRef]
- Bian, W.; Chen, Y.; Ye, X.; Zhang, Q. An Optimization-Based Meta-Learning Model for MRI Reconstruction with Diverse Dataset. J. Imaging 2021, 7, 231. [Google Scholar] [CrossRef]
- Abayazeed, A.H.; Abbassy, A.; Müeller, M.; Hill, M.; Qayati, M.; Mohamed, S.; Mekhaimar, M.; Raymond, C.; Dubey, P.; Nael, K.; et al. NS-HGlio: A Generalizable and Repeatable HGG Segmentation and Volumetric Measurement AI Algorithm for the Longitudinal MRI Assessment to Inform RANO in Trials and Clinics. Neurooncol. Adv. 2022, 5, vdac184. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou-Strauss, A.; Pan, L.; Sachpekidis, C. Kinetic Modeling and Parametric Imaging with Dynamic PET for Oncological Applications: General Considerations, Current Clinical Applications, and Future Perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 21–39. [Google Scholar] [CrossRef]
- Steidl, E.; Langen, K.-J.; Hmeidan, S.A.; Polomac, N.; Filss, C.P.; Galldiks, N.; Lohmann, P.; Keil, F.; Filipski, K.; Mottaghy, F.M.; et al. Sequential Implementation of DSC-MR Perfusion and Dynamic [18F]FET PET Allows Efficient Differentiation of Glioma Progression from Treatment-Related Changes. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1956–1965. [Google Scholar] [CrossRef]
- Pyka, T.; Krzyzanowska, I.; Rominger, A.; Delbridge, C.; Meyer, B.; Boeckh-Behrens, T.; Zimmer, C.; Gempt, J. Multiparametric Characterization of Intracranial Gliomas Using Dynamic [18F]FET-PET and Magnetic Resonance Spectroscopy. Diagnostics 2022, 12, 2331. [Google Scholar] [CrossRef]
- Zaragori, T.; Ginet, M.; Marie, P.-Y.; Roch, V.; Grignon, R.; Gauchotte, G.; Rech, F.; Blonski, M.; Lamiral, Z.; Taillandier, L.; et al. Use of Static and Dynamic [18F]-F-DOPA PET Parameters for Detecting Patients with Glioma Recurrence or Progression. EJNMMI Res. 2020, 10, 56. [Google Scholar] [CrossRef]
- Kebir, S.; Rauschenbach, L.; Galldiks, N.; Schlaak, M.; Hattingen, E.; Landsberg, J.; Bundschuh, R.A.; Langen, K.-J.; Scheffler, B.; Herrlinger, U.; et al. Dynamic O-(2-[18F]Fluoroethyl)-L-Tyrosine PET Imaging for the Detection of Checkpoint Inhibitor-Related Pseudoprogression in Melanoma Brain Metastases. Neuro Oncol. 2016, 18, 1462–1464. [Google Scholar] [CrossRef]
- Tawbi, H.; Boutros, C.; Kok, D.; Robert, C.; Mcarthur, G. New Era in the Management of Melanoma Brain Metastases. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Wardak, M.; Schiepers, C.; Cloughesy, T.F.; Dahlbom, M.; Phelps, M.E.; Huang, S.-C. 18F-FLT and 18F-FDOPA PET Kinetics in Recurrent Brain Tumors. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1199–1209. [Google Scholar] [CrossRef]
- Bolcaen, J.; Lybaert, K.; Moerman, L.; Descamps, B.; Deblaere, K.; Boterberg, T.; Kalala, J.-P.; Van den Broecke, C.; De Vos, F.; Vanhove, C.; et al. Kinetic Modeling and Graphical Analysis of 18F-Fluoromethylcholine (FCho), 18F-Fluoroethyltyrosine (FET) and 18F-Fluorodeoxyglucose (FDG) PET for the Fiscrimination between High-Grade Glioma and Radiation Necrosis in Rats. PLoS ONE 2016, 11, e0161845. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Larimer, B.M.; Wehrenberg-Klee, E.; Caraballo, A.; Mahmood, U. Quantitative CD3 PET Imaging Predicts Tumor Growth Response to Anti-CTLA-4 Therapy. J. Nucl. Med. 2016, 57, 1607–1611. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Tsuji, T.; Luescher, I.F.; Shiku, H.; Mineno, J.; Okamoto, S.; Old, L.J.; Shrikant, P.; Gnjatic, S.; Odunsi, K. Direct Tumor Recognition by a Human CD4+ T-Cell Subset Potently Mediates Tumor Growth Inhibition and Orchestrates Anti-Tumor Immune Responses. Sci. Rep. 2015, 5, 14896. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the Role of CD4+ T Cells in Cancer Immunotherapy—New Insights into Old Paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Kristensen, L.K.; Fröhlich, C.; Christensen, C.; Melander, M.C.; Poulsen, T.T.; Galler, G.R.; Lantto, J.; Horak, I.D.; Kragh, M.; Nielsen, C.H.; et al. CD4+ and CD8a+ PET Imaging Predicts Response to Novel PD-1 Checkpoint Inhibitor: Studies of Sym021 in Syngeneic Mouse Cancer Models. Theranostics 2019, 9, 8221–8238. [Google Scholar] [CrossRef]
- Tavaré, R.; Escuin-Ordinas, H.; Mok, S.; McCracken, M.N.; Zettlitz, K.A.; Salazar, F.B.; Witte, O.N.; Ribas, A.; Wu, A.M. An Effective Immuno-PET Imaging Method to Monitor CD8-Dependent Responses to Immunotherapy. Cancer Res. 2016, 76, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, M.; Ingram, J.R.; Dougan, M.; Dongre, A.; Whang, K.A.; LeGall, C.; Cragnolini, J.J.; Bierie, B.; Gostissa, M.; Gorman, J.; et al. Predicting the Response to CTLA-4 Blockade by Longitudinal Noninvasive Monitoring of CD8 T Cells. J. Exp. Med. 2017, 214, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, M.; LaFleur, M.W.; Verschoor, V.L.; Dongre, A.; Zhang, Y.; Nguyen, T.H.; Kolifrath, S.; Aref, A.R.; Lau, C.J.; Paweletz, C.P.; et al. Immuno-PET Identifies the Myeloid Compartment as a Key Contributor to the Outcome of the Antitumor Response under PD-1 Blockade. Proc. Natl. Acad. Sci. USA 2019, 116, 16971–16980. [Google Scholar] [CrossRef] [PubMed]
- Nagle, V.L.; Henry, K.E.; Hertz, C.A.J.; Graham, M.S.; Campos, C.; Parada, L.F.; Pandit-Taskar, N.; Schietinger, A.; Mellinghoff, I.K.; Lewis, J.S. Imaging Tumor-Infiltrating Lymphocytes in Brain Tumors with [64Cu]Cu-NOTA-Anti-CD8 PET. Clin. Cancer Res. 2021, 27, 1958–1966. [Google Scholar] [CrossRef]
- Kasten, B.B.; Houson, H.A.; Coleman, J.M.; Leavenworth, J.W.; Markert, J.M.; Wu, A.M.; Salazar, F.; Tavaré, R.; Massicano, A.V.F.; Gillespie, G.Y.; et al. Positron Emission Tomography Imaging with 89Zr-Labeled Anti-CD8 Cys-Diabody Reveals CD8+ Cell Infiltration during Oncolytic Virus Therapy in a Glioma Murine Model. Sci. Rep. 2021, 11, 15384. [Google Scholar] [CrossRef]
- Gallegos, C.A.; Lu, Y.; Clements, J.C.; Song, P.N.; Lynch, S.E.; Mascioni, A.; Jia, F.; Hartman, Y.E.; Massicano, A.V.F.; Houson, H.A.; et al. [89Zr]-CD8 ImmunoPET Imaging of Glioblastoma Multiforme Response to Combination Oncolytic Viral and Checkpoint Inhibitor Immunotherapy Reveals CD8 Infiltration Differential Changes in Preclinical Models. Theranostics 2024, 14, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; Postow, M.A.; Hellmann, M.D.; Harding, J.J.; Barker, C.A.; O’Donoghue, J.A.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.K.; Tsai, F.; et al. First-in-Humans Imaging with 89Zr-Df-IAB22M2C Anti-CD8 Minibody in Patients with Solid Malignancies: Preliminary Pharmacokinetics, Biodistribution, and Lesion Targeting. J. Nucl. Med. 2020, 61, 512–519. [Google Scholar] [CrossRef]
- Farwell, M.D.; Gamache, R.F.; Babazada, H.; Hellmann, M.D.; Harding, J.J.; Korn, R.; Mascioni, A.; Le, W.; Wilson, I.; Gordon, M.S.; et al. CD8-Targeted PET Imaging of Tumor-Infiltrating T Cells in Patients with Cancer: A Phase I First-in-Humans Study of 89Zr-Df-IAB22M2C, a Radiolabeled Anti-CD8 Minibody. J. Nucl. Med. 2022, 63, 720–726. [Google Scholar] [CrossRef]
- Kist de Ruijter, L.; van de Donk, P.P.; Hooiveld-Noeken, J.S.; Giesen, D.; Elias, S.G.; Lub-de Hooge, M.N.; Oosting, S.F.; Jalving, M.; Timens, W.; Brouwers, A.H.; et al. Whole-Body CD8+ T Cell Visualization before and during Cancer Immunotherapy: A Phase 1/2 Trial. Nat. Med. 2022, 28, 2601–2610. [Google Scholar] [CrossRef]
- Larimer, B.M.; Bloch, E.; Nesti, S.; Austin, E.E.; Wehrenberg-Klee, E.; Boland, G.; Mahmood, U. The Effectiveness of Checkpoint Inhibitor Combinations and Administration Timing Can Be Measured by Granzyme B PET Imaging. Clin. Cancer Res. 2019, 25, 1196–1205. [Google Scholar] [CrossRef]
- LaSalle, T.; Austin, E.E.; Rigney, G.; Wehrenberg-Klee, E.; Nesti, S.; Larimer, B.; Mahmood, U. Granzyme B PET Imaging of Immune-Mediated Tumor Killing as a Tool for Understanding Immunotherapy Response. J. Immunother. Cancer 2020, 8, e000291. [Google Scholar] [CrossRef]
- Zhang, Y.; Deshane, J.S.; Yang, E.S.; Larimer, B. A Novel Translational PET Imaging Approach to Quantifying Distal Tumor Immune Activation After Targeted Radiation Therapy and Checkpoint Blockade. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 1217–1227. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO Practice Guidelines/SNMMI Procedure Standards for Imaging of Gliomas Using PET with Radiolabelled Amino Acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Lohmann, P.; Aboian, M.; Barajas, R.F.; Breen, W.G.; Ivanidze, J.; Johnson, D.R.; Kaufmann, T.J.; Kim, M.M.; Mair, M.J.; et al. Update to the RANO Working Group and EANO Recommendations for the Clinical Use of PET Imaging in Gliomas. Lancet Oncol. 2025, 26, e436–e447. [Google Scholar] [CrossRef]
- Song, S.; Cheng, Y.; Ma, J.; Wang, L.; Dong, C.; Wei, Y.; Xu, G.; An, Y.; Qi, Z.; Lin, Q.; et al. Simultaneous FET-PET and Contrast-Enhanced MRI Based on Hybrid PET/MR Improves Delineation of Tumor Spatial Biodistribution in Gliomas: A Biopsy Validation Study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1458–1467. [Google Scholar] [CrossRef]
- Harat, M.; Rakowska, J.; Harat, M.; Szylberg, T.; Furtak, J.; Miechowicz, I.; Małkowski, B. Combining Amino Acid PET and MRI Imaging Increases Accuracy to Define Malignant Areas in Adult Glioma. Nat. Commun. 2023, 14, 4572. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino Acid Metabolism in Immune Cells: Essential Regulators of the Effector Functions, and Promising Opportunities to Enhance Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef]
- Hunger, J.; Schregel, K.; Boztepe, B.; Agardy, D.A.; Turco, V.; Karimian-Jazi, K.; Weidenfeld, I.; Streibel, Y.; Fischer, M.; Sturm, V.; et al. In Vivo Nanoparticle-Based T Cell Imaging Can Predict Therapy Response towards Adoptive T Cell Therapy in Experimental Glioma. Theranostics 2023, 13, 5170–5182. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Sanvito, F.; Kaufmann, T.J.; Cloughesy, T.F.; Wen, P.Y.; Ellingson, B.M. Standardized Brain Tumor Imaging Protocols for Clinical Trials: Current Recommendations and Tips for Integration. Front. Radiol. 2023, 3, 1267615. [Google Scholar] [CrossRef]
- Miles, K.A.; Voo, S.A.; Groves, A.M. Additional Clinical Value for PET/MRI in Oncology: Moving Beyond Simple Diagnosis. J. Nucl. Med. 2018, 59, 1028–1032. [Google Scholar] [CrossRef]
- Khan, T.; Khan, F.; Patil, S.; Schaison, F.; Subtirelu, R.; Revheim, M.; Ayubcha, C.; Werner, T.; Singh, S.; Alavi, A. Barriers to Equitable Access: A Systematic Examination of PET/CT Imaging Disparities. J. Nucl. Med. 2025, 66, 251597. [Google Scholar]
- Korn, S.M.; Qian, Z.; Zurl, H.; Hansen, N.; Pohl, K.K.; Stelzl, D.; Dagnino, F.; Lipsitz, S.; Zhang, J.; Kibel, A.S.; et al. Geographic Variability in Contemporary Utilization of PET Imaging for Prostate Cancer: A Medicare Claims Cohort Study. Cancer Imaging 2025, 25, 86. [Google Scholar] [CrossRef] [PubMed]
- Herscovitch, P. Regulatory Agencies and PET/CT Imaging in the Clinic. Curr. Cardiol. Rep. 2022, 24, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Lapi, S.E.; Scott, P.J.H.; Scott, A.M.; Windhorst, A.D.; Zeglis, B.M.; Abdel-Wahab, M.; Baum, R.P.; Buatti, J.M.; Giammarile, F.; Kiess, A.P.; et al. Recent Advances and Impending Challenges for the Radiopharmaceutical Sciences in Oncology. Lancet Oncol. 2024, 25, e236–e249. [Google Scholar] [CrossRef] [PubMed]
| Criteria | Modality | Metric | Criteria for Progressive Disease (PD) | |||
|---|---|---|---|---|---|---|
| Metric Change | New Lesions | Clinical Decline | T2 Worsening | |||
| RECIST [82] | CT, T1 + C MRI | Longest diameter | >20% increase | Considered PD | Not evaluated | Not evaluated |
| McDonald [81] | CT, T1 + C MRI | Largest cross-sectional area | >25% increase | Considered PD | Considered PD | Not evaluated |
| RANO [84] | T1 + C, T2 MRI | Sum of bipendicular diameters | >25% increase | Considered PD | Considered PD | Considered PD |
| RANO 2.0 [85] | T1 + C, T2 MRI | Sum of bipendicular diameters | >25% increase | Considered PD | Considered PD | Considered PD |
| Volume | >40% increase | |||||
| iRANO [90] | T1 + C, T2 MRI | Sum of bipendicular diameters | >25% increase | Not considered PD | Considered PD | Not considered PD |
| TBRmax of lesion | >30% increase | |||||
| PET-RANO [94] | Amino acid PET | TBRmean of lesion | >10% increase | Considered PD | Considered PD | N/A |
| PET-positive volume | >40% increase | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos, C.A.; Lee, B.P.; Kasten, B.B.; Rogers, J.M.; Cardenas, C.E.; Warram, J.M.; Markert, J.M.; Sorace, A.G. Challenges and Opportunities in High-Grade Glioma Management and Imaging-Based Response Monitoring During Novel Immunotherapies. Cancers 2025, 17, 3176. https://doi.org/10.3390/cancers17193176
Gallegos CA, Lee BP, Kasten BB, Rogers JM, Cardenas CE, Warram JM, Markert JM, Sorace AG. Challenges and Opportunities in High-Grade Glioma Management and Imaging-Based Response Monitoring During Novel Immunotherapies. Cancers. 2025; 17(19):3176. https://doi.org/10.3390/cancers17193176
Chicago/Turabian StyleGallegos, Carlos A., Benjamin P. Lee, Benjamin B. Kasten, Jack M. Rogers, Carlos E. Cardenas, Jason M. Warram, James M. Markert, and Anna G. Sorace. 2025. "Challenges and Opportunities in High-Grade Glioma Management and Imaging-Based Response Monitoring During Novel Immunotherapies" Cancers 17, no. 19: 3176. https://doi.org/10.3390/cancers17193176
APA StyleGallegos, C. A., Lee, B. P., Kasten, B. B., Rogers, J. M., Cardenas, C. E., Warram, J. M., Markert, J. M., & Sorace, A. G. (2025). Challenges and Opportunities in High-Grade Glioma Management and Imaging-Based Response Monitoring During Novel Immunotherapies. Cancers, 17(19), 3176. https://doi.org/10.3390/cancers17193176





