The Cost-Effectiveness of Sugemalimab Plus CAPOX in Treating Advanced Gastric Cancer: Analysis from the GEMSTONE-303 Trial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Health Economic Analysis Plan
2.2. Study Population
2.3. Intervention and Comparator
2.4. Clinical Efficacy
2.5. Treatment-Related Adverse Events
2.6. Direct Medical Costs
2.7. Health Utilities
2.8. Model Assumptions
2.9. Sensitivity Analysis
2.10. Scenario Analysis
3. Results
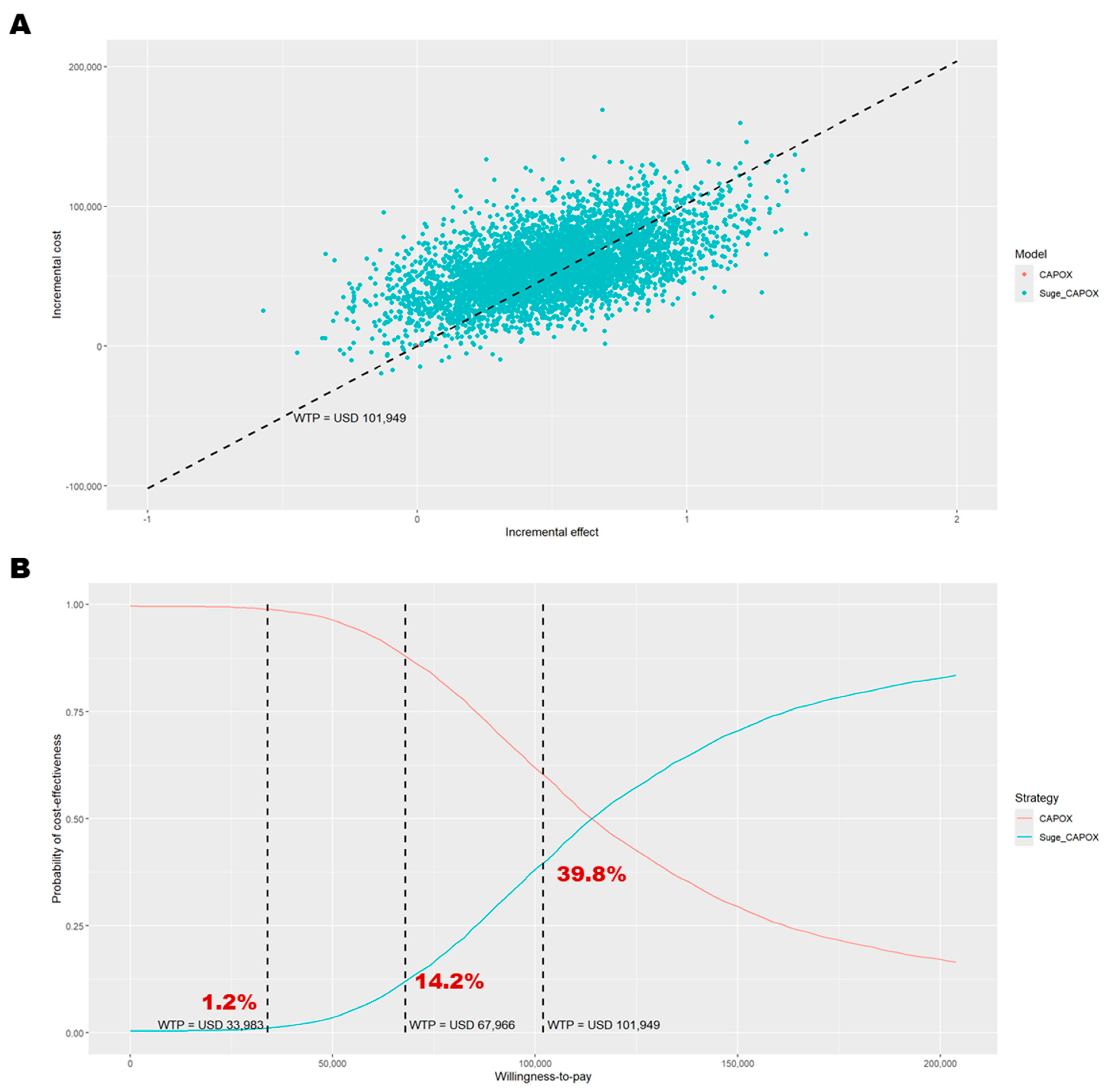
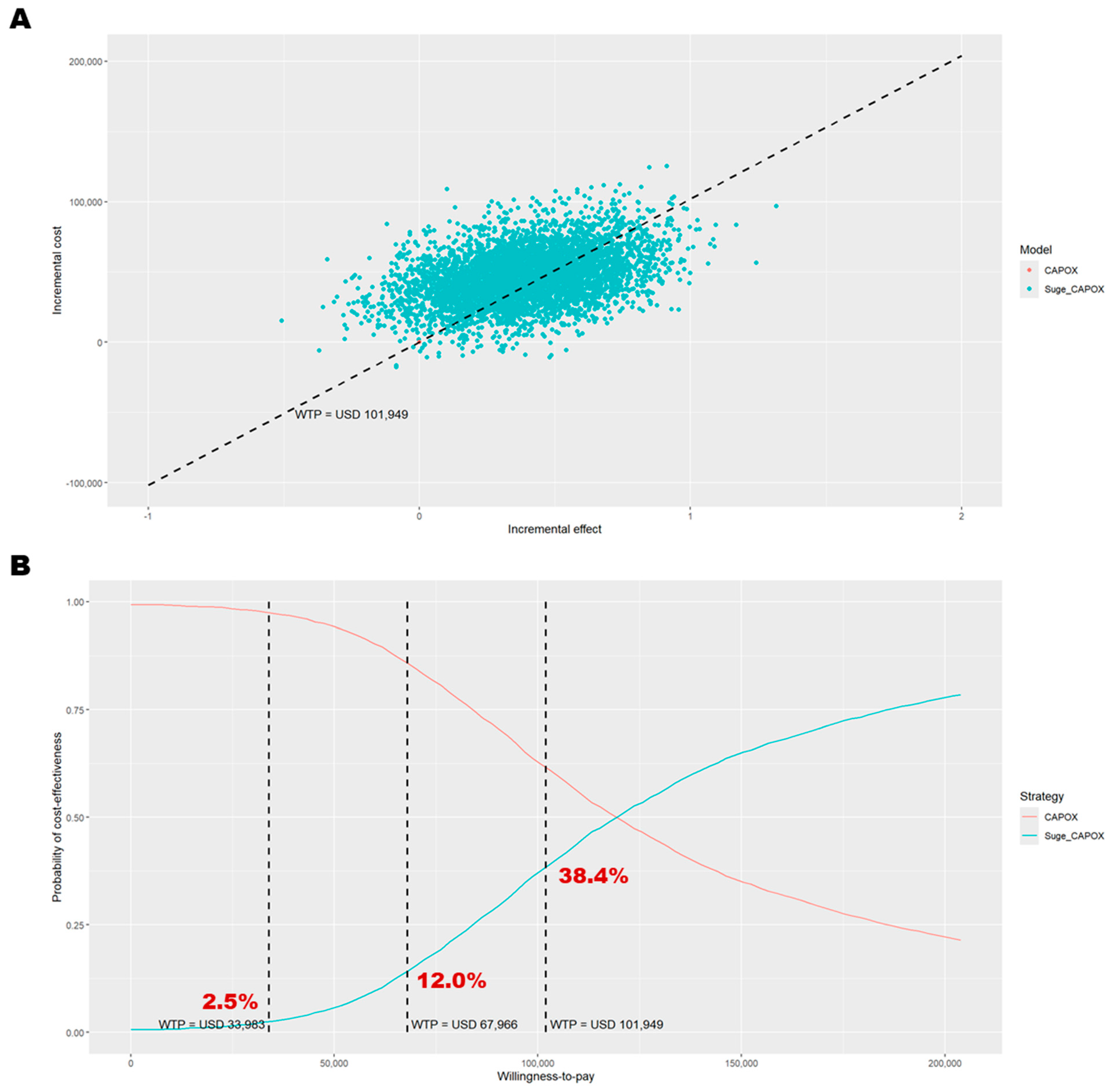
3.1. Base-Case Analysis
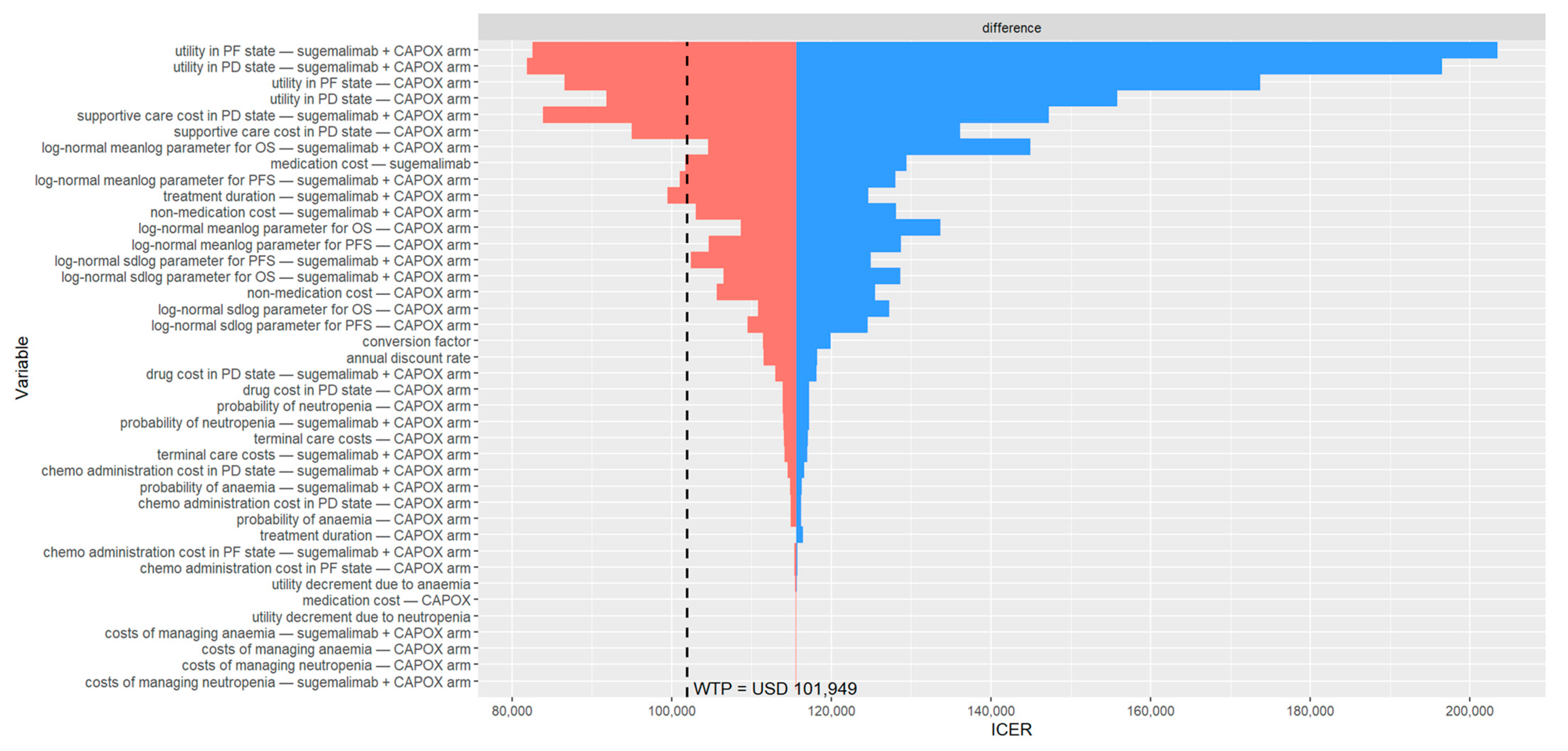
3.2. Base-Case Sensitivity Analysis
3.3. Scenario Analysis
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Topic | No. | Item | Location Where Item Is Reported |
|---|---|---|---|
| Title | |||
| 1 | Identify the study as an economic evaluation and specify the interventions being compared. | Title page | |
| Abstract | |||
| 2 | Provide a structured summary that highlights context, key methods, results, and alternative analyses. | Abstract section | |
| Introduction | |||
| Background and objectives | 3 | Give the context for the study, the study question, and its practical relevance for decision making in policy or practice. | Introduction section |
| Methods | |||
| Health economic analysis plan | 4 | Indicate whether a health economic analysis plan was developed and where available. | Health economic analysis plan subsection |
| Study population | 5 | Describe characteristics of the study population (such as age range, demographics, socioeconomic, or clinical characteristics). | Study population subsection |
| Setting and location | 6 | Provide relevant contextual information that may influence findings. | Health economic analysis plan subsection |
| Comparators | 7 | Describe the interventions or strategies being compared and why chosen. | Intervention and comparator subsection |
| Perspective | 8 | State the perspective(s) adopted by the study and why chosen. | Health economic analysis plan subsection |
| Time horizon | 9 | State the time horizon for the study and why appropriate. | Health economic analysis plan subsection |
| Discount rate | 10 | Report the discount rate(s) and reason chosen. | Health economic analysis plan subsection |
| Selection of outcomes | 11 | Describe what outcomes were used as the measure(s) of benefit(s) and harm(s). | Health economic analysis plan subsection |
| Measurement of outcomes | 12 | Describe how outcomes used to capture benefit(s) and harm(s) were measured. | Clinical efficacy, Treatment-Related adverse events, and Health utilities subsections |
| Valuation of outcomes | 13 | Describe the population and methods used to measure and value outcomes. | Health utilities subsection |
| Measurement and valuation of resources and costs | 14 | Describe how costs were valued. | Direct medical costs subsection |
| Currency, price date, and conversion | 15 | Report the dates of the estimated resource quantities and unit costs, plus the currency and year of conversion. | Health economic analysis plan and Direct medical costs subsections |
| Rationale and description of model | 16 | If modelling is used, describe in detail and why used. Report if the model is publicly available and where it can be accessed. | Health economic analysis plan and Clinical efficacy subsections |
| Analytics and assumptions | 17 | Describe any methods for analysing or statistically transforming data, any extrapolation methods, and approaches for validating any model used. | Model assumptions subsection |
| Characterising heterogeneity | 18 | Describe any methods used for estimating how the results of the study vary for subgroups. | Health economic analysis plan subsection |
| Characterising distributional effects | 19 | Describe how impacts are distributed across different individuals or adjustments made to reflect priority populations. | Not available |
| Characterising uncertainty | 20 | Describe methods to characterise any sources of uncertainty in the analysis. | Sensitivity analysis and Scenario analysis subsections |
| Approach to engagement with patients and others affected by the study | 21 | Describe any approaches to engage patients or service recipients, the general public, communities, or stakeholders (such as clinicians or payers) in the design of the study. | Not available |
| Results | |||
| Study parameters | 22 | Report all analytic inputs (such as values, ranges, references) including uncertainty or distributional assumptions. | Methods section and Table 1 |
| Summary of main results | 23 | Report the mean values for the main categories of costs and outcomes of interest and summarise them in the most appropriate overall measure. | Base-Case analysis and Base-Case sensitivity analysis subsections |
| Effect of uncertainty | 24 | Describe how uncertainty about analytic judgments, inputs, or projections affect findings. Report the effect of choice of discount rate and time horizon, if applicable. | Base-Case sensitivity analysis, Scenario analysis, and Subgroup analysis subsections |
| Effect of engagement with patients and others affected by the study | 25 | Report on any difference patient/service recipient, general public, community, or stakeholder involvement made to the approach or findings of the study | Not available |
| Discussion | |||
| Study findings, limitations, generalisability, and current knowledge | 26 | Report key findings, limitations, ethical or equity considerations not captured, and how these could affect patients, policy, or practice. | Discussion section |
| Other relevant information | |||
| Source of funding | 27 | Describe how the study was funded and any role of the funder in the identification, design, conduct, and reporting of the analysis | Funding section |
| Conflicts of interest | 28 | Report authors’ conflicts of interest according to journal or International Committee of Medical Journal Editors requirements. | Conflict of interest section |
| Regimen | Distribution | Progression-Free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| AIC | BIC | AIC | BIC | ||
| PD-L1 CPS ≥ 5 | |||||
| Sugemalimab + CAPOX | Exponential | 1068.252 | 1071.737 | 1304.787 | 1308.272 |
| Weibull | 1040.966 | 1047.936 | 1286.684 | 1293.653 | |
| Gompertz | 1067.179 | 1074.148 | 1303.422 | 1310.392 | |
| Log-normal | 1006.989 | 1013.959 | 1269.906 | 1276.875 | |
| Log-logistic | 1008.241 | 1015.210 | 1268.815 | 1275.784 | |
| Generalized gamma | 1012.230 | 1022.685 | 1272.836 | 1283.290 | |
| Gamma | 1027.590 | 1034.560 | 1280.048 | 1287.018 | |
| Placebo + CAPOX | Exponential | 1107.486 | 1110.958 | 1386.945 | 1390.418 |
| Weibull | 1064.901 | 1071.846 | 1363.771 | 1370.716 | |
| Gompertz | 1100.335 | 1107.279 | 1383.564 | 1390.508 | |
| Log-normal | 1031.080 | 1038.025 | 1343.358 | 1350.303 | |
| Log-logistic | 1031.023 | 1037.968 | 1344.653 | 1351.597 | |
| Generalized gamma | 1031.948 | 1042.365 | 1345.268 | 1355.685 | |
| Gamma | 1048.522 | 1055.467 | 1355.548 | 1362.492 | |
| PD-L1 CPS ≥ 10 | |||||
| Sugemalimab + CAPOX | Exponential | 556.6350 | 559.5025 | 700.3561 | 703.2237 |
| Weibull | 543.0814 | 548.8165 | 691.7390 | 697.4740 | |
| Gompertz | 556.9605 | 562.6955 | 700.5508 | 706.2858 | |
| Log-normal | 523.6356 | 529.3707 | 681.7808 | 687.5159 | |
| Log-logistic | 526.4785 | 532.2136 | 682.9770 | 688.7121 | |
| Generalized gamma | 527.9641 | 536.5668 | 684.7373 | 693.3399 | |
| Gamma | 535.8626 | 541.5977 | 688.2621 | 693.9972 | |
| Placebo + CAPOX | Exponential | 580.5273 | 583.3794 | 746.9172 | 749.7692 |
| Weibull | 564.0427 | 569.7467 | 740.0807 | 745.7848 | |
| Gompertz | 579.3337 | 585.0377 | 747.3778 | 753.0819 | |
| Log-normal | 549.3813 | 555.0854 | 733.1846 | 738.8886 | |
| Log-logistic | 550.6078 | 556.3118 | 733.3101 | 739.0141 | |
| Generalized gamma | 550.9119 | 559.4680 | 735.0102 | 743.5663 | |
| Gamma | 557.4220 | 563.1261 | 737.2156 | 742.9196 | |
| Scenario | Sugemalimab + CAPOX vs. Placebo + CAPOX | |||||||
|---|---|---|---|---|---|---|---|---|
| Base-Case Analysis | Probabilistic Sensitivity Analysis | |||||||
| Incremental Costs (USD) | Incremental Effectiveness (QALYs) | ICER | INMB (USD) | ICER | INMB (USD) | Probability of Cost-Effectiveness | EVPI/Person | |
| 0. Base case | 58,017 | 0.50 | 115,568 | −6837 | 113,965 | −6098 | 39.8% | 6993 |
| 1. Effectiveness measure: Equal value of life-years gained | 58,017 | 0.61 | 94,385 | 4648 | 93,893 | 4952 | 60.5% | 5024 |
| 2a. Treatment duration: Median PFS (7.92 months vs. 6.93 months) | 62,572 | 0.50 | 124,641 | −11,392 | 122,928 | −10,647 | 32.8% | 5336 |
| 2b. Treatment duration: Protocol-defined maximum | 86,230 | 0.50 | 171,766 | −35,049 | 169,454 | −34,259 | 7.9% | 854 |
| 2c. Treatment duration: Administered until disease progressed | 94,078 | 0.50 | 187,400 | −42,898 | 185,641 | −42,474 | 4.2% | 362 |
| 3a. Time horizons: 5 years | 48,985 | 0.39 | 125,418 | −9166 | 124,393 | −8786 | 34.1% | 4764 |
| 3b. Time horizons: 10 years | 55,509 | 0.47 | 117,607 | −7390 | 116,260 | −6780 | 38.4% | 6383 |
| 3c. Time horizons: 20 years | 57,671 | 0.49 | 115,824 | −6908 | 114,292 | −6199 | 39.7% | 6901 |
| 3d. Time horizons: 30 years | 57,957 | 0.50 | 115,611 | −6849 | 114,026 | −6117 | 39.7% | 6975 |
| 4a. Price: 90% of the hypothesized price of sugemalimab | 55,235 | 0.50 | 110,026 | −4054 | 108,490 | −3320 | 44.4% | 8158 |
| 4b. Price: 80% of the hypothesized price of sugemalimab | 52,453 | 0.50 | 104,484 | −1272 | 103,016 | −541 | 49.4% | 9465 |
| 4c. Price: 70% of the hypothesized price of sugemalimab | 49,671 | 0.50 | 98,943 | 1509 | 97,541 | 2237 | 54.0% | 8663 |
| 4d. Price: 60% of the hypothesized price of sugemalimab | 46,889 | 0.50 | 93,401 | 4291 | 92,066 | 5015 | 58.5% | 7448 |
| 4e. Price: 50% of the hypothesized price of sugemalimab | 44,107 | 0.50 | 87,859 | 7073 | 86,591 | 7794 | 62.9% | 6354 |
| 5. Conversion factor: One point equals TWD 1 | 60,490 | 0.50 | 120,494 | −9310 | 118,856 | −8580 | 36.2% | 6268 |
| 6a. Survival models: Log-logistic | 56,523 | 0.48 | 116,472 | −7048 | 114,450 | −6142 | 40.2% | 7469 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- GBD Compare. Available online: http://vizhub.healthdata.org/gbd-compare (accessed on 20 April 2025).
- Qin, N.; Fan, Y.; Yang, T.; Yang, Z.; Fan, D. The Burden of Gastric Cancer and Possible Risk Factors from 1990 to 2021, and Projections until 2035: Findings from the Global Burden of Disease Study 2021. Biomark. Res. 2025, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Guidelines Detail. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434 (accessed on 21 July 2025).
- Klempner, S.J.; Lee, J.; Sundar, R. PD-1 or PD-L1—A Difference That Makes No Difference? JAMA 2025, 333, 1296. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-Line Nivolumab plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal Junction, and Oesophageal Adenocarcinoma (CheckMate 649): A Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Ajani, J.A.; Moehler, M.; Shen, L.; Garrido, M.; Gallardo, C.; Wyrwicz, L.; Yamaguchi, K.; Cleary, J.M.; Elimova, E.; et al. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. JCO 2024, 42, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.Y.; Wyrwicz, L.S.; Yanez Weber, P.E.; Bai, Y.; Ryu, M.-H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.-K.; et al. Pembrolizumab (Pembro) + Chemotherapy (Chemo) for Advanced HER2-Negative Gastric or Gastroesophageal Junction (G/GEJ) Cancer: Updated Results from the KEYNOTE-859 Study. JCO 2024, 42, 4045. [Google Scholar] [CrossRef]
- Rha, S.Y.; Oh, D.-Y.; Yañez, P.; Bai, Y.; Ryu, M.-H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.-K.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for HER2-Negative Advanced Gastric Cancer (KEYNOTE-859): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, H.; Pan, Y.; Gu, K.; Cang, S.; Han, L.; Shu, Y.; Li, J.; Zhao, J.; Pan, H.; et al. Sintilimab Plus Chemotherapy for Unresectable Gastric or Gastroesophageal Junction Cancer: The ORIENT-16 Randomized Clinical Trial. JAMA 2023, 330, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.-Z.; Oh, D.-Y.; Kato, K.; Arkenau, T.; Tabernero, J.; Correa, M.C.; Zimina, A.V.; Bai, Y.; Shi, J.; Lee, K.-W.; et al. Tislelizumab plus Chemotherapy versus Placebo plus Chemotherapy as First Line Treatment for Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma: RATIONALE-305 Randomised, Double Blind, Phase 3 Trial. BMJ 2024, 385, e078876. [Google Scholar] [CrossRef]
- Lordick, F.; Candia Montero, L.; Castelo-Branco, L.; Pentheroudakis, G.; Sessa, C.; Smyth, E.C. ESMO Gastric Cancer Living Guideline v1.4. Available online: https://www.esmo.org/guidelines/living-guidelines/esmo-living-guideline-gastric-cancer (accessed on 11 August 2025).
- Zhou, Q.; Chen, M.; Jiang, O.; Pan, Y.; Hu, D.; Lin, Q.; Wu, G.; Cui, J.; Chang, J.; Cheng, Y.; et al. Sugemalimab versus Placebo after Concurrent or Sequential Chemoradiotherapy in Patients with Locally Advanced, Unresectable, Stage III Non-Small-Cell Lung Cancer in China (GEMSTONE-301): Interim Results of a Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol. 2022, 23, 209–219. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Sun, Y.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Chang, J.; Chen, J.; et al. Sugemalimab versus Placebo, in Combination with Platinum-Based Chemotherapy, as First-Line Treatment of Metastatic Non-Small-Cell Lung Cancer (GEMSTONE-302): Interim and Final Analyses of a Double-Blind, Randomised, Phase 3 Clinical Trial. Lancet Oncol. 2022, 23, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Z.; Sun, M.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Sun, S.; Chen, J.; et al. Sugemalimab versus Placebo, in Combination with Platinum-Based Chemotherapy, as First-Line Treatment of Metastatic Non-Small-Cell Lung Cancer (GEMSTONE-302): 4-Year Outcomes from a Double-Blind, Randomised, Phase 3 Trial. Lancet Oncol. 2025, 26, 887–897. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Bai, Y.; Liu, B.; Li, Q.; Zhang, J.; Zhou, J.; Deng, T.; Zhou, F.; Gao, S.; et al. First-Line Sugemalimab with Chemotherapy for Advanced Esophageal Squamous Cell Carcinoma: A Randomized Phase 3 Study. Nat. Med. 2024, 30, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Wang, G.; Zhang, Y.; Fan, Q.; Lu, C.; Hu, C.; Sun, M.; Wan, Y.; Sun, S.; et al. First-Line Sugemalimab Plus Chemotherapy for Advanced Gastric Cancer: The GEMSTONE-303 Randomized Clinical Trial. JAMA 2025. [Google Scholar] [CrossRef]
- Cejemly|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cejemly (accessed on 18 September 2025).
- Sugemalimab Approved to Treat Adult Patients with Non-Small Cell Lung Cancer. Available online: https://www.gov.uk/government/news/sugemalimab-approved-to-treat-adult-patients-with-non-small-cell-lung-cancer (accessed on 21 July 2025).
- Dhillon, S.; Duggan, S. Sugemalimab: First Approval. Drugs 2022, 82, 593–599. [Google Scholar] [CrossRef]
- CStone Announces the Fifth Indication Approved for Sugemalimab in China as First-Line Treatment for Gastric Cancer-CStone Pharmaceuticals. Available online: https://www.cstonepharma.com/en/html/news/3668.html (accessed on 21 July 2025).
- Sugemalimab 600 mg (20.0 mL)—Yaozh. Available online: https://db.yaozh.com/pijian/bJmWaWllbmZplWRilJaanDkwMzli.html (accessed on 18 September 2025).
- Tang, L.; Zhu, L.; Zhan, S.; Chen, Y.; Feng, P.-F. Cost-Effectiveness Analysis of Sugemalimab Combined with Chemotherapy as First-Line Treatment for Advanced Gastric Cancer. Front. Public Health 2025, 13, 1620663. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Chen, R.; You, M. Cost-Effective Analysis of Sugemalimab plus Chemotherapy as First-Line Treatment for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma with PD-L1 CPS ≥ 5. Front. Public Health 2025, 13, 1604372. [Google Scholar] [CrossRef] [PubMed]
- Statistical Tables. Available online: https://eng.stat.gov.tw/cp.aspx?n=2334 (accessed on 28 July 2025).
- Huang, W.M.; Chiang, N.J.; Tsai, Y.W. EE836 Cost-Effectiveness of Zolbetuximab Plus Chemotherapy as First-Line Treatment for Advanced Gastric or Gastroesophageal Adenocarcinoma in Taiwan. Value Health 2024, 27, S219. [Google Scholar] [CrossRef]
- Chen, K.-A.; Huang, W.-M.; Chen, E.Y.-T.; Ho, P.-K.; Chueh, C.-H.; Wen, Y.-W.; Chen, M.-H.; Chiang, N.-J.; Tsai, Y.-W. Cost-Effectiveness of Ivosidenib versus Chemotherapy for Previously Treated IDH1-Mutant Advanced Intrahepatic Cholangiocarcinoma in Taiwan. BMC Cancer 2024, 24, 622. [Google Scholar] [CrossRef]
- Chueh, C.-H.; Tsai, Y.-W.; Chen, Z.-R.; Shiu, M.-N.; Wen, Y.-W.; Chiang, N.-J. Cost-Effectiveness Analysis of a New Second-Line Treatment Regimen for Advanced Intrahepatic Cholangiocarcinoma: Biomarker-Driven Targeted Therapy of Pemigatinib Versus 5-FU Chemotherapy. PharmacoEconomics 2023, 41, 307–319. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Chueh, C.-H.; Chiang, N.-J.; Tsai, Y.-W. Cost-Effectiveness of Pemigatinib as a Second-Line Treatment for Advanced Intrahepatic Cholangiocarcinoma with Fibroblast Growth Factor Receptor 2 Fusions in Taiwan: From the Evidence of the Phase II Trial and the Perspective of Taiwan’s National Health Insurance Administration. Cost Eff. Resour. Alloc. 2023, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health 2022, 25, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: A Report of the ISPOR CHEERS II Good Practices Task Force. Value Health 2022, 25, 10–31. [Google Scholar] [CrossRef] [PubMed]
- Filipović-Pierucci, A.; Zarca, K.; Durand-Zaleski, I. Markov Models for Health Economic Evaluations: The R Package Heemod. arXiv 2017, arXiv:1702.03252. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.; Ouwens, M.J.; Welton, N.J. Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Automeris. Io: Computer Vision Assisted Data Extraction from Charts Using WebPlotDigitizer. Available online: https://automeris.io/ (accessed on 28 July 2025).
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct Individual Patient Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef]
- Latimer, N. Nice Dsu Technical Support Document 14: Survival Analysis for Economic Evaluations Alongside Clinical Trials—Extrapolation with Patient-Level Data; Decision Support Unit: Sheffield, UK, 2011. [Google Scholar]
- Drugdataexpy. Available online: https://data.yaozh.com/ (accessed on 17 July 2025).
- Australian Government Department of Health and Aged Care, Pharmaceutical Benefits Scheme (PBS). Nivolumab (HER-2-Neg Gastric Cancer, Gastroesophageal Junction Cancer or Oesophageal Adenocarcinoma): Injection Concentrate for I.V. Infusion 40 Mg in 4 mL, Injection Concentrate for I.V. Infusion 100 Mg in 10 mL; OPDIVO®. Available online: https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2022-03/nivolumab-her-2-neg-gastric-cancer-gastroesophageal-junction-cancer (accessed on 21 July 2025).
- Cai, H.; Xu, B.; Li, N.; Zheng, B.; Zheng, Z.; Liu, M. Cost-Effectiveness Analysis of Camrelizumab Versus Chemotherapy as Second-Line Treatment of Advanced or Metastatic Esophageal Squamous Cell Carcinoma. Front. Pharmacol. 2021, 12, 732912. [Google Scholar] [CrossRef]
- Nafees, B.; Stafford, M.; Gavriel, S.; Bhalla, S.; Watkins, J. Health State Utilities for Non Small Cell Lung Cancer. Health Qual. Life Outcomes 2008, 6, 84. [Google Scholar] [CrossRef]
- Taiwan Center for Drug Evaluation. Methodology Guidelines for Health Technology Assessment. Available online: https://www.cde.org.tw/hta/1444/1834/2155/12989/ (accessed on 8 August 2025).
- National Health Insurance Administration, Ministry of Health and Welfare. Settlement and Estimated Point Value. Available online: https://www.nhi.gov.tw/ch/cp-5773-4d180-2767-1.html (accessed on 12 August 2025).
- Exchange Rates of TWD and Major Trading Partner Currencies Versus USD Over Selected Periods. Available online: https://cpx.cbc.gov.tw/Range/RangeSelect?pxfilename=BP01Y01.px (accessed on 28 July 2025).
- CBC Statistical Database. Available online: https://cpx.cbc.gov.tw/Data/DataMain/?pxfilename=BP06Y01en.px (accessed on 17 August 2025).

| Parameters | Base-Case Analysis | One-Way Sensitivity Analysis | Probabilistic Sensitivity Analysis | Source | ||||
|---|---|---|---|---|---|---|---|---|
| Value | Range (95% CI or ±25%) | Distribution | Parameter 1 | Parameter 2 | ||||
| Overall survival (base-case) | ||||||||
| PD-L1 CPS ≥ 5 | ||||||||
| Sugemalimab + CAPOX | [17] | |||||||
| Log-logistic | Shape | 1.904 | 1.667 | 2.174 | normal (mean, se) | 1.904 | 0.129 | |
| Scale | 16.860 | 14.921 | 19.052 | normal (mean, se) | 16.860 | 1.051 | ||
| CAPOX | [17] | |||||||
| Log-logistic | Shape | 2.004 | 1.772 | 2.266 | normal (mean, se) | 2.004 | 0.126 | |
| Scale | 13.331 | 11.894 | 14.942 | normal (mean, se) | 13.331 | 0.776 | ||
| PD-L1 CPS ≥ 10 | ||||||||
| Sugemalimab + CAPOX | ||||||||
| Log-normal | Mean on log scale | 2.912 | 2.735 | 3.089 | normal (mean, se) | 2.912 | 0.090 | [17] |
| SD on log scale | 0.922 | 0.783 | 1.087 | normal (mean, se) | 0.922 | 0.077 | ||
| CAPOX | ||||||||
| Log-normal | Mean on log scale | 2.538 | 2.363 | 2.712 | normal (mean, se) | 2.538 | 0.089 | [17] |
| SD on log scale | 0.947 | 0.818 | 1.098 | normal (mean, se) | 0.947 | 0.071 | ||
| Progression-free survival (base-case) | ||||||||
| PD-L1 CPS ≥ 5 | ||||||||
| Sugemalimab + CAPOX | [17] | |||||||
| Log-logistic | Shape | 2.212 | 1.936 | 2.528 | normal (mean, se) | 2.212 | 0.15 | |
| Scale | 9.015 | 8.082 | 10.056 | normal (mean, se) | 9.015 | 0.503 | ||
| CAPOX | [17] | |||||||
| Log-logistic | Shape | 2.445 | 2.156 | 2.772 | normal (mean, se) | 2.445 | 0.157 | |
| Scale | 7.305 | 6.629 | 8.05 | normal (mean, se) | 7.305 | 0.362 | ||
| PD-L1 CPS ≥ 10 | ||||||||
| Sugemalimab + CAPOX | ||||||||
| Log-normal | Mean on log scale | 2.278 | 2.123 | 2.433 | normal (mean, se) | 2.278 | 0.079 | [17] |
| SD on log scale | 0.776 | 0.656 | 0.917 | normal (mean, se) | 0.776 | 0.066 | ||
| CAPOX | ||||||||
| Log-normal | Mean on log scale | 2.034 | 1.884 | 2.183 | normal (mean, se) | 2.034 | 0.077 | [17] |
| SD on log scale | 0.777 | 0.666 | 0.906 | normal (mean, se) | 0.777 | 0.061 | ||
| Overall survival (scenario) | ||||||||
| PD-L1 CPS ≥ 5 | ||||||||
| Sugemalimab + CAPOX | [17] | |||||||
| Log-normal | Mean on log scale | 2.838 | 2.708 | 2.967 | normal (mean, se) | 2.838 | 0.066 | |
| SD on log scale | 0.921 | 0.818 | 1.037 | normal (mean, se) | 0.921 | 0.056 | ||
| CAPOX | [17] | |||||||
| Log-normal | Mean on log scale | 2.593 | 2.476 | 2.709 | normal (mean, se) | 2.593 | 0.060 | |
| SD on log scale | 0.862 | 0.773 | 0.961 | normal (mean, se) | 0.862 | 0.048 | ||
| PD-L1 CPS ≥ 10 | ||||||||
| Sugemalimab + CAPOX | ||||||||
| Log-logistic | Shape | 1.870 | 1.557 | 2.246 | normal (mean, se) | 1.870 | 0.175 | [17] |
| Scale | 18.193 | 15.352 | 21.559 | normal (mean, se) | 18.193 | 1.576 | ||
| CAPOX | ||||||||
| Log-logistic | Shape | 1.840 | 1.555 | 2.177 | normal (mean, se) | 1.840 | 0.158 | [17] |
| Scale | 12.808 | 10.823 | 15.159 | normal (mean, se) | 12.808 | 1.101 | ||
| Progression-free survival (scenario) | ||||||||
| PD-L1 CPS ≥ 5 | ||||||||
| Sugemalimab + CAPOX | [17] | |||||||
| Log-normal | Mean on log scale | 2.224 | 2.111 | 2.337 | normal (mean, se) | 2.224 | 0.058 | |
| SD on log scale | 0.785 | 0.698 | 0.884 | normal (mean, se) | 0.785 | 0.048 | ||
| CAPOX | [17] | |||||||
| Log-normal | Mean on log scale | 2.005 | 1.905 | 2.105 | normal (mean, se) | 2.005 | 0.051 | |
| SD on log scale | 0.715 | 0.640 | 0.798 | normal (mean, se) | 0.715 | 0.040 | ||
| PD-L1 CPS ≥ 10 | ||||||||
| Sugemalimab + CAPOX | ||||||||
| Log-logistic | Shape | 2.193 | 1.820 | 2.642 | normal (mean, se) | 2.193 | 0.208 | [17] |
| Scale | 9.503 | 8.156 | 11.072 | normal (mean, se) | 9.503 | 0.741 | ||
| CAPOX | ||||||||
| Log-logistic | Shape | 2.227 | 1.87 | 2.652 | normal (mean, se) | 2.227 | 0.198 | [17] |
| Scale | 7.604 | 6.567 | 8.804 | normal (mean, se) | 7.604 | 0.569 | ||
| Median treatment duration (months) | ||||||||
| Sugemalimab + CAPOX | 6.3 | [17] | ||||||
| CAPOX | 5.2 | [17] | ||||||
| Medication cost (per year, USD) | ||||||||
| Sugemalimab | 59,814 | 44,860 | 74,767 | fixed | [37] | |||
| CAPOX | 8959 | 8474 | 9444 | gamma (μ, s) | 8959 | 247 | [26] | |
| Chemo administration cost (per year, USD) | 1226 | 1148 | 1303 | gamma (μ, s) | 1226 | 39 | [26] | |
| Non-medication cost (per year, USD) | 25,925 | 22,402 | 29,449 | gamma (μ, s) | 25,925 | 1798 | [26] | |
| Supportive care cost (per year, USD) | 63,077 | 41,234 | 84,920 | gamma (μ, s) | 63,077 | 11,144 | [26] | |
| PD drug cost (per year, USD) | 4682 | 3878 | 5485 | gamma (μ, s) | 4682 | 410 | [26] | |
| PD chemo administration cost (per year, USD) | 2018 | 1551 | 2485 | gamma (μ, s) | 2018 | 238 | [26] | |
| Terminal care costs (per year, USD) | 54,893 | 50,519 | 59,266 | gamma (μ, s) | 54,893 | 2231 | [26] | |
| Adverse event cost (per year, USD) | ||||||||
| Neutropenia | 769 | 388 | 1151 | gamma (μ, s) | 769 | 195 | [26] | |
| Anemia | 1494 | 959 | 2029 | gamma (μ, s) | 1494 | 273 | [26] | |
| Adverse event probability | ||||||||
| Sugemalimab + CAPOX | ||||||||
| Neutropenia | 0.141 | 0.10575 | 0.17625 | fixed | [17] | |||
| Anemia | 0.183 | 0.13725 | 0.22875 | fixed | [17] | |||
| CAPOX | ||||||||
| Neutropenia | 0.143 | 0.10725 | 0.17875 | fixed | [17] | |||
| Anemia | 0.16 | 0.12 | 0.2 | fixed | [17] | |||
| Utility | ||||||||
| Sugemalimab (PF state) | 0.812 | 0.609 | 1 | beta (α, β) | 10.74 | 2.49 | [38] | |
| Sugemalimab (PD state) | 0.746 | 0.5595 | 0.9325 | beta (α, β) | 14.87 | 5.06 | [38] | |
| Chemotherapy (PF state) | 0.798 | 0.5985 | 0.9975 | beta (α, β) | 11.62 | 2.94 | [38] | |
| Chemotherapy (PD state) | 0.721 | 0.54075 | 0.90125 | beta (α, β) | 16.43 | 6.36 | [38] | |
| Disutility | ||||||||
| Neutropenia | 0.2 | 0.15 | 0.25 | beta (α, β) | 48.97 | 195.89 | [39] | |
| Anemia | 0.07 | 0.0525 | 0.0875 | beta (α, β) | 57.09 | 758.52 | [40] | |
| Annual discount rate | 0.03 | 0 | 0.05 | fixed | [41] | |||
| Conversion factor | 0.9198 | 0.853 | 0.9913 | uniform (min, max) | 0.853 | 0.9913 | [42] | |
| Outcomes of Partitioned Survival Models | Incremental Changes | |||||
|---|---|---|---|---|---|---|
| Population | PD-L1 CPS ≥ 5 | PD-L1 CPS ≥ 10 | PD-L1 CPS ≥ 5 | PD-L1 CPS ≥ 10 | ||
| Treatment Strategy | Sugemalimab + CAPOX | CAPOX | Sugemalimab + CAPOX | CAPOX | Sugemalimab + CAPOX vs. CAPOX | Sugemalimab + CAPOX vs. CAPOX |
| Cost (USD) | 123,373 | 76,353 | 130,008 | 71,990 | 47,020 | 58,018 |
| Total cost of PF state | 55,015 | 21,951 | 56,316 | 23,068 | 33,064 | 33,248 |
| Medication cost | 30,264 | 2846 | 30,741 | 2830 | 27,418 | 27,911 |
| Non-medication cost | 24,751 | 19,105 | 25,575 | 20,238 | 5646 | 5337 |
| Total cost of PD state | 68,358 | 54,401 | 73,692 | 48,922 | 13,957 | 24,770 |
| Medication cost | 4740 | 3726 | 5127 | 3329 | 1014 | 1798 |
| Non-medication cost | 63,618 | 50,675 | 68,565 | 45,593 | 12,943 | 22,972 |
| LY | 2.05 | 1.59 | 2.17 | 1.56 | 0.46 | 0.61 |
| PF state | 1.03 | 0.79 | 1.06 | 0.84 | 0.24 | 0.22 |
| PD state | 1.02 | 0.80 | 1.11 | 0.72 | 0.22 | 0.39 |
| QALY | 1.56 | 1.17 | 1.65 | 1.15 | 0.39 | 0.50 |
| PF state | 0.80 | 0.59 | 0.83 | 0.64 | 0.21 | 0.19 |
| PD state | 0.76 | 0.58 | 0.82 | 0.51 | 0.18 | 0.31 |
| ICERs | ||||||
| Incremental cost per LY gained | 102,559 | 94,385 | ||||
| Incremental cost per QALY gained | 121,230 | 115,568 | ||||
| INMB | ||||||
| LY | −279 | 4648 | ||||
| QALY | −7478 | −6837 | ||||
| EVPI/person | 6274 | 6993 | ||||
| Scenario | Sugemalimab + CAPOX vs. Placebo + CAPOX | |||||||
|---|---|---|---|---|---|---|---|---|
| Base-Case Analysis | Probabilistic Sensitivity Analysis | |||||||
| Incremental Costs (USD) | Incremental Effectiveness (QALYs) | ICER | INMB (USD) | ICER | INMB (USD) | Probability of Cost-Effectiveness | EVPI/Person | |
| 0. Base case | 47,020 | 0.39 | 121,230 | −7478 | 119,409 | −6837 | 38.4% | 6274 |
| 1. Effectiveness measure: Equal value of life-years gained | 47,020 | 0.46 | 102,559 | −279 | 101,801 | 68 | 49.7% | 6325 |
| 2a. Treatment duration: Median PFS (7.72 months vs. 6.24 months) | 51,380 | 0.39 | 132,470 | −11,838 | 130,523 | −11,189 | 31.2% | 4748 |
| 2b. Treatment duration: Protocol-defined maximum | 72,100 | 0.39 | 185,891 | −32,558 | 183,604 | −31,975 | 8.5% | 873 |
| 2c. Treatment duration: Administered until disease progressed | 81,444 | 0.39 | 209,982 | −41,902 | 208,520 | −41,731 | 3.6% | 303 |
| 3a. Time horizons: 5 years | 39,352 | 0.28 | 140,143 | −10,725 | 138,879 | −10,379 | 29.6% | 3762 |
| 3b. Time horizons: 10 years | 43,824 | 0.34 | 127,221 | −8705 | 125,669 | −8206 | 35.4% | 5236 |
| 3c. Time horizons: 20 years | 46,115 | 0.37 | 122,706 | −7801 | 120,980 | −7206 | 37.7% | 5986 |
| 3d. Time horizons: 30 years | 46,762 | 0.38 | 121,632 | −7567 | 119,842 | −6940 | 38.1% | 6193 |
| 4a. Price: 90% of the hypothesized price of sugemalimab | 44,282 | 0.39 | 114,171 | −4740 | 112,428 | −4103 | 43.0% | 7384 |
| 4b. Price: 80% of the hypothesized price of sugemalimab | 41,545 | 0.39 | 107,112 | −2002 | 105,447 | −1370 | 47.7% | 8622 |
| 4c. Price: 70% of the hypothesized price of sugemalimab | 38,807 | 0.39 | 100,054 | 734 | 98,465 | 1364 | 52.2% | 8626 |
| 4d. Price: 60% of the hypothesized price of sugemalimab | 36,069 | 0.39 | 92,995 | 3472 | 91,484 | 4098 | 57.0% | 7388 |
| 4e. Price: 50% of the hypothesized price of sugemalimab | 33,331 | 0.39 | 85,936 | 6210 | 84,503 | 6831 | 61.6% | 6277 |
| 5. Conversion factor: One point equals TWD 1 | 48,645 | 0.39 | 125,418 | −9102 | 123,542 | −8456 | 36.6% | 5919 |
| 6a. Survival models: Log-logistic | 49,103 | 0.41 | 120,171 | −7445 | 118,633 | −6879 | 37.5% | 5975 |
| 6b. Best-fit survival models based solely on AIC and BIC | 51,384 | 0.43 | 119,602 | −7584 | 118,180 | −7071 | 37.6% | 6028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chueh, C.-H.; Huang, W.-M.; Hong, M.-Y.; Tsai, Y.-W.; Chiang, N.-J.; Chen, H.-L. The Cost-Effectiveness of Sugemalimab Plus CAPOX in Treating Advanced Gastric Cancer: Analysis from the GEMSTONE-303 Trial. Cancers 2025, 17, 3171. https://doi.org/10.3390/cancers17193171
Chueh C-H, Huang W-M, Hong M-Y, Tsai Y-W, Chiang N-J, Chen H-L. The Cost-Effectiveness of Sugemalimab Plus CAPOX in Treating Advanced Gastric Cancer: Analysis from the GEMSTONE-303 Trial. Cancers. 2025; 17(19):3171. https://doi.org/10.3390/cancers17193171
Chicago/Turabian StyleChueh, Chen-Han, Wei-Ming Huang, Ming-Yu Hong, Yi-Wen Tsai, Nai-Jung Chiang, and Hsiao-Ling Chen. 2025. "The Cost-Effectiveness of Sugemalimab Plus CAPOX in Treating Advanced Gastric Cancer: Analysis from the GEMSTONE-303 Trial" Cancers 17, no. 19: 3171. https://doi.org/10.3390/cancers17193171
APA StyleChueh, C.-H., Huang, W.-M., Hong, M.-Y., Tsai, Y.-W., Chiang, N.-J., & Chen, H.-L. (2025). The Cost-Effectiveness of Sugemalimab Plus CAPOX in Treating Advanced Gastric Cancer: Analysis from the GEMSTONE-303 Trial. Cancers, 17(19), 3171. https://doi.org/10.3390/cancers17193171






