Effects of Nordic Walking on Physical Fitness in Patients with Cancer: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- Design: Only randomized controlled trials (RCTs) or quasi-randomized controlled trials (quasi-RCTs) were included to ensure high-quality evidence. Non-controlled studies, observational studies, case reports, and qualitative research were excluded.
- Population: Studies involving participants diagnosed with any type of cancer at any stage were included. There were no restrictions regarding age, sex, or ethnicity. Studies focusing on non-oncological populations or mixed populations where cancer-specific outcomes could not be extracted were excluded.
- Intervention: The intervention of interest was NW either as a standalone intervention or in combination with usual care. Eligible studies had to provide a structured NW program alone or in combination with other exercises.
- Comparator: At least one of the following comparators was required: Nordic Walking versus no intervention (this included usual care and general exercise recommendations but excluded specific exercise programs). Nordic Walking versus another structured exercise program.
- Outcomes: Studies were required to report at least one of the following outcomes: Cardiorespiratory endurance: Assessed via field tests (e.g., six-minute walk distance [6MWD]) or laboratory-based measures (e.g., VO2 max). Muscle strength: Evaluated using direct measures (e.g., handgrip dynamometry) or functional assessments (e.g., sit-to-stand [STS] test). Balance: Quantified through balance assessments (e.g., Berg Balance Scale or timed up-and-go test) or equivalent tools. Physical Activity level: Assessed through self-reported questionnaires (e.g., International Physical Activity Questionnaire [IPAQ]) or objective measures (e.g., accelerometry). Health Related Quality of Life (HRQoL): Evaluated using validated questionnaires specific to oncological or general populations (e.g., 36 Health Survey [SF-36]). Adherence: Evaluated by the number or proportion of participants who completed the intervention period. Safety: Evaluated by the number and type of adverse events.
2.2. Information Sources and Search Strategies
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias for Individual Studies
2.6. Measurements of Treatment
2.6.1. Efficacy Outcomes
2.6.2. Adherence and Safety Outcomes
2.7. Data Synthesis
2.8. Publication Bias
2.9. Summary of Finding and Assessment of the Certainty of the Evidence
3. Results
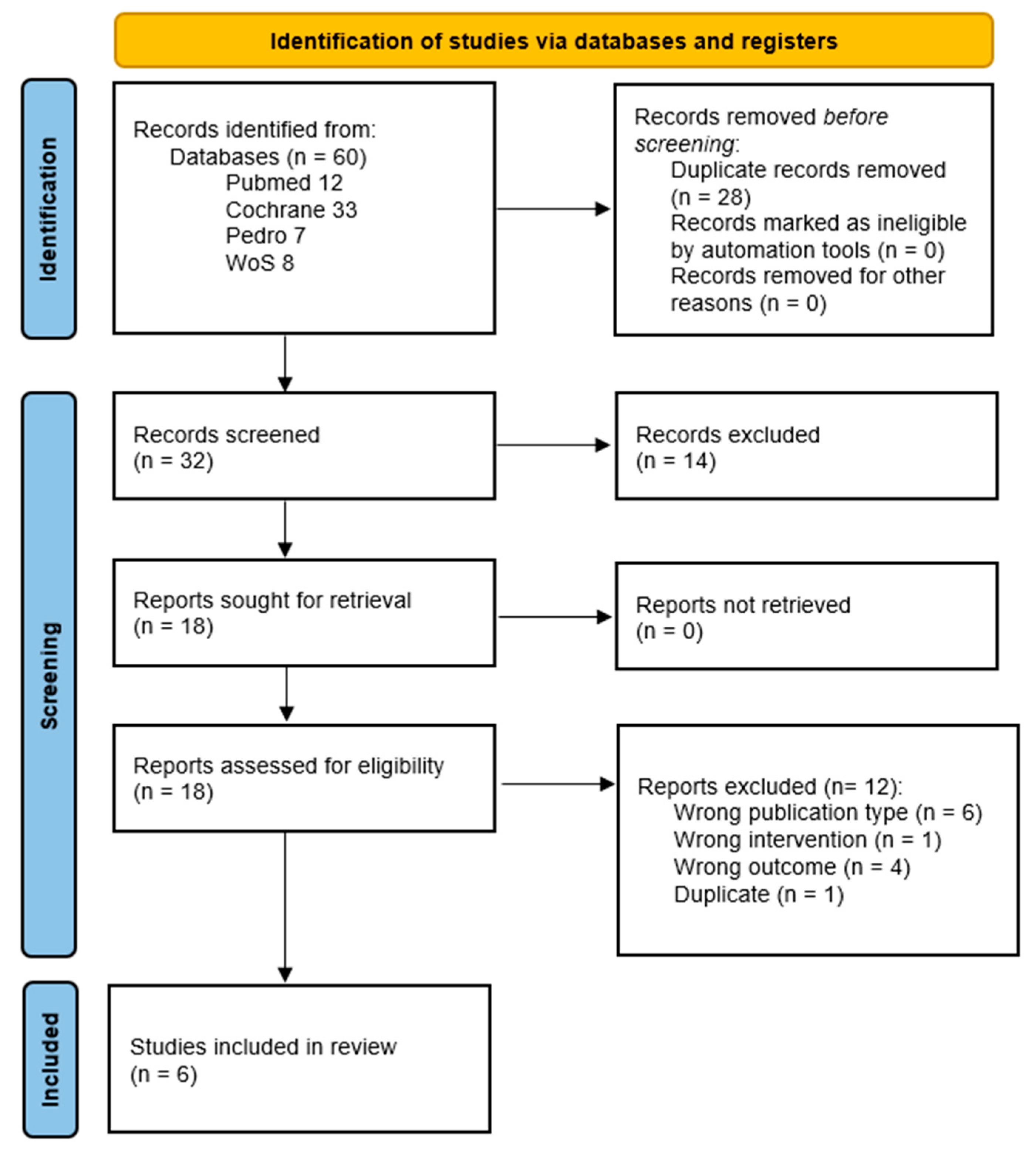
3.1. Search Results
3.2. Characteristics of the Studies Included
3.2.1. Publication Characteristics
3.2.2. Participant Characteristics
3.2.3. Intervention Characteristics
3.2.4. Control Group Characteristics
| Autor Year Country | Study Design | Participants Sample Size Male/Female Age (Mean and SD) Cancer Type Phase of Therapy Others | Intervention | Control | Outcomes and Measurement Tools | Endpoints |
|---|---|---|---|---|---|---|
| Casanovas-Álvarez 2024 Spain [18] | 2-arm parallel RCT Single-center | 61 (30 IG, 31 CG) All female IG: 49.2 (10.9), CG: 54.7 (12.1) Breast cancer In treatment (Scheduled for tumor resection) | Nordic Waling and body weight exercises | Usual care | Cardiorespiratory endurance
HRQoL (EORTC-QLQC30) Adherence (% of session attendance) Safety (number of events) | Post intervention 1-month post intervention 3-months post intervention |
| Cunningham 2020 Canada [19] | 2-arm pilot parallel RCT Single-center | 8 (4 IG and 4 CG) 2 male and 6 female 67 (5.8) Mixed cohort (Lung, Prostate, Colorectal, Endometrial) In treatment or post-treatment: 7; No treatment: 1 | Nordic walking | Usual care | Cardiorespiratory endurance (6MWT) Muscle strength
HRQoL (SF-36) Adherence (number of participants completing the program) Safety (NR) | Post intervention |
| Fields 2016 UK [20] | 2-arm pilot parallel RCT Single-center | 40 (20 IG and 20 CG) All female 63 (8) Breast Cancer Maintenance phase With aromatase inhibitor-associated arthralgia | Nordic Walking only | Enhanced usual care (contacted every 2 weeks and booklet on physical activity) | Physical activity level (GPPAQ) HRQoL (SF-36) Adherence (% of session attendance) Safety (Injury prevalence and type/outcome) | Post intervention |
| Hanuszkiewicz 2021 Poland [21] | 2-arm parallel RCT Single-center | 39 (19 IG and 20 CG) All female 58.8 (7.3) Breast Cancer Post-treatment (≥1 year post-surgery) | Nordic walking and general gymnastics training | General gymnastics based on guidelines for cancer survivors | Muscle strength:
| Post intervention |
| Malicka 2011 Poland [22] | 2-arm parallel RCT Single-center | 38 (23 IG and 15 CG) All female 62.8 (6.1) Breast Cancer Pot-treatment (average time since surgery 7.6 years) | Nordic Walking only | Usual care | Muscle strength:
| Post intervention |
| Rösner 2011 Germany [23] | 2-arm parallel RCT Single-center | 50 (26 IG and 24 CG) IG 52.4 (5.5), CG: 50.8 (5.9) All female Breast Cancer In treatment (Initial diagnosis of tumor stages T1–3, N0-1, and M0) | Nordic Walking only | Usual care | Muscle strength:
| Post intervention |
| Study | Nº Sessions and Supervision | Frequency | Session Description | Intensity | Progression | Session Duration | Length of Intervention |
|---|---|---|---|---|---|---|---|
| Casanovas-Álvarez 2024 [18] | From 24 to 32 supervised group sessions | Twice per week | Warm up (10 min) NW (10 min) Body weight exercises (10 exercises, 15–30 rep per exercise) NW (15 min) Body weight exercises Cool-down (15 min) | Moderate-to-vigorous (RPE of 6–8) | Body weight exercise repetitions were increased every 2 weeks | 75 min | 12 to 16 weeks |
| Cunningham 2020 [19] | 8 supervised group session (1 session/week) 24 non-supervised individual session (up to 3 sessions/week) | One up to four times per week | Individualized prescription depending on the PA level. High active: NW (30–60 min) Minimally active: NW (30–45 min) Inactive: NW (20–30 min) | Moderate intensity (Borg 11–15) | Time was increased from 20–30 min to 60 | 20–60 min | 8 weeks |
| Fields 2016 [20] | 6 Supervised group session (first 6 weeks) Up to 12 non-supervised individual session | One a week for the first 3 weeks and increasing to 2, 3 and 4 times a week every two weeks | Warm-up (10 min) NW (30 min) + Cool-down (10 min) | Moderate intensity (Borg 11–13) | Intensity was increased progressively using on Borg scale | 50 min | 12 weeks |
| Hanuszkiewicz 2020 [21] | 16 supervised group sessions | Twice per week | Warm-up (5 min) NW (35 min) Cool-down (5 min) | 65–70% of maximal HR | Intensity was increased gradually by decreasing the number of rest periods. | 45 min | 8 weeks |
| Malicka 2011 [22] | 16 supervised group sessions | Twice per week | Warm up (10 min) NW (40 min) Cool-down (10 min) | Up to 85% of maximal HR | NR | 60 min | 8 weeks |
| Rösner 2011 [23] | 12 sessions, supervision NR | Tree times per week | Warm-up (NR) Playful forms (NR) Walking without poles (NR) Stretching exercises leg muscles and upper body (NR) | NR | NR | 60 min | 4 weeks |
3.3. Risk of Bias Assessment
| Casanovas-Álvarez 2024 [18] | Cunningham 2020 [19] | Fields 2016 [20] | Hanuszkiewicz 2020 [21] | Malicka 2011 [22] | Rösner 2021 [23] | |
|---|---|---|---|---|---|---|
| Item 1 | YES | YES | YES | UNCLEAR | UNCLEAR | UNCLEAR |
| Item 2 | YES | YES | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR |
| Item 3 | YES | UNCLEAR | YES | YES | YES | YES |
| Item 4 | NO | NO | NO | NO | NO | NO |
| Item 5 | NO | NO | NO | NO | NO | NO |
| Item 6 | UNCLEAR | NO | NO | UNCLEAR | NO | UNCLEAR |
| Item 7 | YES | UNCLEAR | YES | YES | YES | YES |
| Item 8 | YES | YES | YES | YES | UNCLEAR | YES |
| Item 9 | YES | YES | UNCLEAR | YES | UNCLEAR | YES |
| Item 10 | YES | YES | YES | YES | YES | YES |
| Item 11 | YES | YES | YES | YES | YES | YES |
| Item 12 | YES | YES | YES | YES | YES | YES |
| Item 13 | YES | YES | YES | YES | UNCLEAR | YES |
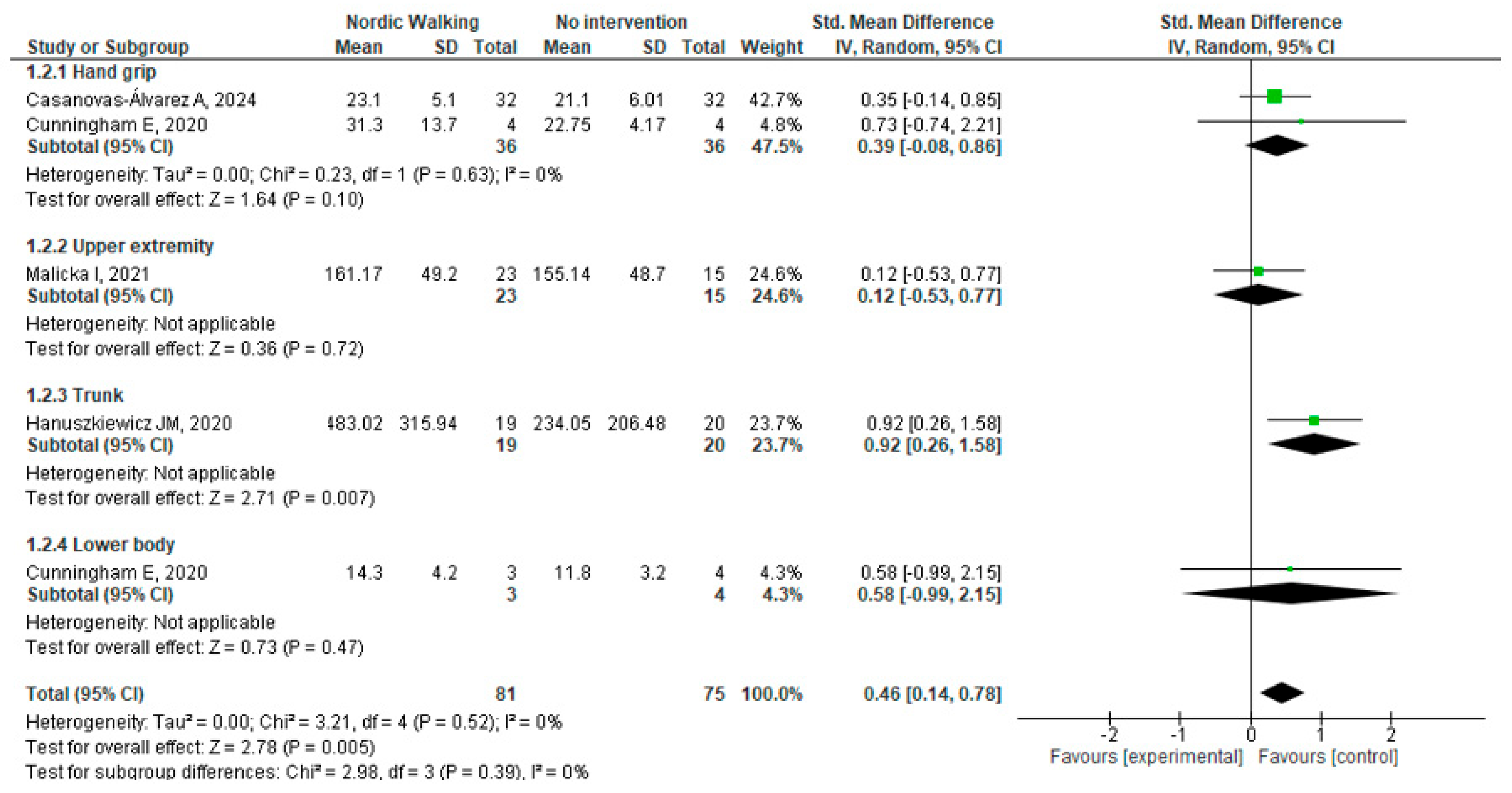
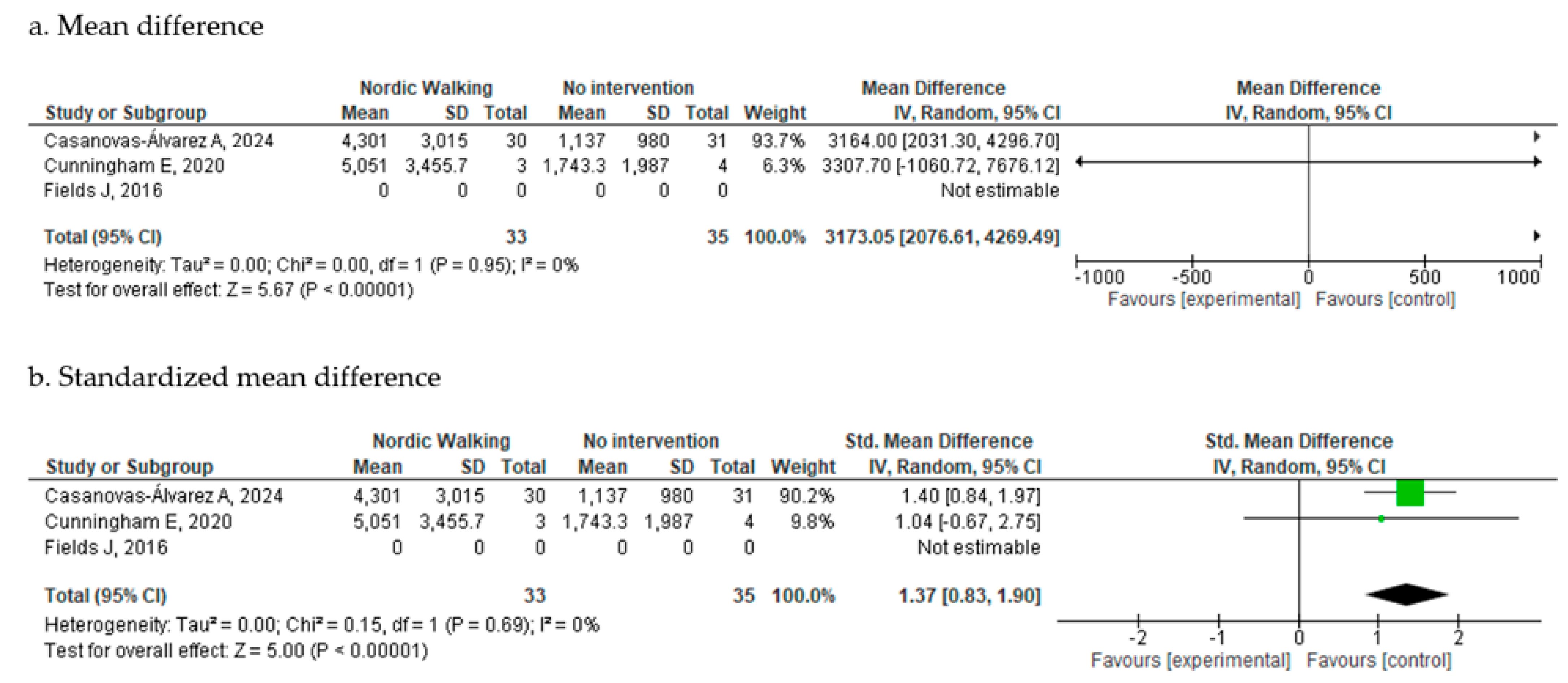
3.4. Efficacy Outcomes
3.4.1. Nordic Walking vs. No Intervention
Cardiorespiratory Endurance
Muscle Strength
Balance
Physical Activity Level
HRQoL
3.4.2. Nordic Walking vs. Other Exercise Programs
3.5. Adherence and Safety
3.5.1. Adherence
3.5.2. Safety
3.6. Reporting Bias
3.7. Cartain of the Evidence
4. Discussion
4.1. Limitations
4.2. Implications for Practice, Policy and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dibben, G.O.; Gardiner, L.; Young, H.M.L.; Wells, V.; Evans, R.A.; Ahmed, Z.; Barber, S.; Dean, S.; Doherty, P.; Gardiner, N.; et al. Evidence for Exercise-Based Interventions across 45 Different Long-Term Conditions: An Overview of Systematic Reviews. EClinicalMedicine 2024, 72, 102599. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef]
- Thraen-Borowski, K.M.; Gennuso, K.P.; Cadmus-Bertram, L. Accelerometer-Derived Physical Activity and Sedentary Time by Cancer Type in the United States. PLoS ONE 2017, 12, e0182554. [Google Scholar] [CrossRef]
- Blaney, J.M.; Lowe-Strong, A.; Rankin-Watt, J.; Campbell, A.; Gracey, J.H. Cancer Survivors’ Exercise Barriers, Facilitators and Preferences in the Context of Fatigue, Quality of Life and Physical Activity Participation: A Questionnaire-Survey. Psychooncology 2013, 22, 186–194. [Google Scholar] [CrossRef]
- Ottenbacher, A.J.; Day, R.S.; Taylor, W.C.; Sharma, S.V.; Sloane, R.; Snyder, D.C.; Kraus, W.E.; Demark-Wahnefried, W. Exercise among Breast and Prostate Cancer Survivors--What Are Their Barriers? J. Cancer Surviv. 2011, 5, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Morgulec-Adamowicz, N.; Marszalek, J.; Jagustyn, P. Nordic Walking—A New Form of Adapted Physical Activity (A Literature Review). Hum. Mov. 2011, 12, 124–132. [Google Scholar] [CrossRef]
- What Is Nordic Walking. Available online: https://www.inwa-nordicwalking.com/nordicwalking (accessed on 11 July 2025).
- Pellegrini, B.; Boccia, G.; Zoppirolli, C.; Rosa, R.; Stella, F.; Bortolan, L.; Rainoldi, A.; Schena, F. Muscular and Metabolic Responses to Different Nordic Walking Techniques, When Style Matters. PLoS ONE 2018, 13, e0195438. [Google Scholar] [CrossRef] [PubMed]
- Bullo, V.; Gobbo, S.; Vendramin, B.; Duregon, F.; Cugusi, L.; Di Blasio, A.; Bocalini, D.S.; Zaccaria, M.; Bergamin, M.; Ermolao, A. Nordic Walking Can Be Incorporated in the Exercise Prescription to Increase Aerobic Capacity, Strength, and Quality of Life for Elderly: A Systematic Review and Meta-Analysis. Rejuvenation Res. 2018, 21, 141–161. [Google Scholar] [CrossRef]
- Reuter, I.; Mehnert, S.; Leone, P.; Kaps, M.; Oechsner, M.; Engelhardt, M. Effects of a Flexibility and Relaxation Programme, Walking, and Nordic Walking on Parkinson’s Disease. J. Aging Res. 2011, 2011, 232473. [Google Scholar] [CrossRef]
- Lacharité-Lemieux, M.; Brunelle, J.-P.; Dionne, I.J. Adherence to Exercise and Affective Responses: Comparison between Outdoor and Indoor Training. Menopause 2015, 22, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.N.; McAuley, E.; Trinh, L. Physical Activity Programming and Counseling Preferences among Cancer Survivors: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 48. [Google Scholar] [CrossRef]
- Sánchez-Lastra, M.A.; Torres, J.; Martínez-Lemos, I.; Ayán, C. Nordic Walking for Women with Breast Cancer: A Systematic Review. Eur. J. Cancer Care 2019, 28, e13130. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Randomized Controlled Trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Review Manager (RevMan) [Computer Program]. Version 5.4. 2024. Available online: http://revman.cochrane.org (accessed on 11 July 2025).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Casanovas-Álvarez, A.; Estanyol, B.; Ciendones, M.; Padròs, J.; Cuartero, J.; Barnadas, A.; García-Valdecasas, B.; González-Colom, R.; Sebio-García, R.; Masià, J. Effectiveness of an Exercise and Educational-Based Prehabilitation Program in Patients With Breast Cancer Receiving Neoadjuvant Chemotherapy (PREOptimize) on Functional Outcomes: A Randomized Controlled Trial. Phys. Ther. 2024, 104, pzae151. [Google Scholar] [CrossRef]
- Cunningham, E.; Weaver, R.; Lemonde, M.; Dogra, S.; Nonoyama, M. Nordic Pole Walking for Individuals with Cancer: A Feasibility Randomized Controlled Trial Assessing Physical Function and Health-Related Quality of Life. Rehabil. Oncol. 2020, 38, 81–91. [Google Scholar] [CrossRef]
- Fields, J.; Richardson, A.; Hopkinson, J.; Fenlon, D. Nordic Walking as an Exercise Intervention to Reduce Pain in Women With Aromatase Inhibitor-Associated Arthralgia: A Feasibility Study. J. Pain Symptom Manag. 2016, 52, 548–559. [Google Scholar] [CrossRef]
- Hanuszkiewicz, J.; WoZniewski, M.; Malicka, I. The Influence of Nordic Walking on Isokinetic Trunk Muscle Endurance and Sagittal Spinal Curvatures in Women after Breast Cancer Treatment. Acta Bioeng. Biomech. 2021, 22, 2409. [Google Scholar] [CrossRef] [PubMed]
- Malicka, I.; Stefanska, M.; Rudziak, M.; Jarmoluk, P.; Pawlowska, K.; Szczepanska-Gieracha, J.; Wozniewski, M. The Influence of Nordic Walking Exercise on Upper Extremity Strength and the Volume of Lymphoedema in Women Following Breast Cancer Treatment. Isokinet. Exerc. Sci. 2011, 19, 295–304. [Google Scholar] [CrossRef]
- Rösner, M. Evaluation of a Nordic Walking Program on Shoulder Joint Mobility and Isometric Force in Breast Cancer Patients. Dtsch. Z. Fur Sportmed. 2011, 62, 120. [Google Scholar]
- Sweegers, M.G.; Altenburg, T.M.; Chinapaw, M.J.; Kalter, J.; Verdonck-de Leeuw, I.M.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; Brug, J.; et al. Which Exercise Prescriptions Improve Quality of Life and Physical Function in Patients with Cancer during and Following Treatment? A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Sports Med. 2018, 52, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, N.; Islam, M.M.; Rogers, M.E.; Rogers, N.L.; Sengoku, N.; Koizumi, D.; Kitabayashi, Y.; Imai, A.; Naruse, A. Effects of Nordic Walking Compared to Conventional Walking and Band-Based Resistance Exercise on Fitness in Older Adults. J. Sports Sci. Med. 2013, 12, 422–430. [Google Scholar] [PubMed]
- Cantarero-Villanueva, I.; Postigo-Martin, P.; Granger, C.L.; Waterland, J.; Galiano-Castillo, N.; Denehy, L. The Minimal Clinically Important Difference in the Treadmill Six-Minute Walk Test in Active Women with Breast Cancer during and after Oncological Treatments. Disabil. Rehabil. 2023, 45, 871–878. [Google Scholar] [CrossRef]
- Sanchez-Lastra, M.A.; Miller, K.J.; Martínez-Lemos, R.I.; Giráldez, A.; Ayán, C. Nordic Walking for Overweight and Obese People: A Systematic Review and Meta-Analysis. J. Phys. Act. Health 2020, 17, 762–772. [Google Scholar] [CrossRef]
- Cugusi, L.; Manca, A.; Yeo, T.J.; Bassareo, P.P.; Mercuro, G.; Kaski, J.C. Nordic Walking for Individuals with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Prev. Cardiol. 2017, 24, 1938–1955. [Google Scholar] [CrossRef]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A Study of Concurrent and Construct Validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Lucia, A.; Silva, M.; Peixoto, A.; Ramalho, R.; da Silva, P.C.; Mota, J.; Macedo, G.; Abreu, S. Physical Fitness and Health-Related Quality of Life in Patients with Colorectal Cancer. Int. J. Sports Med. 2021, 42, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Polat, K.; Karadibak, D.; Güç, Z.G.S.; Yavuzşen, T.; Öztop, İ. The Relationship between Exercise Capacity and Muscle Strength, Physical Activity, Fatigue and Quality of Life in Patients with Cancer Cachexia. Nutr. Cancer 2024, 76, 55–62. [Google Scholar] [CrossRef]
- Bullard, T.; Ji, M.; An, R.; Trinh, L.; Mackenzie, M.; Mullen, S.P. A Systematic Review and Meta-Analysis of Adherence to Physical Activity Interventions among Three Chronic Conditions: Cancer, Cardiovascular Disease, and Diabetes. BMC Public Health 2019, 19, 636. [Google Scholar] [CrossRef]
- Mahdaviani, B.; Selk-Ghaffari, M.; Sarzaeim, M.; Thornton, J.S. Barriers and Enablers of Adherence to High-Intensity Interval Training among Patients with Cancer: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2024, 58, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Statement: Updated Guideline for Reporting Randomised Trials. Lancet 2025, 405, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanovas-Álvarez, A.; Mur-Gimeno, E.; Masià Ayala, J.; Fernández-Jané, C.; Sebio-Garcia, R. Effects of Nordic Walking on Physical Fitness in Patients with Cancer: A Systematic Review. Cancers 2025, 17, 3170. https://doi.org/10.3390/cancers17193170
Casanovas-Álvarez A, Mur-Gimeno E, Masià Ayala J, Fernández-Jané C, Sebio-Garcia R. Effects of Nordic Walking on Physical Fitness in Patients with Cancer: A Systematic Review. Cancers. 2025; 17(19):3170. https://doi.org/10.3390/cancers17193170
Chicago/Turabian StyleCasanovas-Álvarez, Anabel, Esther Mur-Gimeno, Jaume Masià Ayala, Carles Fernández-Jané, and Raquel Sebio-Garcia. 2025. "Effects of Nordic Walking on Physical Fitness in Patients with Cancer: A Systematic Review" Cancers 17, no. 19: 3170. https://doi.org/10.3390/cancers17193170
APA StyleCasanovas-Álvarez, A., Mur-Gimeno, E., Masià Ayala, J., Fernández-Jané, C., & Sebio-Garcia, R. (2025). Effects of Nordic Walking on Physical Fitness in Patients with Cancer: A Systematic Review. Cancers, 17(19), 3170. https://doi.org/10.3390/cancers17193170