Deferred Versus Upfront Cytoreductive Nephrectomy in MetaStatic Renal Cell Carcinoma: Comparative Survival Analysis in the Immunotherapy Era
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
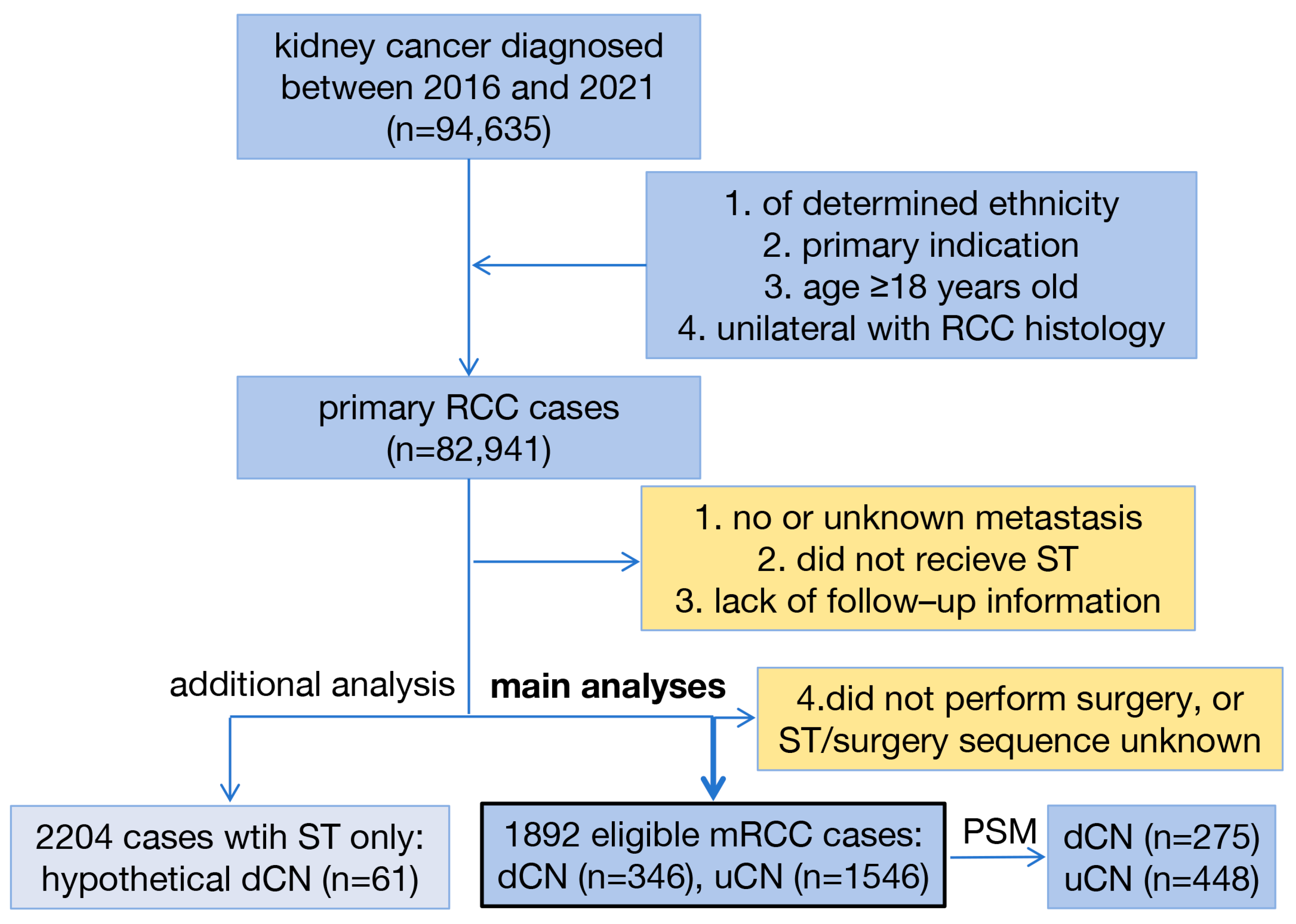
2.1. Study Design
2.2. Patient Selection
2.3. Variables and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
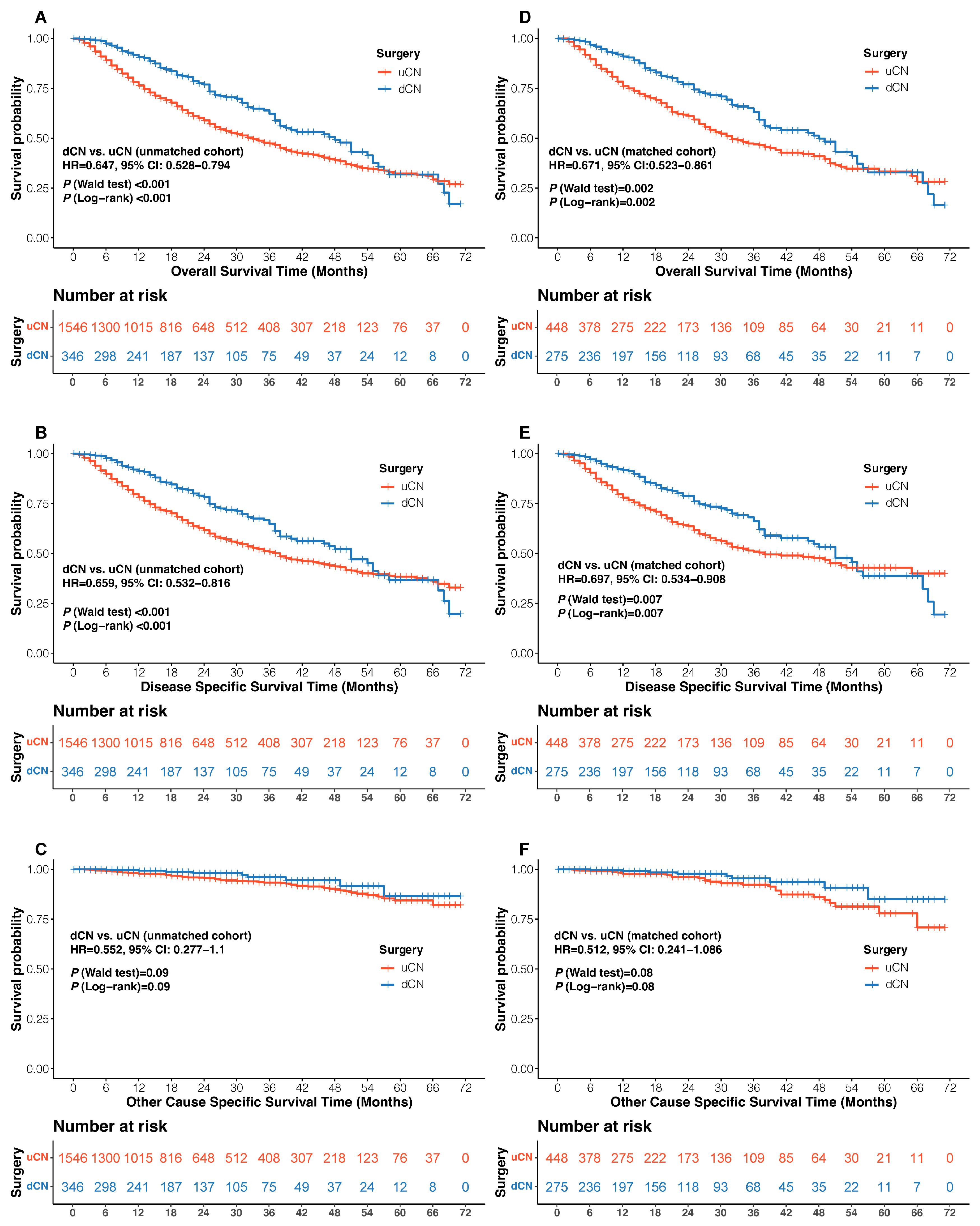
3.2. Clinical Outcomes
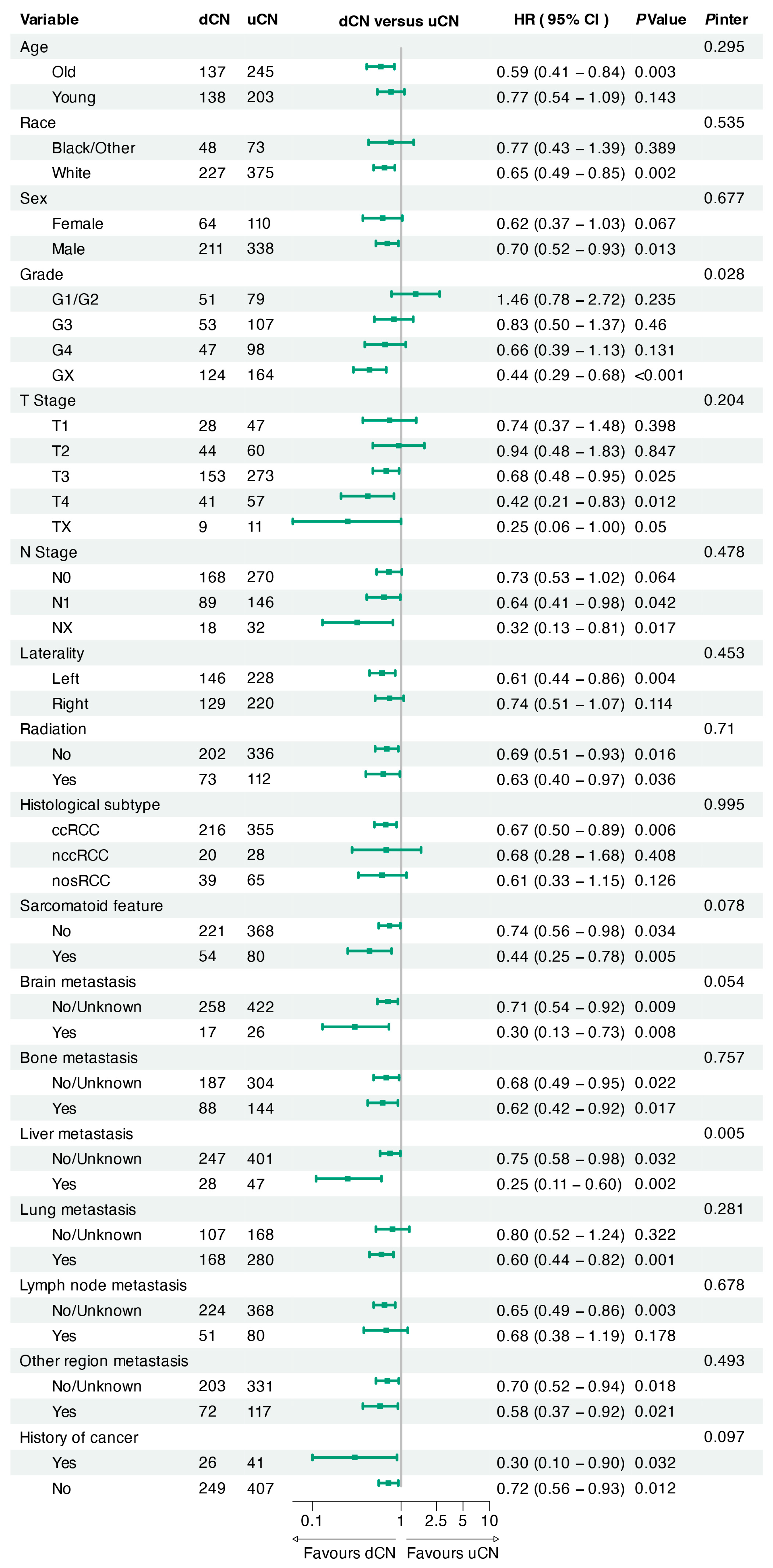
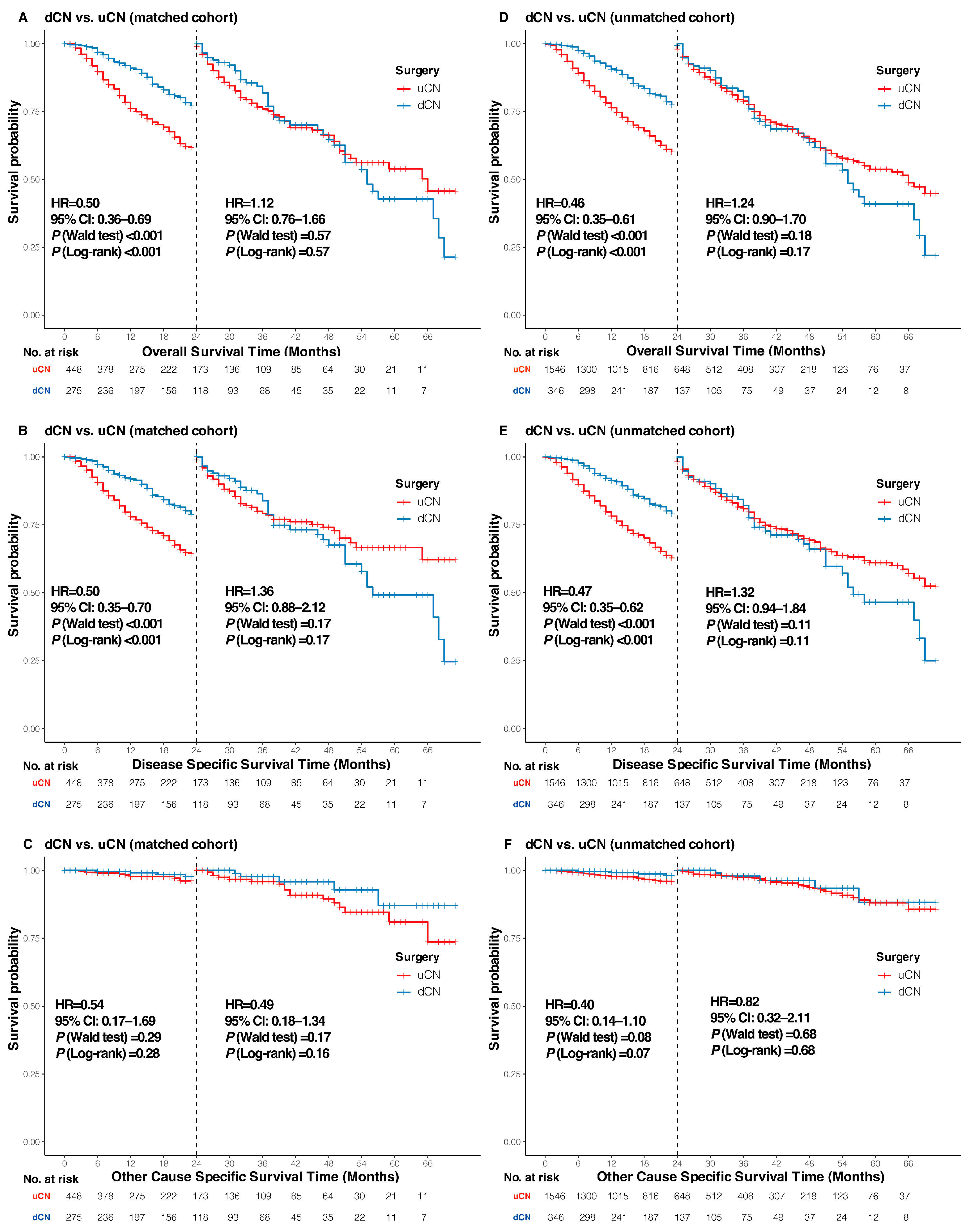
3.3. Sensitivity, Subgroup, and Landmark Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Pineros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef]
- Patel, H.D.; Gupta, M.; Joice, G.A.; Srivastava, A.; Alam, R.; Allaf, M.E.; Pierorazio, P.M. Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. Eur. Urol. Oncol. 2019, 2, 343–348. [Google Scholar] [CrossRef]
- Young, M.; Jackson-Spence, F.; Beltran, L.; Day, E.; Suarez, C.; Bex, A.; Powles, T.; Szabados, B. Renal cell carcinoma. Lancet 2024, 404, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Netti, G.S.; De Luca, F.; Camporeale, V.; Khalid, J.; Leccese, G.; Troise, D.; Sanguedolce, F.; Stallone, G.; Ranieri, E. Liquid Biopsy as a New Tool for Diagnosis and Monitoring in Renal Cell Carcinoma. Cancers 2025, 17, 1442. [Google Scholar] [CrossRef]
- Singer, E.A.; Rumble, R.B.; Rathmell, W.K.; Van Veldhuizen, P.J.; Management of Metastatic Renal Clear Cell Cancer Guideline Expert Panel. Management of Metastatic Renal Clear Cell Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2023, 41, 5184–5186. [Google Scholar] [CrossRef]
- Barragan-Carrillo, R.; Scavuzzo, A.; Jimenez-Rios, M.A.; Sobrevilla-Moreno, N. Management of Metastatic Renal Cell Carcinoma: Guideline Updates. Eur. Urol. Focus. 2025. [Google Scholar] [CrossRef]
- Flanigan, R.C.; Salmon, S.E.; Blumenstein, B.A.; Bearman, S.I.; Roy, V.; McGrath, P.C.; Caton, J.R., Jr.; Munshi, N.; Crawford, E.D. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 2001, 345, 1655–1659. [Google Scholar] [CrossRef]
- Mickisch, G.H.; Garin, A.; van Poppel, H.; de Prijck, L.; Sylvester, R.; The European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet 2001, 358, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Mejean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Mulders, P.; Jewett, M.; Wagstaff, J.; van Thienen, J.V.; Blank, C.U.; van Velthoven, R.; Del Pilar Laguna, M.; Wood, L.; van Melick, H.H.E.; et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019, 5, 164–170. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Barragan-Carrillo, R.; Saad, E.; Saliby, R.M.; Sun, M.; Albiges, L.; Bex, A.; Heng, D.; Mejean, A.; Motzer, R.J.; Plimack, E.R.; et al. First and Second-line Treatments in Metastatic Renal Cell Carcinoma. Eur. Urol. 2025, 87, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Sura, S.; Shinde, R.; Shi, J.; Bupathi, M.; Vickery, D.; Perini, R.; Motzer, R.J. Real-World Treatment Patterns and Clinical Outcomes Among Patients with Metastatic Renal Cell Carcinoma Post-Immune-Oncology and Vascular Endothelial Growth Factor Receptor Targeted Therapies. Cancers 2025, 17, 1434. [Google Scholar] [CrossRef]
- Bell, H.; Cotta, B.H.; Salami, S.S.; Kim, H.; Vaishampayan, U. “PROBE”ing the Role of Cytoreductive Nephrectomy in Advanced Renal Cancer. Kidney Cancer J. 2022, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Iisager, L.; Ahrenfeldt, J.; Donskov, F.; Ljungberg, B.; Bex, A.; Lund, L.; Lyskjaer, I.; Fristrup, N. Multicenter randomized trial of deferred cytoreductive nephrectomy in synchronous metastatic renal cell carcinoma receiving checkpoint inhibitors: The NORDIC-SUN-Trial. BMC Cancer 2024, 24, 260. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.; Jeon, J.; Lee, J.; Jang, W.S.; Lee, S.H.; Han, W.K.; Choi, Y.D.; Koo, K.C.; Cho, K.S.; et al. The role of cytoreductive nephrectomy in metastatic renal cell carcinoma in immune-oncology era (SEVURO-CN): Study protocol for a multi-center, prospective, randomized trial. Trials 2024, 25, 447. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Bamias, A.; Zakopoulou, R.; Myint, Z.W.; Cavasin, N.; Iacovelli, R.; Pichler, M.; Kopecky, J.; Kucharz, J.; Rizzo, M.; et al. Geographical differences in the management of metastatic de novo renal cell carcinoma in the era of immune-combinations. Minerva Urol. Nephrol. 2023, 75, 460–470. [Google Scholar] [CrossRef]
- Bakouny, Z.; El Zarif, T.; Dudani, S.; Connor Wells, J.; Gan, C.L.; Donskov, F.; Shapiro, J.; Davis, I.D.; Parnis, F.; Ravi, P.; et al. Upfront Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors or Targeted Therapy: An Observational Study from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 2023, 83, 145–151. [Google Scholar] [CrossRef]
- Takemura, K.; Ernst, M.S.; Navani, V.; Wells, J.C.; Bakouny, Z.; Donskov, F.; Basappa, N.S.; Wood, L.A.; Meza, L.; Pal, S.K.; et al. Characterization of Patients with Metastatic Renal Cell Carcinoma Undergoing Deferred, Upfront, or No Cytoreductive Nephrectomy in the Era of Combination Immunotherapy: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. Oncol. 2024, 7, 501–508. [Google Scholar] [CrossRef]
- Grimm, M.O.; Oya, M.; Choueiri, T.K.; Motzer, R.J.; Schmidinger, M.; Quinn, D.I.; Gravis-Mescam, G.; Verzoni, E.; Van den Eertwegh, A.J.M.; di Pietro, A.; et al. Impact of Prior Cytoreductive Nephrectomy on Efficacy in Patients with Synchronous Metastatic Renal Cell Carcinoma Treated with Avelumab plus Axitinib or Sunitinib: Post Hoc Analysis from the JAVELIN Renal 101 Phase 3 Trial. Eur. Urol. 2024, 85, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Makrakis, D.; Msaouel, P.; Karam, J.A.; Esagian, S. Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis of Individual Patient Data. Eur. Urol. Focus. 2024. [Google Scholar] [CrossRef]
- Das, A.; Shapiro, D.D.; Craig, J.K.; Abel, E.J. Understanding and integrating cytoreductive nephrectomy with immune checkpoint inhibitors in the management of metastatic RCC. Nat. Rev. Urol. 2023, 20, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Monda, S.M.; Salami, S.S.; Vaishampayan, U.; Morgan, T.M.; Singhal, U. Defining the Role of Surgical Resection in Metastatic Renal Cell Carcinoma: A Mini Review. Eur. Urol. Focus. 2025. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.; Manfredi, C.; Cirillo, L.; Fusco, G.M.; Passaro, F.; Abate, M.; La Rocca, R.; Mastrangelo, F.; Spirito, L.; Pandolfo, S.D.; et al. Cytoreductive Nephrectomy and Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives. Medicina 2023, 59, 767. [Google Scholar] [CrossRef]
- Abu-Ghanem, Y.; van Thienen, J.V.; Blank, C.; Aarts, M.J.B.; Jewett, M.; de Jong, I.J.; Lattouf, J.B.; van Melick, H.H.E.; Wood, L.; Mulders, P.; et al. Cytoreductive nephrectomy and exposure to sunitinib—A post hoc analysis of the Immediate Surgery or Surgery After Sunitinib Malate in Treating Patients With Metastatic Kidney Cancer (SURTIME) trial. BJU Int. 2022, 130, 68–75. [Google Scholar] [CrossRef]
- Gross, E.E.; Li, M.; Yin, M.; Orcutt, D.; Hussey, D.; Trott, E.; Holt, S.K.; Dwyer, E.R.; Kramer, J.; Oliva, K.; et al. A multicenter study assessing survival in patients with metastatic renal cell carcinoma receiving immune checkpoint inhibitor therapy with and without cytoreductive nephrectomy. Urol. Oncol. 2023, 41, 51.e25–51.e31. [Google Scholar] [CrossRef]
- Yoshino, M.; Ishihara, H.; Nemoto, Y.; Nakamura, K.; Nishimura, K.; Tachibana, H.; Fukuda, H.; Toki, D.; Yoshida, K.; Kobayashi, H.; et al. Therapeutic role of deferred cytoreductive nephrectomy in patients with metastatic renal cell carcinoma treated with nivolumab plus ipilimumab. Jpn. J. Clin. Oncol. 2022, 52, 1208–1214. [Google Scholar] [CrossRef]
- Shen, X.P.; Xie, M.; Wang, J.S.; Guo, X. Efficacy of immunotherapy-based immediate cytoreductive nephrectomy vs. deferred cytoreductive nephrectomy in metastatic renal cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5684–5691. [Google Scholar] [CrossRef]
- Meagher, M.F.; Minervini, A.; Mir, M.C.; Cerrato, C.; Rebez, G.; Autorino, R.; Hampton, L.; Campi, R.; Kriegmair, M.; Linares, E.; et al. Does the Timing of Cytoreductive Nephrectomy Impact Outcomes? Analysis of REMARCC Registry Data for Patients Receiving Tyrosine Kinase Inhibitor Versus Immune Checkpoint Inhibitor Therapy. Eur. Urol. Open Sci. 2024, 63, 71–80. [Google Scholar] [CrossRef]
- Singla, N.; Hutchinson, R.C.; Ghandour, R.A.; Freifeld, Y.; Fang, D.; Sagalowsky, A.I.; Lotan, Y.; Bagrodia, A.; Margulis, V.; Hammers, H.J.; et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: An analysis of the National Cancer Database. Urol. Oncol. 2020, 38, 604.e9–604.e17. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, K.; Tian, X.; Chen, P.; Xiong, Q.; Li, G.; Ma, X.; Han, R.; Sun, L.; Shen, Y.; et al. Individualized Prediction of Postoperative Survival in Gallbladder Cancer: A Nomogram Based on SEER Data and External Validation. Cancers 2025, 17, 1919. [Google Scholar] [CrossRef]
- Leone, J.; Hassett, M.J.; Freedman, R.A.; Tolaney, S.M.; Graham, N.; Tayob, N.; Vallejo, C.T.; Winer, E.P.; Lin, N.U.; Leone, J.P. Mortality Risks Over 20 Years in Men With Stage I to III Hormone Receptor-Positive Breast Cancer. JAMA Oncol. 2024, 10, 508–515. [Google Scholar] [CrossRef]
- Liu, N.; Qian, Y.; Fan, Y.; Li, Y.; Ji, C.; Zhao, K.; Jiang, X.; Xiong, Z.; Wang, M.; Xu, Z.; et al. Comparison of Partial Nephrectomy Versus Radical Nephrectomy for Metastatic Renal Cell Carcinoma in the Immunotherapy Era: A Propensity Score Matching, Population-Based Analysis. Ann. Surg. Oncol. 2025. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Adhikari, A.; Sapkota, S.; Gogia, S.; Kc, O. Changes in the overall survival of patients with metastatic renal cell carcinoma in the era of immune-checkpoint inhibitors. Cancer Epidemiol. 2024, 92, 102639. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Amin, M.B.; Berney, D.M.; Comperat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Ingels, A.; Campi, R.; Capitanio, U.; Amparore, D.; Bertolo, R.; Carbonara, U.; Erdem, S.; Kara, O.; Klatte, T.; Kriegmair, M.C.; et al. Complementary roles of surgery and systemic treatment in clear cell renal cell carcinoma. Nat. Rev. Urol. 2022, 19, 391–418. [Google Scholar] [CrossRef]
- Leung, D.K.; Ko, I.C.; Siu, B.W.; Wong, C.H.; Yuen, S.K.; Ng, C.F.; Teoh, J.Y. The Role of Surgery in Metastatic Renal Cell Carcinoma in 2024. Clin. Med. Insights Oncol. 2024, 18, 11795549241272447. [Google Scholar] [CrossRef] [PubMed]
- Esagian, S.M.; Karam, J.A.; Msaouel, P.; Makrakis, D. Upfront Versus Deferred Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-analysis. Eur. Urol. Focus. 2025, 11, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.Y.; Lim, E.J.; Wong, H.C.; Tay, K.J.; Ho, H.S.S.; Yuen, J.S.P.; Aslim, E.; Chen, K.; Gan, V.H.L. Deferred cytoreductive nephrectomy in patients with metastatic renal cell carcinoma: A systematic review and patient-level meta-analysis. Urol. Oncol. 2025, 43, 348–358. [Google Scholar] [CrossRef]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Pauken, K.E.; Alhalabi, O.; Goswami, S.; Sharma, P. Neoadjuvant immune checkpoint therapy: Enabling insights into fundamental human immunology and clinical benefit. Cancer Cell 2025, 43, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, P.; Tian, Y. The Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma: An International Multicenter Study. Cancers 2025, 17, 2419. [Google Scholar] [CrossRef] [PubMed]
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| dCN (n = 346) | uCN (n = 1546) | p Value | dCN (n = 275) | uCN (n = 448) | p Value | |
| Age (years, median [IQR]) | 61.00 [54.00, 68.00] | 62.00 [55.00, 69.00] | 0.065 | 60.00 [54.00, 68.00] | 61.00 [55.00, 68.00] | 0.492 |
| Race | 0.151 | 0.921 | ||||
| Black | 26 (7.5) | 96 (6.2) | 21 (7.6) | 32 (7.1) | ||
| Other | 38 (11.0) | 127 (8.2) | 27 (9.8) | 41 (9.2) | ||
| White | 282 (81.5) | 1323 (85.6) | 227 (82.5) | 375 (83.7) | ||
| Sex | 0.06 | 0.763 | ||||
| Female | 77 (22.3) | 423 (27.4) | 64 (23.3) | 110 (24.6) | ||
| Male | 269 (77.7) | 1123 (72.6) | 211 (76.7) | 338 (75.4) | ||
| Grade | <0.001 | 0.148 | ||||
| G1 | 7 (2.0) | 11 (0.7) | 7 (2.5) | 10 (2.2) | ||
| G2 | 45 (13.0) | 172 (11.1) | 44 (16.0) | 69 (15.4) | ||
| G3 | 53 (15.3) | 500 (32.3) | 53 (19.3) | 107 (23.9) | ||
| G4 | 47 (13.6) | 675 (43.7) | 47 (17.1) | 98 (21.9) | ||
| GX | 194 (56.1) | 188 (12.2) | 124 (45.1) | 164 (36.6) | ||
| T Stage | <0.001 | 0.619 | ||||
| T1 | 36 (10.4) | 134 (8.7) | 28 (10.2) | 47 (10.5) | ||
| T2 | 56 (16.2) | 161 (10.4) | 44 (16.0) | 60 (13.4) | ||
| T3 | 187 (54.0) | 1070 (69.2) | 153 (55.6) | 273 (60.9) | ||
| T4 | 57 (16.5) | 161 (10.4) | 41 (14.9) | 57 (12.7) | ||
| TX | 10 (2.9) | 20 (1.3) | 9 (3.3) | 11 (2.5) | ||
| N Stage | 0.02 | 0.947 | ||||
| N0 | 199 (57.5) | 1010 (65.3) | 168 (61.1) | 270 (60.3) | ||
| N1 | 125 (36.1) | 445 (28.8) | 89 (32.4) | 146 (32.6) | ||
| NX | 22 (6.4) | 91 (5.9) | 18 (6.5) | 32 (7.1) | ||
| Laterality | 0.65 | 0.619 | ||||
| Left | 176 (50.9) | 810 (52.4) | 146 (53.1) | 228 (50.9) | ||
| Right | 170 (49.1) | 736 (47.6) | 129 (46.9) | 220 (49.1) | ||
| Radiation Therapy | 0.981 | 0.708 | ||||
| No | 257 (74.3) | 1152 (74.5) | 202 (73.5) | 336 (75.0) | ||
| Yes | 89 (25.7) | 394 (25.5) | 73 (26.5) | 112 (25.0) | ||
| Histology Subtype | 0.2 | 0.864 | ||||
| ccRCC | 273 (78.9) | 1170 (75.7) | 216 (78.5) | 355 (79.2) | ||
| nccRCC | 22 (6.4) | 144 (9.3) | 20 (7.3) | 28 (6.2) | ||
| nosRCC | 51 (14.7) | 232 (15.0) | 39 (14.2) | 65 (14.5) | ||
| Sarcomatoid Feature | 0.002 | 0.618 | ||||
| No | 287 (82.9) | 1161 (75.1) | 221 (80.4) | 368 (82.1) | ||
| Yes | 59 (17.1) | 385 (24.9) | 54 (19.6) | 80 (17.9) | ||
| Bone Metastasis | 0.909 | 0.995 | ||||
| No | 237 (68.5) | 1077 (69.7) | 185 (67.3) | 301 (67.2) | ||
| Unknown | 2 (0.6) | 8 (0.5) | 2 (0.7) | 3 (0.7) | ||
| Yes | 107 (30.9) | 461 (29.8) | 88 (32.0) | 144 (32.1) | ||
| Brain Metastasis | 0.674 | 0.965 | ||||
| No | 321 (92.8) | 1449 (93.7) | 257 (93.5) | 420 (93.8) | ||
| Unknown | 1 (0.3) | 7 (0.5) | 1 (0.4) | 2 (0.4) | ||
| Yes | 24 (6.9) | 90 (5.8) | 17 (6.2) | 26 (5.8) | ||
| Liver Metastasis | 0.392 | 0.738 | ||||
| No | 302 (87.3) | 1352 (87.5) | 245 (89.1) | 395 (88.2) | ||
| Unknown | 4 (1.2) | 8 (0.5) | 2 (0.7) | 6 (1.3) | ||
| Yes | 40 (11.6) | 186 (12.0) | 28 (10.2) | 47 (10.5) | ||
| Lung Metastasis | 0.366 | 0.664 | ||||
| No | 133 (38.4) | 538 (34.8) | 105 (38.2) | 162 (36.2) | ||
| Unknown | 2 (0.6) | 15 (1.0) | 2 (0.7) | 6 (1.3) | ||
| Yes | 211 (61.0) | 993 (64.2) | 168 (61.1) | 280 (62.5) | ||
| Distant Lymph Node Metastasis | 0.509 | 0.692 | ||||
| No | 286 (82.7) | 1256 (81.2) | 223 (81.1) | 364 (81.2) | ||
| Unknown | 1 (0.3) | 13 (0.8) | 1 (0.4) | 4 (0.9) | ||
| Yes | 59 (17.1) | 277 (17.9) | 51 (18.5) | 80 (17.9) | ||
| Other Organ Metastasis | 0.44 | 0.865 | ||||
| No | 253 (73.1) | 1157 (74.8) | 202 (73.5) | 328 (73.2) | ||
| Unknown | 1 (0.3) | 12 (0.8) | 1 (0.4) | 3 (0.7) | ||
| Yes | 92 (26.6) | 377 (24.4) | 72 (26.2) | 117 (26.1) | ||
| History of Cancer | 1 | 0.997 | ||||
| Yes | 32 (9.2) | 141 (9.1) | 26 (9.5) | 41 (9.2) | ||
| No | 314 (90.8) | 1405 (90.9) | 249 (90.5) | 407 (90.8) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Tuerxun, P.; Liu, N.; Ji, C.; Zhao, K.; Qian, Y.; Abudushataer, A.; Li, Y.; Jiang, X.; Xiong, Z.; et al. Deferred Versus Upfront Cytoreductive Nephrectomy in MetaStatic Renal Cell Carcinoma: Comparative Survival Analysis in the Immunotherapy Era. Cancers 2025, 17, 3136. https://doi.org/10.3390/cancers17193136
Xu T, Tuerxun P, Liu N, Ji C, Zhao K, Qian Y, Abudushataer A, Li Y, Jiang X, Xiong Z, et al. Deferred Versus Upfront Cytoreductive Nephrectomy in MetaStatic Renal Cell Carcinoma: Comparative Survival Analysis in the Immunotherapy Era. Cancers. 2025; 17(19):3136. https://doi.org/10.3390/cancers17193136
Chicago/Turabian StyleXu, Tao, Paerhati Tuerxun, Ning Liu, Chencheng Ji, Kunlun Zhao, Yiguan Qian, Abudukelimu Abudushataer, Yang Li, Xiaotian Jiang, Zhongli Xiong, and et al. 2025. "Deferred Versus Upfront Cytoreductive Nephrectomy in MetaStatic Renal Cell Carcinoma: Comparative Survival Analysis in the Immunotherapy Era" Cancers 17, no. 19: 3136. https://doi.org/10.3390/cancers17193136
APA StyleXu, T., Tuerxun, P., Liu, N., Ji, C., Zhao, K., Qian, Y., Abudushataer, A., Li, Y., Jiang, X., Xiong, Z., Wang, M., Jia, R., & Ge, Y.-Z. (2025). Deferred Versus Upfront Cytoreductive Nephrectomy in MetaStatic Renal Cell Carcinoma: Comparative Survival Analysis in the Immunotherapy Era. Cancers, 17(19), 3136. https://doi.org/10.3390/cancers17193136






