Echolaser Focal Treatment for Prostate Cancer Guided by Fiducial Marker Placement
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Inclusion Criteria
- Histologically confirmed localized prostate adenocarcinoma, confirmed by transperineal targeted biopsy.
- Gleason score ≤ 8, with stratification based on the International Society of Urological Pathology (ISUP) grade grouping.
- TNM staging between T1c and T2cN0M0, indicating no evidence of extraprostatic extension or nodal/metastatic involvement.
- Absence of prior curative treatment, including radical prostatectomy, external beam radiation therapy (EBRT), and brachytherapy.
- Favorable life expectancy (≥10 years) based on Charlson Comorbidity Index (CCI) assessment.
- Ability to undergo multiparametric MRI (mpMRI) for lesion characterization and treatment planning.
- Solitary or multiple localized lesions within the prostate gland.
2.4. Exclusion Criteria
- Contraindications to MRI scanning, such as implanted metallic devices, pacemakers, or severe claustrophobia.
- Uncontrolled coagulopathy or active bleeding disorders, as these increase the risk of hemorrhage following transperineal procedures.
- Severe cardiovascular or pulmonary comorbidities, including advanced heart failure (NYHA Class III-IV) and severe chronic obstructive pulmonary disease (COPD), which may increase procedural risk.
- History of prior prostate interventions, including previous focal therapy and irreversible electroporation (IRE), which may alter prostate anatomy.
- Allergy to the ultrasound contrast agent Sonovue™, used for enhanced imaging visualization.
2.5. Study Groups and Design
2.6. EchoLaser Treatment Protocol
- Laser output: up to 7 watts per fiber,
- Total energy per lesion: up to 1800 J,
- Ablation dimensions: lateral width up to 1.2 cm, transverse dimension up to 1.5 cm and posterior safety margin up to 0.3 cm.
- Software Biopsee
2.7. Perioperative Management
- Propofol 1% at a continuous infusion rate of 3 mL/kg/h, supplemented with 25–30 mg bolus doses as needed.
- Remifentanil (8 µg/mL solution) administered at a rate of 2 µg/kg/h for analgesia.
- Paracetamol (1 g IV) for perioperative pain management.
- Diclofenac (75 mg IV) as an additional anti-inflammatory and analgesic agent.
- Ondansetron (8 mg IV) for prophylaxis against postoperative nausea and vomiting.
- Omeprazole (40 mg IV) for gastric protection.
2.8. Post-Treatment Monitoring and Follow-Up
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wang, P.; Jia, Z.; Zheng, Z.; Wang, J.; Liang, H. Global burden and risk factors of male cancers from 1990 to 2021, with forecasts to 2040. Sci. Rep. 2025, 15, 5123. [Google Scholar] [CrossRef] [PubMed]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors—A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A.M. Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Goggins, W.B.; Wang, H.H.X.; Fung, F.D.H.; Leung, C.; Wong, S.Y.-S.; Ng, C.-F.; Sung, J.J.Y. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Serdà-Ferrer, B.C.; Sanvisens, A.; Fuentes-Raspall, R.; Puigdemont, M.; Farré, X.; Vidal-Vila, A.; Rispau-Pagès, M.; Baltasar-Bagué, A.; Marcos-Gragera, R. Significantly reduced incidence and improved survival from prostate cancer over 25 years. BMC Public Health 2023, 23, 2552. [Google Scholar] [CrossRef]
- Chung, J.S.; Morgan, T.M.; Hong, S.K. Clinical implications of genomic evaluations for prostate cancer risk stratification, screening, and treatment: A narrative review. Prostate Int. 2020, 8, 99–106. [Google Scholar] [CrossRef]
- Olah, C.; Mairinger, F.; Wessolly, M.; Joniau, S.; Spahn, M.; Julio, M.K.-D.; Hadaschik, B.; Soós, A.; Nyirády, P.; Győrffy, B.; et al. Enhancing risk stratification models in localized prostate cancer by novel validated tissue biomarkers. Prostate Cancer Prostatic Dis. 2024. [Google Scholar] [CrossRef]
- Pedrani, M.; Barizzi, J.; Salfi, G.; Nepote, A.; Testi, I.; Merler, S.; Castelo-Branco, L.; Mestre, R.P.; Turco, F.; Tortola, L.; et al. The Emerging Predictive and Prognostic Role of Aggressive-Variant-Associated Tumor Suppressor Genes Across Prostate Cancer Stages. Int. J. Mol. Sci. 2025, 26, 318. [Google Scholar] [CrossRef]
- Ciccarese, C.; Massari, F.; Iacovelli, R.; Fiorentino, M.; Montironi, R.; Di Nunno, V.; Giunchi, F.; Brunelli, M.; Tortora, G. Prostate cancer heterogeneity: Discovering novel molecular targets for therapy. Cancer Treat. Rev. 2017, 54, 68–73. [Google Scholar] [CrossRef]
- Ongün, Ş.; Sarıkaya, A.E.; Yılmaz, S.H.B.; Sevgi, B.; Çelik, S.; Şen, V.; Tuna, B.; Yörükoğlu, K.; Aslan, G.; Mungan, M.U.; et al. Long-term Surveillance Outcomes of Prostate Cancer Patients Eligible for Active Surveillance but Who Underwent Radical Prostatectomy. J. Urol. Surg. 2024, 11, 153–158. [Google Scholar] [CrossRef]
- Eggener, S.E.; Berlin, A.; Vickers, A.J.; Paner, G.P.; Wolinsky, H.; Cooperberg, M.R. Low-Grade Prostate Cancer: Time to Stop Calling It Cancer. J. Clin. Oncol. 2022, 40, 27. [Google Scholar] [CrossRef] [PubMed]
- Baboudjian, M.; Breda, A.; Rajwa, P.; Gallioli, A.; Gondran-Tellier, B.; Sanguedolce, F.; Verri, P.; Diana, P.; Territo, A.; Bastide, C.; et al. Active Surveillance for Intermediate-risk Prostate Cancer: A Systematic Review, Meta-analysis, and Metaregression. Eur. Urol. Oncol. 2022, 5, 617–627. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Sidana, A.; Choyke, P.L.; Wood, B.J.; Pinto, P.A.; Türkbey, İ.B. Multiparametric Magnetic Resonance Imaging for Active Surveillance of Prostate Cancer. Balk. Med. J. 2017, 34, 388. [Google Scholar]
- Chappidi, M.R.; Lin, D.W.; Westphalen, A.C. Role of MRI in Active Surveillance of Prostate Cancer. Semin. Ultrasound CT MRI 2025, 46, 31–44. [Google Scholar] [CrossRef]
- Press, B.H.; Jones, T.; Olawoyin, O.; Lokeshwar, S.D.; Rahman, S.N.; Khajir, G.; Lin, D.W.; Cooperberg, M.R.; Loeb, S.; Darst, B.F.; et al. Association Between a 22-feature Genomic Classifier and Biopsy Gleason Upgrade During Active Surveillance for Prostate Cancer. Eur. Urol. Open Sci. 2022, 37, 113–119. [Google Scholar] [CrossRef]
- Komisarenko, M.; Wong, L.-M.; Richard, P.O.; Timilshina, N.; Toi, A.; Evans, A.; Zlotta, A.; Kulkarni, G.; Hamilton, R.; Fleshner, N.; et al. An Increase in Gleason 6 Tumor Volume While on Active Surveillance Portends a Greater Risk of Grade Reclassification with Further Followup. J. Urol. 2016, 195, 307–312. [Google Scholar] [CrossRef]
- Hagmann, S.; Ramakrishnan, V.; Tamalunas, A.; Hofmann, M.; Vandenhirtz, M.; Vollmer, S.; Hug, J.; Niggli, P.; Nocito, A.; Kubik-Huch, R.A.; et al. Two Decades of Active Surveillance for Prostate Cancer in a Single-Center Cohort: Favorable Outcomes after Transurethral Resection of the Prostate. Cancers 2022, 14, 368. [Google Scholar] [CrossRef]
- Boladeras, A.; Martinez, E.; Ferrer, F.; Gutierrez, C.; Villa, S.; Pera, J.; Guedea, F. Localized prostate cancer treated with external beam radiation therapy: Long-term outcomes at a European comprehensive cancer centre. Rep. Pract. Oncol. Radiother. 2016, 21, 181. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Li, Q.; Wang, Y.; Zhang, Y.; Ma, X. Comparison on efficacy of radical prostatectomy versus external beam radiotherapy for the treatment of localized prostate cancer. Oncotarget 2017, 8, 79854. [Google Scholar] [CrossRef]
- Numakura, K.; Kobayashi, M.; Muto, Y.; Sato, H.; Sekine, Y.; Sobu, R.; Aoyama, Y.; Takahashi, Y.; Okada, S.; Sasagawa, H.; et al. The Current Trend of Radiation Therapy for Patients with Localized Prostate Cancer. Curr. Oncol. 2023, 30, 8092–8110. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Karnes, R.J.; Viterbo, R.; Rangel, L.J.; Bergstralh, E.J.; Horwitz, E.M.; Blute, M.L.; Buyyounouski, M.K. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer 2011, 117, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.L.; Feng, Z.; Hamilton, A.S.; Gilliland, F.D.; Stephenson, R.A.; Eley, J.W.; Albertsen, P.C.; Harlan, L.C.; Potosky, A.L. Urinary and Sexual Function After Radical Prostatectomy for Clinically Localized Prostate Cancer: The Prostate Cancer Outcomes Study. JAMA 2000, 283, 354–360. [Google Scholar] [CrossRef]
- Rossi, F.; Marino, F.; Gandi, C.; Bizzarri, F.P.; Campetella, M.; Bientinesi, R.; Silvaggi, M.; Sacco, E. Relationship Between Post-Prostatectomy urinary Incontinence, Sexual Functions, and Dyadic Adjustment: A Cross-Sectional Study. Urologia 2025, 92, 348–354. [Google Scholar] [CrossRef]
- Haglind, E.; Carlsson, S.; Stranne, J.; Wallerstedt, A.; Wilderäng, U.; Thorsteinsdottir, T.; Lagerkvist, M.; Damber, J.-E.; Bjartell, A.; Hugosson, J.; et al. Urinary Incontinence and Erectile Dysfunction After Robotic Versus Open Radical Prostatectomy: A Prospective, Controlled, Nonrandomised Trial. Eur. Urol. 2015, 68, 216–225. [Google Scholar] [CrossRef]
- Gacci, M.; De Nunzio, C.; Sakalis, V.; Rieken, M.; Cornu, J.N.; Gravas, S. Latest Evidence on Post-Prostatectomy Urinary Incontinence. J. Clin. Med. 2023, 12, 1190. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Wang, J.; Gao, X.; Zhong, Q.; He, L.; Li, C.; Liu, M.; Liu, Y.; Ma, M.; et al. Guidelines for radiotherapy of prostate cancer (2020 edition). Precis. Radiat. Oncol. 2021, 5, 160–182. [Google Scholar] [CrossRef]
- Suriano, F.; Altobelli, E.; Sergi, F.; Buscarini, M. Bladder Cancer After Radiotherapy for Prostate Cancer. Rev. Urol. 2013, 15, 108. [Google Scholar]
- Jahreiß, M.C.; Heemsbergen, W.D.; Aben, K.K.H.; Incrocci, L. Risk factors for secondary bladder cancer following prostate cancer radiotherapy. Transl. Androl. Urol. 2024, 13, 1288–1296. [Google Scholar] [CrossRef]
- Valerio, M.; Ahmed, H.U.; Emberton, M.; Lawrentschuk, N.; Lazzeri, M.; Montironi, R.; Nguyen, P.L.; Trachtenberg, J.; Polascik, T.J. The Role of Focal Therapy in the Management of Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2014, 66, 732. [Google Scholar] [CrossRef]
- Eggener, S.; Salomon, G.; Scardino, P.T.; De la Rosette, J.; Polascik, T.J.; Brewster, S. Focal Therapy for Prostate Cancer: Possibilities and Limitations. Eur. Urol. 2010, 58, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L. Active surveillance and focal therapy for low-intermediate risk prostate cancer. Transl. Androl. Urol. 2015, 4, 342–354. [Google Scholar] [PubMed]
- Nomura, T.; Mimata, H. Focal Therapy in the Management of Prostate Cancer: An Emerging Approach for Localized Prostate Cancer. Adv. Urol. 2012, 2012, 391437. [Google Scholar] [CrossRef]
- Napoli, A.; Alfieri, G.; Scipione, R.; Leonardi, A.; Fierro, D.; Panebianco, V.; De Nunzio, C.; Leonardo, C.; Catalano, C. High-intensity focused ultrasound for prostate cancer. Expert Rev. Med. Devices 2020, 17, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Elhelf, I.A.S.; Albahar, H.; Shah, U.; Oto, A.; Cressman, E.; Almekkawy, M. High intensity focused ultrasound: The fundamentals, clinical applications and research trends. Diagn. Interv. Imaging 2018, 99, 349–359. [Google Scholar] [CrossRef]
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. 2020, 9, 460. [Google Scholar] [CrossRef]
- Aker, M.N.; Brisbane, W.G.; Kwan, L.; Gonzalez, S.; Priester, A.M.; Kinnaird, A.; Delfin, M.K.; Felker, E.; Sisk, A.E.; Kuppermann, D.; et al. Cryotherapy for partial gland ablation of prostate cancer: Oncologic and safety outcomes. Cancer Med. 2023, 12, 9351. [Google Scholar] [CrossRef]
- Kotamarti, S.; Polascik, T.J. Focal cryotherapy for prostate cancer: A contemporary literature review. Ann. Transl. Med. 2022, 11, 26. [Google Scholar] [CrossRef]
- Baust, J.G.; Gage, A.A.; Bjerklund Johansen, T.E.; Baust, J.M. Mechanisms of Cryoablation: Clinical Consequences on Malignant Tumors. Cryobiology 2013, 68, 1. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Ferreira, A.R.; Neves Mda, G.P.M.S.; Ribeiro, D.; Fardilha, M.; Faustino, M.A.F. Photodynamic therapy of prostate cancer using porphyrinic formulations. J. Photochem. Photobiol. B. 2021, 223, 112301. [Google Scholar] [CrossRef]
- Gheewala, T.; Skwor, T.; Munirathinam, G. Photosensitizers in prostate cancer therapy. Oncotarget 2017, 8, 30524. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Scheltema, M.J.; Jung, J.; Bakker, J.; Timmer, F.E.; Cerutti, X.; Katelaris, A.; Doan, P.; Gondoputro, W.; Blazevski, A.; et al. Irreversible electroporation of localised prostate cancer downregulates immune suppression and induces systemic anti-tumour T-cell activation—IRE-IMMUNO study. BJU Int. 2025, 135, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Faiella, E.; Santucci, D.; Vertulli, D.; Vergantino, E.; Vaccarino, F.; Perillo, G.; Zobel, B.B.; Grasso, R.F. Irreversible Electroporation (IRE) for Prostate Cancer (PCa) Treatment: The State of the Art. J. Pers. Med. 2024, 14, 137. [Google Scholar] [CrossRef]
- Iacovelli, V.; Carilli, M.; Bertolo, R.; Forte, V.; Vittori, M.; Filippi, B.; Di Giovanni, G.; Cipriani, C.; Petta, F.; Maiorino, F.; et al. Transperineal Laser Ablation for Focal Therapy of Localized Prostate Cancer: 12-Month Follow-up Outcomes from a Single Prospective Cohort Study. Cancers 2024, 16, 2620. [Google Scholar] [CrossRef]
- Manenti, G.; Perretta, T.; Nezzo, M.; Fraioli, F.R.; Carreri, B.; Gigliotti, P.E.; Micillo, A.; Malizia, A.; Di Giovanni, D.; Ryan, C.P.; et al. Transperineal Laser Ablation (TPLA) Treatment of Focal Low–Intermediate Risk Prostate Cancer. Cancers 2024, 16, 1404. [Google Scholar] [CrossRef]
- Cocci, A.; Pezzoli, M.; Bianco, F.; Blefari, F.; Bove, P.; Cornud, F.; De Rienzo, G.; Destefanis, P.; Di Trapani, D.; Giacobbe, A.; et al. Transperineal laser ablation of the prostate as a treatment for benign prostatic hyperplasia and prostate cancer: The results of a Delphi consensus project. Asian J. Urol. 2023, 11, 271. [Google Scholar] [CrossRef]
- Sessa, F.; Polverino, P.; Bisegna, C.; Siena, G.; Re, M.L.; Spatafora, P.; Pecoraro, A.; Rivetti, A.; Conte, F.L.; Cocci, A.; et al. Transperineal laser ablation of the prostate with EchoLaserTM system: Perioperative and short-term functional and sexual outcomes. Front. Urol. 2022, 2, 969208. [Google Scholar] [CrossRef]
- Tzelves, L.; Nagasubramanian, S.; Pinitas, A.; Juliebø-Jones, P.; Madaan, S.; Sienna, G.; Somani, B. Transperineal laser ablation as a new minimally invasive surgical therapy for benign prostatic hyperplasia: A systematic review of existing literature. Ther. Adv. Urol. 2023, 15, 17562872231198634. [Google Scholar] [CrossRef]
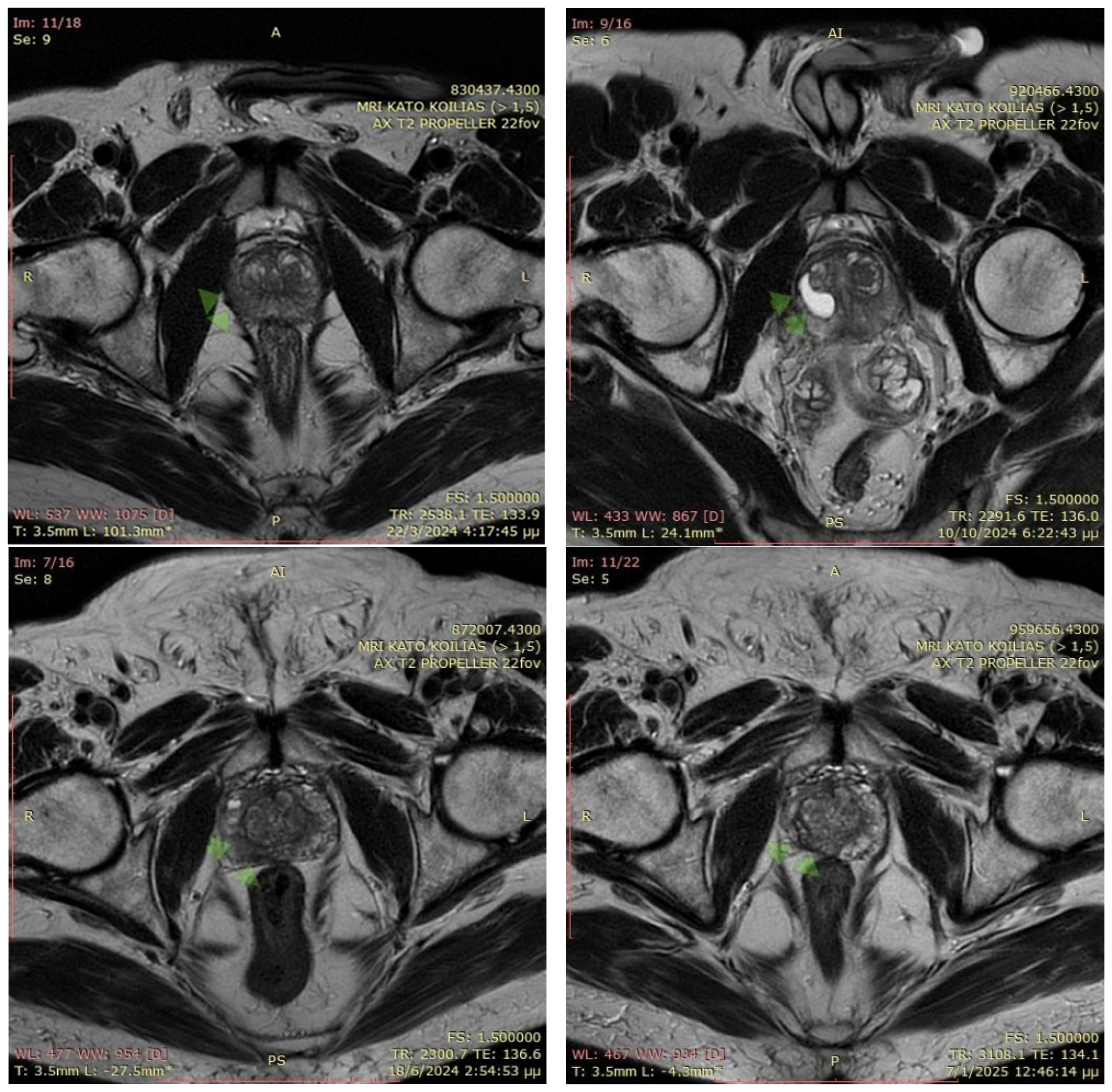
- Osman, S.O.S.; Russell, E.; King, R.B.; Crowther, K.; Jain, S.; McGrath, C.; Hounsell, A.R.; Prise, K.M.; McGarry, C.K. Fiducial markers visibility and artefacts in prostate cancer radiotherapy multi-modality imaging. Radiat. Oncol. 2019, 14, 237. [Google Scholar] [CrossRef]
- Brown, K.; Ghita, M.; Prise, K.M.; Butterworth, K.T. Feasibility and guidelines for the use of an injectable fiducial marker (BioXmark®) to improve target delineation in preclinical radiotherapy studies using mouse models. F1000Research 2023, 12, 526. [Google Scholar] [CrossRef]
- Mahdavi, A.; Mofid, B.; Taghizadeh-Hesary, F. Intra-prostatic gold fiducial marker insertion for image-guided radiotherapy (IGRT): Five-year experience on 795 patients. BMC Med. Imaging 2023, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Gill, S.; Sabet, M.; Ebert, M.A.; Rowshanfarzad, P.; Kendrick, J.; Jacques, A.; Herbert, C.; Croker, J.; Bydder, S.; et al. Treatment efficiency and quality improvement via double imaging modality (DIM) versus single imaging modality (SIM) image-guided radiotherapy for prostate cancer. Tech. Innov. Patient Support Radiat. Oncol. 2025, 33, 100307. [Google Scholar] [CrossRef]
- Gurjar, O.P.; Arya, R.; Goyal, H. A study on prostate movement and dosimetric variation because of bladder and rectum volumes changes during the course of image-guided radiotherapy in prostate cancer. Prostate Int. 2020, 8, 91. [Google Scholar] [CrossRef]
- O’neill, A.G.M.; Jain, S.; Hounsell, A.R.; O’sullivan, J.M. Fiducial marker guided prostate radiotherapy: A review. Br. J. Radiol. 2016, 89, 20160296. [Google Scholar] [CrossRef]
- Elm Evon Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ Br. Med. J. 2007, 335, 806. [Google Scholar]
| Frequency | Percentage | |
|---|---|---|
| Age (in years) * | 72.36 ± 9.53 | |
| Gleason Groups * | 2.36 ± 1.06 | |
| Group 1 | 10 | 20.00 |
| Group 2 | 22 | 44.00 |
| Group 3 | 10 | 20.00 |
| Group 4 | 6 | 12.00 |
| Group 5 | 2 | 4.00 |
| Fiducial | ||
| No | 19 | 38.00 |
| Yes | 31 | 62.00 |
| Duration of operation (in minutes) * | 38.16 ± 6.56 | |
| MRI (after 6 months) | ||
| Negative | 40 | 80.00 |
| Positive | 10 | 20.00 |
| PSA | ||
|---|---|---|
| Pre Biopsy | After 6 Months | |
| Mean ± Standard Deviation | 10.26 ± 14.99 | 2.70 ± 2.67 |
| Median (IQR) | 5.95 (4.34–9.06) | 1.90 (1.11–3.26) |
| Minimum | 2.38 | 0.00 |
| Maximum | 100.00 | 15.00 |
| Fiducial | t(48)/U | p | ||
|---|---|---|---|---|
| No | Yes | |||
| M ± SD | M ± SD | |||
| Age a | 78.37 ± 8.68 | 68.68 ± 8.14 | 3.985 | <0.01 |
| Gleason Groups b | 3.00 ± 1.25 | 1.97 ± 0.71 | 146.500 | <0.01 |
| Duration of operation (in minutes) b | 45.79 ± 2.92 | 33.48 ± 2.41 | 0.000 | <0.01 |
| Fiducial | Total | χ2 | ||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| MRI (after 6 months) | Negative | Count | 12 | 28 | 40 | χ2 = 5.433 p < 0.05 |
| % within MRI (after 6 months) | 30.0% | 70.0% | 100.0% | |||
| Positive | Count | 7 | 3 | 10 | ||
| % within MRI (after 6 months) | 70.0% | 30.0% | 100.0% | |||
| Total | Count | 19 | 31 | 50 | ||
| % within MRI (after 6 months) | 38.0% | 62.0% | 100.0% | |||
| Duration of Operation (in Minutes) | ||
|---|---|---|
| Age | Correlation Coefficient | 0.44 * |
| Sig. (2-tailed) | <0.01 | |
| N | 50 | |
| Gleason Groups | Correlation Coefficient | 0.40 * |
| Sig. (2-tailed) | <0.01 | |
| N | 50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granitsas, T.; Anastassakis, I.; Brempos, S.; Brempos, K. Echolaser Focal Treatment for Prostate Cancer Guided by Fiducial Marker Placement. Cancers 2025, 17, 1707. https://doi.org/10.3390/cancers17101707
Granitsas T, Anastassakis I, Brempos S, Brempos K. Echolaser Focal Treatment for Prostate Cancer Guided by Fiducial Marker Placement. Cancers. 2025; 17(10):1707. https://doi.org/10.3390/cancers17101707
Chicago/Turabian StyleGranitsas, Timoleon, Ioannis Anastassakis, Stamatios Brempos, and Kyriakos Brempos. 2025. "Echolaser Focal Treatment for Prostate Cancer Guided by Fiducial Marker Placement" Cancers 17, no. 10: 1707. https://doi.org/10.3390/cancers17101707
APA StyleGranitsas, T., Anastassakis, I., Brempos, S., & Brempos, K. (2025). Echolaser Focal Treatment for Prostate Cancer Guided by Fiducial Marker Placement. Cancers, 17(10), 1707. https://doi.org/10.3390/cancers17101707






