Dynamics in Quality of Life of Breast Cancer Patients Following Surgery: Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Global Quality of Life
3.1.1. Descriptive Statistics
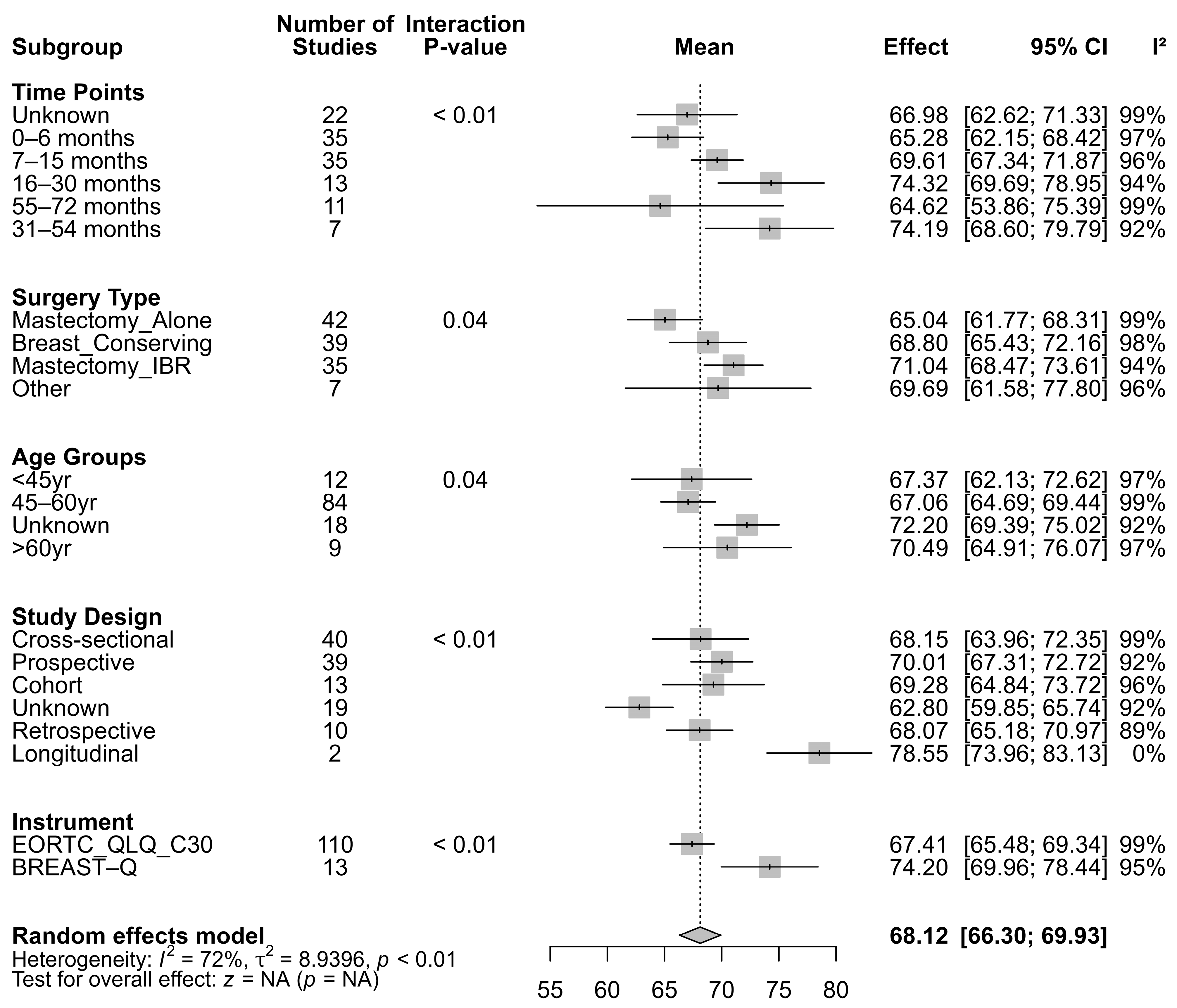
3.1.2. Subgroup Analysis of Global QoL
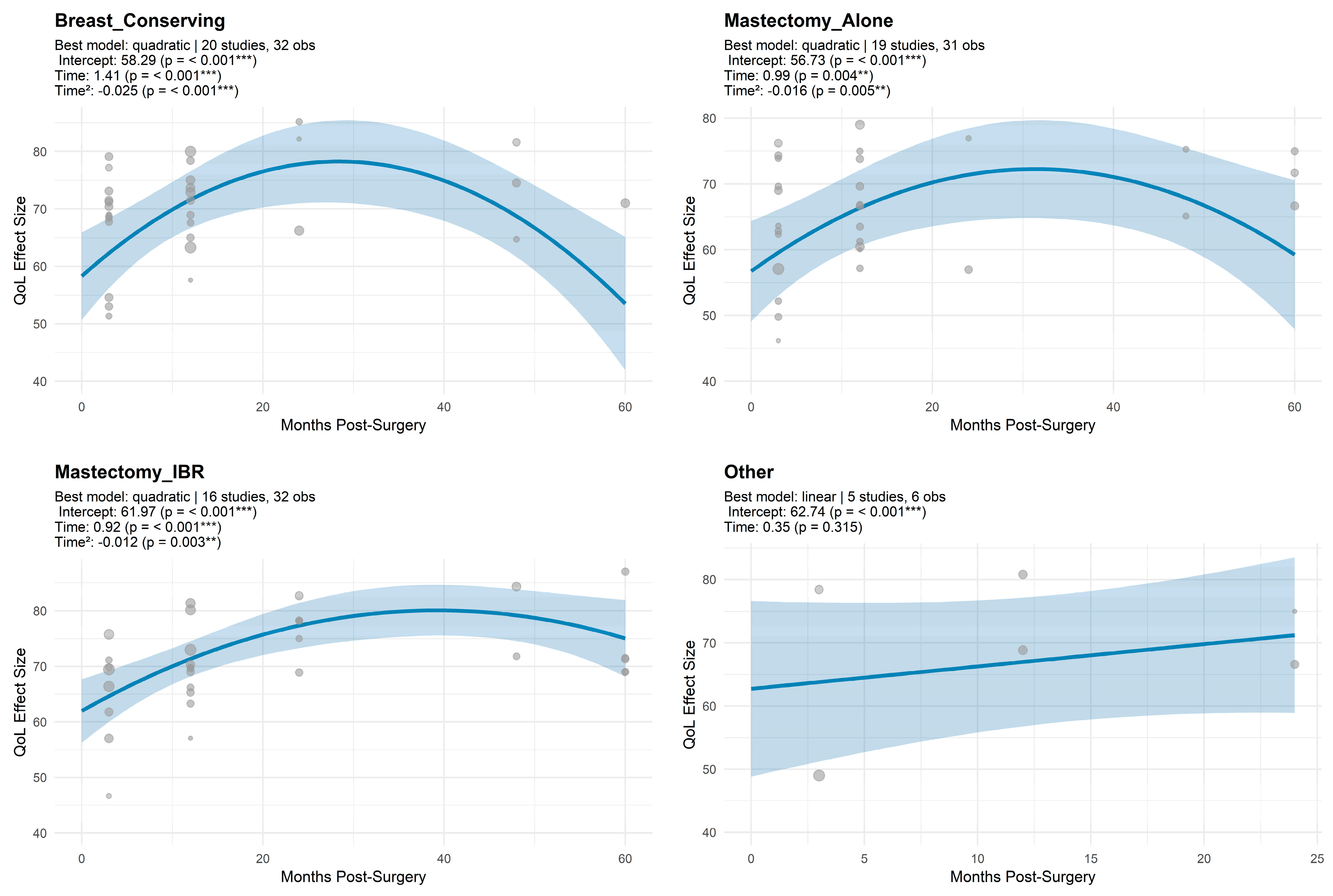
3.1.3. Trajectory Modelling for Global QoL
3.1.4. Trajectory Model Estimates for QoL by Surgical Modality
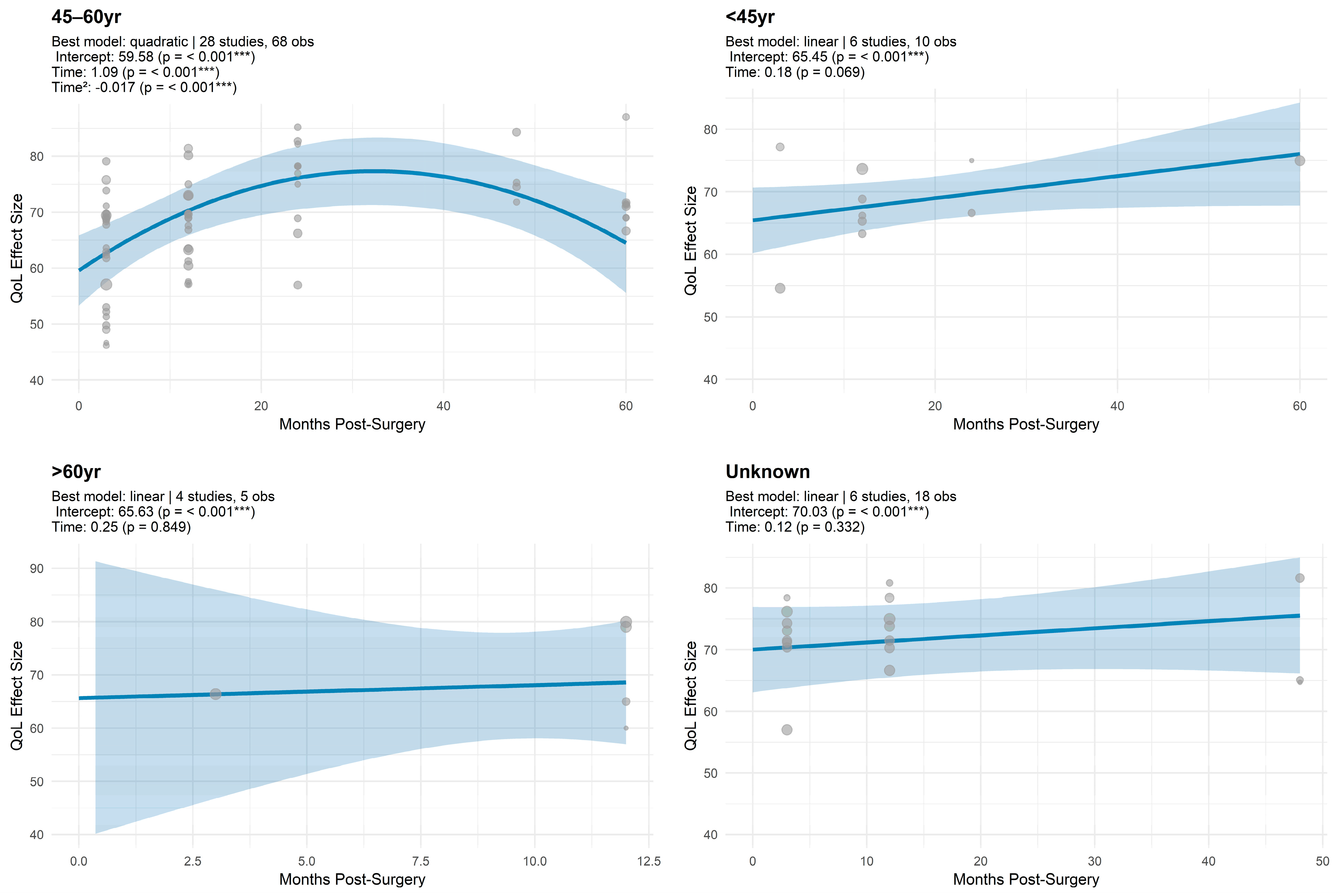
3.1.5. Trajectory Model Estimates for Global QoL by Age
3.1.6. Meta Regression for Global QoL
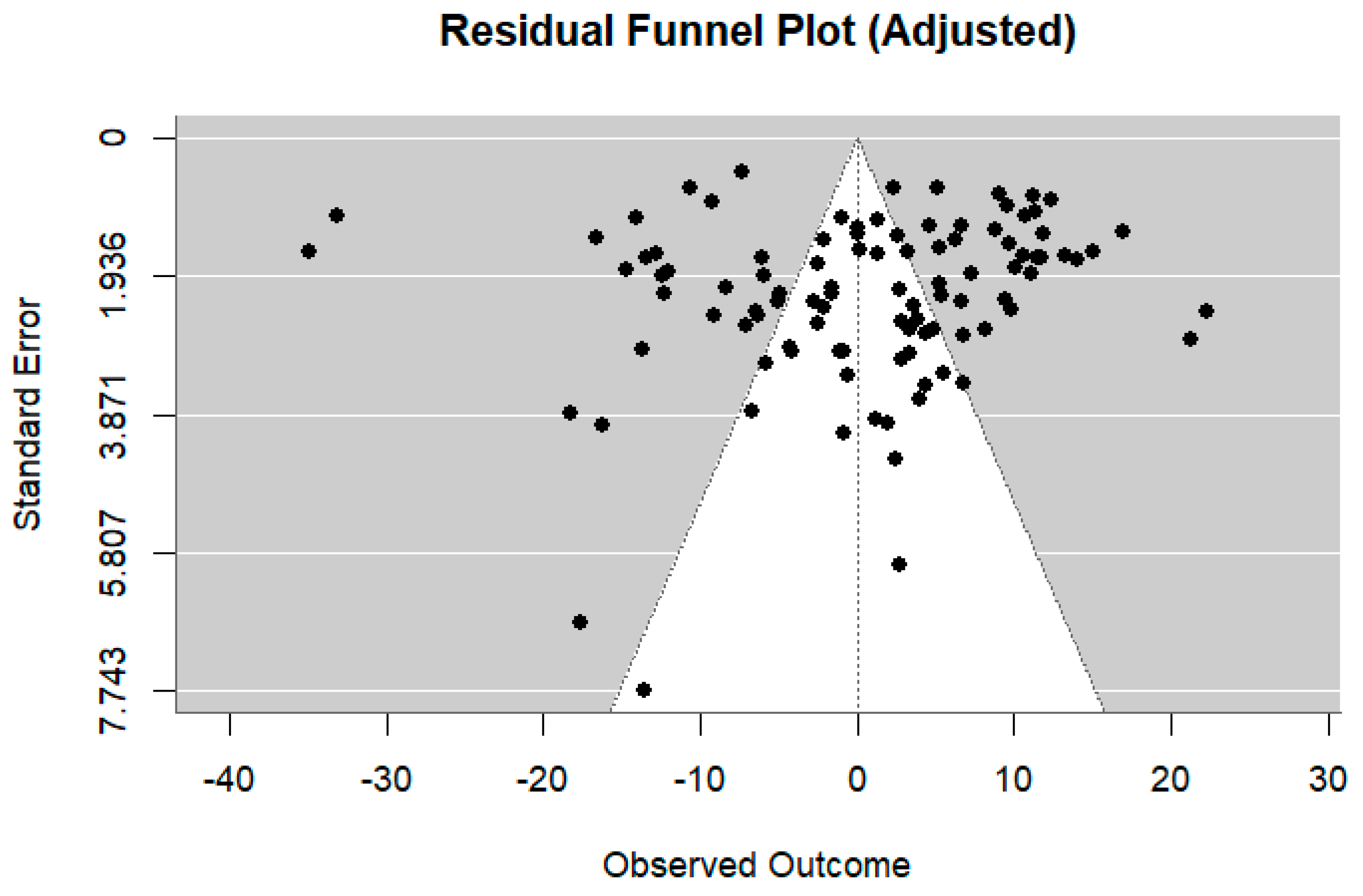
4. Discussion
4.1. Strengths and Limitations
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike information criterion |
| BC | breast cancer |
| BCS | breast-conserving surgery |
| BIC | Bayesian information criterion |
| IBR | immediate breast reconstruction |
| Mx+IR | mastectomy with immediate reconstruction |
| PROMs | patient-reported outcome measures |
| MA | Mastectomy Alone |
| QoL | quality of life |
Appendix A
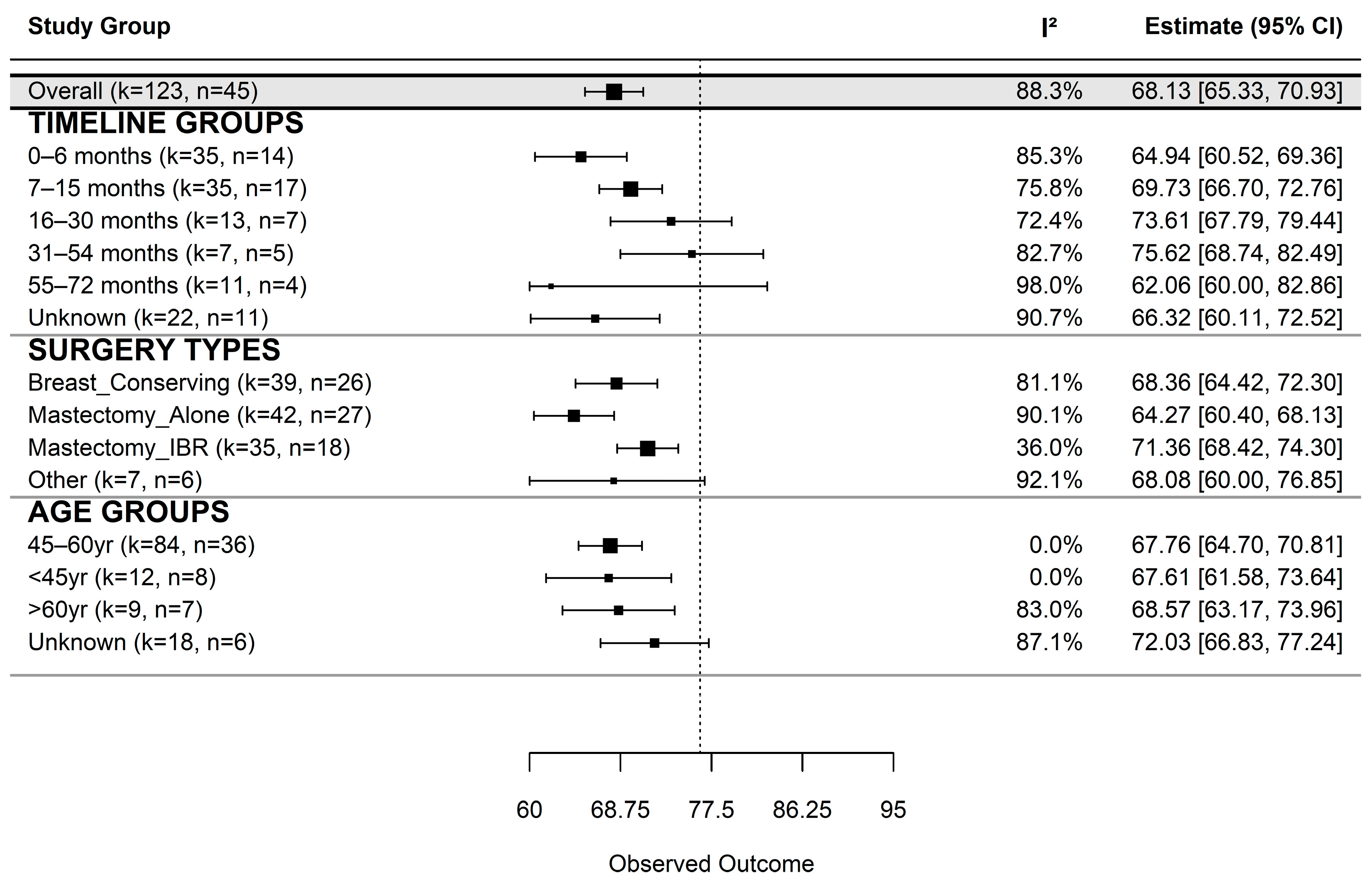
| Group | k | n_Studies | Estimate | SE | 95% CI (LB–UB) | τ2 | I2 (%) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Overall | 123 | 45 | 68.13 | 1.43 | 65.33–70.93 | 47.63 | 88.28 | <0.001 |
| Time since surgery | ||||||||
| 0–6 months | 35 | 14 | 64.94 | 2.26 | 60.52–69.36 | 34.67 | 85.32 | <0.001 |
| 7–15 months | 35 | 17 | 69.73 | 1.55 | 66.70–72.76 | 19.21 | 75.78 | <0.001 |
| 16–30 months | 13 | 7 | 73.61 | 2.97 | 67.79–79.44 | 28.50 | 72.40 | <0.001 |
| 31–54 months | 7 | 5 | 75.62 | 3.51 | 68.74–82.49 | 28.64 | 82.73 | <0.001 |
| 55–72 months | 11 | 4 | 62.06 | 10.61 | 41.26–82.86 | 224.62 | 98.03 | <0.001 |
| Unknown | 22 | 11 | 66.32 | 3.17 | 60.11–72.52 | 53.84 | 90.70 | <0.001 |
| Surgery type | ||||||||
| Breast-Conserving | 39 | 26 | 68.36 | 2.01 | 64.42–72.30 | 19.67 | 81.10 | <0.001 |
| Mastectomy Alone | 42 | 27 | 64.27 | 1.97 | 60.40–68.13 | 40.40 | 90.07 | <0.001 |
| Mastectomy IBR | 35 | 18 | 71.36 | 1.50 | 68.42–74.30 | 5.56 | 36.02 | <0.001 |
| Other | 7 | 6 | 68.08 | 4.47 | 59.32–76.85 | 111.06 | 92.09 | <0.001 |
| Age group | ||||||||
| 45–60 yr | 84 | 36 | 67.76 | 1.56 | 64.71–70.81 | 0.00 | 0.00 | <0.001 |
| <45 yr | 12 | 8 | 67.61 | 3.08 | 61.58–73.64 | 0.00 | 0.00 | <0.001 |
| >60 yr | 9 | 7 | 68.57 | 2.75 | 63.17–73.96 | 24.10 | 83.01 | <0.001 |
| Unknown | 18 | 6 | 72.03 | 2.66 | 66.83–77.24 | 24.78 | 87.05 | <0.001 |
| Model | AIC | BIC | R2 | ΔAIC | ΔBIC |
|---|---|---|---|---|---|
| Quadratic | 972.18 | 985.10 | 1 | 0.00 | 0.00 |
| Logarithmic | 985.20 | 995.58 | 1 | 13.02 | 10.48 |
| Linear | 989.26 | 999.64 | 1 | 17.08 | 14.54 |
| Surgery Type | k | Studies | Intercept | Linear Term | Quadratic Term | R2 (QM p-Value) | QE (p-Value) |
|---|---|---|---|---|---|---|---|
| Breast_Conserving | 32 | 20 | 58.29 ± 3.88 *** | 1.41 ± 0.39 *** | −0.02 ± 0.01 *** | (<0.001) | 1180.85 (<0.001) |
| Mastectomy_Alone | 31 | 19 | 56.73 ± 3.90 *** | 0.99 ± 0.34 ** | −0.02 ± 0.01 ** | (0.015) | 1714.69 (<0.001) |
| Mastectomy_IBR | 32 | 16 | 61.97 ± 2.92 *** | 0.92 ± 0.25 *** | −0.01 ± 0.00 ** | (<0.001) | 371.04 (<0.001) |
| Other | 6 | 5 | 62.74 ± 7.10 *** | 0.35 ± 0.35 | — | (0.315) | 124.30 (<0.001) |
| Age Group | k | Studies | Intercept | Linear Term | Quadratic Term | R2 (QM p-Value) | QE (p-Value) |
|---|---|---|---|---|---|---|---|
| 45–60 yr | 68 | 28 | 59.58 ± 3.20 *** | 1.09 ± 0.29 *** | −0.02 ± 0.00 *** | (<0.001) | 3180.58 (<0.001) |
| <45 yr | 10 | 6 | 65.45 ± 2.68 *** | 0.18 ± 0.10 | — | (0.069) | 101.86 (<0.001) |
| >60 yr | 5 | 4 | 65.63 ± 13.48 *** | 0.25 ± 1.29 | — | (0.849) | 75.31 (<0.001) |
| Unknown | 18 | 6 | 70.03 ± 3.53 *** | 0.12 ± 0.12 | — | (0.332) | 212.75 (<0.001) |
| Moderator | Chi-Square | df | p-Value |
|---|---|---|---|
| Time Points | 25.61 | 4 | <0.0001 |
| Surgery Types | 40.53 | 3 | <0.0001 |
| Age Groups | 67.09 | 3 | <0.0001 |
| Instrument Used | 4.07 | 1 | 0.044 |
| Study Design | 3.73 | 4 | 0.445 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, A. Health-related quality of life in breast cancer patients: A bibliographic review of the literature from 1974 to 2007. J. Exp. Clin. Cancer Res. 2008, 27, 32. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.R.; Rajan, S.; Sudeepthi, B.L.; Abdul Nazir, C.P. Patient-reported outcomes: A new era in clinical research. Perspect. Clin. Res. 2011, 2, 137–144. [Google Scholar] [CrossRef]
- Al-Ghazal, S.; Fallowfield, L.; Blamey, R. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur. J. Cancer 2000, 36, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.A.; Semple, J.; Quan, M.-L.; Vadaparampil, S.T.; Holloway, C.; Brown, M.; Bower, B.; Sun, P.; Narod, S.A. Changes in Psychosocial Functioning 1 Year After Mastectomy Alone, Delayed Breast Reconstruction, or Immediate Breast Reconstruction. Ann. Surg. Oncol. 2011, 19, 233–241. [Google Scholar] [CrossRef]
- Parker, P.A.; Youssef, A.; Walker, S.; Basen-Engquist, K.; Cohen, L.; Gritz, E.R.; Wei, Q.X.; Robb, G.L. Short-Term and Long-Term Psychosocial Adjustment and Quality of Life in Women Undergoing Different Surgical Procedures for Breast Cancer. Ann. Surg. Oncol. 2007, 14, 3078–3089. [Google Scholar] [CrossRef]
- Sisco, M.; Johnson, D.B.; Wang, C.; Rasinski, K.; Rundell, V.L.; Yao, K.A. The quality-of-life benefits of breast reconstruction do not diminish with age. J. Surg. Oncol. 2015, 111, 663–668. [Google Scholar] [CrossRef]
- Nano, M.T.; Gill, P.G.; Kollias, J.; Bochner, M.A.; Malycha, P.; Winefield, H.R. Psychological impact and cosmetic outcome of surgical breast cancer strategies. ANZ J. Surg. 2005, 75, 940–947. [Google Scholar] [CrossRef]
- Brandberg, Y.; Malm, M.; Blomqvist, L. A Prospective and Randomized Study, “SVEA,” Comparing Effects of Three Methods for Delayed Breast Reconstruction on Quality of Life, Patient-Defined Problem Areas of Life, and Cosmetic Result. Plast. Reconstr. Surg. 2000, 105, 66–74. [Google Scholar] [CrossRef]
- Avis, N.E.; Levine, B.; Naughton, M.J.; Case, L.D.; Naftalis, E.; Van Zee, K.J. Age-related longitudinal changes in depressive symptoms following breast cancer diagnosis and treatment. Breast Cancer Res. Treat. 2013, 139, 199–206. [Google Scholar] [CrossRef]
- Champion, V.L.; Wagner, L.I.; Monahan, P.O.; Daggy, J.; Smith, L.; Cohee, A.; Ziner, K.W.; Haase, J.E.; Miller, K.D.; Pradhan, K.; et al. Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer 2014, 120, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Pat-Horenczyk, R.; Kelada, L.; Kolokotroni, E.; Stamatakos, G.; Dahabre, R.; Bentley, G.; Perry, S.; Karademas, E.C.; Simos, P.; Poikonen-Saksela, P.; et al. Trajectories of Quality of Life among an International Sample of Women during the First Year after the Diagnosis of Early Breast Cancer: A Latent Growth Curve Analysis. Cancers 2023, 15, 1961. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Higgins, J.P.T. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Covidence Systematic Review Software. Veritas Health Innovation: Melbourne, Australia. Available online: www.covidence.org (accessed on 20 September 2025).
- Barker, T.H.; Hasanoff, S.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Habibi, N.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Cohort Studies. JBI Evid. Synth. 2025, 23, 441–453. [Google Scholar] [CrossRef]
- Barker, T.H.; Hasanoff, S.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Klugar, M.; Tufanaru, C.; Moola, S.; Liu, X.-L.; et al. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Analytical Cross-Sectional Studies. JBI Evid. Synth. 2025. [Google Scholar] [CrossRef]
- Abebe, E.; Demilie, K.; Lemmu, B.; Abebe, K. Female Breast Cancer Patients, Mastectomy-Related Quality of Life: Experience from Ethiopia. Int. J. Breast Cancer 2020, 2020, 8460374. [Google Scholar] [CrossRef]
- Acil, H.; Cavdar, I. Comparison of Quality of Life of Turkish Breast Cancer Patients Receiving Breast Conserving Surgery or Modified Radical Mastectomy. Asian Pac. J. Cancer Prev. 2014, 15, 5377–5381. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Christiaens, M.; Enzlin, P.; Neven, P.; Amant, F. Sexual functioning in women after mastectomy versus breast conserving therapy for early-stage breast cancer: A prospective controlled study. Breast 2014, 23, 629–636. [Google Scholar] [CrossRef]
- Cherian, K.; Acharya, N.R.; Bhargavan, R.V.; Augustine, P.; Krishnan, J.K. Quality of Life Post Breast Cancer Surgery: Comparison of Breast Conservation Surgery versus Modified Radical Mastectomy in a Developing Country. South Asian J. Cancer 2022, 11, 183–189. [Google Scholar] [CrossRef]
- Cortés-Flores, A.O.; Morgan-Villela, G.; del Valle, C.J.Z.-F.; Jiménez-Tornero, J.; Juárez-Uzeta, E.; Urias-Valdez, D.P.; Garcia-González, L.-A.; Fuentes-Orozco, C.; Chávez-Tostado, M.; Macías-Amezcua, M.D.; et al. Quality of Life Among Women Treated for Breast Cancer: A Survey of Three Procedures in Mexico. Aesthetic Plast. Surg. 2014, 38, 887–895. [Google Scholar] [CrossRef]
- Dahlui, M.; Azzani, M.; Taib, N.A.; Hoong, S.M.; Jamaris, S.; Islam, T. Breast conserving surgery versus mastectomy: The effect of surgery on quality of life in breast cancer survivors in Malaysia. BMC Women’s Health 2023, 23, 607. [Google Scholar] [CrossRef]
- Harcourt, D.M.; Rumsey, N.J.; Ambler, N.R.; Cawthorn, S.J.; Reid, C.D.; Maddox, P.R.; Kenealy, J.M.; Rainsbury, R.M.; Umpleby, H.C. The Psychological Effect of Mastectomy with or without Breast Reconstruction: A Prospective, Multicenter Study. Plast. Reconstr. Surg. 2003, 111, 1060–1068. [Google Scholar] [CrossRef]
- Nowicki, A.; Licznerska, B.; Rhone, P. Evaluation of the quality of life of women treated due to breast cancer using amputation or breast conserving surgery in the early postoperative period. Pol. J. Surg. 2015, 87, 174–180. [Google Scholar] [CrossRef]
- Songtish, D.; Akranurakkul, P. Body Image and Quality of Life Correlation after Treatment in Thai Breast Cancer Patients. J. Med. Assoc. Thail. 2021, 104 (Suppl. S3), S31–S37. [Google Scholar]
- Cohen, L.; Hack, T.F.; de Moor, C.; Katz, J.; Goss, P.E. The Effects of Type of Surgery and Time on Psychological Adjustment in Women After Breast Cancer Treatment. Ann. Surg. Oncol. 2000, 7, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Domenici, L.; Caputo, G.G.; Losco, L.; Di Taranto, G.; Lo Torto, F.; Pierazzi, D.M.; Governa, M.; Panici, P.B.; Ribuffo, D.; Cigna, E. Muscle-Sparing Skin-Reducing Breast Reconstruction with Pre-Pectoral Implants in Breast Cancer Patients: Long-Term Assessment of Patients’ Satisfaction and Quality of Life. J. Investig. Surg. 2022, 35, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Hejl, L.; Raft, J.; Leufflen, L.; Rauch, P.; Buhler, J.; Abel-Decollogne, F.; Routiot, T.; Hotton, J.; Salleron, J.; Marchal, F. Quality of life, anxiety, and postoperative complications of patients undergoing breast cancer surgery as ambulatory surgery compared to non-ambulatory surgery: A prospective non-randomized study. J. Gynecol. Obstet. Hum. Reprod. 2020, 50, 101779. [Google Scholar] [CrossRef]
- Hadi, N.; Soltanipour, S.; Talei, A. Impact of modified radical mastectomy on health-related quality of life in women with early stage breast cancer. Arch. Iran. Med. 2012, 15, 504–507. [Google Scholar]
- Hassan, B.A.R.; Mohammed, A.H.; Al Zobair, A.A.; Wayyes, A.M.; Al-Jawadi, H.K.; Alsammarraie, A.Z.A.; Alabboodi, M.K.; Jiang, J.X. Enhancing Women’s Quality of Life: Exploring the Impact of Mastectomy with and without Breast Reconstruction among Breast Cancer Survivors in Iraq. Asian Pac. J. Cancer Prev. 2024, 25, 1097–1105. [Google Scholar] [CrossRef]
- Jayasinghe, R.; Fernando, A.; Jayarajah, U.; Seneviratne, S. Post treatment quality of life among Sri Lankan women with breast cancer. BMC Cancer 2021, 21, 305. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, M.; Fal, A. The Influence of the Surgical Treatment Method on the Quality of Life of Women With Breast Cancer. Eur. J. Breast Health 2023, 19, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kouwenberg, C.A.E.M.; de Ligt, K.M.; Kranenburg, L.W.; Rakhorst, H.M.; de Leeuw, D.M.; Siesling, S.; Busschbach, J.J.; Mureau, M.A.M.M. Long-Term Health-Related Quality of Life after Four Common Surgical Treatment Options for Breast Cancer and the Effect of Complications: A Retrospective Patient-Reported Survey among 1871 Patients. Plast. Reconstr. Surg. 2020, 146, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moro-Valdezate, D.; Buch-Villa, E.; Peiró, S.; Morales-Monsalve, M.D.; Caballero-Gárate, A.; Martínez-Agulló, Á.; Checa-Ayet, F.; Ortega-Serrano, J. Factors associated with health-related quality of life in a cohort of Spanish breast cancer patients. Breast Cancer 2012, 21, 442–452. [Google Scholar] [CrossRef]
- Nsaful, J.; Nartey, E.T.; Dedey, F.; Bediako-Bowan, A.; Appiah-Danquah, R.; Darko, K.; Ankrah, L.N.A.; Akli-Nartey, C.; Annan, J.Y.; Dei-Asamoa, J.; et al. Quality of Life after Mastectomy with or without Breast Reconstruction and Breast-Conserving Surgery in Breast Cancer Survivors: A Cross-Sectional Study at a Tertiary Hospital in Ghana. Curr. Oncol. 2024, 31, 2952–2962. [Google Scholar] [CrossRef]
- Pačarić, S.; Kristek, J.; Mirat, J.; Kondža, G.; Turk, T.; Farčić, N.; Orkić, Ž.; Nemčić, A. The quality of life of Croatian women after mastectomy: A cross-sectional single-center study. BMC Public Health 2018, 18, 999. [Google Scholar] [CrossRef]
- Camejo, N.; Amarillo, D.; Castillo, C.; Guerrina, M.; Savio, F.; Carrasco, M.; Strazzarino, N.; Hernandez, A.L.; Herrera, G.; Krygier, G. Quality of life in patients treated with breast cancer surgery and adjuvant systemic therapy and/or adjuvant radiotherapy in Uruguay. J. Cancer Res. Ther. 2023, 20, 832–839. [Google Scholar] [CrossRef]
- Enien, M.A.; Ibrahim, N.; Makar, W.; Darwish, D.; Gaber, M. Health-related quality of life. J. Cancer Res. Ther. 2018, 14, 957–963. [Google Scholar] [CrossRef]
- Esgueva, A.J.; Noordhoek, I.; Kranenbarg, E.M.-K.; Espinosa-Bravo, M.; Mátrai, Z.; Zhygulin, A.; Irmejs, A.; Mavioso, C.; Meani, F.; González, E.; et al. Health-Related Quality of Life After Nipple-Sparing Mastectomy: Results from the INSPIRE Registry. Ann. Surg. Oncol. 2021, 29, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.; Tan, K.; Anthony, L.; Miller, F. Effect of Psychosocial, Behavioral, and Disease Characteristics on Health-Related Quality of Life (HRQoL) After Breast Cancer Surgery: A Cross-Sectional Study of a Regional Australian Population. Cureus 2023, 15, e36054. [Google Scholar] [CrossRef]
- Hallberg, H.; Elander, A.; Kölby, L.; Hansson, E. A biological or a synthetic mesh in immediate breast reconstruction? A cohort-study of long-term Health related Quality of Life (HrQoL). Eur. J. Surg. Oncol. 2019, 45, 1812–1816. [Google Scholar] [CrossRef]
- Lagendijk, M.; van Egdom, L.; Richel, C.; van Leeuwen, N.; Verhoef, C.; Lingsma, H.; Koppert, L. Patient reported outcome measures in breast cancer patients. Eur. J. Surg. Oncol. 2018, 44, 963–968. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T.; Moon, H.; Jin, U.; Kim, K.; Kim, J.; Lee, J.; Lee, E.; Yoo, T.; Noh, D.-Y.; et al. Effect of cosmetic outcome on quality of life after breast cancer surgery. Eur. J. Surg. Oncol. 2015, 41, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Löfstrand, J.; Paganini, A.; Lidén, M.; Hansson, E. Comparison of patient-reported achievements of goals and core outcomes with delayed breast reconstruction in irradiated patients: Latissimus dorsi with an implant versus DIEP. J. Plast. Surg. Hand Surg. 2023, 58, 74–81. [Google Scholar] [CrossRef]
- Qin, Q.; Tan, Q.; Lian, B.; Mo, Q.; Huang, Z.; Wei, C. Postoperative outcomes of breast reconstruction after mastectomy. Medicine 2018, 97, e9766. [Google Scholar] [CrossRef]
- Kuroda, F.; Urban, C.; Zucca-Matthes, G.; de Oliveira, V.M.; Arana, G.H.; Iera, M.; Rietjens, M.; Santos, G.; Spagnol, C.; de Lima, R.S. Evaluation of Aesthetic and Quality-of-Life Results after Immediate Breast Reconstruction with Definitive Form-Stable Anatomical Implants. Plast. Reconstr. Surg. 2016, 137, 278e–286e. [Google Scholar] [CrossRef]
- Shi, H.; Uen, Y.; Yen, L.; Culbertson, R.; Juan, C.; Hou, M. Two-year quality of life after breast cancer surgery: A comparison of three surgical procedures. Eur. J. Surg. Oncol. 2011, 37, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kim, S.-W.; Heo, C.Y.; Kim, D.; Hwang, Y.; Yom, C.K.; Kang, E. Comparison of Quality of Life Based on Surgical Technique in Patients with Breast Cancer. Ultrasound Med. Biol. 2013, 44, 22–27. [Google Scholar] [CrossRef]
- Ozmen, V.; Ilgun, S.; Ozden, B.C.; Ozturk, A.; Aktepe, F.; Agacayak, F.; Elbuken, F.; Alco, G.; Ordu, C.; Iyigun, Z.E.; et al. Comparison of breast cancer patients who underwent partial mastectomy (PM) with mini latissimus dorsi flap (MLDF) and subcutaneous mastectomy with implant (M + I) regarding quality of life (QOL), cosmetic outcome and survival rates. World J. Surg. Oncol. 2020, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Spatuzzi, R.; Vespa, A.; Lorenzi, P.; Miccinesi, G.; Ricciuti, M.; Cifarelli, W.; Susi, M.; Fabrizio, T.; Ferrari, M.G.; Ottaviani, M.; et al. Evaluation of Social Support, Quality of Life, and Body Image in Women with Breast Cancer. Breast Care 2016, 11, 28–32. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Kuo, R.N.-C.; Chung, K.-P. Quality of life of breast cancer survivors following breast-conserving therapy versus mastectomy: A multicenter study in Taiwan. Ultrasound Med. Biol. 2017, 47, 909–918. [Google Scholar] [CrossRef]
- Razdan, S.; Ahmed, G.A.; Vishwakarma, G.; Baban, C.; Tenovici, A. Surgical and Patient-Reported Outcomes After Mastectomy and Implant-Based Prepectoral Reconstruction Using TIGR® Synthetic Mesh. Cureus 2024, 16, e61052. [Google Scholar] [CrossRef]
- Volders, J.H.; Negenborn, V.L.; Haloua, M.H.; Krekel, N.M.A.; Jóźwiak, K.; Meijer, S.; Tol, P.M.v.D. Cosmetic outcome and quality of life are inextricably linked in breast-conserving therapy. J. Surg. Oncol. 2017, 115, 941–948. [Google Scholar] [CrossRef]
- von Glinski, M.; Holler, N.; Kümmel, S.; Reinisch, M.; Wallner, C.; Wagner, J.M.; Dadras, M.; Sogorski, A.; Lehnhardt, M.; Behr, B. Autologous vs. implant-based breast reconstruction after skin- and nipple-sparing mastectomy—A deeper insight considering surgical and patient-reported outcomes. Front. Surg. 2022, 9, 903734. [Google Scholar] [CrossRef]
- Janni, W.; Rjosk, D.; Dimpfl, T.; Haertl, K.; Strobl, B.; Hepp, F.; Hanke, A.; Bergauer, F.; Sommer, H. Quality of Life Influenced by Primary Surgical Treatment for Stage I-III Breast Cancer?Long-Term Follow-Up of a Matched-Pair Analysis. Ann. Surg. Oncol. 2001, 8, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Grothuesmann, D.; Neises, M.; Hille, U.; Hillemanns, P. Quality of life and satisfaction after breast cancer operation. Arch. Gynecol. Obstet. 2009, 282, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.A.; Sisco, M.; Yao, K.; Winchester, D.J.; Barrera, E.; Warner, J.; Jaffe, J.; Hulick, P.; Kuchta, K.; Pusic, A.L.; et al. Patient satisfaction with nipple-sparing mastectomy: A prospective study of patient reported outcomes using the BREAST-Q. J. Surg. Oncol. 2016, 114, 416–422. [Google Scholar] [CrossRef] [PubMed]
- King, M.T.; Kenny, P.; Shiell, A.; Hall, J.; Boyages, J. Quality of life three months and one year after first treatment for early stage breast cancer: Influence of treatment and patient characteristics. Qual. Life Res. 2000, 9, 789–800. [Google Scholar] [CrossRef]
- Denis-Katz, H.S.; Ghaedi, B.B.; Fitzpatrick, A.; Zhang, J. Oncological Safety, Surgical Outcome, and Patient Satisfaction of Oncoplastic Breast-Conserving Surgery With Contralateral Balancing Reduction Mammoplasty. Plast. Surg. 2020, 29, 235–242. [Google Scholar] [CrossRef]
- Ticha, P.; Mestak, O.; Wu, M.; Bujda, M.; Sukop, A. Patient-Reported Outcomes of Three Different Types of Breast Reconstruction with Correlation to the Clinical Data 5 Years Postoperatively. Aesthetic Plast. Surg. 2020, 44, 2021–2029. [Google Scholar] [CrossRef]
- Szutowicz-Wydra, B.; Wydra, J.; Kruszewski, W.J.; Ciesielski, M.; Szajewski, M.; Walczak, J.; Hansdorfer-Korzon, R. Same Quality of Life for Polish Breast Cancer Patients Treated with Mastectomy and Breast Reconstruction or Breast-Conserving Therapy. Pol. J. Surg. 2016, 88, 264–269. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf (accessed on 20 September 2025).
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A Re-Evaluation of Random-Effects Meta-Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2008, 172, 137–159. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Arndt, V.; Merx, H.; Stegmaier, C.; Ziegler, H.; Brenner, H. Quality of Life in Patients With Colorectal Cancer 1 Year After Diagnosis Compared With the General Population: A Population-Based Study. J. Clin. Oncol. 2004, 22, 4829–4836. [Google Scholar] [CrossRef]
- Janz, N.K.; Hawley, S.T.; Mujahid, M.S.; Griggs, J.J.; Alderman, A.; Hamilton, A.S.; Graff, J.J.; Jagsi, R.; Katz, S.J. Correlates of worry about recurrence in a multiethnic population-based sample of women with breast cancer. Cancer 2011, 117, 1827–1836. [Google Scholar] [CrossRef]
- Montazeri, A.; Vahdaninia, M.; Harirchi, I.; Ebrahimi, M.; Khaleghi, F.; Jarvandi, S. Quality of life in patients with breast cancer before and after diagnosis: An eighteen months follow-up study. BMC Cancer 2008, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Corica, T.; Nowak, A.K.; Saunders, C.M.; Bulsara, M.; Taylor, M.; Vaidya, J.S.; Baum, M.; Joseph, D.J. Cosmesis and Breast-Related Quality of Life Outcomes After Intraoperative Radiation Therapy for Early Breast Cancer: A Substudy of the TARGIT-A Trial. Int. J. Radiat. Oncol. 2016, 96, 55–64. [Google Scholar] [CrossRef] [PubMed]

| Predictor | Estimate | SE | z-Value | p-Value | 95% CI (LB–UB) |
|---|---|---|---|---|---|
| Intercept | 77.49 | 3.41 | 22.73 | <0.001 | 70.81–84.17 |
| Time (linear) | 7.30 | 3.43 | 2.13 | 0.033 | 0.58–14.02 |
| Time2 (quadratic) | −5.55 | 2.59 | −2.14 | 0.032 | −10.63–−0.47 |
| Age > 60 yr | 5.35 | 5.01 | 1.07 | 0.286 | −4.47–15.17 |
| Age 45–60 yr | 1.42 | 2.68 | 0.53 | 0.596 | −3.83–6.66 |
| Age Unknown | −4.20 | 5.75 | −0.73 | 0.465 | −15.46–7.06 |
| Mastectomy Alone | −6.21 | 0.87 | −7.11 | <0.001 | −7.92–−4.50 |
| Mastectomy with IBR | −4.57 | 2.06 | −2.22 | 0.027 | −8.61–−0.53 |
| Other surgery | −1.29 | 3.45 | −0.37 | 0.709 | −8.06–5.48 |
| Time × Age > 60 yr | 19.97 | 7.48 | 2.67 | 0.008 | 5.31–34.63 |
| Time × Age 45–60 yr | 1.57 | 2.85 | 0.55 | 0.580 | −4.00–7.15 |
| Time × Age Unknown | 1.79 | 4.32 | 0.41 | 0.679 | −6.68–10.26 |
| Time2 × Age 45–60 yr | −3.34 | 2.08 | −1.61 | 0.108 | −7.41–0.74 |
| Time2 × Age Unknown | −2.54 | 4.77 | −0.53 | 0.594 | −11.89–6.81 |
| Time × Mastectomy Alone | −3.19 | 1.13 | −2.81 | 0.005 | −5.41–−0.97 |
| Time × Mastectomy with IBR | −2.14 | 2.49 | −0.86 | 0.391 | −7.02–2.75 |
| Time × Other surgery | −27.82 | 14.11 | −1.97 | 0.049 | −55.47–−0.18 |
| Time2 × Mastectomy Alone | 4.08 | 0.83 | 4.95 | <0.001 | 2.47–5.70 |
| Time2 × Mastectomy with IBR | 2.93 | 1.70 | 1.72 | 0.085 | −0.40–6.27 |
| Time2 × Other surgery | −34.72 | 15.50 | −2.24 | 0.025 | −65.10–−4.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhnevych, I.; Fadl Elseed, M.I.M.; Musa, I.M.A.; Alblooshi, J.J.S.; Smetanina, D.; Tahsin, F.; Statsenko, Y. Dynamics in Quality of Life of Breast Cancer Patients Following Surgery: Systematic Review and Meta-Analysis. Cancers 2025, 17, 3108. https://doi.org/10.3390/cancers17193108
Makhnevych I, Fadl Elseed MIM, Musa IMA, Alblooshi JJS, Smetanina D, Tahsin F, Statsenko Y. Dynamics in Quality of Life of Breast Cancer Patients Following Surgery: Systematic Review and Meta-Analysis. Cancers. 2025; 17(19):3108. https://doi.org/10.3390/cancers17193108
Chicago/Turabian StyleMakhnevych, Iryna, Mussab Ibrahim Mohamed Fadl Elseed, Ibrahim Mohamed Ahmed Musa, Jood Jasem Shaddad Alblooshi, Darya Smetanina, Faisal Tahsin, and Yauhen Statsenko. 2025. "Dynamics in Quality of Life of Breast Cancer Patients Following Surgery: Systematic Review and Meta-Analysis" Cancers 17, no. 19: 3108. https://doi.org/10.3390/cancers17193108
APA StyleMakhnevych, I., Fadl Elseed, M. I. M., Musa, I. M. A., Alblooshi, J. J. S., Smetanina, D., Tahsin, F., & Statsenko, Y. (2025). Dynamics in Quality of Life of Breast Cancer Patients Following Surgery: Systematic Review and Meta-Analysis. Cancers, 17(19), 3108. https://doi.org/10.3390/cancers17193108






