The FSIP Family: Roles in Health and Cancer
Simple Summary
Abstract
1. Introduction
2. Functions of FSIP in Cancers
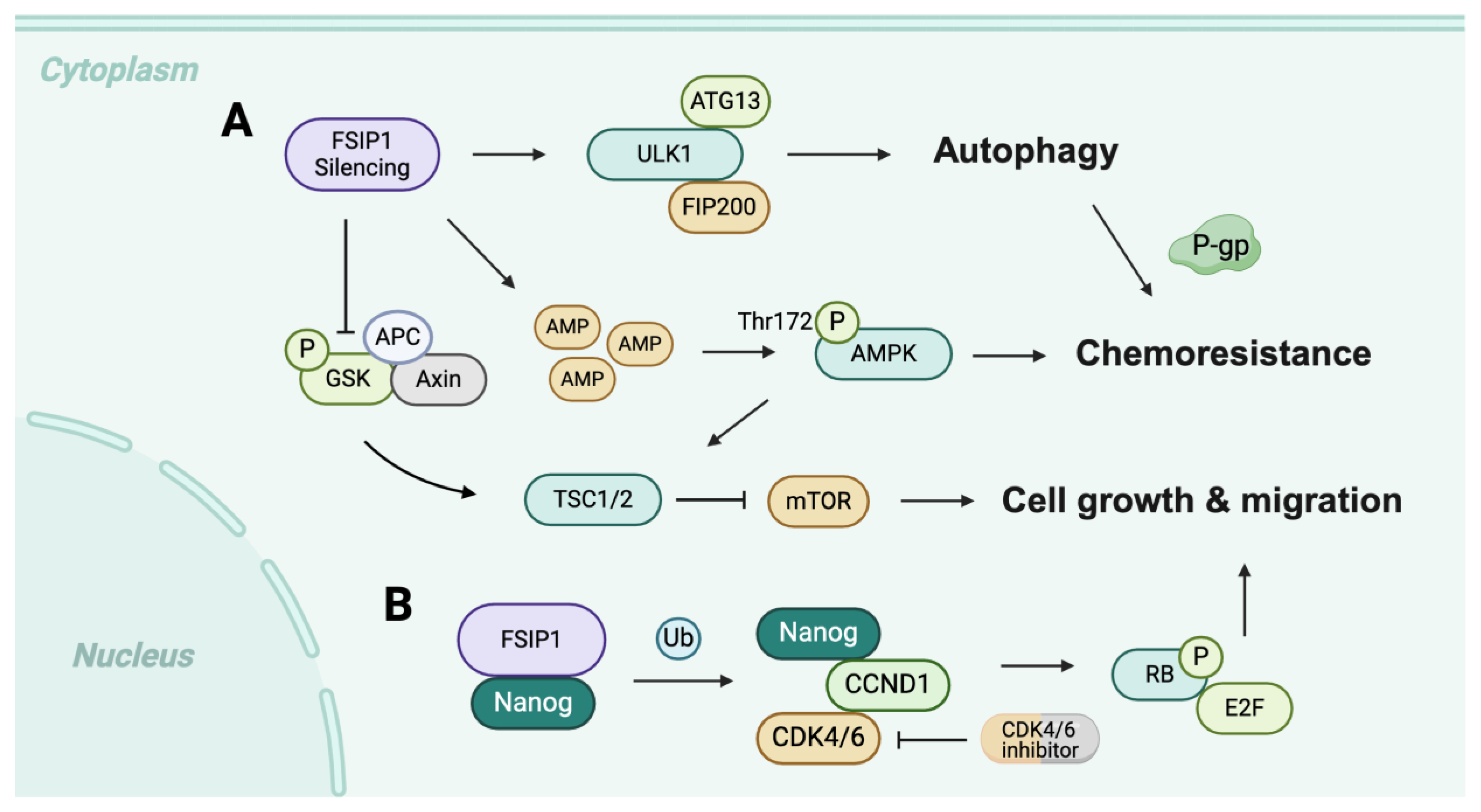
2.1. FSIP in Breast Cancer
2.2. FSIP in Testicular Germ Cell Tumor (TGCT)
2.3. FSIP in Urological Cancer
2.4. FSIP in Gastrointestinal Cancers
2.5. FSIP in Oral Mucosal Melanoma (OMM)
2.6. FSIP in Skin Cutaneous Melanoma (SKCM)
2.7. FSIP in Non-Small Cell Lung Cancer (NSCLC)
3. Opportunities and Challenges
- 1.
- CTA-based immunotherapy:
- Exploits tumor-restricted expression for selective targeting (e.g., cancer vaccines, TCR-engineered T cells, adoptive cell transfer).
- Requires clear evidence of antigen processing and presentation in tumors and sufficient epitope immunogenicity.
- Immune privilege of testes may reduce systemic toxicity, but potential effects on spermatogenesis need careful assessment.
- 2.
- Antibody-based treatment strategies:
- First, determine whether FSIPs or stable extracellular fragments are accessible on the tumor surface.
- If predominantly intracellular, conventional antibodies are limited; alternative approaches such as TCR-mimic antibodies or bispecific T cell engagers may be required.
- Success depends on robust validation of antigen presentation across patient tumors.
- 3.
- Targeted protein degradation (PROTACs/molecular glues):
- Provides a route to pharmacologically target intracellular FSIPs.
- Feasibility depends on identifying small-molecule ligands with adequate affinity and selectivity, which is challenging given the protein size and complexity.
- Structure-guided, peptide-based, or degron-mimetic strategies may serve as starting points for ligand discovery.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKAP4 | A-kinase anchoring protein 4 |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| CCND1 | Cyclin D1 |
| CDK2 | Cyclin-dependent kinase 2 |
| CDK4 | Cyclin-dependent kinase 4 |
| CRC | Colorectal cancer |
| CTA | Cancer/testis antigen |
| DUSP1 | Dual-specificity protein phosphatase 1 |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| ERBB2 | Erb-B2 receptor tyrosine kinase 2 |
| ESCC | Esophageal squamous cell carcinoma |
| FGFR | Fibroblast growth factor receptor |
| FSIP | Fibrous sheath-interacting protein |
| GC | Gastric cancer |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hormone receptor |
| IL | Interleukin |
| MAPK | Mitogen-activated protein kinase |
| MMAF | Multiple morphological abnormalities of the sperm flagella |
| MRP1 | Multidrug resistance-associated protein 1 |
| NSCLC | Non-small cell lung cancer |
| OMM | Oral mucosal melanoma |
| PARP | Poly (ADP-ribose) polymerase |
| PCBP2 | Poly(rC)-binding protein 2 |
| PKAI | Protein kinase A type I |
| PR | Progesterone receptor |
| RB1 | Retinoblastoma protein 1 |
| RCC | Renal cell carcinoma |
| SKCM | Skin cutaneous melanoma |
References
- Liu, T.; Zhang, H.; Sun, L.; Zhao, D.; Liu, P.; Yan, M.; Zaidi, N.; Izadmehr, S.; Gupta, A.; Abu-Amer, W.; et al. FSIP1 binds HER2 directly to regulate breast cancer growth and invasiveness. Proc. Natl. Acad. Sci. USA 2017, 114, 7683–7688. [Google Scholar] [CrossRef]
- Liu, C.; Sun, L.; Yang, J.; Liu, T.; Yang, Y.; Kim, S.-M.; Ou, X.; Wang, Y.; Sun, L.; Zaidi, M.; et al. FSIP1 regulates autophagy in breast cancer. Proc. Natl. Acad. Sci. USA 2018, 115, 13075–13080. [Google Scholar] [CrossRef]
- Brown, P.R.; Miki, K.; Harper, D.B.; Eddy, E.M. A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol. Reprod. 2003, 68, 2241–2248. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, B.; Geng, D.H. Clinical significance of expression of fibrous sheath interacting protein 1 in colon cancer. World. J. Gastrointest. Oncol. 2020, 12, 677–686. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, M.N.; Jin, Z.N.; Wang, D.; Sun, M.; Zhao, X.H.; Zhao, Z.W.; Lei, H.X.; Li, M.; Liu, C.G. Expression and clinicopathological significance of FSIP1 in breast cancer. Oncotarget 2015, 6, 10658–10666. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Yan, X.; Yang, S.; Bian, X.; Wang, Y.; You, Q.; Zhang, L. Elevated mRNA level indicates FSIP1 promotes EMT and gastric cancer progression by regulating fibroblasts in tumor microenvironment. Open Med. 2024, 19, 20240964. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, J.; Ren, Y.; Li, L.; He, W.; Zhang, Y.; Liu, T.; Li, Z. Over-expression of FSIP1 promotes breast cancer progression and confers resistance to docetaxel via MRP1 stabilization. Cell Death Dis. 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Gamallat, Y.; Fang, X.; Mai, H.; Liu, X.; Li, H.; Zhou, P.; Han, D.; Zheng, S.; Liao, C.; Yang, M.; et al. Bi-allelic mutation in Fsip1 impairs acrosome vesicle formation and attenuates flagellogenesis in mice. Redox Biol. 2021, 43, 101969. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y. Biology of spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 1. [Google Scholar] [CrossRef]
- Hai, Y.; Hou, J.; Liu, Y.; Liu, Y.; Yang, H.; Li, Z.; He, Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 66–75. [Google Scholar] [CrossRef]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar]
- Jan, S.Z.; Hamer, G.; Repping, S.; de Rooij, D.G.; van Pelt, A.M.M.; Vormer, T.L. Molecular control of rodent spermatogenesis. Biochim. Et Biophys. Acta-Mol. Basis Dis. 2012, 1822, 1838–1850. [Google Scholar] [CrossRef]
- Holdcraft, R.W.; Braun, R.E. Hormonal regulation of spermatogenesis. Int. J. Androl. 2004, 27, 335–342. [Google Scholar] [CrossRef]
- Fok, K.L.; Chen, H.; Ruan, Y.C.; Chan, H.C. Novel regulators of spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Xi, Q.; Zhu, L.; Jia, W.; Liu, Z.; Wang, C.; Zhou, X.; Zhang, D.; Xing, C.; Peng, X.; et al. Novel Compound Heterozygous Mutation in FSIP2 Causes Multiple Morphological Abnormalities of the Sperm Flagella (MMAF) and Male Infertility. Reprod. Sci. 2022, 29, 2697–2702. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Tang, D.; Yu, H.; Geng, H.; Zhou, Y.; Shao, Z.; Li, K.; Gao, Y.; Guo, S.; Xu, C.; et al. Novel FSIP2 Variants Induce Super-Length Mitochondrial Sheath and Asthenoteratozoospermia in Humans. Int. J. Biol. Sci. 2023, 19, 393–411. [Google Scholar] [CrossRef]
- Wang, W.L.; Tu, C.F.; Tan, Y.Q. Insight on multiple morphological abnormalities of sperm flagella in male infertility: What is new? Asian J. Androl. 2020, 22, 236–245. [Google Scholar] [CrossRef]
- Ray, P.F.; Toure, A.; Metzler-Guillemain, C.; Mitchell, M.J.; Arnoult, C.; Coutton, C. Genetic abnormalities leading to qualitative defects of sperm morphology or function. Clin. Genet. 2017, 91, 217–232. [Google Scholar]
- King, S.M.; Sale, W.S. Fifty years of microtubule sliding in cilia. Mol. Biol. Cell 2018, 29, 698–701. [Google Scholar] [CrossRef]
- Lishko, P.V.; Kirichok, Y.; Ren, D.; Navarro, B.; Chung, J.J.; Clapham, D.E. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 2012, 74, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Lehti, M.S.; Sironen, A. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017, 97, 522–536. [Google Scholar] [CrossRef]
- Vyklicka, L.; Lishko, P.V. Dissecting the signaling pathways involved in the function of sperm flagellum. Curr. Opin. Cell Biol. 2020, 63, 154–161. [Google Scholar] [CrossRef]
- Visconti, P.E.; Johnson, L.R.; Oyaski, M.; Fornes, M.; Moss, S.B.; Gerton, G.L.; Kopf, G.S. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev. Biol. 1997, 192, 351–363. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, S.; Carmona, R.; Guttmann, D.M.; Jain, V.; Freedman, G.M.; Clark, A.S.; Shabason, J.E. Use and Effectiveness of Adjuvant Endocrine Therapy for Hormone Receptor-Positive Breast Cancer in Men. JAMA Oncol. 2018, 4, e181114. [Google Scholar] [CrossRef]
- Huppert, L.A.; Gumusay, O.; Idossa, D.; Rugo, H.S. Systemic therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer. CA Cancer J. Clin. 2023, 73, 480–515. [Google Scholar] [CrossRef]
- Chapman, K.B.; Prendes, M.J.; Kidd, J.L.; Sternberg, H.; West, M.D.; Wagner, J. Elevated expression of cancer/testis antigen FSIP1 in ER-positive breast tumors. Biomark. Med. 2013, 7, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, X.; Ma, J.; Zhao, Y.; Jiang, Q.; Zhao, Z.; Li, M. FSIP1 is correlated with estrogen receptor status and poor prognosis. Mol. Carcinog. 2020, 59, 126–135. [Google Scholar]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.L.; Schillaci, R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers 2023, 15, 1987. [Google Scholar] [CrossRef]
- Essadi, I.; Benbrahim, Z.; Kaakoua, M.; Reverdy, T.; Corbaux, P.; Freyer, G. HER2-Positive Metastatic Breast Cancer: Available Treatments and Current Developments. Cancers 2023, 15, 1738. [Google Scholar] [CrossRef]
- Bai, J.W.; Qiu, S.Q.; Zhang, G.J. Molecular and functional imaging in cancer-targeted therapy: Current applications and future directions. Signal Transduct. Target. Ther. 2023, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; White, J.; Zhou, J. Cancer stem cells in TNBC. Semin. Cancer Biol. 2022, 82, 26–34. [Google Scholar] [CrossRef]
- Song, Z.; Tu, X.; Zhou, Q.; Huang, J.; Chen, Y.; Liu, J.; Lee, S.; Kim, W.; Nowsheen, S.; Luo, K.; et al. A novel UCHL(3) inhibitor, perifosine, enhances PARP inhibitor cytotoxicity through inhibition of homologous recombination-mediated DNA double strand break repair. Cell Death Dis. 2019, 10, 398. [Google Scholar]
- Min, A.; Im, S.A.; Kim, D.K.; Song, S.H.; Kim, H.J.; Lee, K.H.; Kim, T.Y.; Han, S.W.; Oh, D.Y.; Kim, T.Y.; et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2015, 17, 33. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Liu, Y.; Zhang, J.; Zhang, Y.; Dai, Y.; Liu, C. From mechanism to application: Programmed cell death pathways in nanomedicine-driven cancer therapies. Bioact. Mater. 2025, 52, 773–809. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Liu, W.; Chen, G.; Liu, J.; Ma, Q.; Hou, P.; Liang, L.; Liu, C. Polyphenol nanocomplex modulates lactate metabolic reprogramming and elicits immune responses to enhance cancer therapeutic effect. Drug Resist. Updates 2024, 73, 101060. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, B.; Xie, L.; Sang, W.; Tian, H.; Li, J.; Wang, G.; Dai, Y. Metal-Phenolic Network-Enabled Lactic Acid Consumption Reverses Immunosuppressive Tumor Microenvironment for Sonodynamic Therapy. ACS Nano 2021, 15, 16934–16945. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, B.; Liu, Y.; Zhang, K.; Wei, Z.; Dai, Y.; Liu, C. Advances in Nanomedicine for Targeting Cancer Stem Cells and Overcoming Therapeutic Resistance. ACS Nano 2025, 19, 30720–30757. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, T.; Huang, X.; Wu, P.; Shen, L.; Yang, Y.; Wan, W.; Sun, S.; Zhang, Z. Ultrasound-mediated nanomaterials for the treatment of inflammatory diseases. Ultrason. Sonochem. 2025, 114, 107270. [Google Scholar] [CrossRef]
- Mackeh, R.; Perdiz, D.; Lorin, S.; Codogno, P.; Pous, C. Autophagy and microtubules—New story, old players. J. Cell Sci. 2013, 126, 1071–1080. [Google Scholar] [CrossRef]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.Y.; Kho, D.; Reiners, J.J., Jr.; Wu, G.S. Role for DUSP1 (dual-specificity protein phosphatase 1) in the regulation of autophagy. Autophagy 2016, 12, 1791–1803. [Google Scholar] [CrossRef]
- Gao, C.; Cao, W.; Bao, L.; Zuo, W.; Xie, G.; Cai, T.; Fu, W.; Zhang, J.; Wu, W.; Zhang, X.; et al. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol. 2010, 12, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, B.; Cao, W.; Gao, C.; Qi, Z.; Ning, Y.; Chen, Y.G. The Wnt Signaling Antagonist Dapper1 Accelerates Dishevelled2 Degradation via Promoting Its Ubiquitination and Aggregate-induced Autophagy. J. Biol. Chem. 2015, 290, 12346–12354. [Google Scholar] [CrossRef]
- Chen, G.; Sun, L.; Gu, X.; Ai, L.; Yang, J.; Zhang, Z.; Hou, P.; Wang, Y.; Ou, X.; Jiang, X.; et al. FSIP1 enhances the therapeutic sensitivity to CDK4/6 inhibitors in triple-negative breast cancer patients by activating the Nanog pathway. Sci. China Life Sci. 2023, 66, 2805–2817. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16 (Suppl. S1), 1–11. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 161, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, Y.; Fan, C.; Herring, L.E.; Liu, J.; Locasale, J.W.; Takada, M.; Zhou, J.; Zurlo, G.; Hu, L.; et al. Identification of BBOX1 as a Therapeutic Target in Triple-Negative Breast Cancer. Cancer Discov. 2020, 10, 1706–1721. [Google Scholar] [CrossRef]
- Zagorac, I.; Fernandez-Gaitero, S.; Penning, R.; Post, H.; Bueno, M.J.; Mouron, S.; Manso, L.; Morente, M.M.; Alonso, S.; Serra, V.; et al. In vivo phosphoproteomics reveals kinase activity profiles that predict treatment outcome in triple-negative breast cancer. Nat. Commun. 2018, 9, 3501. [Google Scholar] [CrossRef]
- Ozgun, G.; Nappi, L. Primary Mediastinal Germ Cell Tumors: A Thorough Literature Review. Biomedicines 2023, 11, 487. [Google Scholar] [CrossRef]
- Nakhaei-Rad, S.; Soleimani, Z.; Vahedi, S.; Gorjinia, Z. Testicular germ cell tumors: Genomic alternations and RAS-dependent signaling. Crit. Rev. Oncol. Hematol. 2023, 183, 103928. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Kristiansen, K.; Kapp, D.S.; Hu, R.; Preda, O.; Nogales, F.F. Epigenetic Regulation of Driver Genes in Testicular Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 4148. [Google Scholar] [CrossRef]
- Litchfield, K.; Summersgill, B.; Yost, S.; Sultana, R.; Labreche, K.; Dudakia, D.; Renwick, A.; Seal, S.; Al-Saadi, R.; Broderick, P.; et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat. Commun. 2015, 6, 5973. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Maruvada, P.; Milner, J.A. Metabolomics in biomarker discovery: Future uses for cancer prevention. Future Oncol. 2008, 4, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Andrew, A.S.; Nelson, H.H.; Li, Z.; Punshon, T.; Schned, A.; Marsit, C.J.; Morris, J.S.; Moore, J.H.; Tyler, A.L.; et al. SLC39A2 and FSIP1 polymorphisms as potential modifiers of arsenic-related bladder cancer. Hum. Genet. 2012, 131, 453–461. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, W.; Zeng, Y.; Zhang, D.; Chen, Z.; Liu, C.; Wu, B. Fibrous sheath interacting protein 1 overexpression is associated with unfavorable prognosis in bladder cancer: A potential therapeutic target. Onco Targets Ther. 2017, 10, 3949–3956. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Qiao, X.; Sun, L.; Tian, Y.; Yang, Y.; Zhao, Y.; Liu, C. FSIP2 can serve as a predictive biomarker for Clear Cell Renal Cell Carcinoma prognosis. Int. J. Med. Sci. 2020, 17, 2819–2825. [Google Scholar]
- Chen, C.; Lei, J.; Zheng, Q.; Tan, S.; Ding, K.; Yu, C. Poly(rC) binding protein 2 (PCBP2) promotes the viability of human gastric cancer cells by regulating CDK2. FEBS Open Bio 2018, 8, 764–773. [Google Scholar]
- Yan, X.; Dai, J.; Han, Y.; You, Q.; Liu, Y. FSIP1 Is Associated with Poor Prognosis and Can Be Used to Construct a Prognostic Model in Gastric Cancer. Dis. Markers 2022, 2022, 2478551. [Google Scholar] [CrossRef]
- Ding, D.; Zhong, H.; Liang, R.; Lan, T.; Zhu, X.; Huang, S.; Wang, Y.; Shao, J.; Shuai, X.; Wei, B. Multifunctional Nanodrug Mediates Synergistic Photodynamic Therapy and MDSCs-Targeting Immunotherapy of Colon Cancer. Adv. Sci. 2021, 8, e2100712. [Google Scholar]
- Jin, C.; Yu, H.; Ke, J.; Ding, P.; Yi, Y.; Jiang, X.; Duan, X.; Tang, J.; Chang, D.T.; Wu, X.; et al. Predicting treatment response from longitudinal images using multi-task deep learning. Nat. Commun. 2021, 12, 1851. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Tang, P.; Tan, C.; Pang, Q.; Chi, C.W.; Wang, Y.; Yuan, Z.; Huang, Y.C.; Chen, Y.J. Combination of 35-Gene Mutation Profile and Radiotherapy Dosimetry Predicts the Therapeutic Outcome of Definitive Chemoradiation in Patients With Esophageal Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 729418. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Khammissa, R.A.G.; Lemmer, J. A Review of the Aetiopathogenesis and Clinical and Histopathological Features of Oral Mucosal Melanoma. Sci. World J. 2017, 2017, 9189812. [Google Scholar] [CrossRef] [PubMed]
- Thuaire, A.; Nicot, R.; Boileau, M.; Raoul, G.; Descarpentries, C.; Mouawad, F.; Germain, N.; Mortier, L.; Schlund, M. Oral mucosal melanoma—A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e425–e432. [Google Scholar]
- Chen, M.; Wu, Y.; Li, W.; Zhang, X.; Chen, L.; Zheng, X.; Zuo, X.; Zhou, F.; Hong, Y.; Cheng, H.; et al. Loss-of-function variants in FSIP1 identified by targeted sequencing are associated with one particular subtype of mucosal melanoma. Gene 2020, 759, 144964. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- Miao, D.; Margolis, C.A.; Vokes, N.I.; Liu, D.; Taylor-Weiner, A.; Wankowicz, S.M.; Adeegbe, D.; Keliher, D.; Schilling, B.; Tracy, A.; et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat. Genet. 2018, 50, 1271–1281. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, R.; Liu, X.; Shi, W.; Han, Y. Elevated fibrous sheath interacting protein 1 levels are associated with poor prognosis in non-small cell lung cancer patients. Oncotarget 2017, 8, 12186–12193. [Google Scholar] [CrossRef]
- Rawe, V.Y.; Olmedo, S.B.; Benmusa, A.; Shiigi, S.M.; Chemes, H.E.; Sutovsky, P. Sperm ubiquitination in patients with dysplasia of the fibrous sheath. Hum. Reprod. 2002, 17, 2119–2127. [Google Scholar] [CrossRef]
- Hajjar, C.; Sampuda, K.M.; Boyd, L. Dual roles for ubiquitination in the processing of sperm organelles after fertilization. BMC Dev. Biol. 2014, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Nabeel-Shah, S.; Pu, S.; Lee, H.; Braunschweig, U.; Ni, Z.; Ahmed, N.; Marcon, E.; Zhong, G.; Ray, D.; et al. Regulation of alternative polyadenylation by the C2H2-zinc-finger protein Sp1. Mol. Cell 2022, 82, 3135–3150.e9. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Alam, J.; Prakash, O. NF-kappa B site-mediated negative regulation of the HIV-1 promoter by CCAAT/enhancer binding proteins in brain-derived cells. J. Mol. Neurosci. 1994, 5, 241–258. [Google Scholar] [CrossRef]
- Hauschulz, M.; Villwock, S.; Kosinski, J.; Steib, F.; Heij, L.R.; Bednarsch, J.; Knuchel-Clarke, R.; Dahl, E. Identification and Validation of Potentially Clinically Relevant CpG Regions within the Class 2 Tumor Suppressor Gene SFRP1 in Pancreatic Cancer. Cancers 2023, 15, 683. [Google Scholar] [CrossRef]
| Cancer Type | FSIP1 Expression | FSIP2 Expression | Clinical Correlations | Prognostic Implications |
|---|---|---|---|---|
| Breast cancer | Overexpressed in multiple subtypes; correlates with ER/PR status and HER2 levels | – | Promotes proliferation, migration, EMT, autophagy inhibition, drug resistance; interacts with HER2; may stabilize Nanog/CDK4/6 | High FSIP1 linked to poor survival; predictive marker for drug resistance and CDK4/6 inhibitor sensitivity |
| Testicular germ cell tumor | – | High copy number amplification in TGCT | Anchors AKAP4 to fibrous sheath; associated with germ cell biology | Potential oncogenic role; limited prognostic data |
| Bladder cancer | Upregulated in advanced stages with lymph node metastasis | – | SNP variants linked to arsenic-related susceptibility | High FSIP1 = independent predictor of poor prognosis |
| Clear cell renal cell carcinoma | – | Elevated compared to normal tissue | Associated with abnormal platelet count, distant metastasis | High FSIP2 expression linked to reduced survival |
| Gastric cancer | Elevated in tumor vs. normal tissue | – | Correlates with advanced stage, nervous system invasion, EMT marker expression | High FSIP1 = poor disease-specific and progression-free survival |
| Colorectal cancer | Strong cytoplasmic expression in tumor vs. normal | – | Correlates with T stage, N stage, histological stage | High FSIP1 = poor overall survival |
| Esophageal squamous cell carcinoma | – | Elevated in tumor | Associated with gross type, lymphatic vascular invasion, T stage | High FSIP2 = poor prognosis |
| Oral mucosal melanoma | Upregulated compared to other subtypes | – | Relative scarcity of LOF mutations suggests upregulation of FSIP1 | High FSIP1 may contribute to OMM progression |
| Skin cutaneous melanoma | – | FSIP2 mutations detected | Linked to reduced Treg infiltration, high tumor mutational burden, altered MAPK/FGFR pathways | FSIP2 mutation associated with improved immunotherapy responsiveness |
| Non-small cell lung cancer | Overexpressed vs. adjacent tissue | – | Correlates with advanced TNM stage | High FSIP1 = poor 5-year survival |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liu, Y.; Liu, C.; Qin, L.; Zaidi, M.; Liu, C. The FSIP Family: Roles in Health and Cancer. Cancers 2025, 17, 3107. https://doi.org/10.3390/cancers17193107
Zhang Z, Liu Y, Liu C, Qin L, Zaidi M, Liu C. The FSIP Family: Roles in Health and Cancer. Cancers. 2025; 17(19):3107. https://doi.org/10.3390/cancers17193107
Chicago/Turabian StyleZhang, Zhan, Yunfan Liu, Chao Liu, Lujia Qin, Mone Zaidi, and Caigang Liu. 2025. "The FSIP Family: Roles in Health and Cancer" Cancers 17, no. 19: 3107. https://doi.org/10.3390/cancers17193107
APA StyleZhang, Z., Liu, Y., Liu, C., Qin, L., Zaidi, M., & Liu, C. (2025). The FSIP Family: Roles in Health and Cancer. Cancers, 17(19), 3107. https://doi.org/10.3390/cancers17193107






