Discrepancies Between the Tennessee Nomogram and Oncotype DX: Implications for the Korean Breast Cancer Population—The BRAIN Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinicopathologic Evaluation
2.3. Model Validation and Statistical Analysis
3. Results
3.1. Patient Characteristics
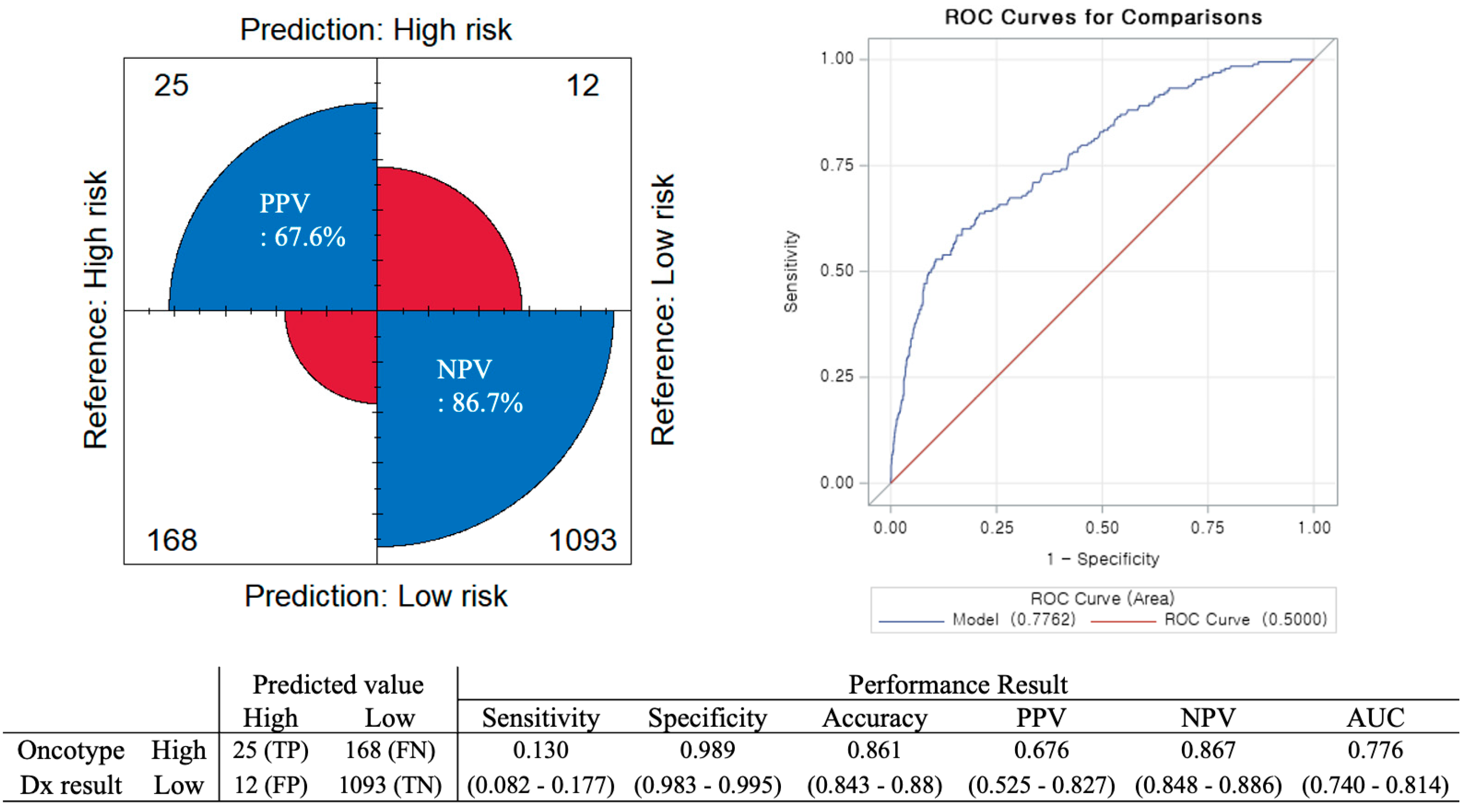
3.2. Accuracy of the Tennessee Nomogram in the Korean Population
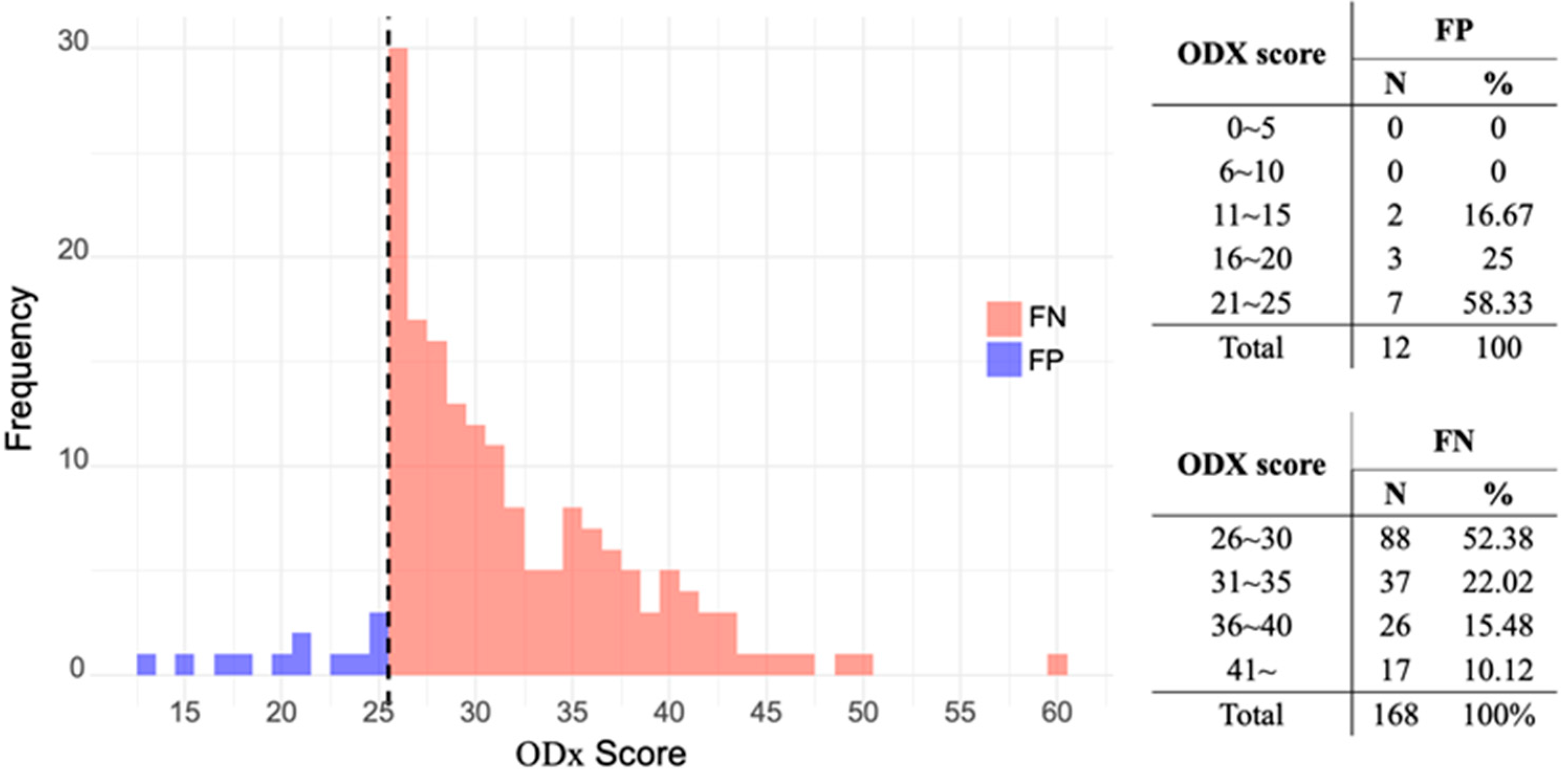
3.3. Characteristics of Discordant Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paik, S.; Tang, G.; Shak, S.; Kim, C.; Baker, J.; Kim, W.; Cronin, M.; Baehner, F.L.; Watson, D.; Bryant, J.; et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006, 24, 3726–3734. [Google Scholar] [CrossRef]
- Carlson, J.J.; Roth, J.A. The impact of the oncotype dx breast cancer assay in clinical practice: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 141, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Crager, M.R.; Tang, G.; Gray, R.J.; Stemmer, S.M.; Shak, S. Development and validation of a tool integrating the 21-gene recurrence score and clinical-pathological features to individualize prognosis and prediction of chemotherapy benefit in early breast cancer. J. Clin. Oncol. 2021, 39, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Albain, K.S.; Saphner, T.J.; Badve, S.S.; Wagner, L.I.; Kaklamani, V.G.; Keane, M.M.; Gomez, H.L.; et al. Clinical outcomes in early breast cancer with a high 21-gene recurrence score of 26 to 100 assigned to adjuvant chemotherapy plus endocrine therapy: A secondary analysis of the tailorx randomized clinical trial. JAMA Oncol. 2020, 6, 367–374. [Google Scholar] [CrossRef]
- Sparano, J.A. Tailorx: Trial assigning individualized options for treatment (rx). Clin. Breast Cancer 2006, 7, 347–350. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef]
- Cho, S.; Joo, B.; Park, M.; Ahn, S.J.; Suh, S.H.; Park, Y.W.; Ahn, S.S.; Lee, S.-K. A radiomics-based model for potentially more accurate identification of subtypes of breast cancer brain metastases. Yonsei Med. J. 2023, 64, 573–580. [Google Scholar] [CrossRef]
- Yang, G.; Kim, J.W.; Lee, I.J.; Jeong, J.; Ahn, S.G.; Bae, S.J.; Kim, J.H.; Cho, Y. Feasibility of intraoperative radiotherapy tumor bed boost in patients with breast cancer after neoadjuvant chemotherapy. Yonsei Med. J. 2024, 65, 129–136. [Google Scholar] [CrossRef]
- NSABP study confirms Oncotype DX predicts chemotherapy benefit in breast cancer patients. Oncology 2006, 20, 789–790. Available online: https://www.cancernetwork.com/view/nsabp-study-confirms-oncotype-dx-predicts-chemotherapy-benefit-breast-cancer-patients (accessed on 16 September 2025).
- Sparano, J.A.; Gray, R.J.; Ravdin, P.M.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N. Engl. J. Med. 2019, 380, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.G.; Lee, H.M.; Lee, S.A.; Jeong, J.; Lee, H.-D. Long-term survival analysis of korean breast cancer patients at a single center: Improving outcome over time. Yonsei Med. J. 2014, 55, 1187–1195. [Google Scholar] [CrossRef]
- Albanell, J.; Svedman, C.; Gligorov, J.; Holt, S.D.; Bertelli, G.; Blohmer, J.U.; Rouzier, R.; Lluch, A.; Eiermann, W. Pooled analysis of prospective european studies assessing the impact of using the 21-gene recurrence score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur. J. Cancer 2016, 66, 104–113. [Google Scholar]
- Orucevic, A.; Heidel, R.E.; Bell, J.L. Utilization and impact of 21-gene recurrence score assay for breast cancer in clinical practice across the United States: Lessons learned from the 2010 to 2012 national cancer data base analysis. Breast Cancer Res. Treat. 2016, 157, 427–435. [Google Scholar] [CrossRef]
- Iles, K.; Roberson, M.L.; Spanheimer, P.; Gallagher, K.; Ollila, D.W.; Strassle, P.D.; Downs-Canner, S. The impact of age and nodal status on variations in oncotype dx testing and adjuvant treatment. npj Breast Cancer 2022, 8, 27. [Google Scholar] [CrossRef]
- Chen, S.; Thacker, C.; Wang, S.; Young, K.A.; Hoffman, R.L.; Blansfield, J.A. Adherence disparities and utilization trends of oncotype dx assay: A national cancer database study. J. Surg. Res. 2023, 286, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, F.; Masetti, R.; Fabi, A.; Bianchi, G.; Santini, D.; Rognone, A.; Catania, G.; Angelucci, D.; Naso, G.; Giuliano, M.; et al. Pondx: Real-life utilization and decision impact of the 21-gene assay on clinical practice in italy. NPJ Breast Cancer 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-H.; Ahn, S.G.; Yoo, Y.; Park, S.-Y.; Kim, J.-H.; Jeong, J.-Y.; Park, S.; Lee, I. Prediction of a multi-gene assay (oncotype dx and mammaprint) recurrence risk group using machine learning in estrogen receptor-positive, her2-negative breast cancer—The brain study. Cancers 2024, 16, 774. [Google Scholar] [CrossRef]
- Orucevic, A.; Bell, J.L.; McNabb, A.P.; Heidel, R.E. Oncotype dx breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res. Treat. 2017, 163, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Orucevic, A.; Bell, J.L.; King, M.; McNabb, A.P.; Heidel, R.E. Nomogram update based on tailorx clinical trial results–oncotype dx breast cancer recurrence score can be predicted using clinicopathologic data. Breast 2019, 46, 116–125. [Google Scholar] [CrossRef]
- Kantor, O.; King, T.A.; Freedman, R.A.; Mayer, E.L.; Chavez-MacGregor, M.; Korde, L.A.; Sparano, J.A.; Mittendorf, E.A. Racial and ethnic disparities in locoregional recurrence among patients with hormone receptor-positive, node-negative breast cancer: A post hoc analysis of the tailorx randomized clinical trial. JAMA Surg. 2023, 158, 583–591. [Google Scholar] [CrossRef]
- Teichgraeber, D.C.; Guirguis, M.S.; Whitman, G.J. Breast cancer staging: Updates in the AJCC cancer staging manual, 8th edition, and current challenges for radiologists, from the ajr special series on cancer staging. Am. J. Roentgenol 2021, 217, 278–290. [Google Scholar] [CrossRef]
- Faghani, S.; Khosravi, B.; Zhang, K.; Moassefi, M.; Jagtap, J.M.; Nugen, F.; Vahdati, S.; Kuanar, S.P.; Rassoulinejad-Mousavi, S.M.; Singh, Y.; et al. Mitigating bias in radiology machine learning: 3. Performance metrics. Radiol. Artif. Intell. 2022, 4, e220061. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Ryu, J.M.; Kim, I.; Choi, H.J.; Nam, S.J.; Kim, S.W.; Yu, J.; Lee, S.K.; Lee, J.E. Verification of a western nomogram for predicting oncotype dx™ recurrence scores in Korean patients with breast cancer. J. Breast Cancer 2018, 21, 222–226. [Google Scholar] [CrossRef]
- Berdunov, V.; Millen, S.; Paramore, A.; Griffin, J.; Reynia, S.; Fryer, N.; Brown, R.; Longworth, L. Cost-effectiveness analysis of the oncotype dx breast recurrence score® test in node-negative early breast cancer. Clin. Outcomes Res. 2022, 14, 619–633. [Google Scholar] [CrossRef]
- Berdunov, V.; Cuyun Carter, G.; Laws, E.; Luo, R.; Russell, C.A.; Campbell, S.; Abdou, Y.; Force, J. Cost-effectiveness analysis of the oncotype dx breast recurrence score® test from a us societal perspective. Clin. Outcomes Res. 2024, 16, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Anderson, W.F.; Rosenberg, P.S.; Prat, A.; Perou, C.M.; Sherman, M.E. How many etiological subtypes of breast cancer: Two, three, four, or more? J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Choi, J.E.; Youn, H.J.; Park, H.S.; Kim, D.; Oh, S.J.; Lee, H.J.; Lee, J.; Sun, W.Y. Clinicopathological features and prognosis associated with breast cancer laterality: A nationwide study from the korean breast cancer society. Ann. Surg. Treat. Res. 2022, 103, 119–128. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (tripod): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.W.; Chung, M.S.; Choi, J.G.; Sim, S.H.; Kim, H.J.; Kim, J.E.; Lee, K.E.; Park, Y.H.; Kang, M.J.; et al. Age—and ethnic—driven molecular and clinical disparity of east asian breast cancers. BMC Med. 2024, 22. [Google Scholar] [CrossRef]
- Shaw, V.R.; Amos, C.I.; Cheng, C. Predicting chemotherapy benefit across different races in early-stage breast cancer patients using the oncotype dx score. Cancers 2023, 15, 3217. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, S.J.; Ahn, J.H.; Yoon, C.S.; Park, S. Characteristics of premenopausal breast cancer patients with a midrange 21-gene recurrence score. Ann. Surg. Treat. Res. 2025, 108, 219–230. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 1298) | ||
|---|---|---|
| N | % | |
| Age | ||
| <50 | 683 | 52.6 |
| ≥50 | 615 | 47.4 |
| T stage | ||
| T1b | 232 | 17.9 |
| T1c | 764 | 58.8 |
| T2 | 302 | 23.3 |
| N stage | ||
| N0 | 1298 | 100.0 |
| Histologic type | ||
| IDC | 1160 | 89.4 |
| ILC | 138 | 10.6 |
| Histologic grade | ||
| 1 | 379 | 29.2 |
| 2 | 812 | 62.6 |
| 3 | 107 | 8.2 |
| Progesterone receptor | ||
| Negative | 174 | 13.4 |
| Positive | 1124 | 86.6 |
| HER2 | ||
| 0 | 307 | 23.7 |
| 1+ | 600 | 46.2 |
| 2+, SISH no amplification | 391 | 30.1 |
| Ki-67 | ||
| <20% | 907 | 70.1 |
| ≥20% | 387 | 29.9 |
| Missing | 4 | |
| Recurrence risk | ||
| Low (≤25) | 1105 | 85.1 |
| High (≥25) | 193 | 14.9 |
| FN | (n = 168) | TP | (n = 25) | p | |
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age | 0.504 | ||||
| <50 | 65 | 38.7 | 12 | 48.0 | |
| ≥50 | 103 | 61.3 | 13 | 52.0 | |
| T stage | 0.397 | ||||
| T1 | 131 | 78.0 | 17 | 68.0 | |
| T2 | 37 | 22.0 | 8 | 32.0 | |
| Histologic type | >0.999 | ||||
| IDC | 156 | 92.9 | 24 | 96.0 | |
| ILC | 12 | 7.1 | 1 | 4.0 | |
| Histologic grade | <0.001 | ||||
| 1 | 17 | 10.1 | 0 | 0 | |
| 2 | 117 | 69.6 | 5 | 20.0 | |
| 3 | 34 | 20.3 | 20 | 80.0 | |
| Progesterone receptor | <0.001 | ||||
| negative | 46 | 27.4 | 24 | 96.0 | |
| Positive | 122 | 72.6 | 1 | 4.0 | |
| HER2 | 0.339 | ||||
| 0 | 40 | 23.8 | 4 | 16.0 | |
| 1+ | 66 | 39.3 | 8 | 32.0 | |
| 2+ | 62 | 36.9 | 13 | 52.0 | |
| Ki-67 | 0.017 | ||||
| <20% | 80 | 47.6 | 5 | 20.0 | |
| ≥20% | 88 | 52.4 | 20 | 80.0 |
| FN | (n = 168) | TN | (n = 1093) | p | |
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age | <0.001 | ||||
| <50 | 65 | 38.7 | 601 | 55.0 | |
| ≥50 | 103 | 61.3 | 492 | 45.0 | |
| T stage | 0.154 | ||||
| T1 | 131 | 78.0 | 902 | 82.5 | |
| T2 | 37 | 22.0 | 191 | 17.5 | |
| Histologic type | 0.102 | ||||
| IDC | 156 | 92.9 | 969 | 88.6 | |
| ILC | 12 | 7.1 | 124 | 11.3 | |
| Histologic grade | <0.001 | ||||
| 1 | 17 | 10.1 | 362 | 33.1 | |
| 2 | 117 | 69.6 | 687 | 62.9 | |
| 3 | 34 | 20.3 | 44 | 4.0 | |
| Progesterone receptor | <0.001 | ||||
| negative | 46 | 27.4 | 93 | 8.5 | |
| positive | 122 | 72.6 | 1000 | 91.5 | |
| HER2 | 0.053 | ||||
| 0 | 40 | 23.8 | 260 | 23.8 | |
| 1+ | 66 | 39.3 | 523 | 47.6 | |
| 2+ | 62 | 36.9 | 310 | 28.4 | |
| Ki-67 | <0.001 | ||||
| <20% | 80 | 47.6 | 818 | 75.1 | |
| ≥20% | 88 | 52.4 | 271 | 24.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Kim, J.H.; Ahn, J.H.; Gwon, S.H.; Lee, I.; Park, S.; Son, N.-H. Discrepancies Between the Tennessee Nomogram and Oncotype DX: Implications for the Korean Breast Cancer Population—The BRAIN Study. Cancers 2025, 17, 3083. https://doi.org/10.3390/cancers17183083
Lee SJ, Kim JH, Ahn JH, Gwon SH, Lee I, Park S, Son N-H. Discrepancies Between the Tennessee Nomogram and Oncotype DX: Implications for the Korean Breast Cancer Population—The BRAIN Study. Cancers. 2025; 17(18):3083. https://doi.org/10.3390/cancers17183083
Chicago/Turabian StyleLee, Suk Jun, Joo Heung Kim, Jee Hyun Ahn, So Hyeon Gwon, Ilkyun Lee, Seho Park, and Nak-Hoon Son. 2025. "Discrepancies Between the Tennessee Nomogram and Oncotype DX: Implications for the Korean Breast Cancer Population—The BRAIN Study" Cancers 17, no. 18: 3083. https://doi.org/10.3390/cancers17183083
APA StyleLee, S. J., Kim, J. H., Ahn, J. H., Gwon, S. H., Lee, I., Park, S., & Son, N.-H. (2025). Discrepancies Between the Tennessee Nomogram and Oncotype DX: Implications for the Korean Breast Cancer Population—The BRAIN Study. Cancers, 17(18), 3083. https://doi.org/10.3390/cancers17183083






