Cervical Cancer Treatment and Fertility: What We Know and What We Do
Simple Summary
Abstract
1. Background
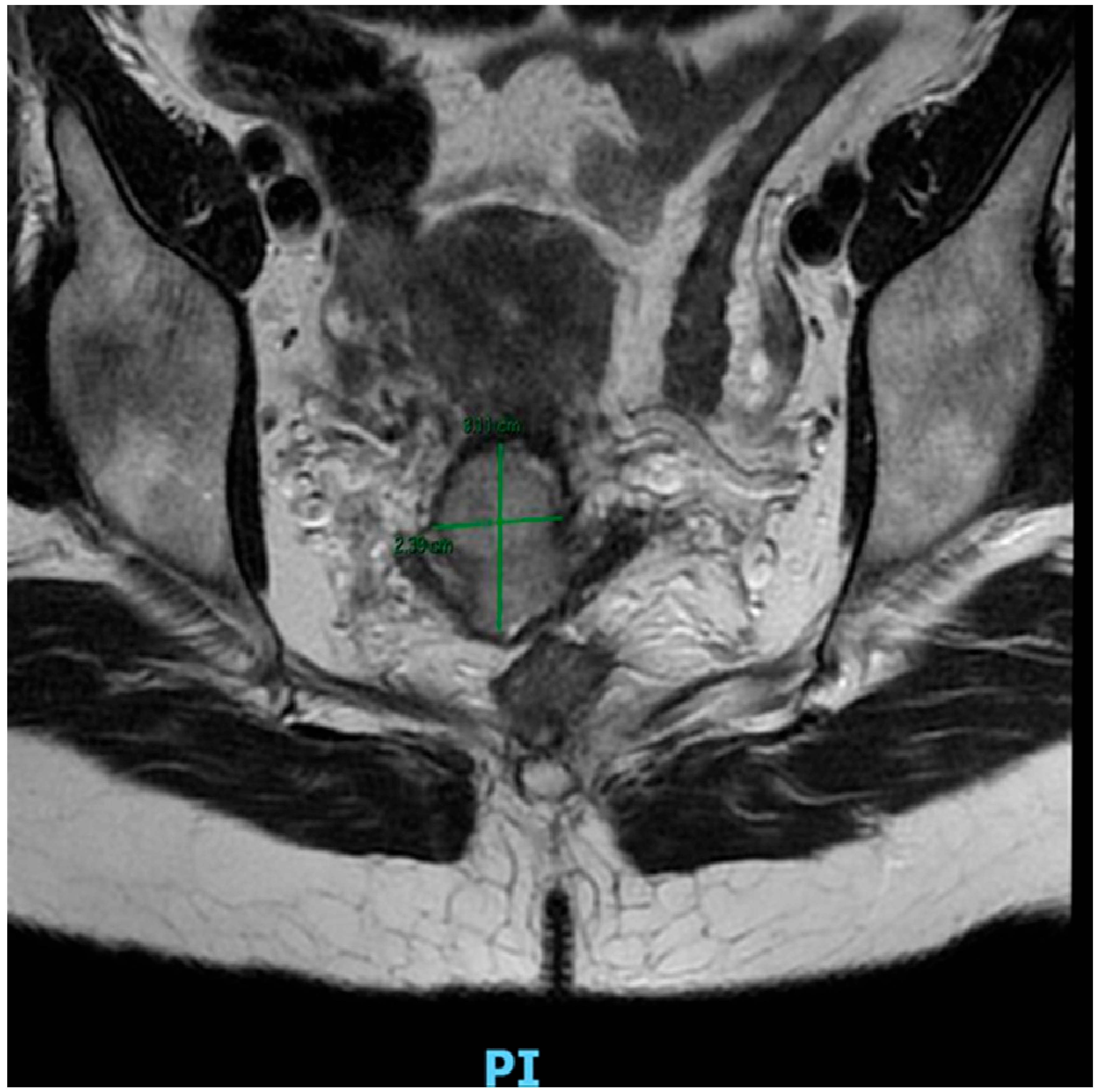
2. Cervical Cancer Treatment
- IA: Invasive carcinoma that can only be diagnosed microscopically, with a depth of stromal invasion < 5 mm.
- ○
- IA1: Stromal invasion < 3 mm in depth.
- ○
- IA2: Stromal invasion > 3 mm but <5 mm in depth.
- IB: Invasive carcinoma with stromal invasion > 5 mm (greater than stage IA), lesion still limited to the uterine cervix.
- ○
- IB1: Invasive carcinoma with stromal invasion > 5 mm and maximum tumor diameter < 2 cm.
- ○
- IB2: Invasive carcinoma with tumor diameter > 2 cm and <4 cm.
- ○
- IB3: Invasive carcinoma with tumor diameter > 4 cm.
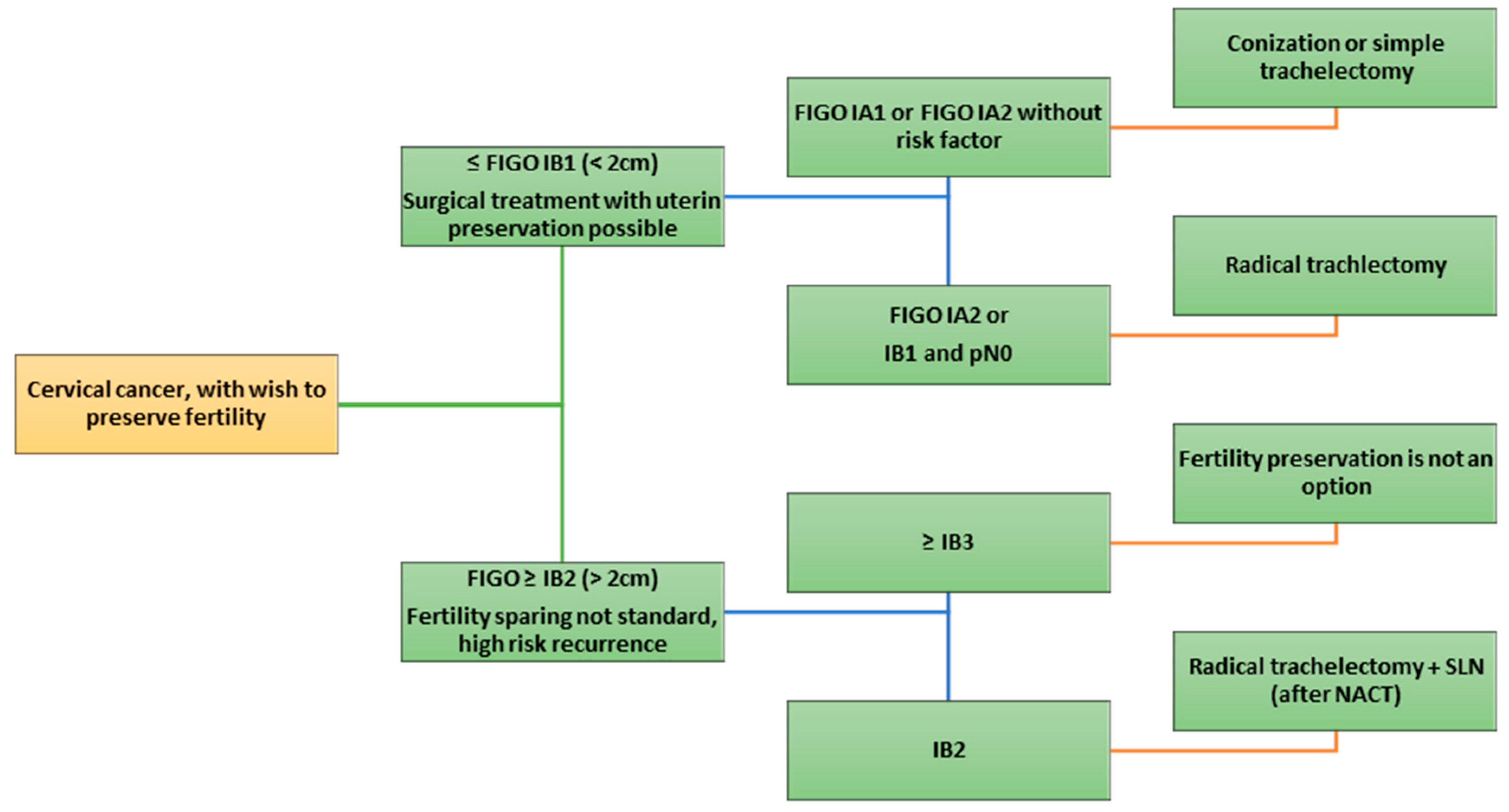
3. Surgical Techniques
3.1. Conization and Simple Trachelectomy
3.2. Vaginal Radical Trachelectomy (VRT, Dargent Procedure)
3.3. Abdominal Radical Trachelectomy (ART)
3.4. Laparoscopic and Robotic Radical Trachelectomy (LRT/RRT)
4. Neoadjuvant Chemotherapy
5. Radiation Therapy
6. Ovarian Transposition
7. Indication for Closure Treatment
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADK | Adenocarcinoma |
| ART | Abdominal Radical Trachelectomy |
| ASC | Adenosquamous Carcinoma |
| CCR | Concurrent Chemoradiotherapy |
| CRT | Chemoradiotherapy |
| EBRT | External Beam Radiation Therapy |
| ESGO | European Society of Gynaecological Oncology |
| FIGO | Fédération Internationale de Gynécologie et d’Obstétrique |
| HPV | Human Papillomavirus |
| IGBT | Image-Guided Brachytherapy |
| IMRT | Intensity-Modulated Radiation Therapy |
| LEEP | Loop Electrosurgical Excision Procedure |
| LN | Lymph Node |
| LND | Lymph Node Dissection |
| LVSI | Lymphovascular Space Invasion |
| MIS | Minimally Invasive Surgery |
| NACT | Neoadjuvant Chemotherapy |
| OT | Ovarian Transposition |
| PMA | Procreative Medical Assistance (e.g., IVF, IUI) |
| PLND | Pelvic Lymph Node Dissection |
| pCR | Pathological Complete Response |
| PR | Partial Response |
| PROM | Premature Rupture of Membranes |
| RRT | Robotic Radical Trachelectomy |
| SCC | Squamous Cell Carcinoma |
| SD | Stable Disease |
| SLN | Sentinel Lymph Node |
| SIR | Standardized Incidence Rate |
| TEP | Paclitaxel-Epirubicin-Cisplatin |
| TIP | Paclitaxel-Ifosfamide-Cisplatin |
| TLRT | Total Laparoscopic Radical Trachelectomy |
| TP | Paclitaxel-Cisplatin |
| VRT | Vaginal Radical Trachelectomy |
| 3D | Three-Dimensional |
References
- Wu, J.; Jin, Q.; Zhang, Y.; Ji, Y.; Li, J.; Liu, X.; Duan, H.; Feng, Z.; Liu, Y.; Zhang, Y.; et al. Global burden of cervical cancer: Current estimates, temporal trend and future projections based on the GLOBOCAN 2022. J. Natl. Cancer Cent. 2025, 5, 322–329. [Google Scholar] [CrossRef]
- Panorama Des Cancers En France—Édition 2023. Available online: https://www.cancer.fr/catalogue-des-publications/panorama-des-cancers-en-france-edition-2023 (accessed on 30 April 2025).
- Les Traitements Des Cancers Invasifs Du Col de L’utérus—2022 | PDF | Cancer | Spécialités médicales. Available online: https://fr.scribd.com/document/681801130/Les-Traitements-Des-Cancers-Invasifs-Du-Col-de-l-Uterus-2022 (accessed on 30 April 2025).
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Futcher, F.; Tran, P.; Ah-Kit, X.; Habib, N.; Von Theobald, P.; Birsan, A.; Boukerrou, M.; Balaya, V. Histological results of para-aortic lymph node dissection in patients with negative PET-CT for locally advanced cervical cancer in Reunion Island. J. Gynecol. Obstet. Hum. Reprod. 2025, 54, 102934. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and Cervical Cancer: A Review of Epidemiology and Screening Uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.E.L.; Zielonke, N.; Gini, A.; Anttila, A.; Segnan, N.; Vokó, Z.; Ivanuš, U.; McKee, M.; De Koning, H.J.; De Kok, I.M.C.M.; et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: A systematic review. Eur. J. Cancer 2020, 127, 207–223. [Google Scholar] [CrossRef]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.-C.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA. Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Ethics Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org Fertility preservation and reproduction in patients facing gonadotoxic therapies: An Ethics Committee opinion. Fertil. Steril. 2018, 110, 380–386. [Google Scholar] [CrossRef]
- Su, H.I.; Lacchetti, C.; Letourneau, J.; Partridge, A.H.; Qamar, R.; Quinn, G.P.; Reinecke, J.; Smith, J.F.; Tesch, M.; Wallace, W.H.; et al. Fertility Preservation in People with Cancer: ASCO Guideline Update. J. Clin. Oncol. 2025, 43, 1488–1515. [Google Scholar] [CrossRef]
- Rebolj, M.; Rimmer, J.; Denton, K.; Tidy, J.; Mathews, C.; Ellis, K.; Smith, J.; Evans, C.; Giles, T.; Frew, V.; et al. Primary cervical screening with high risk human papillomavirus testing: Observational study. BMJ 2019, 364, l240. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; Ali, H.; Boily, M.-C.; Baldo, V.; Brassard, P.; Brotherton, J.M.L.; Callander, D.; et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Wallberg, K.A.; Oktay, K. Fertility Preservation Medicine: Options for Young Adults and Children With Cancer. J. Pediatr. Hematol. Oncol. 2010, 32, 390. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health 2022, 10, e1115–e1127. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.; Covens, A. Fertility Preservation in Cervical Cancer—Treatment Strategies and Indications. Curr. Oncol. 2024, 31, 296–306. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Ferrandina, G.; Salutari, V.; Petrillo, M.; Carbone, A.; Scambia, G. Conservatively treated glassy cell carcinoma of the cervix. World J. Surg. Oncol. 2008, 6, 92. [Google Scholar] [CrossRef]
- Ho, C.-M.; Chien, T.-Y.; Huang, S.-H.; Wu, C.-J.; Shih, B.-Y.; Chang, S.-C. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol. Oncol. 2004, 93, 458–464. [Google Scholar] [CrossRef]
- Helpman, L.; Grisaru, D.; Covens, A. Early adenocarcinoma of the cervix: Is radical vaginal trachelectomy safe? Gynecol. Oncol. 2011, 123, 95–98. [Google Scholar] [CrossRef]
- Plante, M.; Gregoire, J.; Renaud, M.-C. The vaginal radical trachelectomy: An update of a series of 125 cases and 106 pregnancies. Gynecol. Oncol. 2011, 121, 290–297. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Yashar, C.M.; Bean, S.; Bradley, K.; Campos, S.M.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 660–666. [Google Scholar] [CrossRef]
- Balaya, V.; Guani, B.; Morice, P.; Querleu, D.; Fourchotte, V.; Leblanc, E.; Daraï, E.; Baron, M.; Marret, H.; Levêque, J.; et al. Long-term oncological safety of sentinel lymph node biopsy in early-stage cervical cancer: A post-hoc analysis of SENTICOL I and SENTICOL II cohorts. Gynecol. Oncol. 2022, 164, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Schmeler, K.M.; Pareja, R.; Lopez Blanco, A. ConCerv: A prospective trial of conservative surgery for low-risk early-stage cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 1317–1325. [Google Scholar] [CrossRef]
- Stegeman, M.; Louwen, M.; van der Velden, J.; ten Kate, F.J.W.; den Bakker, M.A.; Burger, C.W.; Ansink, A.C. The incidence of parametrial tumor involvement in select patients with early cervix cancer is too low to justify parametrectomy. Gynecol. Oncol. 2007, 105, 475–480. [Google Scholar] [CrossRef]
- For the Groupe de Recherche FRANCOGYN; Dabi, Y.; Willecocq, C.; Ballester, M.; Carcopino, X.; Bendifallah, S.; Ouldamer, L.; Lavoue, V.; Canlorbe, G.; Raimond, E.; et al. Identification of a low risk population for parametrial invasion in patients with early-stage cervical cancer. J. Transl. Med. 2018, 16, 163. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Q.; He, X. Retrospective analysis of the incidence and predictive factors of parametrial involvement in FIGO IB1 cervical cancer. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102145. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Renaud, M.-C.; Sebastianelli, A.; Gregoire, J. Simple vaginal trachelectomy in women with early-stage low-risk cervical cancer who wish to preserve fertility: The new standard of care? Int. J. Gynecol. Cancer 2020, 30, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Mathevet, P.; Chemali, E.; Roy, M.; Dargent, D. Long-term outcome of a randomized study comparing three techniques of conization: Cold knife, laser, and LEEP. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 106, 214–218. [Google Scholar] [CrossRef]
- Athanasiou, A.; Veroniki, A.A.; Efthimiou, O. Comparative effectiveness and risk of preterm birth of local treatments for cervical intraepithelial neoplasia and stage IA1 cervical cancer: A systematic review and network meta-analysis. Lancet Oncol. 2022, 23, 1097–1108. [Google Scholar] [CrossRef]
- Zhuang, H.; Hong, S.; Zheng, L. Effects of cervical conisation on pregnancy outcome: A meta-analysis. J. Obstet. Gynaecol. 2019, 39, 74–81. [Google Scholar] [CrossRef]
- Kuznicki, M.L.; Chambers, L.M.; Morton, M.; Son, J.; Horowitz, M.; Crean-Tate, K.K.; Hackett, L.; Rose, P.G. Fertility-Sparing Surgery for Early-Stage Cervical Cancer: A Systematic Review of the Literature. J. Minim. Invasive Gynecol. 2021, 28, 513–526.e1. [Google Scholar] [CrossRef]
- Theofanakis, C.; Koulakmanidis, A.-M.; Prodromidou, A.; Haidopoulos, D.; Rodolakis, A.; Thomakos, N. Fertility-Sparing Treatment for Young Patients with Early-Stage Cervical Cancer: A Dawn of a New Era. Front. Surg. 2022, 9, 867993. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Kanis, M.J.; Qi, G.; Li, M.; Yang, X.; Kong, B. Oncologic and obstetrical outcomes with fertility-sparing treatment of cervical cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 46580–46592. [Google Scholar] [CrossRef]
- Marchiole, P.; Benchaib, M.; Buenerd, A. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent’s operation): A comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH). Gynecol. Oncol. 2007, 106, 132–141. [Google Scholar] [CrossRef]
- Hauerberg, L.; Høgdall, C.; Loft, A.; Ottosen, C.; Bjoern, S.F.; Mosgaard, B.J.; Nedergaard, L.; Lajer, H. Vaginal Radical Trachelectomy for early stage cervical cancer. Results of the Danish National Single Center Strategy. Gynecol. Oncol. 2015, 138, 304–310. [Google Scholar] [CrossRef]
- Dargent, D.; Martin, X.; Mathevet, P. Laparoscopic assessment of the sentinel lymph node in early stage cervical cancer. Gynecol. Oncol. 2000, 79, 411–415. [Google Scholar] [CrossRef]
- Mathevet, P.; Dargent, D. Hystérectomie élargie par voie basse ou opération de Schauta-Stoeckel. EMC Chir. 2005, 2, 630–643. [Google Scholar] [CrossRef]
- Querleu, D.; Leblanc, E.; Castelain, B. Laparoscopic pelvic lymphadenectomy in the staging of early carcinoma of the cervix. Am. J. Obstet. Gynecol. 1991, 164, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Ottosen, C. Trachelectomy for cancer of the cervix: Dargent’s operation. Vaginal hysterectomy for early cancer of the cervix stage IA1 and CIN III. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Chi, D.S.; Carter, J.; Barakat, R.R.; Abu-Rustum, N.R. Initial experience with Dargent’s operation: The radical vaginal trachelectomy. Gynecol. Oncol. 2008, 108, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Zusterzeel, P.L.M.; Pol, F.J.M.; van Ham, M.; Zweemer, R.P.; Bekkers, R.L.M.; Massuger, L.F.A.G.; Verheijen, R.H.M. Vaginal Radical Trachelectomy for Early-Stage Cervical Cancer: Increased Recurrence Risk for Adenocarcinoma. Int. J. Gynecol. Cancer 2016, 26, 1293–1299. [Google Scholar] [CrossRef]
- Rizzuto, I.; MacNab, W.; Nicholson, R.; Nalam, M.; Rufford, B. Less radical surgery for women with early stage cervical cancer: Our experience on radical vaginal trachelectomy and laparoscopic pelvic lymphadenectomy. Gynecol. Oncol. Rep. 2019, 28, 65–67. [Google Scholar] [CrossRef]
- Xu, L.; Sun, F.-Q.; Wang, Z.-H. Radical trachelectomy versus radical hysterectomy for the treatment of early cervical cancer: A systematic review. Acta Obstet. Gynecol. Scand. 2011, 90, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Speiser, D.; Köhler, C.; Schneider, A.; Mangler, M. Radical vaginal trachelectomy: A fertility-preserving procedure in early cervical cancer in young women. Dtsch. Arztebl. Int. 2013, 110, 289–295. [Google Scholar] [CrossRef][Green Version]
- Plante, M.; Renaud, M.-C.; François, H. Vaginal radical trachelectomy: An oncologically safe fertility-preserving surgery. An updated series of 72 cases and review of the literature. Gynecol. Oncol. 2004, 94, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.F.; Roman, L.D.; O’Meara, A.T. Radical vaginal trachelectomy and pelvic lymphadenectomy for preservation of fertility in early cervical carcinoma. Gynecol. Oncol. 2003, 88, 419–423. [Google Scholar] [CrossRef]
- Schlaerth, J.B.; Spirtos, N.M.; Schlaerth, A.C. Radical trachelectomy and pelvic lymphadenectomy with uterine preservation in the treatment of cervical cancer. Am. J. Obs. Gynecol. 2003, 188, 29–34. [Google Scholar] [CrossRef]
- Kasuga, Y.; Hasegawa, K.; Hamuro, A.; Fukuma, Y.; Tamai, J.; Tanaka, Y.; Ikenoue, S.; Tanaka, M. Pregnancy outcomes following radical trachelectomy for early-stage cervical cancer: A retrospective observational study in the Kanto area, Japan. Int. J. Gynecol. Obstet. 2024, 164, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Prodromidou, A.; Iavazzo, C.; Fotiou, A.; Psomiadou, V.; Douligeris, A.; Vorgias, G.; Kalinoglou, N. Short- and long term outcomes after abdominal radical trachelectomy versus radical hysterectomy for early stage cervical cancer: A systematic review of the literature and meta-analysis. Arch. Gynecol. Obstet. 2019, 300, 25–31. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Chen, X.; Sun, L.; Chen, K.; Sheng, X. Surgical and Oncologic Outcomes of Radical Abdominal Trachelectomy Versus Hysterectomy for Stage IA2-IB1 Cervical Cancer. J. Minim. Invasive Gynecol. 2019, 26, 484–491. [Google Scholar] [CrossRef]
- Smith, E.S.; Moon, A.S.; O’Hanlon, R.; Leitao, M.M.; Sonoda, Y.; Abu-Rustum, N.R.; Mueller, J.J. Radical Trachelectomy for the Treatment of Early-Stage Cervical Cancer: A Systematic Review. Obstet. Gynecol. 2020, 136, 533–542. [Google Scholar] [CrossRef]
- Morice, P.; Dargent, D.; Haie-Meder, C.; Duvillard, P.; Castaigne, D. First case of a centropelvic recurrence after radical trachelectomy: Literature review and implications for the preoperative selection of patients. Gynecol. Oncol. 2004, 92, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Priore, G.D.; Ungar, L.; Smith, J.R.; Heller, P.B. Letter to the editor regarding Morice P., Dargent D., Haie-Meder C., Duvillard P., Castaigne D. Related Articles, Links First case of a centropelvic recurrence afetr radical trachelectomy: Literature review and implications for the preoperative selection of patients. Gynecol. Oncol. 2004 Mar. 92(3): 1002-5. Gynecol. Oncol. 2004, 95, 414. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.; Weekes, A.; Trappen, P. Central pelvic recurrence 7 years after radical vaginal trachelectomy. Gynecol. Oncol. 2005, 96, 854–856. [Google Scholar] [CrossRef]
- Dargent, D.; Franzosi, F.; Ansquer, Y. Extended trachelectomy relapse: Plea for patient involvement in the medical decision. Bull. Du Cancer 2002, 89, 1027–1030. [Google Scholar]
- Nezhat, C.; Roman, R.A.; Rambhatla, A.; Nezhat, F. Reproductive and oncologic outcomes after fertility-sparing surgery for early stage cervical cancer: A systematic review. Fertil. Steril. 2020, 113, 685–703. [Google Scholar] [CrossRef]
- Kohler, C.; Plaikner, A.; Siegler, K.; Hertel, H.; Hasenbein, K.; Petzel, A.; Schubert, M.; Blohmer, J.-U.; Böhmer, G.; Stolte, C.; et al. Radical vaginal trachelectomy: Long-term oncologic and fertility outcomes in patients with early cervical cancer. Int. J. Gynecol. Cancer 2024, 34, 799–805. [Google Scholar] [CrossRef]
- Smits, A.; Wolswinkel, J.T.; ten Eikelder, M.L.G.; Abu-Rustum, N.R.; Baiocchi, G.; Beltman, J.J.; Covens, A.; Cornel, K.M.C.; Falconer, H.; Fotopoulou, C.; et al. Trachelectomy and Cerclage Placement as Fertility-Sparing Surgery for Cervical Cancer—An Expert Survey. J. Pers. Med. 2025, 15, 77. [Google Scholar] [CrossRef]
- Klemm, P.; Tozzi, R.; Köhler, C. Does radical trachelectomy influence uterine blood supply? Gynecol. Oncol. 2005, 96, 283–286. [Google Scholar] [CrossRef]
- Lintner, B.; Saso, S.; Tarnai, L.; Novak, Z.; Palfalvi, L.; Del Priore, G.; Smith, J.R.; Ungar, L. Use of abdominal radical trachelectomy to treat cervical cancer greater than 2 cm in diameter. Int. J. Gynecol. Cancer 2013, 23, 1065–1070. [Google Scholar] [CrossRef]
- Căpîlna, M.E.; Loanid, N.; Scripcariu, V.; Gavrilescu, M.M.; Szabo, B. Abdominal Radical Trachelectomy: A Romanian Series. Int. J. Gynecol. Cancer 2014, 24, 615–619. [Google Scholar] [CrossRef]
- Beiner, M.E.; Hauspy, J.; Rosen, B.; Murphy, J.; Laframboise, S.; Nofech-Mozes, S.; Ismiil, N.; Rasty, G.; Khalifa, M.A.; Covens, A. Radical vaginal trachelectomy vs. radical hysterectomy for small early stage cervical cancer: A matched case-control study. Gynecol. Oncol. 2008, 110, 168–171. [Google Scholar] [CrossRef]
- Danisch, M.; Kranawetter, M.; Bartl, T.; Postl, M.; Grimm, C.; Langthaler, E.; Polterauer, S. Oncologic and Obstetric Outcomes Following Radical Abdominal Trachelectomy in Non-Low-Risk Early-Stage Cervical Cancers: A 10-Year Austrian Single-Center Experience. J. Pers. Med. 2024, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Kannisto, P.; Bossmar, T. Robot-assisted abdominal laparoscopic radical trachelectomy. Gynecol. Oncol. 2008, 111, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Vieira, M.A.; Rendón, G.J.; Munsell, M.; Echeverri, L.; Frumovitz, M.; Schmeler, K.M.; Pareja, R.; Escobar, P.F.; Reis, R.D.; Ramirez, P.T. Radical trachelectomy in early-stage cervical cancer: A comparison of laparotomy and minimally invasive surgery. Gynecol. Oncol. 2015, 138, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Nick, A.M.; Frumovitz, M.M.; Soliman, P.T.; Schmeler, K.M.; Ramirez, P.T. Fertility sparing surgery for treatment of early-stage cervical cancer: Open vs. robotic radical trachelectomy. Gynecol. Oncol. 2012, 124, 276–280. [Google Scholar] [CrossRef]
- Salvo, G.; Ramirez, P.T.; Leitao, M.M.; Cibula, D.; Wu, X.; Falconer, H.; Persson, J.; Perrotta, M.; Mosgaard, B.J.; Kucukmetin, A.; et al. Open vs. minimally invasive radical trachelectomy in early-stage cervical cancer: International Radical Trachelectomy Assessment Study. Am. J. Obstet. Gynecol. 2022, 226, 97.e1–97.e16. [Google Scholar] [CrossRef]
- Gwacham, N.I.; McKenzie, N.D.; Fitzgerald, E.R. Neoadjuvant chemotherapy followed by fertility sparing surgery in cervical cancers size 2–4 cm; emerging data and future perspectives. Gynecol. Oncol. 2021, 162, 809–815. [Google Scholar] [CrossRef]
- Buda, A.; Fossati, R.; Colombo, N. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: The SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study. J. Clin. Oncol. 2005, 23, 4137–4145. [Google Scholar] [CrossRef]
- Zhang, A.; Song, J.; Ma, Z. Combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging to predict neoadjuvant chemotherapy effect in FIGO stage IB2–IIA2 cervical cancers. La Radiol. Medica 2020, 125, 1233–1242. [Google Scholar] [CrossRef]
- Albuquerque, K.; Tumati, V.; Lea, J. A Phase II Trial of Stereotactic Ablative Radiation Therapy as a Boost for Locally Advanced Cervical Cancer. Int. J. Radiat. Oncol. 2020, 106, 464–471. [Google Scholar] [CrossRef]
- Koka, K.; Verma, A.; Dwarakanath, B.S. Technological Advancements in External Beam Radiation Therapy (EBRT): An Indispensable Tool for Cancer Treatment. Cancer Manag. Res. 2022, 14, 1421–1429. [Google Scholar] [CrossRef]
- Rigaud, B.; Anderson, B.M.; Yu, Z.H. Automatic Segmentation Using Deep Learning to Enable Online Dose Optimization During Adaptive Radiation Therapy of Cervical Cancer. Int. J. Radiat. Oncol. 2021, 109, 1096–1110. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Li, Y. Imaging-guided brachytherapy for locally advanced cervical cancer: The main process and common techniques. Am. J. Cancer Res. 2020, 10, 4165–4177. [Google Scholar]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Mayadev, J.S.; Ke, G.; Mahantshetty, U. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.W.; Sirák, I.; Xu, R. Positron Emission Tomography-Guided Bone Marrow-Sparing Radiation Therapy for Locoregionally Advanced Cervix Cancer: Final Results From the INTERTECC Phase II/III Trial. Int. J. Radiat. Oncol. 2022, 112, 169–178. [Google Scholar] [CrossRef]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wallace, W.H.B.; Thomson, A.B.; Saran, F. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat. Oncol. 2005, 62, 738–744. [Google Scholar] [CrossRef]
- Buonomo, B.; Multinu, F.; Casarin, J. Ovarian transposition in patients with cervical cancer prior to pelvic radiotherapy: A systematic review. Int. J. Gynecol. Cancer 2021, 31, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Batten, R.; Brown, D.E.M. Protection of ovaries from radiation. Lancet 1956, 270, 939–940. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.L.; Keaty, E.C.; Thompson, J.D. Conservation of ovarian tissue in the treatment of carcinoma of the cervix with radical surgery. Am. J. Obstet. Gynecol. 1958, 75, 590–600. [Google Scholar] [CrossRef]
- Moawad, N.S.; Santamaria, E.; Rhoton-Vlasak, A. Laparoscopic Ovarian Transposition Before Pelvic Cancer Treatment: Ovarian Function and Fertility Preservation. J. Minim. Invasive Gynecol. 2017, 24, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Mossa, B.; Schimberni, M.; Di Benedetto, L.; Mossa, S. Ovarian transposition in young women and fertility sparing. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3418–3425. [Google Scholar]
- Marchocki, Z.; May, T. High laparoscopic bilateral ovarian transposition to the upper abdomen prior to pelvic radiotherapy. Int. J. Gynecol. Cancer 2021, 31, 1384–1385. [Google Scholar] [CrossRef]
- Arian, S.E.; Goodman, L.; Flyckt, R.L. Ovarian transposition: A surgical option for fertility preservation. Fertil. Steril. 2017, 107, E15. [Google Scholar] [CrossRef]
- Hoekman, E.J.; Broeders, E.A.B.J.; Louwe, L.A.; Nout, R.A.; Jansen, F.W.; de Kroon, C.D. Ovarian function after ovarian transposition and additional pelvic radiotherapy: A systematic review. Eur. J. Surg. Oncol. 2019, 45, 1328–1340. [Google Scholar] [CrossRef]
- Hwang, J.H.; Yoo, H.J.; Park, S.H. Association between the location of transposed ovary and ovarian function in patients with uterine cervical cancer treated with (postoperative or primary) pelvic radiotherapy. Fertil. Steril. 2012, 97, 1387–1393. [Google Scholar] [CrossRef]
- Tessier, L.; McKechnie, T.; Lee, Y.; Park, L.J.; Gangam, N.; Eskicioglu, C. Laparoscopic ovarian transposition prior to pelvic radiation in young women with anorectal malignancies: A systematic review and meta-analysis of prevalence. Color. Dis. 2023, 25, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Duarte Portela, S.; Papadopoulou, A. Ovarian transposition and cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 75, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Gubbala, K.; Laios, A.; Gallos, I.; Pathiraja, P.; Haldar, K.; Ind, T. Outcomes of ovarian transposition in gynaecological cancers; a systematic review and meta-analysis. J. Ovarian Res. 2014, 7, 69. [Google Scholar] [CrossRef]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: A randomized trial of the Gynecologic Oncology Group☆. Gynecol. Oncol. 2003, 89, 343–353. [Google Scholar] [CrossRef]
- Lavoué, V.; Voguet, L.; Bertel, C.; Mesbah, H.; Williaume, D.; Laguerre, B.; Porée, P.; Foucher, F.; Montpetit, E.; Leblanc, M.; et al. Place de la chirurgie avant et après radiochimiothérapie des cancers du col localement évolués: À propos de 102 cas. J. Gynécologie Obs. Biol. Reprod. 2011, 40, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Rouanet, P.; Rey, A.; Romestaing, P.; Houvenaeghel, G.; Boulanger, J.C.; Leveque, J.; Cowen, D.; Mathevet, P.; Malhaire, J.P.; et al. Results of the GYNECO 02 Study, an FNCLCC Phase III Trial Comparing Hysterectomy with No Hysterectomy in Patients with a (Clinical and Radiological) Complete Response After Chemoradiation Therapy for Stage IB2 or II Cervical Cancer. Oncologist 2012, 17, 64–71. [Google Scholar] [CrossRef]
- Azria, E.; Morice, P.; Haie-Meder, C.; Thoury, A.; Pautier, P.; Lhomme, C.; Duvillard, P.; Castaigne, D. Results of Hysterectomy in Patients With Bulky Residual Disease at the End of Chemoradiotherapy for Stage IB2/II Cervical Carcinoma. Ann. Surg. Oncol. 2005, 12, 332–337. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Lelievre, L.; Buttarelli, M.; Jacquemier, J.; Carcopino, X.; Viens, P.; Gonzague-Casabianca, L. Contribution of surgery in patients with bulky residual disease after chemoradiation for advanced cervical carcinoma. Eur. J. Surg. Oncol. EJSO 2007, 33, 498–503. [Google Scholar] [CrossRef]
- Schorge, J.O.; Lee, K.R.; Lee, S.J.; Flynn, C.E.; Goodman, A.; Sheets, E.E. Early cervical adenocarcinoma: Selection criteria for radical surgery. Obstet. Gynecol. 1999, 94, 386–390. [Google Scholar] [CrossRef]
- Jefferis, H.; Price, N.; Jackson, S. Pregnancy following laparoscopic hysteropexy—A case series. Gynecol. Surg. 2017, 14, 16. [Google Scholar] [CrossRef]
- Covens, A.; Shaw, P.; Murphy, J.; DePetrillo, D.; Lickrish, G.; Laframboise, S.; Rosen, B. Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA-B carcinoma of the cervix? Cancer 1999, 86, 2273–2279. [Google Scholar] [CrossRef]
- Cervical Cancer Prognosis and Survival Rates—NCI 2020. Available online: https://www.cancer.gov/types/cervical/survival (accessed on 21 April 2025).
- Zhang, J.; Liu, J.; Zhu, C.; He, J.; Chen, J.; Liang, Y.; Yang, F.; Wu, X.; Ma, X. Prognostic role of vascular endothelial growth factor in cervical cancer: A meta-analysis. Oncotarget 2017, 8, 24797–24803. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.C.; Ozgul, N.; Yuce, K. Widespread recurrence 7 years after radical abdominal trachelectomy for early cervical adenocarcinoma. Case Rep. Obstet. Gynecol. 2015, 2015, 517496. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Renaud, M.-C.; Roy, M. Sentinel node evaluation in gynecologic cancer. Oncol. Williston Park N 2004, 18, 75–87; discussion 88–90, 95–96. [Google Scholar]
- Fuoco, V.; Sassano, S.; Fragomeni, S.M.; Bizzarri, N.; Arciuolo, D.; Bruno, I.; Di Giuda, D.; Collarino, A. Sentinel node biopsy in gynaecological cancers: State of art and future perspectives. Clin. Transl. Imaging 2024, 12, 403–411. [Google Scholar] [CrossRef]
| Advantages | Limitations | ||
|---|---|---|---|
| Maximal Fertility Preservation | Leaves most of the reproductive tract intact. Menstrual and reproductive functions are generally unharmed, and conception rates are high. | Restricted Indications | Only suitable for very early-stage cancers with favorable features. |
| Minimal Morbidity | Conization is typically an outpatient or short-stay procedure with quick recovery. | Need for Careful Pathologic Assessment | Margins must be clear and lymph nodes free of metastasis. Close coordination with pathology is required. |
| Low Complication Risk | Lower rates of blood loss, infection, and surgical complications. | Risk of Re-intervention | If final pathology reveals unexpected adverse features, patients may need a second surgery or adjuvant therapy. |
| Oncologic Safety in Low-Risk Cases | Outcomes are equivalent to radical surgery. | Cervical Insufficiency | Removing all or part of the cervix increases the risk of miscarriage or preterm birth. |
| Avoidance of Parametrial Resection | Spares the autonomic nerves and vascular supply that would be removed in a radical procedure and improves postoperative quality of life. | Surveillance Burden | Patients must adhere to intensive follow-up. Any sign of recurrence mandates prompt intervention, and the emotional burden of ongoing surveillance can be high. |
| Advantages | Limitations | ||
|---|---|---|---|
| Avoidance of Laparotomy | All radical resection performed through the vagina. | Technical Complexity and Expertise | Technical Complexity and Technical Stewardship: Because VRT is a technically challenging operation, it requires a significant degree of surgical experience in radical pelvic surgery in the vaginal approach. |
| Organ Preservation with Radical Oncologic Control | Patients keep potential for fertility and have regular menstrual function. Survival outcomes are similar to radical hysterectomy for appropriate tumors. | Tumor Size Limitations | The vaginal approach is typically only safe for small tumors (≤2 cm). |
| Higher Pregnancy Rates | Compared with abdominal or minimally invasive radical trachelectomy, VRT has more favorable fertility outcomes. | Need for Combined Approach | VRT needs a combined approach for lymph node dissection with laparoscopy (or robotically assisted), adding to the complexity of the procedure. |
| Lower Surgical Morbidity | Some studies demonstrated shorter operating time and reduced blood loss with VRT compared to an abdominal approach. | Conversion Risk | Intraoperative findings may lead to conversion to non-fertility-sparing procedure. |
| Better Obstetric Outcomes | All radical trachelectomy have obstetric risks, but patients undergoing VRT demonstrate less severe prematurity | Obstetric Challenges | Despite being the most fertility-friendly radical option, there is still a significant risk of miscarriage and preterm birth. Each patient is likely to need significant monitoring in pregnancy, which may require even more specialist care. |
| FIGO Stage | Tumor Characteristics (LVSI, Invasion, Size) | Fertility-Sparing Procedures | Eligibility Criteria and Surgical Notes |
|---|---|---|---|
| IA1 (≤3 mm) | LVSI: absent or present (microinvasive ≤ 3 mm) |
|
|
| IA2 (3–5 mm) | LVSI: absent or present (stromal invasion 3–5 mm) |
|
|
| IB1 (≤2 cm) | LVSI: absent or present (tumor ≤ 2 cm) |
|
|
| IB2 (2–4 cm) | Tumor ~2–4 cm |
|
|
| IB3 (>4 cm) | Tumor > 4 cm |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, N.; Idoubba, S.; Futcher, F.; Pieri, E.; Schettini, G.; Giorgi, M.; Negre, R.R.; Gabriele, C. Cervical Cancer Treatment and Fertility: What We Know and What We Do. Cancers 2025, 17, 3057. https://doi.org/10.3390/cancers17183057
Habib N, Idoubba S, Futcher F, Pieri E, Schettini G, Giorgi M, Negre RR, Gabriele C. Cervical Cancer Treatment and Fertility: What We Know and What We Do. Cancers. 2025; 17(18):3057. https://doi.org/10.3390/cancers17183057
Chicago/Turabian StyleHabib, Nassir, Salwa Idoubba, Francoise Futcher, Emilio Pieri, Giorgia Schettini, Matteo Giorgi, Ramon Rovira Negre, and Centini Gabriele. 2025. "Cervical Cancer Treatment and Fertility: What We Know and What We Do" Cancers 17, no. 18: 3057. https://doi.org/10.3390/cancers17183057
APA StyleHabib, N., Idoubba, S., Futcher, F., Pieri, E., Schettini, G., Giorgi, M., Negre, R. R., & Gabriele, C. (2025). Cervical Cancer Treatment and Fertility: What We Know and What We Do. Cancers, 17(18), 3057. https://doi.org/10.3390/cancers17183057






