Defining Standard Data Reporting in Pelvic Exenterations for Non-Rectal Cancers: A Systematic Review of Current Data Reporting
Abstract
Simple Summary
Abstract
1. Introduction
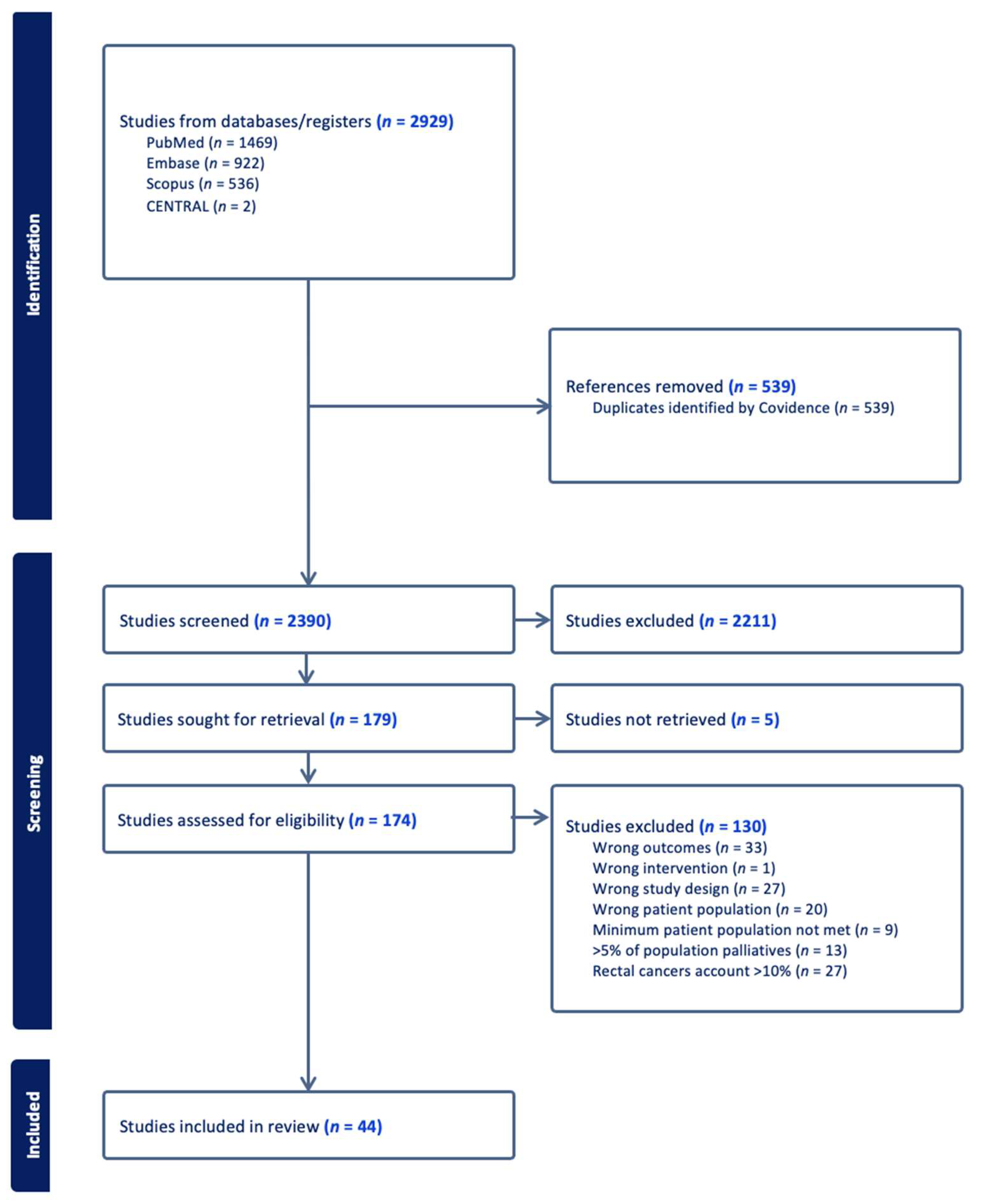
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Data Cataloging
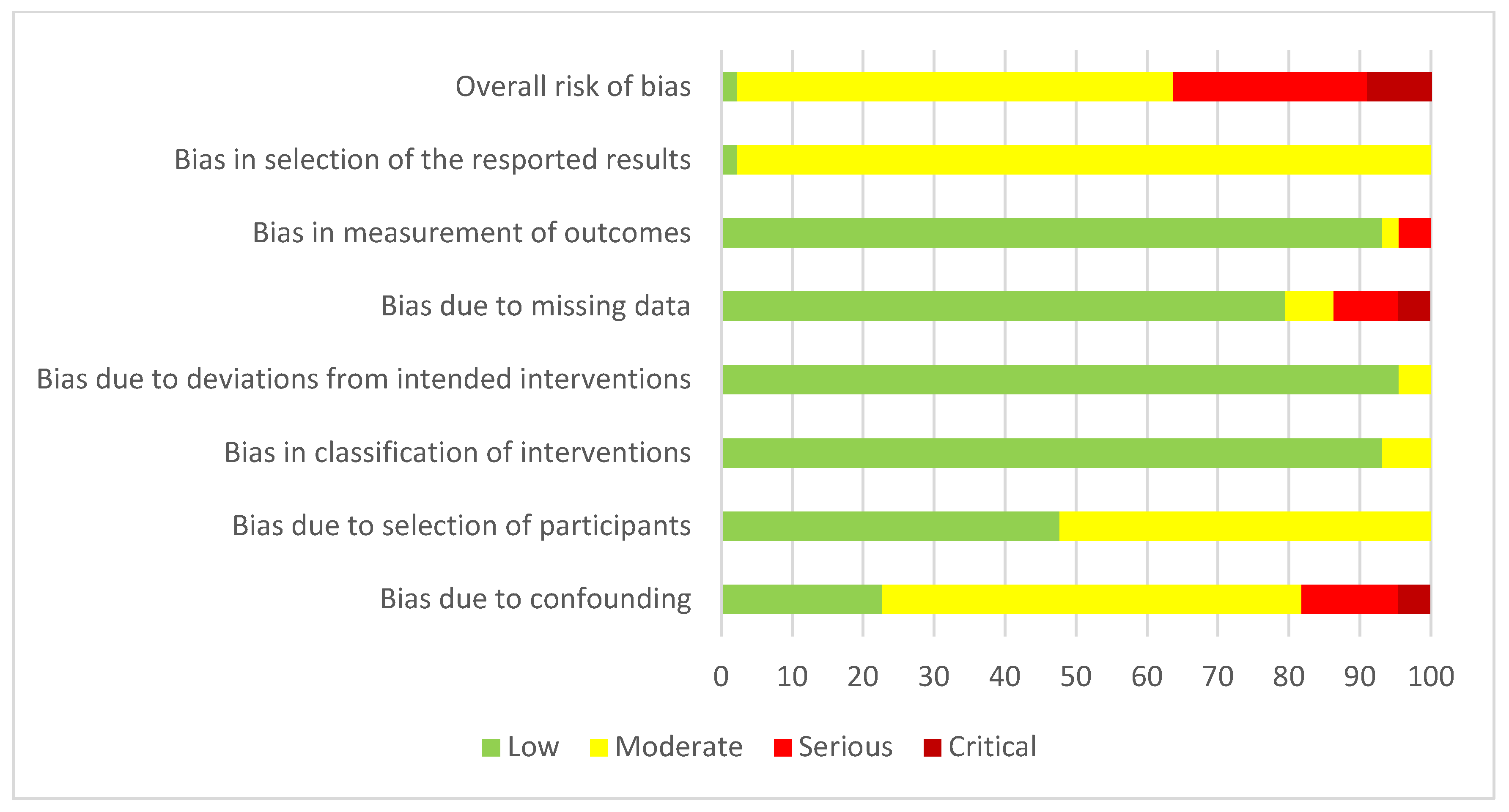
2.6. Bias Assessment
3. Results
3.1. Data Element Reporting
3.2. Study Bias
3.3. Patient Characteristics/Demographics
3.4. Pre-Operative Assessment and Anesthetic Parameters
3.5. Non-Operative Treatments
3.6. Intra-Operative/Surgical Outcomes
3.7. Pathological Outcomes
3.8. Reconstructive Outcomes
3.9. Post-Operative Outcomes
3.10. Patient-Reported and Functional Outcomes
3.11. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Full Authorship
References
- Brunschwig, A. Complete excision of pelvic viscera for advanced carcinoma.A one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948, 1, 177–183. [Google Scholar]
- Brown, K.; Solomon, M.; Koh, C. Pelvic Exenteration Surgery: The Evolution of Radical Surgical Techniques for Advanced and Recurrent Pelvic Malignancy. Dis. Colon. Rectum 2017, 60, 745–754. [Google Scholar]
- Steffens, D.; Solomon, M.J.; Young, J.M.; Koh, C.; Venchiarutti, R.L.; Lee, P.; Austin, K. Cohort study of long-term survival and quality of life following pelvic exenteration. BJS Open 2018, 2, 328–335. [Google Scholar] [CrossRef]
- PelvEx Collaborative. Contemporary Management of Locally Advanced and Recurrent Rectal Cancer: Views from the PelvEx Collaborative. Cancers 2022, 14, 1161. [Google Scholar] [CrossRef]
- Lampe, B.; Luengas-Würzinger, V.; Weitz, J.; Roth, S.; Rawert, F.; Schuler, E.; Classen-von Spee, S.; Fix, N.; Baransi, S.; Dizdar, A.; et al. Opportunities and Limitations of Pelvic Exenteration Surgery. Cancers 2021, 13, 6162. [Google Scholar] [CrossRef] [PubMed]
- Webbe, J.; Sinha, I.; Gale, C. Core Outcome Sets. Arch. Dis. Child. Educ. Pract. Ed. 2018, 103, 63–166. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Abdulrahman, G.O.; Das, N.; Chandrasekaran, T.V.; Khot, U.; Drew, P.J.; Bose, P.; Vet, J.N.; Tofazzal, N.; Roberts, S.; Lutchman Singh, K. Pelvic Exenteration for the Treatment of Locally Advanced Vulvar Cancer in South West Wales. Cancers 2022, 14, 1767. [Google Scholar] [CrossRef] [PubMed]
- Andikyan, V.; Khoury-Collado, F.; Sonoda, Y.; Gerst, S.R.; Alektiar, K.M.; Sandhu, J.S.; Bochner, B.H.; Barakat, R.R.; Boland, P.J.; Chi, D.S. Extended pelvic resections for recurrent or persistent uterine and cervical malignancies: An update on out of the box surgery. Gynecol. Oncol. 2012, 125, 404–408. [Google Scholar] [CrossRef]
- Benn, T.; Brooks, R.; Zhang, Q.; Powell, M.; Thaker, P.; Mutch, D.; Zighelboim, I. Pelvic exenteration in gynecologic oncology: A single institution study over 20 years. Gynecol. Oncol. 2011, 122, 14–188. [Google Scholar] [CrossRef]
- Berek, J.S.; Howe, C.; Lagasse, L.D.; Hacker, N.F. Pelvic exenteration for recurrent gynecologic malignancy: Survival and morbidity analysis of the 45-year experience at UCLA. Gynecol. Oncol. 2005, 99, 153–159. [Google Scholar] [CrossRef]
- Căpîlna, M.; Szabo, B.; Becsi, J.; Morariu, M.; Gheorghe, M.; Kiss, S.; Moldovan, B. Surgical complications and survival after pelvic exenteration: Our experience following 60 procedures. Eur. J. Gynaecol. Oncol. 2020, 41, 171–175. [Google Scholar] [CrossRef]
- de Gregorio, N.; de Gregorio, A.; Ebner, F.; Friedl, T.W.P.; Huober, J.; Hefty, R.; Wittau, M.; Janni, W.; Widschwendter, P. Pelvic exenteration as ultimate ratio for gynecologic cancers: Single-center analyses of 37 cases. Arch. Gynecol. Obstet. 2019, 300, 161–168. [Google Scholar] [CrossRef] [PubMed]
- de Wilt, J.H.; van Leeuwen, D.H.; Logmans, A.; Verhoef, C.; Kirkels, W.J.; Vermaas, M.; Ansink, A.C. Pelvic exenteration for primary and recurrent gynaecological malignancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 134, 243–248. [Google Scholar] [CrossRef]
- Forner, D.M.; Lampe, B. Exenteration in the treatment of Stage III/IV vulvar cancer. Gynecol. Oncol. 2012, 124, 87–91. [Google Scholar] [CrossRef]
- Gheorghe, M.; Cozlea, A.L.; Kiss, S.L.; Stanca, M.; Căpîlna, M.E.; Bacalbașa, N.; Moldovan, A.A. Primary pelvic exenteration: Our experience with 23 patients from a single institution. Exp. Ther. Med. 2021, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Glane, L.T.; Hegele, A.; Wagner, U.; Boekhoff, J. Gynecologic Oncology: Pelvic Exenteration for Advanced or Recurring Cervical Cancer—A Single Center Analysis. Cancer Diagn. Progn. 2022, 2, 308–315. [Google Scholar] [CrossRef]
- Haidopoulos, D.; Pergialiotis, V.; Aggelou, K.; Thomakos, N.; Alexakis, N.; Stamatakis, E.; Rodolakis, A. Pelvic exenteration for gynecologic malignancies: The experience of a tertiary center from Greece. Surg. Oncol. 2022, 40, 101702. [Google Scholar] [CrossRef]
- Huepenbecker, S.P.; Soliman, P.T.; Meyer, L.A.; Iniesta, M.D.; Chisholm, G.B.; Taylor, J.S.; Wilke, R.N.; Fleming, N.D. Perioperative outcomes in gynecologic pelvic exenteration before and after implementation of an enhanced recovery after surgery program. Gynecol. Oncol. 2024, 189, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Jalloul, R.J.; Nick, A.M.; Munsell, M.F.; Westin, S.N.; Ramirez, P.T.; Frumovitz, M.; Soliman, P.T. The influence of surgeon volume on outcomes after pelvic exenteration for a gynecologic cancer. J. Gynecol. Oncol. 2018, 29, e68. [Google Scholar] [CrossRef]
- Jurado, M.; Alcázar, J.L.; Martinez-Monge, R. Resectability rates of previously irradiated recurrent cervical cancer (PIRCC) treated with pelvic exenteration: Is still the clinical involvement of the pelvis wall a real contraindication? A twenty-year experience. Gynecol. Oncol. 2010, 116, 38–43. [Google Scholar] [CrossRef]
- Kanao, H.; Aoki, Y.; Omi, M.; Nomura, H.; Tanigawa, T.; Okamoto, S.; Chang, E.J.; Kurita, T.; Netsu, S.; Matoda, M.; et al. Laparoscopic pelvic exenteration and laterally extended endopelvic resection for postradiation recurrent cervical carcinoma: Technical feasibility and short-term oncologic outcome. Gynecol. Oncol. 2021, 161, 34–38. [Google Scholar] [CrossRef]
- Khoury-Collado, F.; Einstein, M.H.; Bochner, B.H.; Alektiar, K.M.; Sonoda, Y.; Abu-Rustum, N.R.; Brown, C.L.; Gardner, G.J.; Barakat, R.R.; Chi, D.S. Pelvic exenteration with curative intent for recurrent uterine malignancies. Gynecol. Oncol. 2012, 124, 42–47. [Google Scholar] [CrossRef]
- Li, L.; Ma, S.-Q.; Tan, X.-J.; Zhong, S.; Wu, M. Pelvic Exenteration for Recurrent and Persistent Cervical Cancer. Chin. Med. J. 2018, 131, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.; Roviglione, G.; Landoni, F.; Zanagnolo, V.; Peiretti, M.; Colombo, N.; Bocciolone, L.; Biffi, R.; Minig, L.; Morrow, C.P. Pelvic exenteration: Ten-year experience at the European Institute of Oncology in Milan. Gynecol. Oncol. 2009, 114, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Nedyalkov, K.; Magunska, N.; Bechev, B.; Kostov, I. Survival rate and complications after different types of pelvic exenteration for gynecological cancer. EJGO 2019, 40, 69–73. [Google Scholar]
- Martínez, A.; Filleron, T.; Vitse, L.; Querleu, D.; Mery, E.; Balague, G.; Delannes, M.; Soulie, M.; Pomel, C.; Ferron, G. Laparoscopic pelvic exenteration for gynaecological malignancy: Is there any advantage? Gynecol. Oncol. 2011, 120, 374–379. [Google Scholar] [CrossRef]
- Matsuo, K.; Mandelbaum, R.S.; Adams, C.L.; Roman, L.D.; Wright, J.D. Performance and outcome of pelvic exenteration for gynecologic malignancies: A population-based study. Gynecol. Oncol. 2019, 153, 368–375. [Google Scholar] [CrossRef]
- Matsuo, K.; Matsuzaki, S.; Mandelbaum, R.S.; Kanao, H.; Chang, E.J.; Klar, M.; Roman, L.D.; Wright, J.D. Utilization and perioperative outcome of minimally invasive pelvic exenteration in gynecologic malignancies: A national study in the United States. Gynecol. Oncol. 2021, 161, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Matsuzaki, S.; Mandelbaum, R.S.; Matsushima, K.; Klar, M.; Grubbs, B.H.; Roman, L.D.; Wright, J.D. Hospital surgical volume and perioperative mortality of pelvic exenteration for gynecologic malignancies. J. Surg. Oncol. 2019, 121, 402–409. [Google Scholar] [CrossRef]
- McLean, K.A.; Zhang, W.; Dunsmoor-Su, R.F.; Shah, C.A.; Gray, H.J.; Swensen, R.E.; Goff, B.A. Pelvic exenteration in the age of modern chemoradiation. Gynecol. Oncol. 2011, 121, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, L.; van Rangelrooij, L.; van Poelgeest, M.; van Beurden, M.; van Driel, W.; van Lonkhuijzen, L.; Mom, C.; Zaal, A. Clinical outcomes of pelvic exenteration for gynecologic malignancies. Gynecol. Oncol. 2023, 171, 114–120. [Google Scholar] [CrossRef]
- PelvEx Collaborative. Pelvic Exenteration for Advanced Nonrectal Pelvic Malignancy. Ann. Surg. 2019, 270, 899–905. [Google Scholar] [CrossRef]
- Petruzziello, A.; Kondo, W.; Hatschback, S.B.; A Guerreiro, J.; Filho, F.P.; Vendrame, C.; Luz, M.; Ribeiro, R. Surgical results of pelvic exenteration in the treatment of gynecologic cancer. World J. Surg. Oncol. 2014, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, Y.; Puntambekar, S.; Sharma, V.; Joshi, S.; Naval, S. Our experience of Laparoscopic Anterior Exenteration in Locally Advanced Cervical Carcinoma. J. Minim. Invasive Gynecol. 2015, 22, S201. [Google Scholar] [CrossRef]
- Rios-Doria, E.; Filippova, O.T.; Straubhar, A.M.; Chi, A.; Awowole, I.; Sandhu, J.; Broach, V.; Mueller, J.J.; Gardner, G.J.; Jewell, E.L.; et al. A modern-day experience with Brunschwig's operation: Outcomes associated with pelvic exenteration. Gynecol. Oncol. 2022, 167, 277–282. [Google Scholar] [CrossRef]
- Roos, E.; Degraeff, A.; Vaneijkeren, M.; Boon, T.; Heintz, A. Quality of life after pelvic exenteration. Gynecol. Oncol. 2004, 93, 610–614. [Google Scholar] [CrossRef]
- Roos, E.J.; Van Eijkeren, M.A.; Boon, T.A.; Heintz, A.P. Pelvic exenteration as treatment of recurrent or advanced gynecologic and urologic cancer. Int. J. Gynecol. Cancer 2005, 15, 624–629. [Google Scholar] [CrossRef]
- Seagle, B.-L.L.; Dayno, M.; Strohl, A.E.; Graves, S.; Nieves-Neira, W.; Shahabi, S. Survival after pelvic exenteration for uterine malignancy: A National Cancer Data Base study. Gynecol. Oncol. 2016, 143, 472–478. [Google Scholar] [CrossRef]
- Spahn, M.; Weiss, C.; Bader, P.; Frohneberg, D.; Studer, U.; Burkhard, F. The Role of Exenterative Surgery and Urinary Diversion in Persistent or Locally Recurrent Gynecological Malignancy: Complications and Survival. Urol. Int. 2010, 85, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Stanca, M.; Căpîlna, D.M.; Căpîlna, M.E. Long-Term Survival, Prognostic Factors, and Quality of Life of Patients Undergoing Pelvic Exenteration for Cervical Cancer. Cancers 2022, 14, 2346. [Google Scholar] [CrossRef] [PubMed]
- Terán-Porcayo, M.A.; Zeichner-Gancz, I.; Del-Castillo, R.A.C.G.; Beltrán-Ortega, A.; Solorza-Luna, G. Pelvic Exenteration for Recurrent or Persistent Cervical Cancer: Experience of Five Years at the National Cancer Institute in Mexico. Med. Oncol. 2006, 23, 219–224. [Google Scholar] [CrossRef]
- Tixier, H.; Fraisse, J.; Chauffert, B.; Mayer, F.; Causeret, S.; Loustalot, C.; Deville, C.; Bonnetain, F.; Sagot, P.; Douvier, S.; et al. Evaluation of pelvic posterior exenteration in the management of advanced-stage ovarian cancer. Arch. Gynecol. Obstet. 2009, 281, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, L.; Casarin, J.; Mara, K.; Weaver, A.; Multinu, F.; Glaser, G.; Cliby, W.; Scambia, G.; Mariani, A.; Kumar, A. Prediction of short-term surgical complications in women undergoing pelvic exenteration for gynecological malignancies. Gynecol. Oncol. 2019, 152, 151–156. [Google Scholar] [CrossRef]
- Tortorella, L.; Marco, C.; Loverro, M.; Carmine, C.; Persichetti, E.; Bizzarri, N.; Barbara, C.; Francesco, S.; Foschi, N.; Gallotta, V.; et al. Predictive factors of surgical complications after pelvic exenteration for gynecological malignancies: A large single-institution experience. J. Gynecol. Oncol. 2024, 35, e4. [Google Scholar] [CrossRef] [PubMed]
- Uehara, K.; Nakamura, H.; Yoshino, Y.; Arimoto, A.; Kato, T.; Yokoyama, Y.; Ebata, T.; Nagino, M. Initial experience of laparoscopic pelvic exenteration and comparison with conventional open surgery. Surg. Endosc. 2015, 30, 132–138. [Google Scholar] [CrossRef]
- Ungar, L.; Palfalvi, L.; Novak, Z. Primary pelvic exenteration in cervical cancer patients. Gynecol. Oncol. 2008, 111, S9–S12. [Google Scholar] [CrossRef]
- Van Trappen, P.; Walgraeve, M.-S.; Roels, S.; Claes, N.; De Cuypere, E.; Baekelandt, F.; Arentsen, H. Robotic-Assisted Pelvic Exenteration for Cervical Cancer: A Systematic Review and Novel Insights into Compartment-Based Imaging. J. Clin. Med. 2024, 13, 3673. [Google Scholar] [CrossRef]
- Westin, S.N.; Rallapalli, V.; Fellman, B.; Urbauer, D.L.; Pal, N.; Frumovitz, M.M.; Ramondetta, L.M.; Bodurka, D.C.; Ramirez, P.T.; Soliman, P.T. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecol. Oncol. 2014, 134, 546–551. [Google Scholar] [CrossRef]
- Yoo, H.J.; Lim, M.C.; Seo, S.-S.; Kang, S.; Yoo, C.W.; Kim, J.-Y.; Park, S.-Y. Pelvic exenteration for recurrent cervical cancer: Ten-year experience at National Cancer Center in Korea. J. Gynecol. Oncol. 2012, 23, 242–250. [Google Scholar] [CrossRef]
- Yu, J.-H.; Tong, C.-J.; Huang, Q.-D.; Ye, Y.-L.; Chen, G.; Li, H.; Wen, Y.-S.; Yang, F.; Luo, N.-B.; Xu, G.-Y.; et al. Long-term outcomes of pelvic exenterations for gynecological malignancies: A single-center retrospective cohort study. BMC Cancer 2024, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.J.; Brown, K.G.M. Extended Radical Resection: The Standard of Care for Patients with Advanced Pelvic Malignancy. Ann. Surg. Oncol. 2019, 27, 323–324. [Google Scholar] [CrossRef]
- Brown, K.G.M.; Pisaniello, J.; Ng, K.; Solomon, M.J.; Sutton, P.A.; Hatcher, S.; Steffens, D. Variation in outcome measurement and reporting in studies of pelvic exenteration for locally advanced and recurrent rectal cancer: A systematic review. Color. Dis. 2023, 26, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.G.M.; Solomon, M.J.; Koh, C.E.; Sutton, P.A.; Aguiar, S., Jr.; Bezerra, T.S.; Clouston, H.W.; Desouza, A.; Dozois, E.J.; Ersryd, A.L.; et al. Defining Benchmarks for Pelvic Exenteration Surgery: A Multicentre Analysis of Patients with Locally Advanced and Recurrent Rectal Cancer. Ann. Surg. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Publication Period | |
| 2000–2005 | 3 |
| 2006–2010 | 7 |
| 2011–2015 | 9 |
| 2016–2020 | 11 |
| 2021–2024 | 14 |
| Geographical Distribution | |
| North America | 16 |
| South America | 1 |
| Europe | 20 |
| Asia | 6 |
| International multi-center | 1 |
| Domain | Number of Outcomes |
|---|---|
| Patient Characteristics and Demographics | 9 |
| Pre-operative Assessment and Anesthetic Parameters | 8 |
| Non-operative Treatment | 9 |
| Intra-operative/Surgical | 12 |
| Pathological Outcomes | 11 |
| Reconstructive Outcomes | 10 |
| Post-Operative Outcomes | 31 |
| Patient Reported and Functional Outcomes | 11 |
| Survival Outcomes | 10 |
| Domain | Outcome | Number (%) |
|---|---|---|
| Patient Characteristics and Demographics | Age | 41 (93.2) |
| Ethnicity | 9 (20.5) | |
| Gender | 24 (54.5) | |
| Socioeconomic status | 5 (11.4) | |
| BMI | 23 (52.3) | |
| Tumor origin | 44 (100) | |
| Recurrent vs. Primary tumor | 38 (86.4) | |
| Presenting symptom | 5 (11.4) | |
| Previous pelvic surgery | 7 (15.9) | |
| Pre-operative Assessment and Anesthetic Parameters | Serum Markers | |
| Albumin level pre-op | 2 (4.5) | |
| Hemoglobin level pre-op | 2 (4.5) | |
| Creatinine level pre-op | 2 (4.5) | |
| Pre-operative Anesthetic | ||
| ASA status | 7 (15.9) | |
| ECOG | 1 (2.3) | |
| Charlson Comorbidity Index | 5 (11.4) | |
| Smoking status | 5 (11.4) | |
| Individual co-morbidities listed | 4 (9.1) | |
| Non-operative Treatment | Neo-adjuvant Treatment | |
| Neo-adjuvant Chemotherapy | 15 (34.1) | |
| Neo-adjuvant Radiotherapy | 25 (56.8) | |
| Neo-adjuvant chemotherapy + radiotherapy | 12 (27.3) | |
| Adjuvant Treatment | ||
| Adjuvant Chemotherapy | 16 (36.4) | |
| Adjuvant Radiotherapy | 13 (29.5) | |
| Adjuvant Chemotherapy + Radiotherapy | 14 (31.8) | |
| Hormone Therapy | 2 (4.5) | |
| Treatment Regimens | ||
| Radiotherapy regimen/dose | 10 (22.7) | |
| Chemotherapy Regimen | 6 (13.6) | |
| Intra-operative/Surgical | Surgical Outcomes | |
| Compartments/Exenteration Type | 44 (100) | |
| MIS; Open vs. Laparoscopic (vs robotic) | 5 (11.4) | |
| Bone resection (pelvis/sacrum) | 3 (6.8) | |
| Major nerve resection | 4 (9.1) | |
| Major vessel resection | 1 (2.3) | |
| Major muscle resection | 1 (2.3) | |
| operative time | 31 (70.5) | |
| Blood loss | 29 (65.9) | |
| Volume of transfusion intra-op | 22 (50) | |
| Intra-operative Mortality | 5 (11.4) | |
| Intra-operative Complications | 14 (31.8) | |
| Adjuncts to Operation | ||
| IORT | 8 (18.2) | |
| Pathological Outcomes | R0 resection | 29 (65.9) |
| R1 resection | 27 (61.4) | |
| R2 resection | 19 (43.2) | |
| Tumor size | 18 (40.9) | |
| Tumor Grade | 8 (18.2) | |
| Tumor Stage | 15 (34.1) | |
| FIGO Stage | 11 (25) | |
| Histological Subtype | 25 (56.8) | |
| Nodal status | 20 (45.5) | |
| Lymphovascular invasion | 6 (13.6) | |
| Perineural invasion | 3 (6.8) | |
| Reconstructive Outcomes | Flap reconstruction | 21 (47.7) |
| Flap reconstruction technique | 18 (40.9) | |
| Bladder reconstruction | 25 (56.8) | |
| Bladder reconstruction technique | 25 (56.8) | |
| Bowel reconstruction | 21 (47.7) | |
| Vaginal reconstruction | 19 (43.2) | |
| Reconstructive complications | ||
| Flap complications | 7 (15.9) | |
| Urinary conduit complications | 16 (36.4) | |
| Anastomotic leak | 12 (27.3) | |
| Bowel reconstruction complications (non-leak) | 6 (13.6) | |
| Post-Operative Outcomes | Complications | |
| Major complications (as defined by paper) | 23 (52.3) | |
| Minor complications (as defined by paper) | 12 (27.3) | |
| Re-operation | 31 (70.5) | |
| Infection | 29 (65.9) | |
| Pneumonia | 11 (25) | |
| Sepsis | 24 (54.5) | |
| Wound complications | 21 (47.7) | |
| Urinary tract infection | 17 (38.6) | |
| Abscess (abdominal or pelvic) | 26 (59.1) | |
| SBO/Ileus | 33 (75) | |
| Fistula | 30 (68.2) | |
| Bowel perforation | 9 (20.5) | |
| Bleeding/Hematoma | 10 (22.7) | |
| Thrombosed Vascular Grafts | 1 (2.3) | |
| Ulcers | 4 (9.1) | |
| Renal Complications | 20 (45.5) | |
| Neurological complications | 9 (20.5) | |
| Cardiovascular complications | 15 (34.1) | |
| Respiratory complications (non-infectious) | 7 (15.9) | |
| Stoma Complications | 12 (27.3) | |
| DVT | 20 (45.5) | |
| PE | 20 (45.5) | |
| Urethral obstruction | 9 (20.5) | |
| Hernia | 1 (2.3) | |
| Chyle leak | 1 (2.3) | |
| Shock | 3 (6.8) | |
| Psychiatric | 1 (2.3) | |
| Other: | ||
| Length of hospital stay | 32 (72.7) | |
| ICU stay / ICU Admission | 11 (25) | |
| Post-operative mortality (within 90 days) | 31 (70.5) | |
| Re-admission (within 90 days) | 6 (13.6) | |
| Patient Reported and Functional Outcomes | QoL (Using QoL Instruments) | 2 (4.5) |
| Physical wellbeing | 2 (4.5) | |
| Sexual wellbeing | 2 (4.5) | |
| Social/Role Functioning | 2 (4.5) | |
| Cognitive Functioning | 2 (4.5) | |
| Emotional Wellbeing | 2 (4.5) | |
| GI Symptom Burden | 2 (4.5) | |
| Respiratory Symptom Burden | 2 (4.5) | |
| Unspecified patient dissatisfaction | 3 (6.8) | |
| Neo-vagina Satisfaction | 2 (4.5) | |
| Body Image | 2 (4.5) | |
| Survival Outcomes | Median (OR mean) time of follow up | 23 (52.3) |
| Recurrence overall | 19 (43.2) | |
| Local recurrence | 8 (18.2) | |
| Distant recurrence | 7 (15) | |
| Overall survival | 32 (72.7) | |
| Median survival | 12 (27.3) | |
| Disease-free survival | 13 (29.5) | |
| Disease-specific survival | 2 (4.5) | |
| Progression-free survival | 7 (15.9) | |
| Time to recurrence | 9 (20.5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
PelvEx Collaborative. Defining Standard Data Reporting in Pelvic Exenterations for Non-Rectal Cancers: A Systematic Review of Current Data Reporting. Cancers 2025, 17, 3049. https://doi.org/10.3390/cancers17183049
PelvEx Collaborative. Defining Standard Data Reporting in Pelvic Exenterations for Non-Rectal Cancers: A Systematic Review of Current Data Reporting. Cancers. 2025; 17(18):3049. https://doi.org/10.3390/cancers17183049
Chicago/Turabian StylePelvEx Collaborative. 2025. "Defining Standard Data Reporting in Pelvic Exenterations for Non-Rectal Cancers: A Systematic Review of Current Data Reporting" Cancers 17, no. 18: 3049. https://doi.org/10.3390/cancers17183049
APA StylePelvEx Collaborative. (2025). Defining Standard Data Reporting in Pelvic Exenterations for Non-Rectal Cancers: A Systematic Review of Current Data Reporting. Cancers, 17(18), 3049. https://doi.org/10.3390/cancers17183049




