Evolution of Potentially Actionable Genomic Alterations in Advanced Prostate Cancer: A Real-World Analysis of Serial Circulating Tumor DNA Testing
Simple Summary
Abstract
1. Introduction
2. Methods
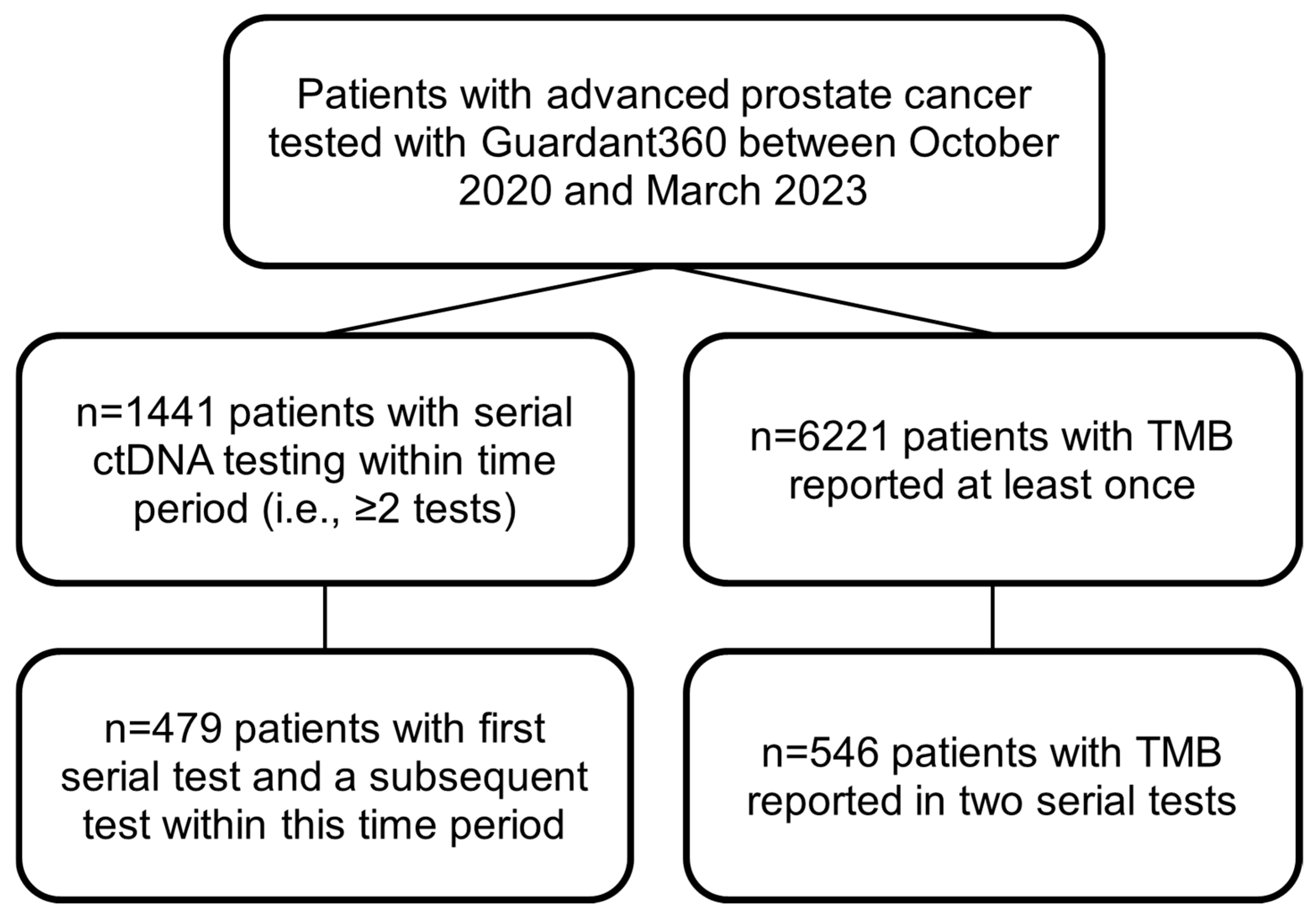
2.1. Overall Cohort
2.2. Mayo Clinic Cohort
2.3. Statistical Analysis
3. Results
3.1. Overall Cohort
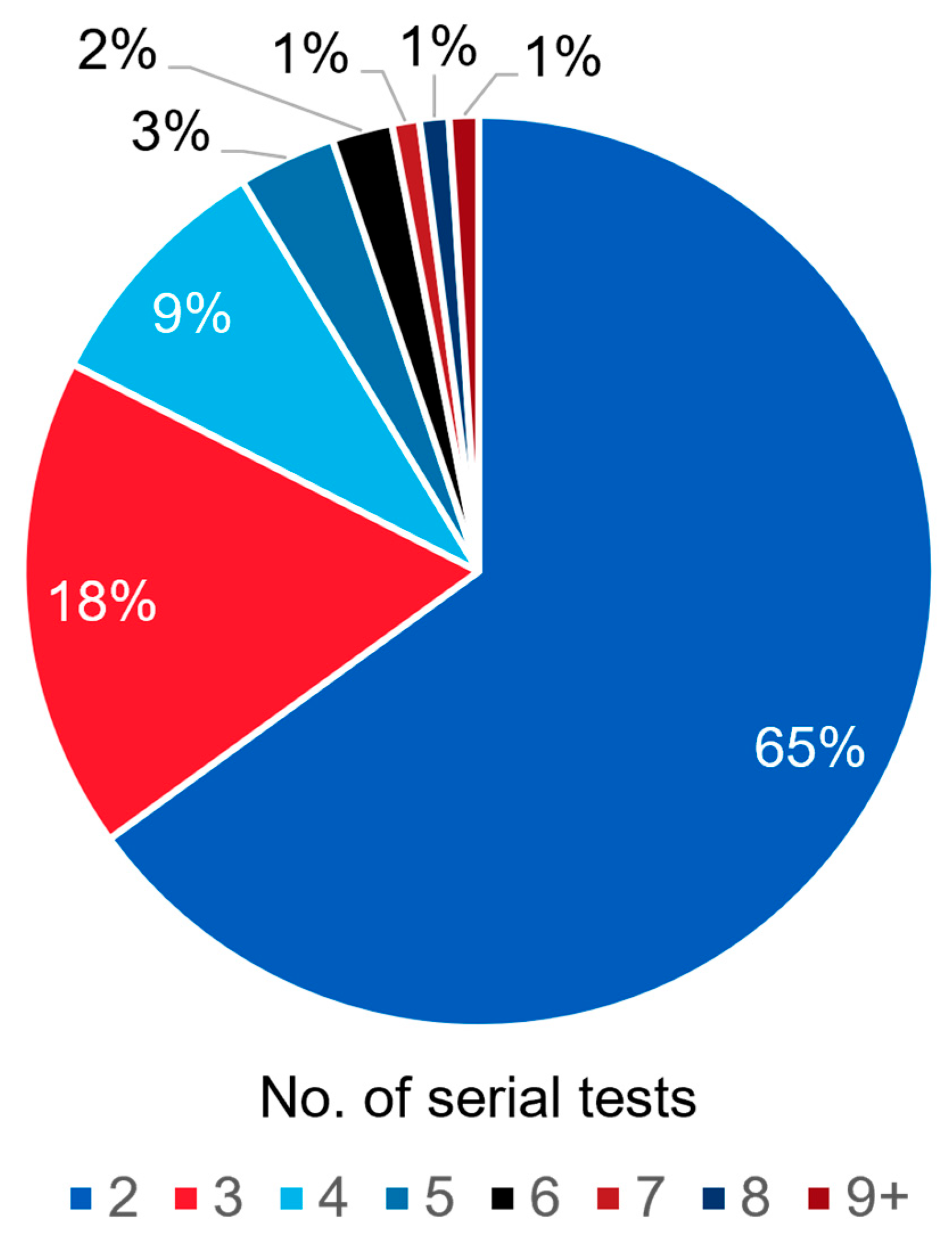
3.1.1. Frequency of Serial ctDNA Testing
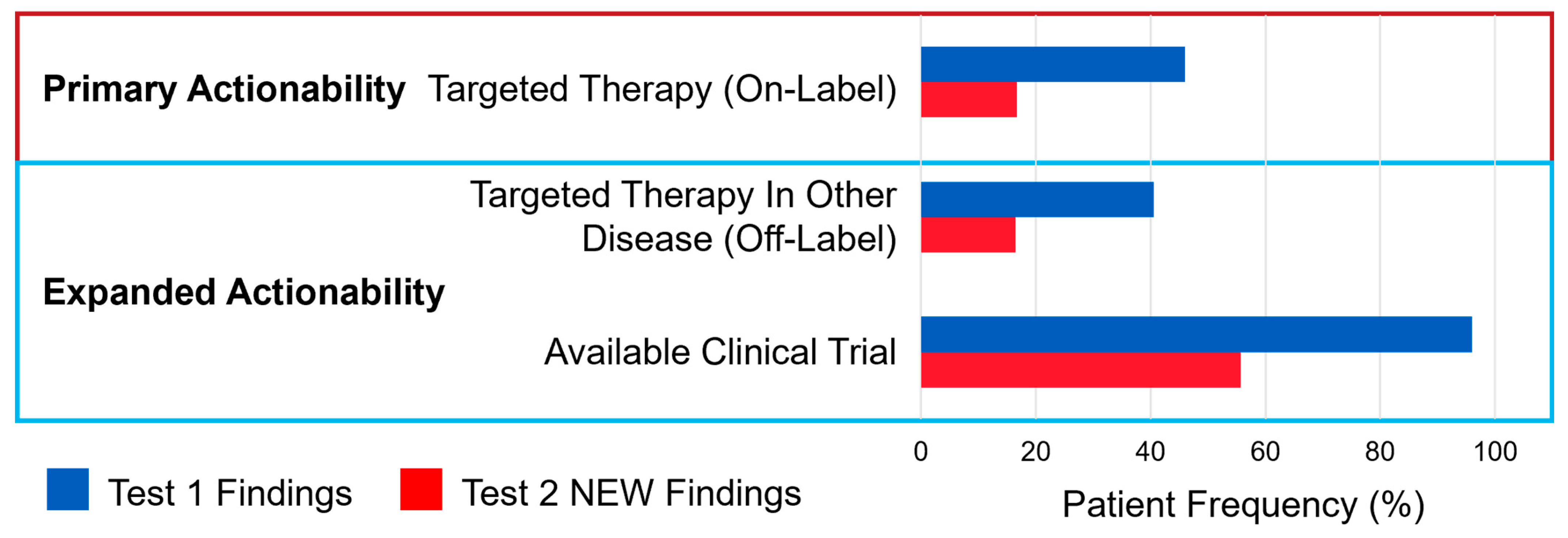
3.1.2. Actionability of Results from Serial ctDNA Testing
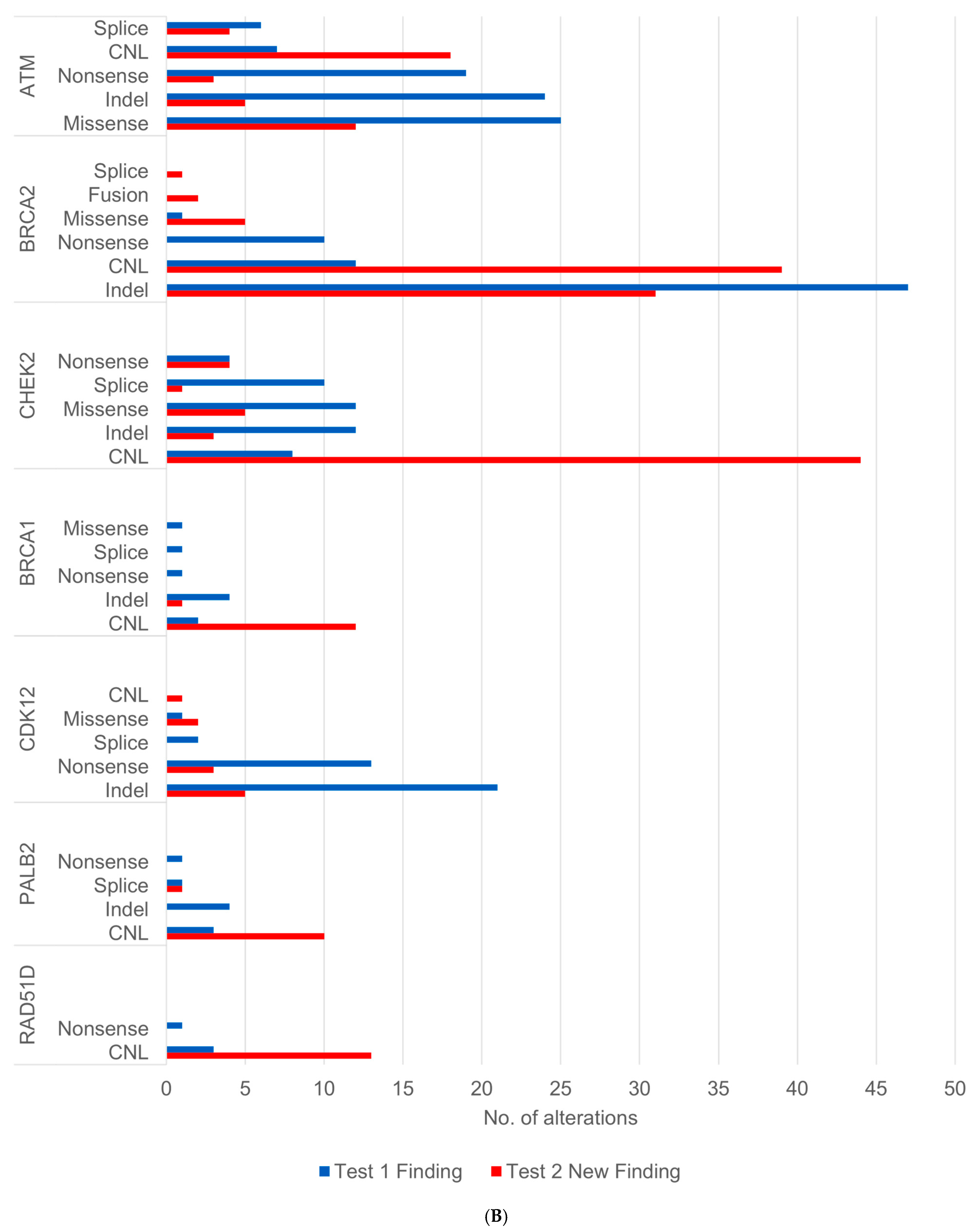
3.1.3. Acquisition of Alterations Impacting Homologous Recombination Repair (HRR) Genes
3.1.4. Changes in Tumor Mutational Burden
3.2. Outcomes for the Mayo Clinic Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| bTMB | blood-based tumor mutational burden |
| CH | clonal hematopoiesis |
| CHIP | clonal hematopoiesis of indeterminate potential |
| CNL | copy number loss |
| CONSORT | Consolidated Standards of Reporting Trials |
| DDR | DNA damage repair |
| ctDNA | circulating tumor DNA |
| HRR | homologous recombination repair |
| IQR | interquartile range |
| IRB | Institutional Review Board |
| LOF | loss of function |
| mCRPC | metastatic castration-resistant prostate cancer |
| MSI | microsatellite instability |
| MSI-H | microsatellite instability–high |
| NGS | next-generation sequencing |
| PARPi | poly (ADP-ribose) polymerase inhibitor(s) |
| PSA | prostate-specific antigen |
| TMB | tumor mutational burden |
| TMB-H | tumor mutational burden–high |
| VAF | variant allele fraction |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Abeshouse, A.; Ahn, J.; Akbani, R.; Ally, A.; Amin, S.; Andry, C.D.; Annala, M.; Aprikian, A.; Armenia, J.; Arora, A.; et al. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.D.; Werner, L.; Francini, E.; Wei, X.X.; Ha, G.; Freeman, S.S.; Rhoades, J.; Reed, S.C.; Gydush, G.; Rotem, D.; et al. Tumor fraction in cell-free DNA as a biomarker in prostate cancer. JCI Insight 2018, 3, e122109. [Google Scholar] [CrossRef]
- Si, H.; Kuziora, M.; Quinn, K.J.; Helman, E.; Ye, J.; Liu, F.; Scheuring, U.; Peters, S.; Rizvi, N.A.; Brohawn, P.Z.; et al. A Blood-based Assay for Assessment of Tumor Mutational Burden in First-line Metastatic NSCLC Treatment: Results from the MYSTIC Study. Clin. Cancer Res. 2021, 27, 1631–1640. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.-J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non–Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef]
- Drusbosky, L.; Bilen, M.A.; Azzi, G.; Barata, P.C.; Boland, P.M.; Bryce, A.H.; Chae, Y.K.; Force, J.M.; Gutierrez, M.; Kasi, P.M.; et al. Blood-based tumor mutational burden from circulating tumor DNA (ctDNA) across advanced solid malignancies using a commercially available liquid biopsy assay. J. Clin. Oncol. 2021, 39, 3040. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Bitting, R.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; Desai, N.; Dorff, T.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 3.2024. J. Natl. Compr. Canc Netw. 2024, 22, 140–150. [Google Scholar] [CrossRef]
- Yu, E.Y.; Rumble, R.B.; Agarwal, N.; Cheng, H.H.; Eggener, S.E.; Bitting, R.L.; Beltran, H.; Giri, V.N.; Spratt, D.; Mahal, B.; et al. Germline and Somatic Genomic Testing for Metastatic Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2025, 43, 748–758. [Google Scholar] [CrossRef]
- Kostos, L.; Fettke, H.; Kwan, E.M.; Azad, A.A. Utility and Clinical Application of Circulating Tumor DNA (ctDNA) in Advanced Prostate Cancer. Soc. Int. Urol. J. 2023, 4, 273–286. [Google Scholar] [CrossRef]
- Chakravarty, D.; Solit, D.B. Clinical cancer genomic profiling. Nat. Rev. Genet. 2021, 22, 483–501. [Google Scholar] [CrossRef]
- Herberts, C.; Annala, M.; Sipola, J.; Sarret, S.; Nurminen, A.; Kettunen, K.; Rintala, N.; Välimäki, N.; Lahnalampi, J.; Savytskyi, O.; et al. Deep whole-genome ctDNA chronology of treatment-resistant prostate cancer. Nature 2022, 608, 199–208. [Google Scholar] [CrossRef]
- Annala, M.; Taavitsainen, S.; Khalaf, D.J.; Vandekerkhove, G.; Beja, K.; Sipola, J.; Warner, E.W.; Herberts, C.; Wong, A.; Fu, S.; et al. Evolution of Castration-Resistant Prostate Cancer in ctDNA during Sequential Androgen Receptor Pathway Inhibition. Clin. Cancer Res. 2021, 27, 4610–4623. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Seed, G.; Bertan, C.; Rescigno, P.; Dolling, D.; Figueiredo, I.; Miranda, S.; Nava Rodrigues, D.; Gurel, B.; Clarke, M.; et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J. Clin. Investig. 2020, 130, 1743–1751. [Google Scholar] [CrossRef]
- Dasari, S.; McCarthy, M.R.; Wojcik, A.A.; Pitel, B.A.; Samaddar, A.; Tekin, B.; Whaley, R.D.; Raghunathan, A.; Hernandez, L.H.; Jimenez, R.E.; et al. Genomic attributes of prostate cancer across primary and metastatic noncastrate and castrate resistant disease states: A next generation sequencing study of 183 patients. Prostate Cancer Prostatic Dis. 2024, 28, 506–508. [Google Scholar] [CrossRef]
- Zhao, Y.; Ramesh, N.; Xu, P.; Sei, E.; Hu, M.; Bai, S.; Troncoso, P.; Aparicio, A.M.; Logothetis, C.J.; Corn, P.G.; et al. Longitudinal Profiling of Circulating Tumor DNA Reveals the Evolutionary Dynamics of Metastatic Prostate Cancer during Serial Therapy. Cancer Res. 2025, 85, 1680–1695. [Google Scholar] [CrossRef]
- Fallah, J.; Xu, J.; Weinstock, C.; Gao, X.; Heiss, B.L.; Maguire, W.F.; Chang, E.; Agrawal, S.; Tang, S.; Amiri-Kordestani, L.; et al. Efficacy of Poly(ADP-ribose) Polymerase Inhibitors by Individual Genes in Homologous Recombination Repair Gene-Mutated Metastatic Castration-Resistant Prostate Cancer: A US Food and Drug Administration Pooled Analysis. J. Clin. Oncol. 2024, 42, 1687–1698. [Google Scholar] [CrossRef]
- Saad, F.; Clarke, N.W.; Oya, M.; Shore, N.; Procopio, G.; Guedes, J.D.; Arslan, C.; Mehra, N.; Parnis, F.; Brown, E.; et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): Final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Rathkopf, D.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; Pereira de Santana Gomes, A.J.; Roubaud, G.; et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2023, 41, 3339–3351. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Young Joung, J.; Fong, P.C.C.; et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomised, placebo-controlled, phase 3 trial. Lancet 2023, 402, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Konnick, E.Q.; Schweizer, M.T.; Sokolova, A.O.; Grivas, P.; Cheng, H.H.; Klemfuss, N.M.; Beightol, M.; Yu, E.Y.; Nelson, P.S.; et al. Association of Clonal Hematopoiesis in DNA Repair Genes With Prostate Cancer Plasma Cell-free DNA Testing Interference. JAMA Oncol. 2021, 7, 107–110. [Google Scholar] [CrossRef]
- Merker, J.D.; Oxnard, G.R.; Compton, C.; Diehn, M.; Hurley, P.; Lazar, A.J.; Lindeman, N.; Lockwood, C.M.; Rai, A.J.; Schilsky, R.L.; et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. 2018, 36, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Sumiyoshi, T.; Okegawa, T.; Terada, N.; Ishitoya, S.; Miyazaki, Y.; Kojima, T.; Katayama, H.; Fujimoto, N.; Hatakeyama, S.; et al. Clinical Impact of Detecting Low-Frequency Variants in Cell-Free DNA on Treatment of Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2021, 27, 6164–6173. [Google Scholar] [CrossRef]
- Jacobs, M.T.; Mohindra, N.A.; Shantzer, L.; Chen, I.L.; Phull, H.; Mitchell, W.; Raymond, V.M.; Banks, K.C.; Nagy, R.J.; Lanman, R.B.; et al. Use of Low-Frequency Driver Mutations Detected by Cell-Free Circulating Tumor DNA to Guide Targeted Therapy in Non-Small-Cell Lung Cancer: A Multicenter Case Series. JCO Precis. Oncol. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Nassar, A.; Tambaoan, C.F.; Tukachinsky, H.; Hernandez, A.G.; Schrock, A.B.; Childress, M.; Huang, R.S.P.; Cortellini, A.; Madison, R.W.; Naqash, A.R. Association of circulating tumor DNA (ctDNA) variant allelic frequency (VAF) with outcomes on matched targeted therapies (TT) in advanced non-small cell lung cancer (aNSCLC). J. Clin. Oncol. 2025, 43, 8628. [Google Scholar] [CrossRef]
- Sisoudiya, S.D.; Tukachinsky, H.; Keller-Evans, R.B.; Schrock, A.B.; Huang, R.S.P.; Gjoerup, O.; Pishvaian, M.J.; Shroff, R.; Sokol, E.S.; Dennis, L.; et al. Tissue-based genomic profiling of 300,000 tumors highlights the detection of variants with low allele fraction. NPJ Precis. Oncol. 2025, 9, 190. [Google Scholar] [CrossRef]
- Abida, W.; Campbell, D.; Patnaik, A.; Bryce, A.H.; Shapiro, J.; Bambury, R.M.; Zhang, J.; Burke, J.M.; Castellano, D.; Font, A.; et al. Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur. Urol. 2023, 84, 321–330. [Google Scholar] [CrossRef]
- Mosalem, O.; Tan, W.; Bryce, A.H.; Dronca, R.S.; Childs, D.S.; Pagliaro, L.C.; Orme, J.J.; Kase, A.M. A real-world experience of pembrolizumab monotherapy in microsatellite instability-high and/or tumor mutation burden-high metastatic castration-resistant prostate cancer: Outcome analysis. Prostate Cancer Prostatic Dis. 2024, 28, 138–144. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.; Mazor, T.; Siegel, E.; Trukhanov, P.; Ovalle, A.; Vecchio Fitz, C.D.; Zwiesler, Z.; Kumari, P.; Van Der Veen, B.; Marriott, E.; et al. MatchMiner: An open-source platform for cancer precision medicine. NPJ Precis. Oncol. 2022, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Peppercorn, J.; Burstein, H.; Miller, F.G.; Winer, E.; Joffe, S. Self-reported practices and attitudes of US oncologists regarding off-protocol therapy. J. Clin. Oncol. 2008, 26, 5994–6000. [Google Scholar] [CrossRef]
- Larson, K.L.; Huang, B.; Weiss, H.L.; Hull, P.; Westgate, P.M.; Miller, R.W.; Arnold, S.M.; Kolesar, J.M. Clinical Outcomes of Molecular Tumor Boards: A Systematic Review. JCO Precis. Oncol. 2021, 5, 1122–1132. [Google Scholar] [CrossRef]
- Angel, M.; Demiray, M.; Dişel, U.; Passos, J. The value of virtual molecular tumor boards for informed clinical decision-making. Oncologist 2024, 29, 554–559. [Google Scholar] [CrossRef]
- Bartels, M.; Chibaudel, B.; Dienstmann, R.; Lehtiö, J.; Piccolo, A.; Michielin, O.; O’Kane, G.; Pruneri, G. Evolving and Improving the Sustainability of Molecular Tumor Boards: The Value and Challenges. Cancers 2024, 16, 2888. [Google Scholar] [CrossRef]
- Cobain, E.F.; Wu, Y.M.; Vats, P.; Chugh, R.; Worden, F.; Smith, D.C.; Schuetze, S.M.; Zalupski, M.M.; Sahai, V.; Alva, A.; et al. Assessment of Clinical Benefit of Integrative Genomic Profiling in Advanced Solid Tumors. JAMA Oncol. 2021, 7, 525–533. [Google Scholar] [CrossRef]
- Klute, K.A.; Rothe, M.; Garrett-Mayer, E.; Mangat, P.K.; Nazemzadeh, R.; Yost, K.J.; Duvivier, H.L.; Ahn, E.R.; Cannon, T.L.; Alese, O.B.; et al. Cobimetinib Plus Vemurafenib in Patients With Colorectal Cancer With BRAF Mutations: Results From the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis. Oncol. 2022, 6, e2200191. [Google Scholar] [CrossRef]

| Characteristic | ||
|---|---|---|
| Median age, years | 73 | |
| Median Baseline bTMB, mut/Mb | 8.61 (0.01–1164.75) | |
| Median time between serial tests in days | 141 | |
| Pattern of bTMB Change over Time | Number of Patients, n (%) | Median Change in TMB (T1 to Tlast) |
| Increasing | 292 (53%) | +2.87 mut/Mb |
| Decreasing | 216 (40%) | −2.38 mut/Mb |
| Stable/no change | 38 (7%) | N/A |
| Total | 546 | +0.72 mut/Mb |
| Patient ID | Prior Lines of Therapy | Acquired ctDNA Alteration(s) Qualifying for Treatment (%cfDNA) | Drug | Baseline PSA, ng/mL | Best PSA Response, % of Baseline | Duration of Therapy, Weeks |
|---|---|---|---|---|---|---|
| 1 | Abiraterone, Enzalutamide, Docetaxel, Carboplatin | BRCA2 CNL CHEK2 CNL | Olaparib | 185 | +253 | 6 |
| 2 | Abiraterone, Enzalutamide, Darolutamide, Docetaxel, Carboplatin | BRCA2 CNL CHEK2 CNL | Olaparib | 61 | +293 | 7 |
| 3 | Abiraterone, Enzalutamide, Apalutamide, Docetaxel, Cabazitaxel, Carboplatin | CHEK2 D6fs (0.3%) | Olaparib | 90 | +218 | 11.1 |
| 4 | Abiraterone, Enzalutamide, Darolutamide, Docetaxel, Carboplatin, LuPSMA | CHEK2 CNL | Olaparib | 40 | +115 | 16.4 |
| 5 | Abiraterone, Enzalutamide, Apalutamide, Docetaxel, Cabazitaxel, Carboplatin, LuPSMA, Sipuleucel-T, Cisplatin + Etoposide | ATM SS SNV (1%) | Olaparib | 36 | +299 | 4 |
| 6 | Abiraterone, Enzalutamide, Docetaxel, Carboplatin, LuPSMA, Ac-225 | ATM CNL CHEK2 CNL | Olaparib | 455 | +154 | 11.1 |
| 7 | Abiraterone, Enzalutamide, Docetaxel, Cabazitaxel, Carboplatin, LuPSMA | BRCA1 CNL CHEK2 CNL RAD51D CNL | Olaparib | 101 | +211 | 6.6 |
| 8 | Abiraterone, Enzalutamide, Docetaxel, Cabazitaxel, Carboplatin, LuPSMA, Ac-225 | CHEK2 D347A (0.5%) | Olaparib | 98 | +285 | 7.1 |
| 9 | Abiraterone, Docetaxel | BRCA2 CNL | Olaparib | 0.12 | +192 | 61.1 |
| 10 | Abiraterone, Docetaxel, LuPSMA | BRCA2 SCD | Olaparib | 0.1 | +100 | 8.6 |
| 11 | Abiraterone, Apalutamide, Docetaxel, LuPSMA | TMB 15.3, MSS, pMMR | Pembrolizumab | 121 | +862 | 6.4 |
| 12 | Abiraterone, Apalutamide, Docetaxel, Cabazitaxel, Carboplatin, LuPSMA | TMB 24.9, MSS, pMMR | Pembrolizumab | 25 | +141 | 9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniz, M.; Tsai, L.J.; Orme, J.J.; Bucheit, L.A.; Basourakos, S.P.; Wei, N.; Koch, R.M.; Scharf, Z.; Gupta, S.; Kase, A.M.; et al. Evolution of Potentially Actionable Genomic Alterations in Advanced Prostate Cancer: A Real-World Analysis of Serial Circulating Tumor DNA Testing. Cancers 2025, 17, 3048. https://doi.org/10.3390/cancers17183048
Muniz M, Tsai LJ, Orme JJ, Bucheit LA, Basourakos SP, Wei N, Koch RM, Scharf Z, Gupta S, Kase AM, et al. Evolution of Potentially Actionable Genomic Alterations in Advanced Prostate Cancer: A Real-World Analysis of Serial Circulating Tumor DNA Testing. Cancers. 2025; 17(18):3048. https://doi.org/10.3390/cancers17183048
Chicago/Turabian StyleMuniz, Miguel, L. Jill Tsai, Jacob J. Orme, Leslie A. Bucheit, Spyridon P. Basourakos, Nancy Wei, Regina M. Koch, Zachary Scharf, Sounak Gupta, Adam M. Kase, and et al. 2025. "Evolution of Potentially Actionable Genomic Alterations in Advanced Prostate Cancer: A Real-World Analysis of Serial Circulating Tumor DNA Testing" Cancers 17, no. 18: 3048. https://doi.org/10.3390/cancers17183048
APA StyleMuniz, M., Tsai, L. J., Orme, J. J., Bucheit, L. A., Basourakos, S. P., Wei, N., Koch, R. M., Scharf, Z., Gupta, S., Kase, A. M., Rodrigues Pessoa, R., Riaz, I. B., Kwon, E. D., Andrews, J. R., & Childs, D. S. (2025). Evolution of Potentially Actionable Genomic Alterations in Advanced Prostate Cancer: A Real-World Analysis of Serial Circulating Tumor DNA Testing. Cancers, 17(18), 3048. https://doi.org/10.3390/cancers17183048






