Alterations in 13C and 15N Isotope Abundance as Potential Biomarkers for Tumor Biology and Risk Factors for Cervical Lymph Node Metastases in Oral Squamous Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Cohort
2.2. Preparation of the Samples
2.3. IRMS Procedure
2.4. Statistical Analysis
3. Results
3.1. Lymph Node Status (pN) and Neck Dissections
3.2. Multivariate Logistic Regression Analysis of the Risk Factors of Cervical Lymph Node Metastasis
3.3. IRMS Measurements of Nitrogen 15N and Carbon 13C in Tumor Tissues
3.4. Correlation Between IRMS Measurements and Risk Factors of Cervical Lymph Node Metastasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rozanova, S.; Barkovits, K.; Nikolov, M.; Schmidt, C.; Urlaub, H.; Marcus, K. Quantitative mass spectrometry-based proteomics: An overview. Methods Mol. Biol. 2021, 2228, 85–116. [Google Scholar] [CrossRef]
- Leslie, A.; Teh, E.; Druker, A.; Pinto, D.M. A targeted isotope dilution mass spectrometry assay for osteopontin quantification in plasma of metastatic breast cancer patients. PLoS ONE 2023, 18, e0281491. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Piper, T.; Ekström, L.; Hirschberg, A.L.; Thevis, M. Usefulness of serum androgen isotope ratio mass spectrometry (IRMS) to detect testosterone supplementation in women. Drug Test. Anal. 2023, 15, 465–469. [Google Scholar] [CrossRef]
- Bartman, C.R.; Faubert, B.; Rabinowitz, J.D.; DeBerardinis, R.J. Metabolic Pathway Analysis Using Stable Isotopes in Patients with Cancer. Nat. Rev. Cancer 2023, 23, 863–878. [Google Scholar] [CrossRef]
- Hilovsky, D.; Hartsell, J.; Young, J.D.; Liu, X. Stable Isotope Tracing Analysis in Cancer Research: Advancements and Challenges in Identifying Dysregulated Cancer Metabolism and Treatment Strategies. Metabolites 2024, 14, 318. [Google Scholar] [CrossRef]
- Tea, I.; De Luca, A.; Schiphorst, A.M.; Grand, M.; Barillé-Nion, S.; Mirallié, E.; Drui, D.; Krempf, M.; Hankard, R.; Tcherkez, G. Stable isotope abundance and fractionation in human diseases. Metabolites 2021, 11, 370. [Google Scholar] [CrossRef]
- Cichoń, M.J.; Gąsior, K.J.; Hincz, A.; Taran, K. The first pyrolysis protocol based on experimental measurements in the atomic level structured cancer studies. J. Health Study Med. 2022, 1, 5–18. [Google Scholar] [CrossRef]
- Zuzak, T.; Bogaczyk, A.; Krata, A.A.; Kamiński, R.; Paneth, P.; Kluz, T. Isotopic Composition of C, N, and S as an Indicator of Endometrial Cancer. Cancers 2024, 16, 3169. [Google Scholar] [CrossRef]
- Straub, M.; Auderset, A.; de Leval, L.; Piazzon, N.; Maison, D.; Vozenin, M.-C.; Ollivier, J.; Petit, B.; Sigman, D.M.; Martínez-García, A. Nitrogen Isotopic Composition as a Gauge of Tumor Cell Anabolism-to-Catabolism Ratio. Sci. Rep. 2023, 13, 19796. [Google Scholar] [CrossRef] [PubMed]
- Taran, K.; Frączek, T.; Sikora-Szubert, A.; Sitkiewicz, A.; Młynarski, W.; Kobos, J. The first investigation of Wilms’ tumor atomic structure-nitrogen and carbon isotopic composition as a novel biomarker. Oncotarget 2016, 7, 76726–76734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tea, I.; Martineau, E.; Antheaume, I.; Domanski, D.; Tcherkez, G. 13C and 15N natural isotope abundance reflects breast cancer cell metabolism. Sci. Rep. 2016, 6, 34251. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.C.; Lee, M.H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Gonçalves, V.; Lameirinhas, A.; Henrique, R.; Jerónimo, C. Metabolism and epigenetic interplay in cancer: Regulation and putative therapeutic targets. Front. Genet. 2018, 9, 427. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Madej, A.; Forma, E.; Golberg, M.; Kamiński, R.; Paneth, P.; Kobos, J.; Różański, W.; Lipiński, M. 13C natural isotope abundance in urothelium as a new marker in the follow-up of patients with bladder cancer. Cancers 2022, 14, 2423. [Google Scholar] [CrossRef] [PubMed]
- Haidari, S.; Obermeier, K.T.; Kraus, M.; Otto, S.; Probst, F.A.; Liokatis, P. Nodal disease and survival in oral cancer: Is occult metastasis a burden factor compared to preoperatively nodal positive neck? Cancers 2022, 14, 4241. [Google Scholar] [CrossRef]
- Dammann, F.; Horger, M.; Mueller-Berg, M.; Schlemmer, H.; Claussen, C.; Hoffman, J.; Eschmann, S.; Bares, R. Rational diagnosis of squamous cell carcinoma of the head and neck region: Comparative evaluation of CT, MRI and 18 FDG PET. AJR Am. J. Roentgenol. 2005, 184, 1326–1331. [Google Scholar] [CrossRef]
- He, T.; Sun, J.; Wu, J.; Wang, H.; Li, S.; Su, S. PET-CT versus MRI in the diagnosis of lymph node metastasis of cervical cancer: A meta-analysis. Microsc. Res. Tech. 2022, 85, 1791–1798. [Google Scholar] [CrossRef]
- Madsen, C.B.; Rohde, M.; Gerke, O.; Godballe, C.; Sørensen, J.A. Diagnostic Accuracy of Up-Front PET/CT and MRI for Detecting Cervical Lymph Node Metastases in T1–T2 Oral Cavity Cancer—A Prospective Cohort Study. Diagnostics 2023, 13, 3414. [Google Scholar] [CrossRef]
- Alsibani, A.; Alqahtani, A.; Almohammadi, R.; Islam, T.; Alessa, M.; Aldhahri, S.F.; Al-Qahtani, K.H. Comparing the Efficacy of CT, MRI, PET-CT, and US in the Detection of Cervical Lymph Node Metastases in Head and Neck Squamous Cell Carcinoma with Clinically Negative Neck Lymph Node: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 7622. [Google Scholar] [CrossRef]
- Deng, C.; Hu, J.; Tang, P.; Xu, T.; He, L.; Zeng, Z.; Sheng, J. Application of CT and MRI Images Based on Artificial Intelligence to Predict Lymph Node Metastases in Patients with Oral Squamous Cell Carcinoma: A Subgroup Meta-Analysis. Front. Oncol. 2024, 14, 1395159. [Google Scholar] [CrossRef]
- Mashberg, A.; Samit, A. Early diagnosis of asymptomatic oral and oropharyngeal squamous cancers. CA Cancer J. Clin. 1995, 45, 328–351. [Google Scholar] [CrossRef]
- Goekerm, M.; Braun, J.; Stoeckli, S.J. Evaluation of clinical and histomorphological parameters as potential predictors of occult metastases in sentinel lymph nodes of early squamous cell carcinoma of the oral cavity. Ann. Surg. Oncol. 2010, 17, 527–535. [Google Scholar] [CrossRef]
- Ionna, F.; Pace, U.; Colella, G.; Favia, G.; Lozito, A.; Baudin, F.; Feroce, F.; La Porta, F.A.; Troiano, G. Sentinel Lymph Node Biopsy in Oral Cavity Squamous Cell Carcinoma: Evidence from the First 20 Years of Study. Cancers 2024, 16, 1153. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers. Version 4.2025. Plymouth Meeting (PA): National Comprehensive Cancer Network. 2025. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437 (accessed on 31 July 2025).
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; Available online: https://cancerstaging.org/references-tools/deskreferences/Pages/8EUpdates.aspx (accessed on 31 July 2025).
- Bogusiak, K.; Puch, A.; Mostowski, R.; Kozakiewicz, M.; Paneth, P.; Kobos, J. Characteristic of oral squamous cell carcinoma tissues using isotope ratio mass spectrometry. J. Clin. Med. 2020, 9, 3760. [Google Scholar] [CrossRef]
- Bogusiak, K.; Kozakiewicz, M.; Puch, A.; Mostowski, R.; Paneth, P.; Kobos, J. Oral Cavity Cancer Tissues Differ in Isotopic Composition Depending on Location and Staging. Cancers 2023, 15, 4610. [Google Scholar] [CrossRef]
- Ludwig, R.; Werlen, S.; Barbatei, D.; Widmer, L.; Pouymayou, B.; Balermpas, P.; Elicin, O.; Dettmer, M.; Zrounba, P.; Giger, R.; et al. Patterns of Lymph Node Involvement for Oral Cavity Squamous Cell Carcinoma. Radiother. Oncol. 2024, 200, 110474. [Google Scholar] [CrossRef]
- Alqutub, S.; Alqutub, A.; Bakhshwin, A.; Mofti, Z.; Alqutub, S.; Alkhamesi, A.A.; Nujoom, M.A.; Rammal, A.; Merdad, M.; Marzouki, H.Z. Histopathological Predictors of Lymph Node Metastasis in Oral Cavity Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2024, 14, 1401211. [Google Scholar] [CrossRef]
| Characteristics | Number of Patients | |
|---|---|---|
| n | % | |
| Gender | ||
| Male | 37 | 60.7 |
| Female | 24 | 39.3 |
| Age | ||
| <65 years | 23 | 37.7 |
| ≥65 years | 38 | 62.3 |
| Smoking | ||
| yes | 37 | 60.7 |
| no | 24 | 39.3 |
| Alcohol consumption | ||
| yes | 17 | 27.9 |
| no | 44 | 72.1 |
| pT stage | ||
| T1 | 2 | 3.3 |
| T2 | 12 | 19.7 |
| T3 | 19 | 31.1 |
| T4 | 28 | 45.9 |
| pN stage | ||
| N0 | 27 | 44.2 |
| N1 | 2 | 3.3 |
| N2 | 4 | 6.6 |
| N3 | 28 | 45.9 |
| Grading | ||
| G1 | 11 | 18.0 |
| G2 | 40 | 65.6 |
| G3 | 10 | 16.4 |
| AJCC stage | ||
| I | 2 | 3.3 |
| II | 4 | 6.6 |
| III | 8 | 13.1 |
| IV | 47 | 77.0 |
| Number of Patients | cT | cN | pT | pN | Ipsilateral Side | Contralateral Side |
|---|---|---|---|---|---|---|
| 2 | 1 | 0 | 1 | 0 | SND | SND |
| 1 | 1 | 0 | 2 | + | SND | SND |
| 1 | 1 | + | 2 | + | MRND/RND | MRND/RND |
| 4 | 2 | 0 | 2 | 0 | SND | SND |
| 2 | 2 | 0 | 3 | 0 | SND | SND |
| 2 | 2 | 0 | 3 | + | SND | SND |
| 1 | 2 | 0 | 2 | + | SND | SND |
| 1 | 2 | 0 | 3 | + | SND | |
| 5 | 2 | + | 2 | + | MRND/RND | SND |
| 2 | 2 | + | 3 | + | MRND/RND | SND |
| 5 | 3 | 0 | 3 | 0 | SND | SND |
| 4 | 3 | 0 | 3 | + | SND | SND |
| 1 | 3 | 0 | 3 | 0 | SND | |
| 1 | 3 | 0 | 4 | 0 | SND | |
| 1 | 3 | 0 | 4 | + | SND | SND |
| 1 | 3 | 0 | 4 | + | SND | |
| 1 | 3 | + | 3 | + | MRND/RND | MRND/RND |
| 1 | 3 | + | 3 | + | MRND/RND | SND |
| 1 | 3 | + | 4 | + | MRND/RND | SND |
| 9 | 4 | 0 | 4 | 0 | SND | SND |
| 3 | 4 | 0 | 4 | 0 | SND | |
| 3 | 4 | 0 | 4 | + | SND | SND |
| 7 | 4 | + | 4 | + | MRND/RND | SND |
| 2 | 4 | + | 4 | + | MRND/RND | MRND/RND |
| Mean | Median | SD | Min | Max | |
|---|---|---|---|---|---|
| Lymph node yield | 36.9 | 36 | 10.5 | 21 | 59 |
| Number of positive lymph nodes | 3.7 | 3 | 2.4 | 1 | 9 |
| Lymph node ratio | 0.10690 | 0.09090 | 0.07334 | 0.02222 | 0.36 |
| Characteristics | Number of Patients | Lymph Node Metastasis | χ2 Value | p Value | |
|---|---|---|---|---|---|
| n | Yes | No | |||
| Gender | 4.390 | p < 0.05 | |||
| Male | 37 | 24 | 13 | ||
| Female | 24 | 9 | 15 | ||
| Age | 5.838 | p < 0.05 | |||
| <65 years | 23 | 17 | 6 | ||
| ≥65 years | 38 | 16 | 22 | ||
| Smoking | 4.390 | p < 0.05 | |||
| Yes | 37 | 24 | 13 | ||
| No | 24 | 9 | 15 | ||
| Alcohol consumption | 1.068 | p > 0.05 | |||
| Yes | 17 | 11 | 6 | ||
| No | 44 | 22 | 22 | ||
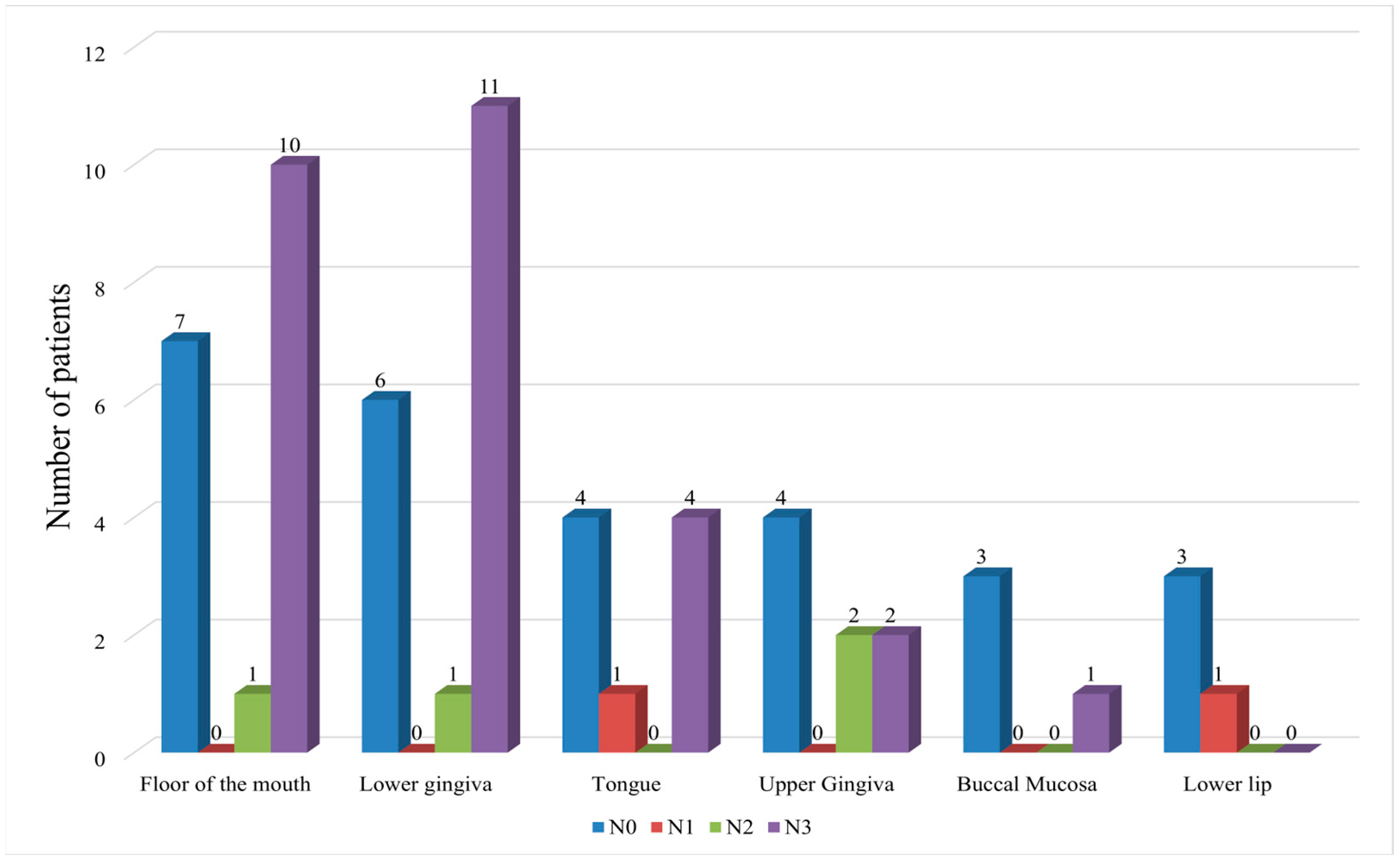
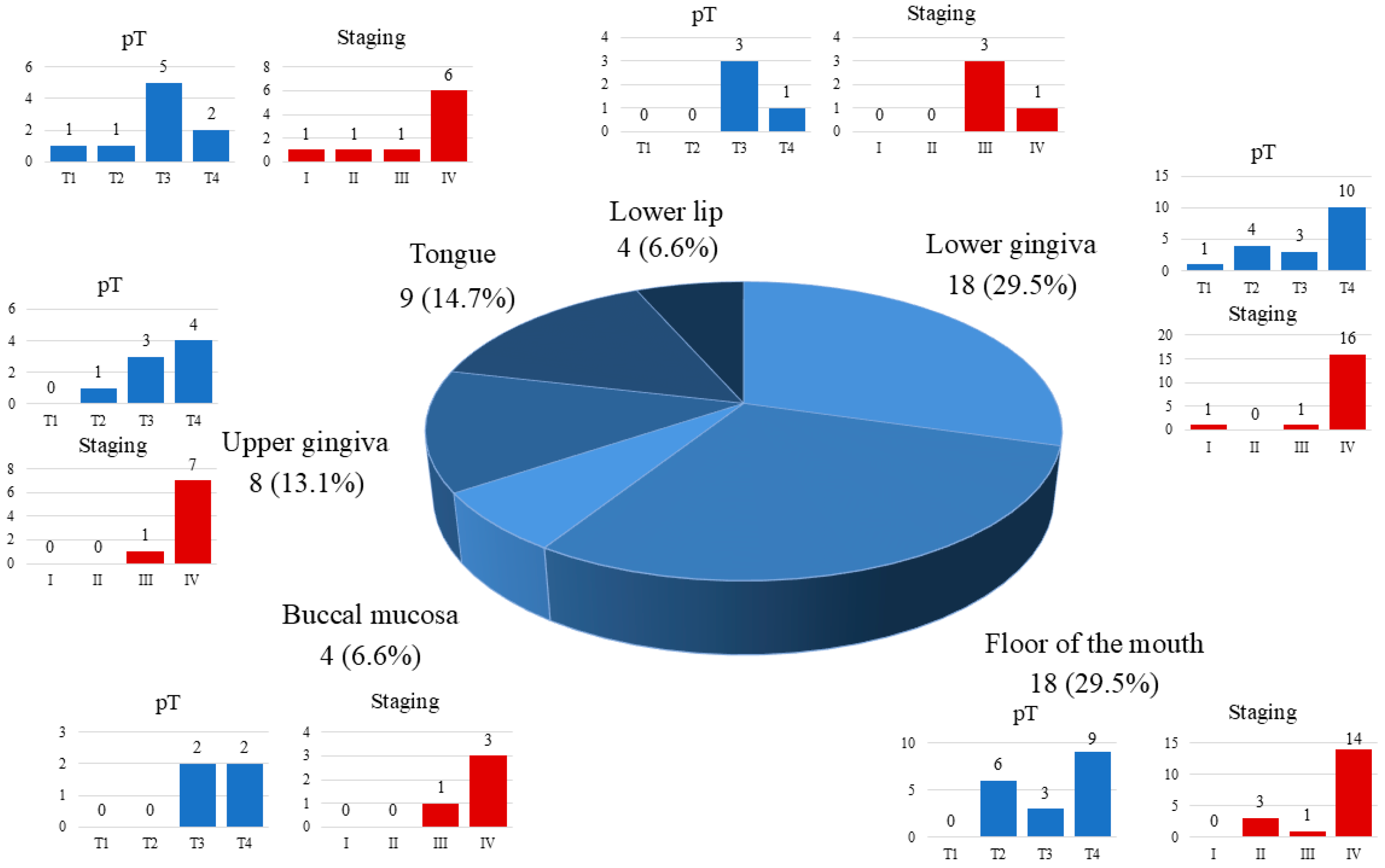
| Primary tumor site ** | 49.969 ** | p < 0.05 | |||
| Buccal mucosa | 4 | 1 | 3 | ||
| Floor of the mouth | 18 | 11 | 7 | ||
| Lower lip | 4 | 0 | 4 | ||
| Lower gingiva | 18 | 12 | 6 | ||
| Upper gingiva | 8 | 4 | 4 | ||
| Tongue | 9 | 5 | 4 | ||
| Ulceration | 0.236 | p > 0.05 | |||
| Yes | 35 | 18 | 17 | ||
| No | 26 | 15 | 11 | ||
| pT stage | 0.100 | p > 0.05 | |||
| T1 + T2 | 14 | 8 | 6 | ||
| T3 | 19 | 11 | 8 | ||
| T4 | 28 | 14 | 14 | ||
| Depth of infiltration | 4.573 | p < 0.05 | |||
| ≤10 mm * | 28 | 11 | 17 | ||
| >10 mm | 33 | 22 | 11 | ||
| Stage (AJCC 8th edition) | 22.881 | p < 0.05 | |||
| I + II + III | 14 | 0 | 14 | ||
| IV | 47 | 33 | 14 | ||
| Angioinvasion and/or neuroinvasion | 6.573 | p < 0.05 | |||
| Yes | 26 | 19 | 7 | ||
| No | 35 | 14 | 21 | ||
| ENE | N/A | N/A | |||
| Yes | 27 | 27 | 0 | ||
| No | 34 | 6 | 28 | ||
| Keratosis | 6.416 | p < 0.05 | |||
| Yes | 53 | 32 | 21 | ||
| No | 8 | 1 | 7 | ||
| Grade ** | 39.165 | p < 0.05 | |||
| G1 | 11 | 4 | 7 | ||
| G2 | 40 | 24 | 16 | ||
| G3 | 10 | 5 | 5 | ||
| Variables | B Value | S.E. Value | Wald χ2 Value | OR (95% CI) | p Value |
|---|---|---|---|---|---|
| Age | 0.869 | 0.056 | 8.898 | 0.869 (0.779–0.970) | 0.0029 |
| Gender | 0.220 | 0.770 | 4.133 | 0.22 (0.049–0.997) | 0.0420 |
| Tumor stage | |||||
| I | 2.236 | 223.609 | 34.396 | 2.236 (1.030–4.854) | 0.0000 |
| II | 1.379 | 158.117 | 1.379 (3.546–5.364) | ||
| III | 2.896 | 111.805 | 2.896 (1.865–4.263) |
| Lymph Node Metastasis | Lymph Node Metastasis | χ2 Value | p Value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Nitrogen (%) | Min–Max | 3.10–13.40 | 6.40–13.10 | 0.195 | p > 0.05 |
| Median | 12.70 | 12.50 | |||
| IQR | 0.60 | 0.80 | |||
| Carbon (%) | Min–Max | 44.00–69.50 | 44.10–63.90 | 0.103 | p > 0.05 |
| Median | 46.20 | 46.20 | |||
| IQR | 1.40 | 2.00 | |||
| [N]/[C] | Min–Max | 0.045–0.316 | 0.104–0.291 | 0.641 | p > 0.05 |
| Median | 0.274 | 0.272 | |||
| IQR | 0.014 | 0.024 | |||
| δ15N(‰) | Min–Max | 7.240–10.218 | 7.500–10.838 | 0.064 | p > 0.05 |
| Median | 8.800 | 8.700 | |||
| IQR | 1.117 | 0.945 | |||
| δ13C(‰) | Min–Max | −26.506–−20.072 | −25.780–−20.858 | 0.103 | p > 0.05 |
| Median | −22.304 | −22.746 | |||
| IQR | 1.145 | 0.653 | |||
| (a) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | Nitrogen (%) | Carbon (%) | |||||||||||||||||||||||
| Min–Max | Median | IQR | χ2 | p Value | Min–Max | Median | IQR | χ2 | p Value | ||||||||||||||||
| Age | |||||||||||||||||||||||||
| <65 years | 3.10–13.30 | 12.0 | 2.10 | 0.15 | p > 0.05 | 44.0–69.50 | 46.0 | 14.40 | 0.02 | p > 0.05 | |||||||||||||||
| ≥65 years | 3.10–13.10 | 13.0 | 0.70 | 44.0–69.50 | 46.0 | 1.40 | |||||||||||||||||||
| Gender | |||||||||||||||||||||||||
| Male | 6.30–13.40 | 13.0 | 0.90 | 0.01 | p > 0.05 | 44.0–63.90 | 46.0 | 2.60 | 0.04 | p > 0.05 | |||||||||||||||
| Female | 3.10–13.0 | 13.0 | 0.90 | 44.0–69.50 | 46.0 | 2.10 | |||||||||||||||||||
| Stage | |||||||||||||||||||||||||
| I + II + III | 6.40–13.40 | 12.0 | 6.10 | 4.12 | p < 0.05 | 44.80–63.90 | 46.0 | 15.70 | 0.09 | p > 0.05 | |||||||||||||||
| IV | 3.10–13.40 | 13.0 | 0.90 | 44.0–69.50 | 46.0 | 1.70 | |||||||||||||||||||
| Smoking | |||||||||||||||||||||||||
| Yes | 3.10–13.40 | 13.0 | 0.90 | 0.03 | p > 0.05 | 44.0–69.50 | 46.0 | 2.00 | 0.06 | p > 0.05 | |||||||||||||||
| No | 3.10–13.40 | 13.0 | 1.40 | 44.0–69.50 | 46.0 | 5.30 | |||||||||||||||||||
| DOI (mm) | |||||||||||||||||||||||||
| ≤10 mm | 6.50–13.20 | 13.0 | 1.00 | 0.05 | p > 0.05 | 44.0–63.90 | 46.0 | 5.40 | 0.08 | p > 0.05 | |||||||||||||||
| >10 mm | 3.10–13.40 | 13.0 | 0.80 | 44.0–69.50 | 46.0 | 1.60 | |||||||||||||||||||
| Angioinvasion or neuroinvasion | |||||||||||||||||||||||||
| Yes | 3.10–13.0 | 12.0 | 0.70 | 0.07 | p > 0.05 | 44.0–69.50 | 46.0 | 1.60 | 0.03 | p > 0.05 | |||||||||||||||
| No | 5.50–13.40 | 13.0 | 1.40 | 44.0–69.50 | 46.0 | 3.60 | |||||||||||||||||||
| ENE (+) (ipsilateral or contralateral) | |||||||||||||||||||||||||
| Yes | 10.30–13.40 | 13.0 | 0.90 | 0.09 | p > 0.05 | 44.0–50.80 | 46.0 | 1.40 | 0.12 | p > 0.05 | |||||||||||||||
| No | 3.10–13.40 | 12.0 | 1.10 | 44.0–69.50 | 46.0 | 3.60 | |||||||||||||||||||
| Keratosis | |||||||||||||||||||||||||
| Yes | 3.10–13.40 | 13.0 | 0.90 | 0.04 | p > 0.05 | 44.0–69.50 | 46.0 | 1.60 | 0.05 | p > 0.05 | |||||||||||||||
| No | 6.60–13.40 | 12.0 | 5.20 | 45.80–63.70 | 46.0 | 13.70 | |||||||||||||||||||
| Grade | |||||||||||||||||||||||||
| G1 | 6.40–13.10 | 13.0 | 6.20 | 0.06 | p > 0.05 | 45.40–63.90 | 46.0 | 15.70 | 0.08 | p > 0.05 | |||||||||||||||
| G2 | 3.10–13.40 | 13.0 | 0.90 | 44.0–69.50 | 46.0 | 1.70 | |||||||||||||||||||
| G3 | 3.10–12.70 | 12.0 | 9.40 | 45.80–69.50 | 46.0 | 23.70 | |||||||||||||||||||
| (b) | |||||||||||||||||||||||||
| Category | [N]/[C] | δ15N(‰) | δ13C(‰) | ||||||||||||||||||||||
| Min–Max | Median | IQR | χ2 | p Value | Min–Max | Median | IQR | χ2 | p Value | Min–Max | Median | IQR | χ2 | p Value | |||||||||||
| Age | |||||||||||||||||||||||||
| <65 years | 0.045–0.316 | 0.27 | 0.066 | 0.08 | p > 0.05 | 7.24–10.25 | 8.99 | 1.19 | 0.73 | p > 0.05 | −26.51–−20.07 | −22.70 | 2.48 | 0.26 | p > 0.05 | ||||||||||
| ≥65 years | 0.045–0.286 | 0.27 | 0.009 | 7.74–10.84 | 8.65 | 1.34 | −26.51–−20.86 | −22.41 | 1.04 | ||||||||||||||||
| Gender | |||||||||||||||||||||||||
| Male | 0.104–0.316 | 0.27 | 0.034 | 0.02 | p > 0.05 | 7.24–10.25 | 8.65 | 1.01 | 0.12 | p > 0.05 | −24.69–−20.07 | −22.44 | 1.52 | 0.03 | p > 0.05 | ||||||||||
| Female | 0.045–0.291 | 0.27 | 0.010 | 8.19–10.22 | 8.83 | 1.31 | −26.51–−21.29 | −22.48 | 0.76 | ||||||||||||||||
| Stage | |||||||||||||||||||||||||
| I + II + III | 0.104–0.291 | 0.27 | 0.168 | 0.07 | p > 0.05 | 8.13–10.84 | 8.83 ± 0.87 | 1.02 | 0.11 | p > 0.05 | −24.68–−22.01 | −22.88 | 1.68 | 4.25 | p < 0.05 | ||||||||||
| IV | 0.045–0.316 | 0.27 | 0.013 | 7.24–10.22 | 8.86 ± 0.67 | 1.35 | −26.51–−20.07 | −22.40 | 2.48 | ||||||||||||||||
| Smoking | |||||||||||||||||||||||||
| Yes | 0.045–0.316 | 0.27 | 0.012 | 0.04 | p > 0.05 | 7.24–10.25 | 8.81 ± 0.76 | 1.33 | 0.09 | p > 0.05 | −26.51–−20.07 | −22.45 | 1.44 | 0.02 | p > 0.05 | ||||||||||
| No | 0.045–0.291 | 0.27 | 0.030 | 7.74–10.84 | 8.91 ± 0.64 | 1.35 | −26.51–−20.86 | −22.47 | 2.68 | ||||||||||||||||
| DOI (mm) | |||||||||||||||||||||||||
| ≤10 mm | 0.104–0.312 | 0.27 | 0.011 | 0.03 | p > 0.05 | 7.24–10.84 | 8.81 ± 0.84 | 1.08 | 0.14 | p > 0.05 | −25.76–−20.07 | −22.70 | 1.62 | 0.04 | p > 0.05 | ||||||||||
| >10 mm | 0.045–0.316 | 0.27 | 0.010 | 7.74–9.86 | 8.88 ± 0.60 | 1.17 | −26.51–−21.29 | −22.70 | 1.52 | ||||||||||||||||
| Angioinvasion or neuroinvasion | |||||||||||||||||||||||||
| Yes | 0.045–0.280 | 0.27 | 0.009 | 0.06 | p > 0.05 | 7.90–10.22 | 8.92 ± 0.56 | 1.38 | 0.18 | p > 0.05 | −26.51–−20.07 | −22.26 | 1.15 | 4.03 | p < 0.05 | ||||||||||
| No | 0.045–0.316 | 0.27 | 0.034 | 7.24–10.84 | 8.80 ± 0.81 | 1.27 | −26.51–−20.86 | −22.75 | 2.68 | ||||||||||||||||
| ENE(+) (ipsilateral or contralateral) | |||||||||||||||||||||||||
| Yes | 0.214–0.316 | 0.28 | 0.018 | 0.08 | p > 0.05 | 7.24–10.22 | 8.87 ± 0.74 | 1.19 | 0.16 | p > 0.05 | −23.92–−20.07 | −22.38 | 1.15 | 0.05 | p > 0.05 | ||||||||||
| No | 0.045–0.291 | 0.27 | 0.026 | 7.74–10.84 | 8.83 ± 0.69 | 1.47 | −26.51–−20.86 | −22.71 | 2.68 | ||||||||||||||||
| Keratosis | |||||||||||||||||||||||||
| Yes | 0.045–0.316 | 0.27 | 0.011 | 0.03 | p > 0.05 | 7.24–10.22 | 8.84 ± 0.68 | 1.27 | 0.07 | p > 0.05 | −26.51–−20.07 | −22.44 | 2.16 | 0.06 | p > 0.05 | ||||||||||
| No | 0.104–0.291 | 0.27 | 0.150 | 7.50–10.84 | 8.89 ± 0.93 | 2.12 | −24.69–−22.01 | −22.72 | 2.02 | ||||||||||||||||
| Grade | |||||||||||||||||||||||||
| G1 | 0.104–0.312 | 0.27 | 0.168 | 0.05 | p > 0.05 | 7.24–10.84 | 8.63 ± 0.95 | 1.09 | 0.13 | p > 0.05 | −24.68–−21.37 | −22.70 | 1.83 | 0.07 | p > 0.05 | ||||||||||
| G2 | 0.045–0.316 | 0.27 | 0.014 | 7.50–10.22 | 8.90 ± 0.65 | 1.27 | −26.51–−20.07 | −22.41 | 2.48 | ||||||||||||||||
| G3 | 0.045–0.278 | 0.27 | 0.229 | 8.55–9.66 | 8.89 ± 0.62 | 1.06 | −26.51–−21.29 | −22.58 | 4.21 | ||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogusiak, K.; Paneth, P.; Kobos, J.; Kozakiewicz, M. Alterations in 13C and 15N Isotope Abundance as Potential Biomarkers for Tumor Biology and Risk Factors for Cervical Lymph Node Metastases in Oral Squamous Cell Carcinoma. Cancers 2025, 17, 3047. https://doi.org/10.3390/cancers17183047
Bogusiak K, Paneth P, Kobos J, Kozakiewicz M. Alterations in 13C and 15N Isotope Abundance as Potential Biomarkers for Tumor Biology and Risk Factors for Cervical Lymph Node Metastases in Oral Squamous Cell Carcinoma. Cancers. 2025; 17(18):3047. https://doi.org/10.3390/cancers17183047
Chicago/Turabian StyleBogusiak, Katarzyna, Piotr Paneth, Józef Kobos, and Marcin Kozakiewicz. 2025. "Alterations in 13C and 15N Isotope Abundance as Potential Biomarkers for Tumor Biology and Risk Factors for Cervical Lymph Node Metastases in Oral Squamous Cell Carcinoma" Cancers 17, no. 18: 3047. https://doi.org/10.3390/cancers17183047
APA StyleBogusiak, K., Paneth, P., Kobos, J., & Kozakiewicz, M. (2025). Alterations in 13C and 15N Isotope Abundance as Potential Biomarkers for Tumor Biology and Risk Factors for Cervical Lymph Node Metastases in Oral Squamous Cell Carcinoma. Cancers, 17(18), 3047. https://doi.org/10.3390/cancers17183047









