Prediction of Occult Cervical Lymph Node Metastasis in Bone-Invasive pT4a cN0 Oral Squamous Cell Carcinoma in Relation to Tumor Size: A Retrospective Observational Cohort Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Patient Cohort Characteristics
3.2. Cervical Lymph Node Metastasis
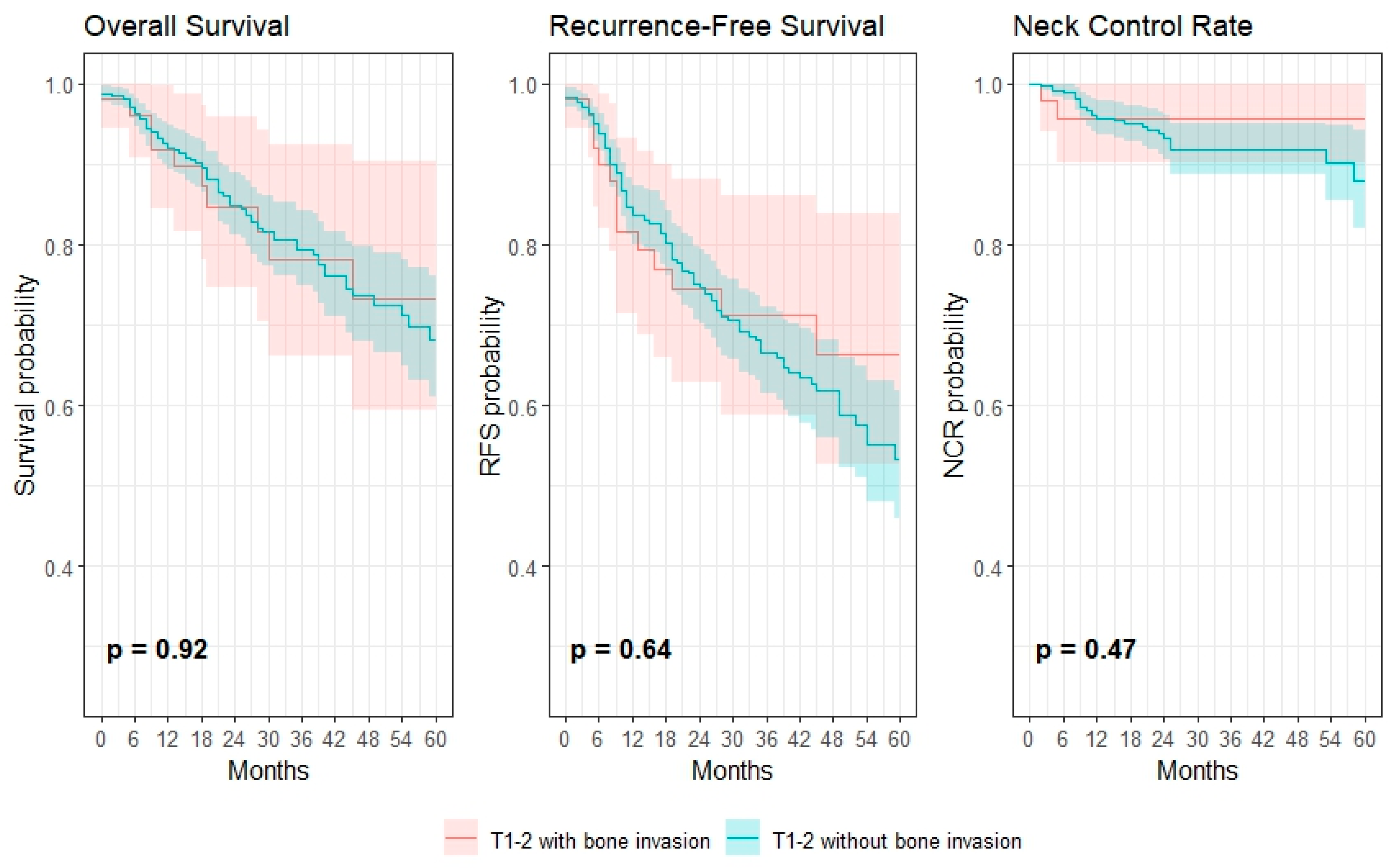
3.3. Survival and Regional Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Lee, N.C.J.; Eskander, A.; Park, H.S.; Mehra, S.; Burtness, B.A.; Husain, Z. Pathologic staging changes in oral cavity squamous cell carcinoma: Stage migration and implications for adjuvant treatment. Cancer 2019, 125, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.A.; Rahim, A.U.; Iqbal, S.; Batool, H.; Younas, S.; Jawaad Manzoor, H.M. The frequency of occult cervical metastasis in oral squamous cell carcinoma patients—A cross sectional study. J. Pak. Med. Assoc. 2022, 72, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Massey, C.; Dharmarajan, A.; Bannuru, R.R.; Rebeiz, E. Management of N0 neck in early oral squamous cell carcinoma: A systematic review and meta-analysis. Laryngoscope 2019, 129, E284–E298. [Google Scholar] [CrossRef]
- Mrosk, F.; Krom, V.; Doll, C.; Mödl, L.; Kreutzer, K.; Voss, J.; Rendenbach, C.; Heiland, M.; Koerdt, S. Prediction of nodal disease in oral squamous cell carcinoma of the tongue: Histopathological risk assessment with the focus on depth of invasion. Clin. Oral Investig. 2024, 28, 1–8. [Google Scholar] [CrossRef]
- Doll, C.; Mrosk, F.; Wuester, J.; Runge, A.-S.; Neumann, F.; Rubarth, K.; Heiland, M.; Kreutzer, K.; Voss, J.; Raguse, J.-D.; et al. Pattern of cervical lymph node metastases in squamous cell carcinoma of the upper oral cavity—How to manage the neck. Oral Oncol. 2022, 130, 105898. [Google Scholar] [CrossRef]
- Garrel, R.; Poissonnet, G.; Plana, A.M.; Fakhry, N.; Dolivet, G.; Lallemant, B.; Sarini, J.; Vergez, S.; Guelfucci, B.; Choussy, O.; et al. Equivalence Randomized Trial to Compare Treatment on the Basis of Sentinel Node Biopsy Versus Neck Node Dissection in Operable T1-T2N0 Oral and Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, 4010–4018. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Tsukahara, K.; Yoshimoto, S.; Miura, K.; Yokoyama, J.; Hirano, S.; Uemura, H.; Sugasawa, M.; Yoshizaki, T.; Homma, A.; et al. Neck Dissections Based on Sentinel Lymph Node Navigation Versus Elective Neck Dissections in Early Oral Cancers: A Randomized, Multicenter, and Noninferiority Trial. J. Clin. Oncol. 2021, 39, 2025–2036. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.; Stoeckli, S.J.; Haerle, S.K.; Broglie, M.A.; Huber, G.F.; Sorensen, J.A.; Bakholdt, V.; Krogdahl, A.; von Buchwald, C.; Bilde, A.; et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur. J. Cancer 2015, 51, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Head and Neck Cancers (Version 1.2025); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Jang, S.S.; Davis, M.E.; Vera, D.R.; Lai, S.Y.; Guo, T.W. Role of sentinel lymph node biopsy for oral squamous cell carcinoma: Current evidence and future challenges. Head Neck 2023, 45, 251–265. [Google Scholar] [CrossRef]
- du Bois, H.; Heim, T.A.; LundAW. Tumor-draining lymph nodes: At the crossroads of metastasis and immunity. Sci. Immunol. 2021, 6, eabg3551. [Google Scholar] [CrossRef]
- Bittar, R.F.; Ferraro, H.P.; Ribas, M.H.; Lehn, C.N. Predictive factors of occult neck metastasis in patients with oral squamous cell carcinoma. Braz. J. Otorhinolaryngol. 2016, 82, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Arora, S.; Grover, K.; Agarwal, A.; Garg, C.; Katyal, R.; Mishra, B.P.; Sharma, H. Clinico-Pathological Predictors Affecting Lymph Node Status in Oral Squamous Cell Carcinoma. Indian J. Otolaryngol. Head Neck Surg. 2025, 77, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, S.; Pandiar, D.; Krishnan, R.P.; Ramalingam, K.; Bologna-Molina, R. Correlation of Bony Invasion With Nodal Metastasis, Pattern of Invasion and Survival in Oral Squamous Cell Carcinoma: A Retrospective Analysis of 122 Primary Cases from Oral Cancer Centre of South India. Cureus 2023, 15, e42887. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; de Oliveira Santos, A.B.; e Silva, E.L.; de Matos, L.L.; Moyses, R.A.; Kulcsar, M.A.V.; Pinto, F.R.; Brandão, L.G.; Cernea, C.R. Tumor volume as an independent predictive factor of worse survival in patients with oral cavity squamous cell carcinoma. Head Neck 2017, 39, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Aldosimani, M.; Verdonschot, R.G.; Iwamoto, Y.; Nakazawa, M.; Mallya, S.M.; Kakimoto, N.; Toyosawa, S.; Kreiborg, S.; Murakami, S. Prognostic factors for lymph node metastasis from upper gingival carcinomas. Oral Radiol. 2022, 38, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Brown, J.S.; Woolgar, J.A.; Lowe, D.; Rogers, S.N.; Vaughan, E.D. The influence of the pattern of mandibular invasion on recurrence and survival in oral squamous cell carcinoma. Head Neck 2004, 26, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Kuk, S.K.; Yoon, H.J.; Hong, S.D.; Hong, S.P.; Lee, J.I. Staging significance of bone invasion in small-sized (4 cm or less) oral squamous cell carcinoma as defined by the American Joint Committee on Cancer. Oral Oncol. 2016, 55, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Murali, R.; Gao, K.; Elliott, M.S.; Clark, J.R. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer 2011, 117, 4460–4467. [Google Scholar] [CrossRef]
| All (n = 642, %) | pT4a (n = 117, %) | |
|---|---|---|
| Age (mean ± SD) | 64.5 ± 12.2 | 66.7 ± 11.7 |
| Sex | ||
| Female | 266 (41.4) | 45 (38.5) |
| Male | 376 (58.6) | 72 (61.5) |
| T-stage | ||
| pT1 | 275 (42.8) | 9 (7.7) |
| pT2 | 193 (30.1) | 44 (37.6) |
| pT3 | 57 (8.9) | 64 (54.7) |
| pT4a | 117 (18.2) | - |
| N-stage | ||
| pN0 | 512 (79.8) | 82 (70.1) |
| pN+ | 130 (20.2) | 35 (29.9) |
| Extracapsular spread | ||
| Yes | 23 (3.6) | 9 (7.7) |
| No | 619 (96.4) | 108 (92.3) |
| Grade of differentiation | ||
| I | 87 (13.6) | 4 (3.4) |
| II | 490 (76.3) | 98 (83.8) |
| III | 65 (10.1) | 15 (12.8) |
| Vascular infiltration | ||
| Yes | 10 (1.6) | 2 (1.7) |
| No | 632 (98.4) | 115 (98.3) |
| Lymphatic infiltration | ||
| Yes | 26 (4.0) | 7 (6.0) |
| No | 616 (96.0) | 110 (94.0) |
| Perineural invasion | ||
| Yes | 30 (4.7) | 6 (5.1) |
| No | 612 (95.3) | 111 (94.9) |
| Occult Cervical Lymph Node Metastasis | |
|---|---|
| pT1 | 20/275 (7.3%) |
| pT2 | 52/193 (26.9%) |
| pT3 | 23/57 (40.4%) |
| pT4a | 35/117 (29.9%) |
| T1 size | 3/9 (33.3%) |
| T2 size | 10/44 (22.7%) |
| T3 size | 22/64 (34.4%) |
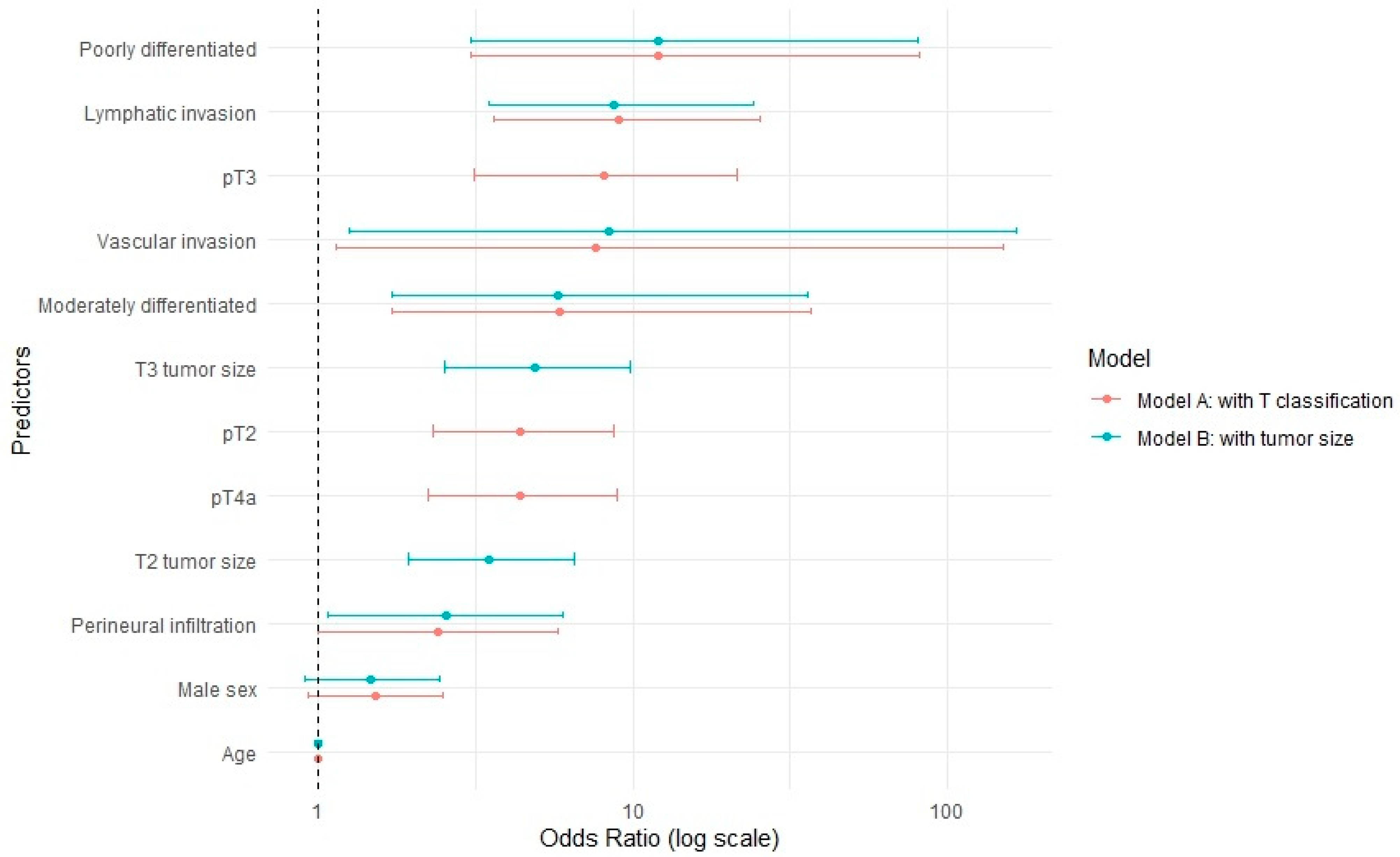
| Predictor | OR (95% CI) (Model A) | OR (95% CI) (Model B) |
|---|---|---|
| Age (continous) | 1 (0.98–1.02) | 1 (0.98–1.01) |
| Sex | ||
| Female | 1 (Reference) | 1 (Reference) |
| Male | 1.53 (0.93–2.53) | 1.56 (1.05–2.36) |
| T-stage | ||
| pT1 | 1 (Reference) | |
| pT2 | 9.81 (1.59–53.39) | |
| pT3 | 11.89 (1.82–68.36) | |
| pT4a | 7.22 (1.4–31.05) | |
| Tumor size | ||
| T1 size | 1 (Reference) | |
| T2 size | 4.02 (2.43–6.85) | |
| T3 size | 6.72 (3.86–11.97) | |
| Vascular invasion | 7.92 (1.18–156.9) | 37.12 (6.88–687.75) |
| Lymphatic invasion | 8.98 (3.55–25.14) | 12.06 (5.16–31.51) |
| Perineural infiltration | 2.41 (1–5.82) | 4.04 (1.9–8.61) |
| Grade of differentiation | ||
| Well differentiated | 1 (Reference) | 1 (Reference) |
| Moderately differentiated | 5.81 (1.69–36.54) | 11.17 (3.45–68.57) |
| Poorly differentiated | 12.16 (3.06–82.02) | 28.33 (7.93–181.44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrosk, F.; Vertic, V.; Richter, M.; Sprünken, E.; Mödl, L.; Voss, J.O.; Sofroniou, A.; Rendenbach, C.; Heiland, M.; Koerdt, S. Prediction of Occult Cervical Lymph Node Metastasis in Bone-Invasive pT4a cN0 Oral Squamous Cell Carcinoma in Relation to Tumor Size: A Retrospective Observational Cohort Study. Cancers 2025, 17, 3044. https://doi.org/10.3390/cancers17183044
Mrosk F, Vertic V, Richter M, Sprünken E, Mödl L, Voss JO, Sofroniou A, Rendenbach C, Heiland M, Koerdt S. Prediction of Occult Cervical Lymph Node Metastasis in Bone-Invasive pT4a cN0 Oral Squamous Cell Carcinoma in Relation to Tumor Size: A Retrospective Observational Cohort Study. Cancers. 2025; 17(18):3044. https://doi.org/10.3390/cancers17183044
Chicago/Turabian StyleMrosk, Friedrich, Victoria Vertic, Maximilian Richter, Erin Sprünken, Lukas Mödl, Jan Oliver Voss, Anna Sofroniou, Carsten Rendenbach, Max Heiland, and Steffen Koerdt. 2025. "Prediction of Occult Cervical Lymph Node Metastasis in Bone-Invasive pT4a cN0 Oral Squamous Cell Carcinoma in Relation to Tumor Size: A Retrospective Observational Cohort Study" Cancers 17, no. 18: 3044. https://doi.org/10.3390/cancers17183044
APA StyleMrosk, F., Vertic, V., Richter, M., Sprünken, E., Mödl, L., Voss, J. O., Sofroniou, A., Rendenbach, C., Heiland, M., & Koerdt, S. (2025). Prediction of Occult Cervical Lymph Node Metastasis in Bone-Invasive pT4a cN0 Oral Squamous Cell Carcinoma in Relation to Tumor Size: A Retrospective Observational Cohort Study. Cancers, 17(18), 3044. https://doi.org/10.3390/cancers17183044







