Outcomes of Robotic Pancreatectomy in the Octogenarian: A Multicenter Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Collection
2.3. Definition of Postoperative Complications
2.4. Surgical Technique
2.5. Selection Criteria for Surgery
2.6. Statistical Analysis
3. Results
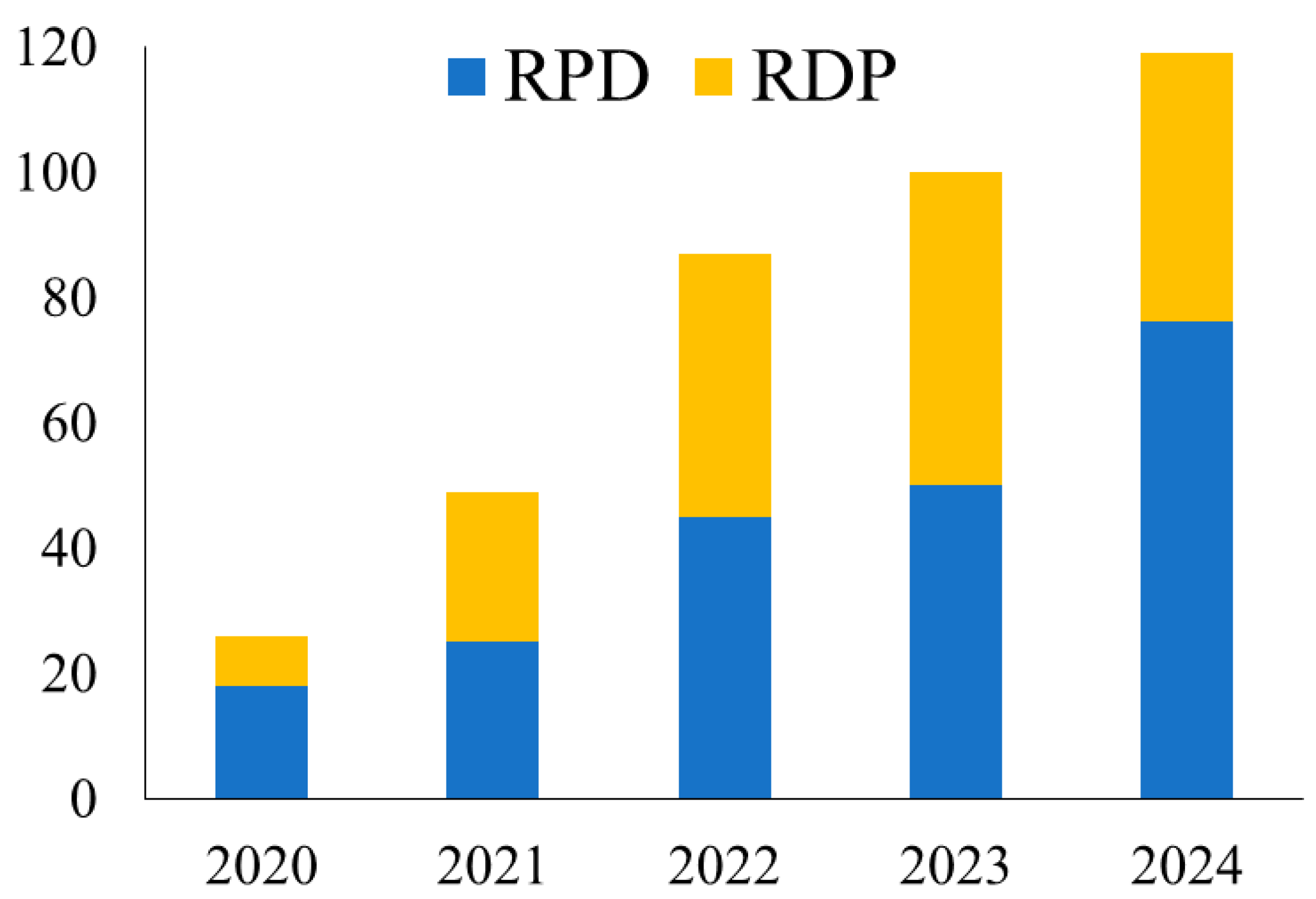
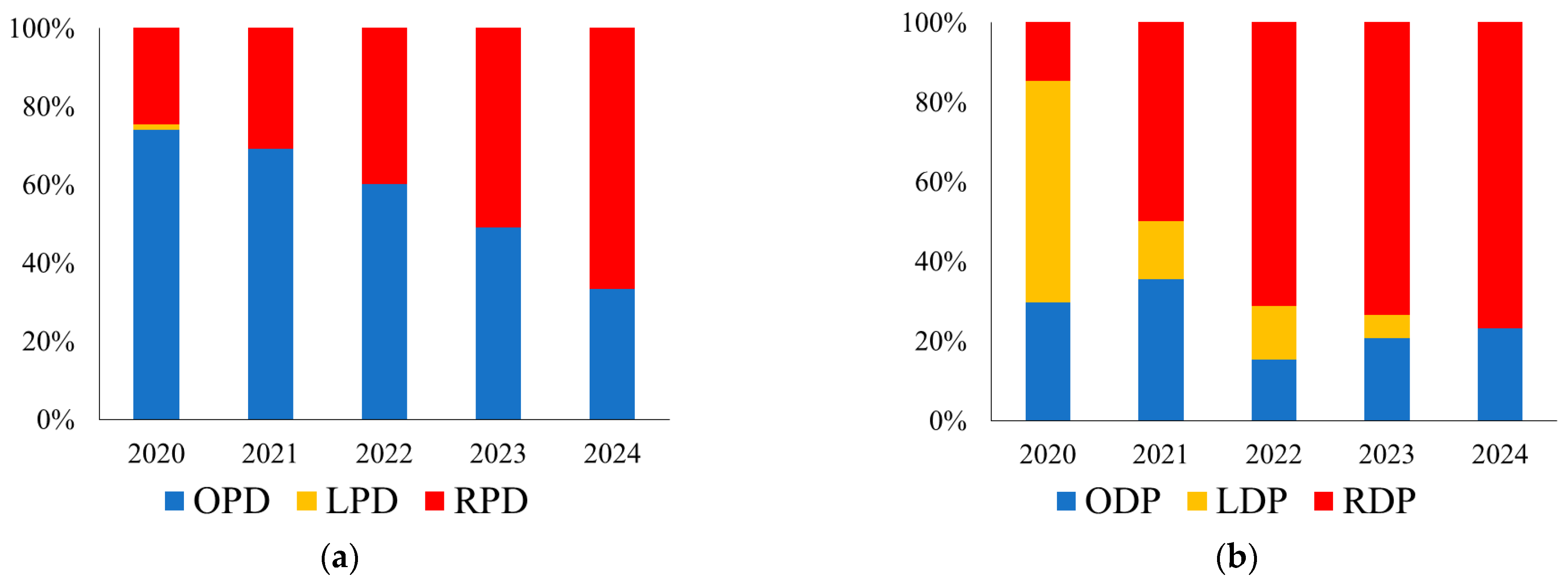
3.1. Implementation
3.2. Patient Demographics
3.3. Outcomes of Robotic Pancreatectomy
3.3.1. Robotic Pancreatoduodenectomy
3.3.2. Robotic Distal Pancreatectomy
3.4. Outcomes of Robotic Pancreatectomy Stratified by Age
3.4.1. Robotic Pancreatoduodenectomy
3.4.2. Robotic Distal Pancreatectomy
3.5. Risk Factors for Postoperative Major Complications After Robotic Pancreatectomy
3.6. Long-Term Outcomes After Robotic Pancreatectomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, M.; Tomioka, S.; Barber, S.L. Research to inform health systems’ responses to rapid population ageing: A collection of studies funded by the WHO Centre for Health Development in Kobe, Japan. Health Res. Policy Syst. 2022, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Tan, E.; Song, J.; Lam, S.; D’Souza, M.; Crawford, M.; Sandroussi, C. Postoperative outcomes in elderly patients undergoing pancreatic resection for pancreatic adenocarcinoma: A systematic review and meta-analysis. Int. J. Surg. 2019, 72, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Asbun, H.J.; Moekotte, A.L.; Vissers, F.L.; Kunzler, F.; Cipriani, F.; Alseidi, A.; D’Angelica, M.I.; Balduzzi, A.; Bassi, C.; Björnsson, B.; et al. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann. Surg. 2020, 271, 1–14. [Google Scholar] [CrossRef]
- Abu Hilal, M.; van Ramshorst, T.M.E.; Boggi, U.; Dokmak, S.; Edwin, B.; Keck, T.; Khatkov, I.; Ahmad, J.; Al Saati, H.; Alseidi, A.; et al. The Brescia Internationally Validated European Guidelines on Minimally Invasive Pancreatic Surgery (EGUMIPS). Ann. Surg. 2024, 279, 45–57. [Google Scholar] [CrossRef]
- Hariri, H.M.; Perez, S.B.; Turner, K.M.; Wilson, G.C. Minimally Invasive Pancreas Surgery: Is There a Benefit? Surg. Clin. N. Am. 2024, 104, 1083–1093. [Google Scholar] [CrossRef]
- Buchs, N.C.; Addeo, P.; Bianco, F.M.; Gangemi, A.; Ayloo, S.M.; Giulianotti, P.C. Outcomes of robot-assisted pancreaticoduodenectomy in patients older than 70 years: A comparative study. World J. Surg. 2010, 34, 2109–2114. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Z.; Zhang, X.; Zhao, G.; Tan, X.; Gao, Y.; Lau, W.Y.; Liu, R. Robotic pancreaticoduodenectomy in elderly and younger patients: A retrospective cohort study. Int. J. Surg. 2020, 81, 61–65. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Garden, O.J.; Padbury, R.; Rahbari, N.N.; Adam, R.; Capussotti, L.; Fan, S.T.; Yokoyama, Y.; Crawford, M.; Makuuchi, M.; et al. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011, 149, 680–688. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Uchida, Y.; Takahara, T.; Mizumoto, T.; Nishimura, A.; Mii, S.; Iwama, H.; Kojima, M.; Uyama, I.; Suda, K. Task division by multiple console surgeons is beneficial for safe robotic pancreaticoduodenectomy implementation and education. Surg. Endosc. 2024, 38, 4712–4721. [Google Scholar] [CrossRef]
- Mizumoto, T.; Takahara, T.; Nishimura, A.; Mii, S.; Uchida, Y.; Iwama, H.; Kojima, M.; Kato, Y.; Uyama, I.; Suda, K. Robot-assisted approach using a laparoscopic articulating vessel-sealing device versus pure-robotic approach during distal pancreatectomy. J. Robot. Surg. 2024, 18, 263. [Google Scholar] [CrossRef]
- Takagi, K.; Umeda, Y.; Yoshida, R.; Yagi, T.; Fujiwara, T.; Zureikat, A.H.; Hogg, M.E.; Koerkamp, B.G. Surgical training model and safe implementation of robotic pancreatoduodenectomy in Japan: A technical note. World J. Surg. Oncol. 2021, 19, 55. [Google Scholar] [CrossRef]
- Takagi, K.; Kumano, K.; Umeda, Y.; Yoshida, R.; Fuji, T.; Yasui, K.; Yagi, T.; Fujiwara, T. Surgical Strategies to Approaching the Splenic Artery in Robotic Distal Pancreatectomy. Anticancer. Res. 2022, 42, 4471–4476. [Google Scholar] [CrossRef]
- Zwart, M.J.W.; van den Broek, B.; de Graaf, N.; Suurmeijer, J.A.; Augustinus, S.; Te Riele, W.W.; van Santvoort, H.C.; Hagendoorn, J.; Borel Rinkes, I.H.M.; van Dam, J.L.; et al. The Feasibility, Proficiency, and Mastery Learning Curves in 635 Robotic Pancreatoduodenectomies Following a Multicenter Training Program: “Standing on the Shoulders of Giants”. Ann. Surg. 2023, 278, e1232–e1241. [Google Scholar] [CrossRef] [PubMed]
- Zureikat, A.H.; Beane, J.D.; Zenati, M.S.; Al Abbas, A.I.; Boone, B.A.; Moser, A.J.; Bartlett, D.L.; Hogg, M.E.; Zeh, H.J., 3rd. 500 Minimally Invasive Robotic Pancreatoduodenectomies: One Decade of Optimizing Performance. Ann. Surg. 2021, 273, 966–972. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, P.; Muller, X.; Malleo, G.; Park, J.S.; Hwang, H.K.; Napoli, N.; Javed, A.A.; Inoue, Y.; Beghdadi, N.; Kalisvaart, M.; et al. Benchmarks in Pancreatic Surgery: A Novel Tool for Unbiased Outcome Comparisons. Ann. Surg. 2019, 270, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.C.; Breuer, E.; Nickel, F.; Zani, S., Jr.; Kauffmann, E.; De Franco, L.; Tschuor, C.; Krohn, P.S.; Burgdorf, S.K.; Jonas, J.P.; et al. Robotic Distal Pancreatectomy: A Novel Standard of Care? Benchmark Values for Surgical Outcomes from 16 International Expert Centers. Ann. Surg. 2023, 278, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, R.; Esposito, G.; Guerrini, G.P.; Magistri, P.; Catellani, B.; Guidetti, C.; Di Sandro, S.; Di Benedetto, F. Minimally Invasive Pancreaticoduodenectomy in Elderly versus Younger Patients: A Meta-Analysis. Cancers 2024, 16, 323. [Google Scholar] [CrossRef]
- Sato, M.; Tateishi, R.; Yasunaga, H.; Horiguchi, H.; Yoshida, H.; Matsuda, S.; Koike, K. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: A national survey of 54,145 patients. J. Gastroenterol. 2012, 47, 1125–1133. [Google Scholar] [CrossRef]
- Sperti, C.; Moletta, L.; Pozza, G. Pancreatic resection in very elderly patients: A critical analysis of existing evidence. World J. Gastrointest. Oncol. 2017, 9, 30–36. [Google Scholar] [CrossRef]
- Melloul, E.; Lassen, K.; Roulin, D.; Grass, F.; Perinel, J.; Adham, M.; Wellge, E.B.; Kunzler, F.; Besselink, M.G.; Asbun, H.; et al. Guidelines for Perioperative Care for Pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) Recommendations 2019. World J. Surg. 2020, 44, 2056–2084. [Google Scholar] [CrossRef]
- Joliat, G.R.; Kobayashi, K.; Hasegawa, K.; Thomson, J.E.; Padbury, R.; Scott, M.; Brustia, R.; Scatton, O.; Tran Cao, H.S.; Vauthey, J.N.; et al. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations 2022. World J. Surg. 2023, 47, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Rostoft, S.; van Leeuwen, B. Frailty assessment tools and geriatric assessment in older patients with hepatobiliary and pancreatic malignancies. Eur. J. Surg. Oncol. 2021, 47, 514–518. [Google Scholar] [CrossRef]
- Deng, Y.; Liao, R.; Hu, X.; Zhang, K.; Zhu, J.; Sato, N. Prevalence of physical frailty and its associated factors among elderly patients undergoing hepatobiliary pancreatic surgery in China. Glob. Health Med. 2024, 6, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nakagawa, S.; Miyata, T.; Yamashita, Y.I.; Baba, H. Frailty and surgical outcomes in gastrointestinal cancer: Integration of geriatric assessment and prehabilitation into surgical practice for vulnerable patients. Ann. Gastroenterol. Surg. 2023, 7, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Mederos, M.A.; Starr, S.; Park, J.Y.; King, J.C.; Tomlinson, J.S.; Hines, O.J.; Donahue, T.R.; Girgis, M.D. Robotic versus open pancreaticoduodenectomy in elderly patients: A propensity score-matched analysis. HPB 2023, 25, 301–310. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, N.; Tian, E.; Li, M.; Zhang, H.; Zhao, G.; Tan, X.; Wang, W.; Han, B.; Yuan, J.; et al. Short-term outcomes of robotic versus open pancreaticoduodenectomy in elderly patients: A multicenter retrospective cohort study. Int. J. Surg. 2022, 104, 106819. [Google Scholar] [CrossRef]

| Variables | n (%) or Median (IQR) | Younger Patients (<80 Years) | Octogenarian Patients (≥80 Years) | p Value |
|---|---|---|---|---|
| No. of patients | 380 | 321 | 59 | |
| Age, years | 72 (61–77) | 70 (59–75) | 82 (81–85) | <0.001 |
| Sex | ||||
| Men | 196 (51.6%) | 166 (51.7%) | 30 (50.9%) | 0.903 |
| Women | 184 (48.4%) | 155 (48.3%) | 29 (49.1%) | |
| BMI, kg/m2 | 22.4 (20.4–24.7) | 22.3 (20.4–24.9) | 22.7 (20.9–24.0) | 0.970 |
| ASA score | ||||
| 1–2 | 325 (85.5%) | 279 (86.9%) | 46 (78.0%) | 0.087 |
| 3–4 | 55 (14.5%) | 42 (13.1%) | 13 (22.0%) | |
| Comorbidity | ||||
| Hypertension | 172 (45.3%) | 138 (43.0%) | 34 (57.6%) | 0.038 |
| Diabetes | 118 (31.1%) | 92 (28.7%) | 26 (44.1%) | 0.022 |
| Neoadjuvant chemotherapy | 72 (18.9%) | 68 (21.2%) | 4 (6.8%) | 0.004 |
| Primary diseases | ||||
| Pancreatic cancer | 115 (30.3%) | 92 (28.7%) | 23 (39.0%) | <0.001 |
| Bile duct cancer | 30 (7.9%) | 17 (5.3%) | 13 (22.0%) | |
| IPMN/IPMC | 83 (21.8%) | 76 (23.7%) | 7 (11.9%) | |
| PNEN | 40 (10.5%) | 37 (11.5%) | 3 (5.1%) | |
| Ampullary carcinoma | 24 (6.3%) | 19 (5.9%) | 5 (8.5%) | |
| Duodenal carcinoma | 25 (6.6%) | 20 (6.2%) | 5 (8.5%) | |
| Others | 63 (16.6%) | 60 (18.7%) | 3 (5.1%) | |
| Malignant diseases | 229 (60.3%) | 179 (55.8%) | 50 (84.8%) | <0.001 |
| Type of procedure | ||||
| RPD | 213 (56.1%) | 177 (55.1%) | 36 (61.0%) | 0.401 |
| RDP | 167 (43.9%) | 144 (44.9%) | 23 (39.0%) | |
| Institution | ||||
| Fujita Health University Hospital | 199 (52.4%) | 165 (51.4%) | 34 (57.6%) | 0.378 |
| Okayama University Hospital | 181 (47.6%) | 156 (48.6%) | 25 (42.4%) |
| Variables | n (%) or Median (IQR) | Younger Patients (<80 Years) | Octogenarian Patients (≥80 Years) | p Value |
|---|---|---|---|---|
| No. of patients | 213 | 177 | 36 | |
| Preoperative biliary drainage | 68 (31.9%) | 48 (27.1) | 20 (55.6) | 0.001 |
| Operative factors | ||||
| Operative time, min | 490 (410–632) | 478 (406–633) | 516 (444–606) | 0.256 |
| Blood loss, mL | 100 (38–242) | 100 (30–228) | 117 (72–338) | 0.077 |
| Conversion to open surgery, n (%) | 3 (1.4%) | 3 (1.7%) | 0 (0%) | 0.290 |
| Transfusion, n (%) | 17 (8.1%) | 10 (5.7%) | 7 (20.0%) | 0.012 |
| Pancreatic texture, n (%) | ||||
| Soft | 170 (80.2%) | 140 (79.6%) | 30 (83.3%) | 0.597 |
| Hard | 42 (19.8%) | 36 (20.4%) | 6 (16.7%) | |
| MPD diameter, mm | 3 (2–4) | 2.5 (2–4) | 3 (2–4.8) | 0.224 |
| Postoperative factors | ||||
| Postoperative hospital stay, days | 16 (11–23) | 14 (10–22) | 22 (16–27) | <0.001 |
| 30-day mortality, n (%) | 2 (0.9%) | 2 (1.1%) | 0 (0%) | 0.388 |
| 90-day mortality, n (%) | 2 (0.9%) | 2 (1.1%) | 0 (0%) | 0.388 |
| Re-operation, n (%) | 9 (5.0%) | 7 (4.0%) | 2 (5.6%) | 0.675 |
| Major complications, n (%) | 37 (17.4%) | 30 (17.0%) | 7 (19.4%) | 0.722 |
| Incisional SSI, n (%) | 9 (4.2%) | 9 (5.1%) | 0 (0%) | 0.065 |
| Organ/space SSI, n (%) | 18 (8.5%) | 14 (7.9%) | 4 (11.1%) | 0.543 |
| POPF (≥grade B), n (%) | 25 (11.7%) | 21 (11.9%) | 4 (11.1%) | 0.898 |
| Bile leakage, n (%) | 5 (2.3%) | 5 (2.8%) | 0 (0%) | 0.171 |
| DGE (≥grade B), n (%) | 11 (5.2%) | 6 (3.4%) | 5 (13.9%) | 0.023 |
| Postoperative pancreatitis | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Variables | n (%) or Median (IQR) | Younger Patients (<80 Years) | Octogenarian Patients (≥80 Years) | p Value |
|---|---|---|---|---|
| No. of patients | 167 | 144 | 23 | |
| Operative factors | ||||
| Type of procedure | ||||
| RDP with splenectomy | 119 (71.3%) | 100 | 19 | 0.207 |
| Spleen-preserving technique | 48 (28.7%) | 44 | 4 | |
| Kimura technique | 25 | 24 | 1 | |
| Warshaw technique | 23 | 20 | 3 | |
| Operative time, min | 281 (219–372) | 280 (217–372) | 297 (238–398) | 0.555 |
| Blood loss, mL | 50 (9–110) | 35 (5–106) | 58 (22–120) | 0.144 |
| Conversion to open surgery, n (%) | 1 (0.6%) | 1 (0.7%) | 0 (0%) | 0.586 |
| Transfusion, n (%) | 4 (2.4%) | 3 (2.1%) | 1 (4.3%) | 0.523 |
| Postoperative factors | ||||
| Postoperative hospital stay, days | 11 (9–15) | 10.5 (9–14.8) | 14 (9–21) | 0.023 |
| 30-day mortality, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| 90-day mortality, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Re-operation, n (%) | 1 (0.6%) | 0 (0%) | 1 (4.3%) | 0.045 |
| Major complications, n (%) | 13 (7.8%) | 9 (6.3%) | 4 (17.4%) | 0.097 |
| Incisional SSI, n (%) | 6 (3.6%) | 5 (3.5%) | 1 (4.3%) | 0.838 |
| Organ/space SSI, n (%) | 16 (9.6%) | 12 (8.3%) | 4 (17.4%) | 0.205 |
| POPF (≥grade B), n (%) | 28 (16.8%) | 23 (16.0%) | 5 (21.7%) | 0.504 |
| Postoperative pancreatitis | 2 (1.2%) | 2 (1.4%) | 0 (0%) | 0.440 |
| Variables | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age (≥80 years) | 1.66 | 0.76–3.37 | 0.193 | 1.33 | 0.59–2.84 | 0.479 |
| Sex (male) | 2.73 | 1.45–5.41 | 0.002 | 2.53 | 1.31–5.10 | 0.005 |
| BMI (≥25 kg/m2) | 1.17 | 0.58–2.27 | 0.647 | |||
| ASA (3–4) | 0.96 | 0.38–2.13 | 0.918 | |||
| Hypertension | 1.03 | 0.57–1.88 | 0.911 | |||
| Neoadjuvant chemotherapy | 0.24 | 0.06–0.69 | 0.005 | 0.26 | 0.06–0.77 | 0.012 |
| Preoperative biliary drainage | 1.17 | 0.53–2.40 | 0.681 | |||
| Etiology of disease | ||||||
| Pancreatic cancer | 1 | |||||
| Bile duct cancer | 3.86 | 1.15–12.6 | 0.030 | |||
| IPMN/IPMC | 1.88 | 0.67–5.46 | 0.229 | |||
| PNEN | 1.67 | 0.42–5.85 | 0.445 | |||
| Ampullary carcinoma | 4.06 | 1.10–14.1 | 0.036 | |||
| Duodenal carcinoma | 3.86 | 1.05–13.3 | 0.042 | |||
| Others | 4.41 | 1.72–12.3 | 0.002 | |||
| Procedure (RPD) | 2.49 | 1.31–5.02 | 0.005 | 2.02 | 1.04–4.16 | 0.038 |
| Operative time (>500/280 min) * | 0.98 | 0.54–1.78 | 0.949 | |||
| Blood loss (>500 mL) | 2.80 | 1.11–6.52 | 0.031 | 2.27 | 0.85–5.62 | 0.099 |
| Institution (Okayama) | 1.11 | 0.61–2.03 | 0.719 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, K.; Uchida, Y.; Fuji, T.; Takahara, T.; Yasui, K.; Nishiyama, T.; Uyama, I.; Suda, K.; Fujiwara, T. Outcomes of Robotic Pancreatectomy in the Octogenarian: A Multicenter Retrospective Cohort Study. Cancers 2025, 17, 3038. https://doi.org/10.3390/cancers17183038
Takagi K, Uchida Y, Fuji T, Takahara T, Yasui K, Nishiyama T, Uyama I, Suda K, Fujiwara T. Outcomes of Robotic Pancreatectomy in the Octogenarian: A Multicenter Retrospective Cohort Study. Cancers. 2025; 17(18):3038. https://doi.org/10.3390/cancers17183038
Chicago/Turabian StyleTakagi, Kosei, Yuichiro Uchida, Tomokazu Fuji, Takeshi Takahara, Kazuya Yasui, Takeyoshi Nishiyama, Ichiro Uyama, Koichi Suda, and Toshiyoshi Fujiwara. 2025. "Outcomes of Robotic Pancreatectomy in the Octogenarian: A Multicenter Retrospective Cohort Study" Cancers 17, no. 18: 3038. https://doi.org/10.3390/cancers17183038
APA StyleTakagi, K., Uchida, Y., Fuji, T., Takahara, T., Yasui, K., Nishiyama, T., Uyama, I., Suda, K., & Fujiwara, T. (2025). Outcomes of Robotic Pancreatectomy in the Octogenarian: A Multicenter Retrospective Cohort Study. Cancers, 17(18), 3038. https://doi.org/10.3390/cancers17183038






