Comparing the Perioperative and Oncological Outcomes of Open Versus Minimally Invasive Inguinal Lymphadenectomy in Penile Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment and Risk of Bias
2.4. Meta-Analysis
3. Results
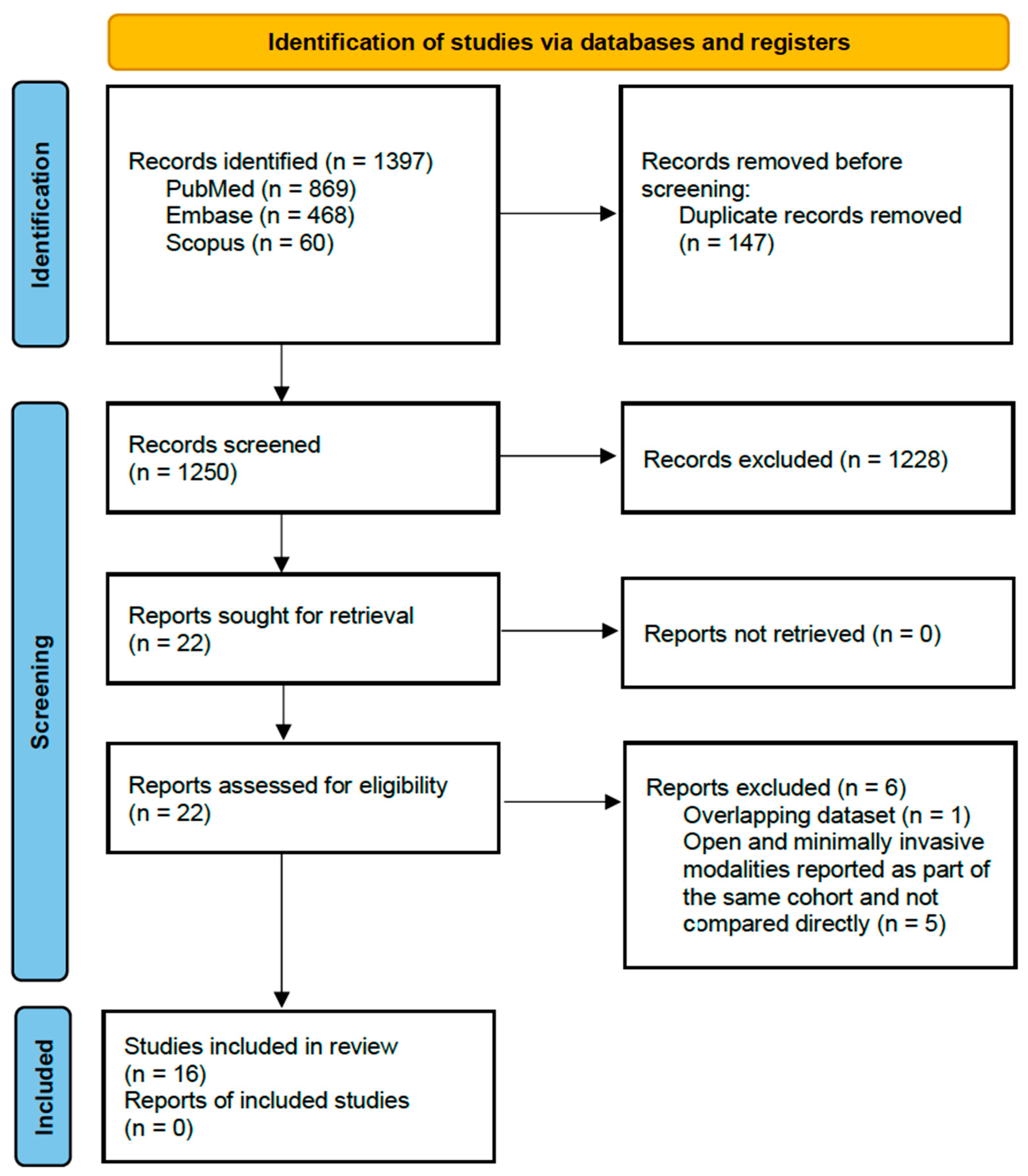
3.1. Study Selection
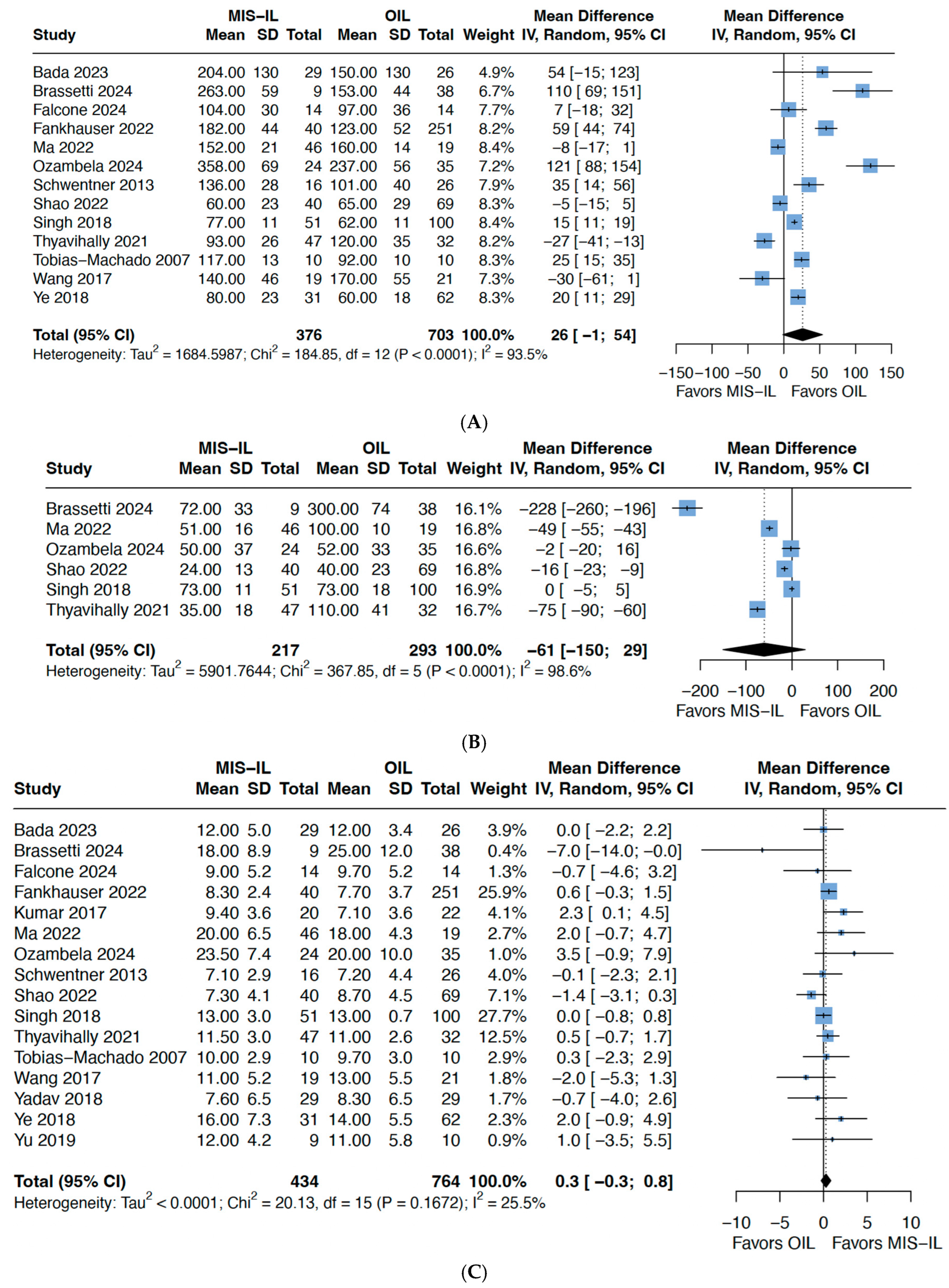
3.2. Operative/Perioperative Outcomes
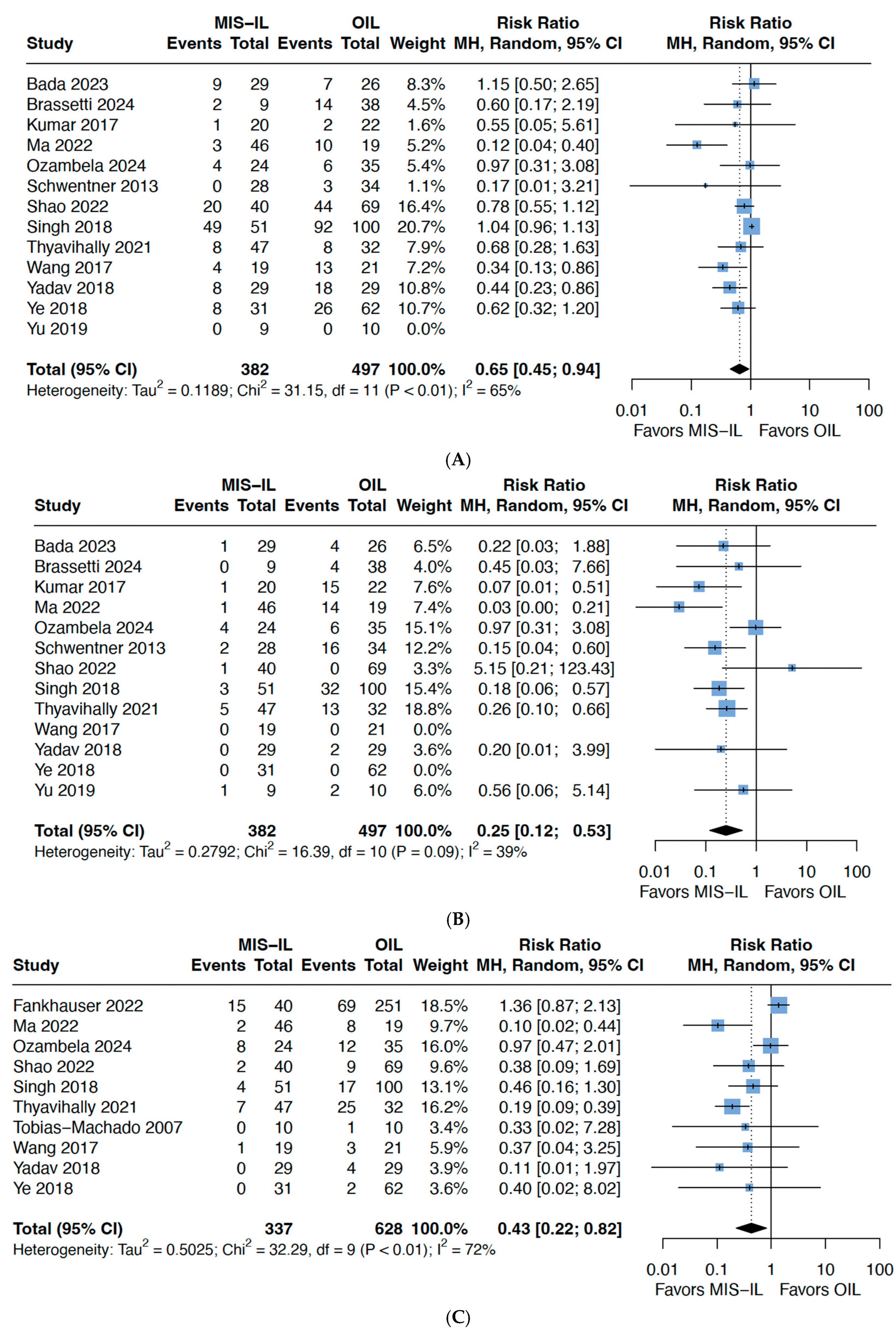
3.3. Complications
3.4. Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skeppner, E.; Andersson, S.-O.; Johansson, J.-E.; Windahl, T. Initial symptoms and delay in patients with penile carcinoma. Scand. J. Urol. Nephrol. 2012, 46, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Pow-Sang, M.R.; Ferreira, U.; Pow-Sang, J.M.; Nardi, A.C.; Destefano, V. Epidemiology and natural history of penile cancer. Urology 2010, 76, S2–S6. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Rumble, R.B.; Ayres, B.; Sánchez Martínez, D.F.; Oliveira, P.; Spiess, P.E.; Johnstone, P.A.S.; Crook, J.; Pettaway, C.A.; Tagawa, S.T.; et al. Penile Cancer: EAU-ASCO Collaborative Guidelines Update Q and A. JCO Oncol. Pract. 2024, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Akduman, B.; Bouchot, O.; Palou, J.; Tobias-Machado, M. Prognostic Factors in Penile Cancer. Urology 2010, 76, S66–S73. [Google Scholar] [CrossRef]
- Sachdeva, A.; McGuiness, L.; Zapala, L.; Greco, I.; Garcia-Perdomo, H.A.; Kailavasan, M.; Antunes-Lopes, T.; Ayres, B.; Barreto, L.; Campi, R.; et al. Management of lymph node-positive penile cancer: A systematic review. Eur. Urol. 2024, 85, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Teh, J.; Duncan, C.; Qu, L.; Guerra, G.; Narasimhan, V.; Pham, T.; Lawrentschuk, N. Inguinal lymph node dissection for penile cancer: A contemporary review. Transl. Androl. Urol. 2020, 9, 3210–3218. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef]
- Tobias-Machado, M.; Tavares, A.; Silva, M.N.R.; Molina, J.W.R.; Forseto, P.H.; Juliano, R.V.; Wroclawski, E.R. Can Video Endoscopic Inguinal Lymphadenectomy Achieve a Lower Morbidity Than Open Lymph Node Dissection in Penile Cancer Patients? J. Endourol. 2008, 22, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.S.; Tomar, V.; Bhattar, R.; Jha, A.K.; Priyadarshi, S. Video Endoscopic Inguinal Lymphadenectomy vs Open Inguinal Lymphadenectomy for Carcinoma Penis: Expanding Role and Comparison of Outcomes. Urology 2018, 113, 79–84. [Google Scholar] [CrossRef]
- Falcone, M.; Gül, M.; Peretti, F.; Preto, M.; Cirigliano, L.; Scavone, M.; Sedigh, O.; Oderda, M.; Gontero, P. Inguinal lymphadenectomy for penile cancer: An interim report from a trial comparing open versus videoendoscopic surgery using a within-patient design. Eur. Urol. Open Sci. 2024, 63, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Schwentner, C.; Todenhöfer, T.; Seibold, J.; Alloussi, S.H.; Mischinger, J.; Aufderklamm, S.; Stenzl, A.; Gakis, G. Endoscopic inguinofemoral lymphadenectomy—Extended follow-up. J. Endourol. 2013, 27, 497–503. [Google Scholar] [CrossRef]
- Brassetti, A.; Pallares-Mendez, R.; Bove, A.M.; Misuraca, L.; Anceschi, U.; Tuderti, G.; Mastroianni, R.; Licari, L.C.; Bologna, E.; Cartolano, S.; et al. Comparing Outcomes of Open and Robot-Assisted Inguinal Lymphadenectomy for the Treatment of cN2 Squamous Cell Carcinoma of the Penis: A Retrospective Single-Center Analysis. Cancers 2024, 16, 3921. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, K.; Li, R.; Lu, J.; Wu, T.; Liu, Z.; Fu, X.; Tang, Q.; Ma, J. (Eds.) Bilateral inguinal lymphadenectomy using simultaneous double laparoscopies for penile cancer: A retrospective study. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Singh, A.; Jaipuria, J.; Goel, A.; Shah, S.; Bhardwaj, R.; Baidya, S.; Jain, J.; Jain, C.; Rawal, S. Comparing Outcomes of Robotic and Open Inguinal Lymph Node Dissection in Patients with Carcinoma of the Penis. J. Urol. 2018, 199, 1518–1525. [Google Scholar] [CrossRef]
- Yu, H.; Lu, Y.; Xiao, Y.; Guo, J.; Yin, X.; Yang, Y.; Wang, H.; Gao, J. Robot-assisted laparoscopic antegrade versus open inguinal lymphadenectomy: A retrospective controlled study. BMC Urol. 2019, 19, 135. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Lee, E.W.; Issa, A.; Oliveira, P.; Lau, M.; Sangar, V.; Parnham, A. Saphenous-sparing ascending video endoscopic inguinal lymph node dissection using a leg approach: Surgical technique and perioperative and pathological outcomes. Eur. Urol. Open Sci. 2022, 35, 9–13. [Google Scholar] [CrossRef]
- Kumar, V.; Sethia, K.K. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int. 2017, 119, 530–534. [Google Scholar] [CrossRef]
- Ozambela, M., Jr.; McCormick, B.Z.; Rudzinski, J.K.; Pieretti, A.C.; González, G.M.N.; Meissner, M.A.; Papadopoulos, J.N.; Adibi, M.; Matin, S.F.; Dahmen, A.S.; et al. (Eds.) Robotic or open superficial inguinal lymph node dissection as staging procedures for clinically node negative high risk penile cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Bada, M.; Crocetto, F.; Nyirady, P.; Pagliarulo, V.; Rapisarda, S.; Aliberti, A.; Boccasile, S.; Ferro, M.; Barone, B.; Celia, A. Inguinal lymphadenectomy in penile cancer patients: A comparison between open and video endoscopic approach in a multicenter setting. J. Basic Clin. Physiol. Pharmacol. 2023, 34, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hu, X.; Ren, S.; Liao, D.; Yang, Z.; Liu, Y.; Lia, T.; Wu, K.; Xiong, S.; Yang, W.; et al. Comparison of different surgical methods and strategies for inguinal lymph node dissection in patients with penile cancer. Sci. Rep. 2022, 12, 2560. [Google Scholar] [CrossRef]
- Thyavihally, Y.B.; Dev, P.; Waigankar, S.S.; Pednekar, A.; Kulkarni, B.; Sharma, A.; Maheshwari, S.; Roy, D.; Agarwal, V.; Khandekar, A.A.; et al. Comparative study of perioperative and survival outcomes after video endoscopic inguinal lymphadenectomy (VEIL) and open inguinal lymph node dissection (O-ILND) in the management of inguinal lymph nodes in carcinoma of the penis. J. Robot. Surg. 2021, 15, 905–914. [Google Scholar] [CrossRef]
- Ye, Y.L.; Guo, S.J.; Li, Z.S.; Yao, K.; Chen, D.; Wang, Y.J.; Chen, P.; Han, H.; Zhou, F.J. Radical videoscopic inguinal lymphadenectomies: A matched pair analysis. J. Endourol. 2018, 32, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, P.; Tang, X.; An, C.; Zhang, N.; Yang, Y. Comparison of Efficiency of Video Endoscopy and Open inguinal lymph node dissection. Anticancer Res. 2017, 37, 4623–4628. [Google Scholar]
- Hu, J.; Li, H.; Cui, Y.; Liu, P.; Zhou, X.; Liu, L.; Chen, H.; Chen, J.; Zu, X. Comparison of clinical feasibility and oncological outcomes between video endoscopic and open inguinal lymphadenectomy for penile cancer. Medicine 2019, 98, e15862. [Google Scholar] [CrossRef]
- Patel, K.N.; Salunke, A.; Bakshi, G.; Jayaprakash, D.; Pandya, S.J. Robotic-Assisted Video-Endoscopic Inguinal Lymphadenectomy (RAVEIL) and Video-Endoscopic Inguinal Lymphadenectomy (VEIL) versus Open Inguinal Lymph-Node Dissection (OILND) in carcinoma of penis: Comparison of perioperative outcomes, complications and onco. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 112.e11–112.e22. [Google Scholar]
- Ge, S.; Zheng, L.; Li, Y.; Gan, L.; Wang, Z.; Zeng, Z.; Meng, C.; Li, K.; Ma, J.; Wang, D.; et al. Comparing the safety and effectiveness of minimally invasive surgery and open inguinal lymph node dissection in penile cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2024, 50, 108553. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, R.; Petrinec, B.; Nabavizadeh, B.; Singh, A.; Rawal, S.; Master, V. Inguinal lymph node dissection in the era of minimally invasive surgical technology. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 1–14. [Google Scholar] [CrossRef]
- Lupinacci, R.M.; Benoît, O.; Peschaud, F. Inguinal lymph node dissection. J. Visc. Surg. 2023, 160, 127–133. [Google Scholar] [CrossRef]
- Fraley, E.E.; Hutchens, H.C. Radical Ilio-Inguinal Node Dissection: The Skin Bridge Technique. A New Procedure. J. Urol. 1972, 108, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.D.; Jakhetiya, A.; Kumar, S.; Mishra, A.; Singh, S.; Shukla, N.K. Minimizing Post-operative Complications of Groin Dissection Using Modified Skin Bridge Technique: A Single-Centre Descriptive Study Showing Post-operative and Early Oncological Outcomes. World J. Surg. 2018, 42, 3196–3201. [Google Scholar] [CrossRef]
- Bertheuil, N.; Sulpice, L.; Sandri, G.L.; Lavoué, V.; Watier, E.; Meunier, B. Inguinal lymphadenectomy for stage III melanoma: A comparative study of two surgical approaches at the onset of lymphoedema. Eur. J. Surg. Oncol. (eJSO) 2015, 41, 215–219. [Google Scholar] [CrossRef]
- Ma, Y.; Hao, J.; Yin, H.; Zhu, M.; Guan, B.; Zhu, C.; Dong, B.; Zhao, S.; He, Z.; Yang, T. A laparoscopic radical inguinal lymphadenectomy approach partly preserving great saphenous vein branches can benefit for patients with penile carcinoma. BMC Surg. 2022, 22, 138. [Google Scholar] [CrossRef]
- Alqudrah, F.; Passarelli, R.; Sykes, J.; Islam, R.; Chua, K.; Ghodoussipour, S. Video-endoscopic inguinal lymphadenectomy (VEIL) oncological and surgical benefits compared to open inguinal lymph node dissection (ILND). J. Men’s Health 2024, 20, 20–25. [Google Scholar]
- Spillane, A.J.; Cheung, B.L.; Stretch, J.R.; Scolyer, R.A.; Shannon, K.F.; Quinn, M.J.; Saw, R.P.; McCarthy, W.H.; Thompson, J.F. Proposed quality standards for regional lymph node dissections in patients with melanoma. Ann. Surg. 2009, 249, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Spillane, A.J.; Winstanley, J.; Thompson, J.F. Lymph node ratio in melanoma: A marker of variation in surgical quality? Cancer 2009, 115, 2384–2387. [Google Scholar] [CrossRef] [PubMed]
- Master, V.A.; Jafri, S.M.A.; Moses, K.A.; Ogan, K.; Kooby, D.A.; Delman, K.A. Minimally invasive inguinal lymphadenectomy via endoscopic groin dissection: Comprehensive assessment of immediate and long-term complications. J. Urol. 2012, 188, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Lyu, J.; Bai, Y.; Jiang, J.; Liu, F. Single-position robot-assisted versus laparoscopic antegrade bilateral inguinal lymphadenectomy for penile cancer: A retrospective controlled study. Asian J. Surg. 2022, 45, 1530–1534. [Google Scholar] [CrossRef]


| Study, Year | Study Design | Arm | Number of Patients | Same Patients | Age | Smoker, % | DM, % | BMI, kg/m2 | Follow-Up, Months | Histology, % | Index Penile Surgery, % | Indication for ILND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tobias-Machado 2008 [11] | Prospective randomised | OILND | 10 | Yes (one on each side; 20 groins) | 48 (39–60) | NR | NR | NR | 19 (12–31) | SCC | Penile amputation | Prophylactic (cN0) |
| VEIL | 19 (12–31) | SCC | ||||||||||
| Yadav 2018 [12] | Prospective randomised | OILND | 29 | Yes (one on each side; 58 groins) | 52.4 | NR | NR | NR | 14 (7–28) | SCC | Partial or total | Mixed (therapeutic and prophylactic) |
| Schwentner 2013 [14] | Retrospective | OILND | 26 (34 cases) | No | 59 | NR | NR | NR | 56 (2–87) | Penile 64%, melanoma 29% | NR | NR |
| VEIL | 16 (28 cases) | 63 | NR | NR | NR | |||||||
| Falcone 2024 [13] | Prospective randomised | OILND | 14 | Yes (one on each side; 28 groins) | 63 (57–69) | 14 | NR | 29 (26–32) | 14 (12–17) | NR | NR | Mixed (therapeutic and prophylactic) |
| VEIL | 14 | |||||||||||
| Brassetti 2024 [15] | Retrospective | OILND | 38 | No | 58 (53–67] | NR | 34 | 26 (24–30) | 96 | SCC | Partial or total | Therapeutic (cN2) |
| RA-VEIL | 9 | 68 (52–73) | NR | 33 | 26 (25–29) | SCC | ||||||
| Ma 2022 [16] | Retrospective | OILND | 19 | No | 52 ± 13 | NR | NR | 26 (23–28) | 48 (34–60) | SCC | NR | Therapeutic (cN1/2) |
| S-VEIL | 24 | 56 ± 9.3 | NR | NR | 24 (22–26) | 36 (30–42) | SCC | |||||
| D-VEIL | 22 | 55 ± 11 | NR | NR | 23 (22–25] | 34 (26–47) | SCC | |||||
| Singh 2018 [17] | Retrospective | OILND | 100 | No | 54 (45–64) | 37 | 30 | 25 (23–29] | 40 (26–59) | SCC | NR | Therapeutic (cN1/2) |
| RA-VEIL | 51 | 58 (50–68) | 41 | 33 | 26 (23–31) | 41 (28–57) | SCC | |||||
| Yu 2019 [18] | Retrospective | OILND | 10 | No | 55 ± 13 | NR | NR | 27 (22–30) | 53 (25–70) | SCC | NR | Therapeutic (cN1–3) |
| RA-VEIL | 9 | 50 ± 7.2 | NR | NR | 27 (22–33) | 25 (15–29) | SCC | |||||
| Fankhauser 2022 [19] | Retrospective | OILND | 251 | No | 63 ± 13 | NR | 13 | 29 (19–47) | 21 (8–54) | SCC 85, basaloid 10, sarcomatoid 3% | Circumcision/WLE 17, glansectomy 15, partial 48, total 18 | Therapeutic (Positive FNAC/DSNB) |
| VEIL | 40 | 29 (19–47) | 12 (4–17) | |||||||||
| Kumar 2017 [20] | Retrospective | OILND | 22 | No | 70 | NR | NR | NR | 71 (30–99) | NR | NR | Therapeutic (Positive DSNB) |
| VEIL | 20 | 66 | NR | NR | NR | 16 (4–35) | NR | |||||
| Ozambela 2024 [21] | Retrospective | OILND | 35 | No | 68 (51–74) | 54 | NR | 31 (26–34) | 33 | SCC | Partial 66, radical 14, WLE 20 | Therapeutic (Positive FNAC/DSNB) |
| RA-VEIL | 24 | 65 (54–71) | 67 | NR | 30 (28–38) | 40 | SCC | Partial 83, radical 13, WLE 4 | ||||
| Bada 2023 [22] | Retrospective | OILND | 26 | No | 59 ± 9.9 | 62 | 61 | 26 (23–31) | 60 | SCC | Glansectomy 35, partial 31, total 7.7 | Mixed (therapeutic and prophylactic) |
| VEIL | 29 | 62 ± 12 | 24 | 34 | 26 (23–29) | SCC | Glansectomy 24, partial 17, total 17 | |||||
| Shao 2022 [23] | Retrospective | OILND | 69 | No | 51 ± 13 | 57 | NR | NR | 43 (15–87) | SCC | Partial or radical penectomy | Mixed (therapeutic and prophylactic) |
| VEIL | 40 | 51 ± 13 | 48 | NR | NR | SCC | ||||||
| Thyavihally 2021 [24] | Retrospective | OILND | 32 | No | 60 (54–62) | 50 | NR | 25 (24–28) | 51 (26–76) | SCC | Partial 66, total 34 | Mixed (therapeutic and prophylactic) |
| VEIL | 47 | 58 (50–62) | 34 | NR | 26 (24–28) | 42 (21–62) | SCC | Partial 60, total 40 | ||||
| Ye 2018 [25] | Retrospective | OILND | 62 | No | 54 (33–82) | NR | NR | 23 (16–34) | 22 (14–47) | NR | NR | Mixed (therapeutic and prophylactic) |
| VEIL | 31 | 54 (34–79) | NR | NR | 24 (17–32) | NR | ||||||
| Wang 2017 [26] | Retrospective | OILND | 18 (3 bilateral) | No | 59 ± 8.4 | NR | NR | NR | 12 | SCC | Penile amputation or radical resection | Mixed (therapeutic and prophylactic) |
| VEIL | 16 (3 bilateral) | 54 ± 9.9 | NR | NR | NR | SCC |
| Outcome | Relative Effect (95% CI) | N (Studies) | p-Value | Heterogeneity (%) | Certainty of Evidence (GRADE) |
|---|---|---|---|---|---|
| Recurrence (overall) | RR 0.77 (0.64–0.92) | 593 (7 studies) | 0.01 | 0 | ⊕⊕⊕⊝ Moderate * |
| Recurrence (local) | RR 0.85 (0.38–1.92) | 559 (7 studies) | 0.65 | 0 | ⊕⊕⊕⊝ Moderate * |
| Total operative time | MD 26 (−1; 54) | 1079 (13 studies) | 0.06 | 94 | ⊕⊕⊝⊝ Low *^ |
| Estimated blood loss | MD −61 (−150; 29) | 510 (6 studies) | 0.14 | 99 | ⊕⊕⊝⊝ Low *^ |
| Lymph node yield | MD 0.3 (−0.3; 0.8) | 1198 (16 studies) | 0.31 | 26 | ⊕⊕⊕⊝ Moderate * |
| Lymph node positivity | RR 0.98 (0.88–1.10) | 831 (13 studies) | 0.75 | 5.5 | ⊕⊕⊕⊝ Moderate * |
| Clavien–Dindo 1–2 complications | RR 0.65 (0.45–0.94) | 860 (12 studies) | 0.02 | 65 | ⊕⊕⊝⊝ Low *^ |
| Clavien–Dindo 3–4 complications | RR 0.25 (0.12–0.53) | 746 (11 studies) | 0.002 | 39 | ⊕⊕⊕⊝ Moderate * |
| Wound infection | RR 0.43 (0.22–0.82) | 965 (10 studies) | 0.02 | 72 | ⊕⊕⊝⊝ Low *^ |
| Lymphedema | RR 0.77 (0.43–1.39) | 1051 (12 studies) | 0.36 | 62 | ⊕⊕⊝⊝ Low *^ |
| Lymphocele/seroma | RR 0.96 (0.71–1.30) | 1104 (14 studies) | 0.10 | 34 | ⊕⊕⊕⊝ Moderate * |
| Deep venous thrombosis | RR 0.31 (0.07–1.41) | 629 (3 studies) | 0.10 | 0 | ⊕⊕⊕⊝ Moderate * |
| Skin/flap necrosis | RR 0.40 (0.12–1.33) | 599 (7 studies) | 0.11 | 56 | ⊕⊕⊝⊝ Low *^ |
| Length of hospital stay | MD −4 (−6; −2) | 757 (8 studies) | 0.005 | 94 | ⊕⊕⊝⊝ Low *^ |
| Time to drain removal | MD −1 (−6; 4) | 903 (10 studies) | 0.79 | 89 | ⊕⊕⊝⊝ Low *^ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.G.; Fong, K.Y.; Goh, N.K.-J.; Lee, A.Y.; Tay, K.J.; Yuen, J.S.; Abern, M.R.; Chen, K. Comparing the Perioperative and Oncological Outcomes of Open Versus Minimally Invasive Inguinal Lymphadenectomy in Penile Cancer: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 3035. https://doi.org/10.3390/cancers17183035
Tan YG, Fong KY, Goh NK-J, Lee AY, Tay KJ, Yuen JS, Abern MR, Chen K. Comparing the Perioperative and Oncological Outcomes of Open Versus Minimally Invasive Inguinal Lymphadenectomy in Penile Cancer: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(18):3035. https://doi.org/10.3390/cancers17183035
Chicago/Turabian StyleTan, Yu Guang, Khi Yung Fong, Nathanael Kai-Jun Goh, Alvin YM Lee, Kae Jack Tay, John SP Yuen, Michael R. Abern, and Kenneth Chen. 2025. "Comparing the Perioperative and Oncological Outcomes of Open Versus Minimally Invasive Inguinal Lymphadenectomy in Penile Cancer: A Systematic Review and Meta-Analysis" Cancers 17, no. 18: 3035. https://doi.org/10.3390/cancers17183035
APA StyleTan, Y. G., Fong, K. Y., Goh, N. K.-J., Lee, A. Y., Tay, K. J., Yuen, J. S., Abern, M. R., & Chen, K. (2025). Comparing the Perioperative and Oncological Outcomes of Open Versus Minimally Invasive Inguinal Lymphadenectomy in Penile Cancer: A Systematic Review and Meta-Analysis. Cancers, 17(18), 3035. https://doi.org/10.3390/cancers17183035








