Centralized Surgical Care Improves Survival in Non-Functional Well-Differentiated Pancreatic Neuroendocrine Tumors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Study Population
2.3. Variables and Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival Outcomes by Treatment Setting
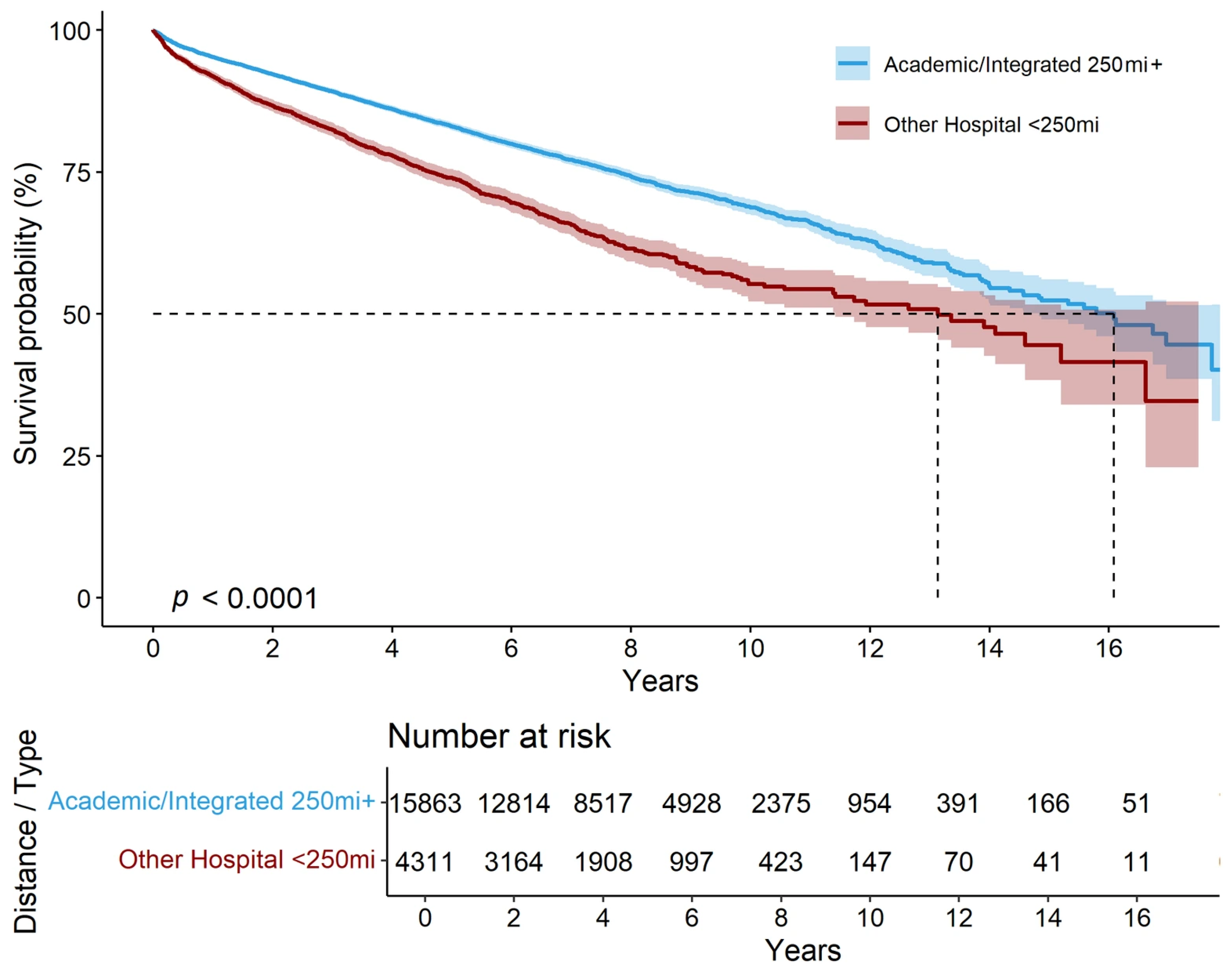
3.3. Impact of Primary Tumor Resection and Systemic Therapies
3.4. Cox Proportional Hazards Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Assi, H.A.; Mukherjee, S.; Kunz, P.L.; Machiorlatti, M.; Vesely, S.; Pareek, V.; Hatoum, H. Surgery Versus Surveillance for Well-Differentiated, Nonfunctional Pancreatic Neuroendocrine Tumors: An 11-Year Analysis of the National Cancer Database. Oncologist 2020, 25, e276–e283. [Google Scholar] [CrossRef]
- de Wilde, R.F.; Edil, B.H.; Hruban, R.H.; Maitra, A. Well-differentiated pancreatic neuroendocrine tumors: From genetics to therapy. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 199–208. [Google Scholar] [CrossRef]
- Sugawara, T.; Rodriguez Franco, S.; Kirsch, M.J.; Colborn, K.L.; Ishida, J.; Grandi, S.; Al-Musawi, M.H.; Gleisner, A.; Del Chiaro, M.; Schulick, R.D. Evaluation of Survival Following Surgical Resection for Small Nonfunctional Pancreatic Neuroendocrine Tumors. JAMA Netw. Open 2023, 6, e234096. [Google Scholar] [CrossRef] [PubMed]
- Perren, A.; Couvelard, A.; Scoazec, J.Y.; Costa, F.; Borbath, I.; Delle Fave, G.; Gorbounova, V.; Gross, D.; Grossma, A.; Jense, R.T.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology 2017, 105, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Masui, T.; Komoto, I.; Doi, R.; Osamura, R.Y.; Sakurai, A.; Ikeda, M.; Takano, K.; Igarashi, H.; Shimatsu, A.; et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: Diagnosis, treatment, and follow-up: A synopsis. J. Gastroenterol. 2021, 56, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Patel, R.K.; Sutton, T.L.; Schwantes, I.R.; Behrens, S.; Pommier, R.F.; Rocha, F.G.; Sheppard, B.C. Care at high-volume centers is associated with improved outcomes for patients with pancreatic neuroendocrine tumors: A population-level analysis. J. Surg. Oncol. 2023, 127, 956–965. [Google Scholar] [CrossRef]
- Underwood, P.W.; Riner, A.N.; Neal, D.; Cameron, M.E.; Yakovenko, A.; Reddy, S.; Rose, J.B.; Hughes, S.J.; Trevino, J.G. It’s more than just cancer biology: Health disparities in patients with pancreatic neuroendocrine tumors. J. Surg. Oncol. 2021, 124, 1390–1401. [Google Scholar] [CrossRef]
- Sakowitz, S.; Bakhtiyar, S.S.; Mallick, S.; Yanagawa, J.; Benharash, P. Travel to High-Volume Centers and Survival After Esophagectomy for Cancer. JAMA Surg. 2025, 160, 19–27. [Google Scholar] [CrossRef]
- Alnajar, A.; Kareff, S.A.; Razi, S.S.; Rao, J.S.; De Lima Lopes, G.; Nguyen, D.M.; Villamizar, N.; Rodriguez, E. Disparities in Survival Due to Social Determinants of Health and Access to Treatment in US Patients with Operable Malignant Pleural Mesothelioma. JAMA Netw. Open 2023, 6, e234261. [Google Scholar] [CrossRef]
- Daruvala, A.; Lucas, F.L.; Sammon, J.; Darus, C.; Bradford, L. Impact of geography and travel distance on outcomes in epithelial ovarian cancer: A national cancer database analysis. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 209–214. [Google Scholar] [CrossRef]
- Vetterlein, M.W.; Löppenberg, B.; Karabon, P.; Dalela, D.; Jindal, T.; Sood, A.; Chun, F.K.; Trinh, Q.D.; Menon, M.; Abdollah, F. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer 2017, 123, 3241–3252. [Google Scholar] [CrossRef]
- Xu, Z.; Becerra, A.Z.; Justiniano, C.F.; Boodry, C.I.; Aquina, C.T.; Swanger, A.A.; Temple, L.K.; Fleming, F.J. Is the Distance Worth It? Patients With Rectal Cancer Traveling to High-Volume Centers Experience Improved Outcomes. Dis. Colon Rectum 2017, 60, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Suss, N.R.; Abou Azar, S.; Memeh, K.; Shogan, B.D.; Keutgen, X.M.; Vaghaiwalla, T.M. Treatment at Academic Facilities Is Associated With Improved Survival in Late-Stage Colonic Neuroendocrine Tumors. J. Surg. Res. 2025, 310, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lidsky, M.E.; Sun, Z.; Nussbaum, D.P.; Adam, M.A.; Speicher, P.J.; Blazer, D.G., 3rd. Going the Extra Mile: Improved Survival for Pancreatic Cancer Patients Traveling to High-volume Centers. Ann. Surg. 2017, 266, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Onega, T.; Duell, E.J.; Shi, X.; Wang, D.; Demidenko, E.; Goodman, D. Geographic access to cancer care in the US. Cancer 2008, 112, 909–918. [Google Scholar] [CrossRef]
- Perry, R.R.; Feliberti, E.C.; Hughes, M.S. Management of Pancreatic Neuroendocrine Tumors: Surgical Strategies and Controversies. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2024, 30, 908–916. [Google Scholar] [CrossRef]
- Wu, C.; Song, Z.; Balachandra, S.; Dream, S.; Chen, H.; Rose, J.B.; Bhatia, S.; Gillis, A. Charting the Course: Insights into Neuroendocrine Tumor Dynamics in the United States. Ann. Surg. 2025, 281, 968–975. [Google Scholar] [CrossRef]
- Blank, L.; Baxter, S.; Woods, H.B.; Goyder, E.; Lee, A.; Payne, N.; Rimmer, M. Referral interventions from primary to specialist care: A systematic review of international evidence. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2014, 64, e765–e774. [Google Scholar] [CrossRef]
- Forrest, L.F.; Sowden, S.; Rubin, G.; White, M.; Adams, J. Socio-economic inequalities in patient, primary care, referral, diagnostic, and treatment intervals on the lung cancer care pathway: Protocol for a systematic review and meta-analysis. Syst. Rev. 2014, 3, 30. [Google Scholar] [CrossRef]
- Stitzenberg, K.B.; Sigurdson, E.R.; Egleston, B.L.; Starkey, R.B.; Meropol, N.J. Centralization of cancer surgery: Implications for patient access to optimal care. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4671–4678. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Ko, C.Y.; Tomlinson, J.S.; Stewart, A.K.; Talamonti, M.S.; Hynes, D.L.; Winchester, D.P.; Bentrem, D.J. Wait times for cancer surgery in the United States: Trends and predictors of delays. Ann. Surg. 2011, 253, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Kurita, Y.; Hara, K.; Kuwahara, T.; Mizuno, N.; Okuno, N.; Haba, S.; Okuno, M.; Natsume, S.; Senda, Y.; Kubota, K.; et al. Comparison of prognosis between observation and surgical resection groups with small sporadic non-functional pancreatic neuroendocrine neoplasms without distant metastasis. J. Gastroenterol. 2020, 55, 543–552. [Google Scholar] [CrossRef]
- Arra, D.A.S.M.; Ribeiro, H.S.C.; Henklain, G.; Barbosa, A.; Torres, S.M.; Diniz, A.L.; Godoy, A.L.; Farias, I.C.; Costa, W.L., Jr.; Coimbra, F.J.F. Surgery or active surveillance for pNETs < 2 cm: Preliminary results from a single center Brazilian cohort. J. Surg. Oncol. 2022, 126, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Barenboim, A.; Lahat, G.; Nachmany, I.; Nakache, R.; Goykhman, Y.; Geva, R.; Osher, E.; Scapa, E.; Wolf, I.; Orbach, L.; et al. Resection Versus Observation of Small Asymptomatic Nonfunctioning Pancreatic Neuroendocrine Tumors. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2020, 24, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, S.M.; In, H.; Winchester, D.J.; Talamonti, M.S.; Baker, M.S. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2015, 19, 117–123. [Google Scholar] [CrossRef]
- Chivukula, S.V.; Tierney, J.F.; Hertl, M.; Poirier, J.; Keutgen, X.M. Operative resection in early stage pancreatic neuroendocrine tumors in the United States: Are we over- or undertreating patients? Surgery 2020, 167, 180–186. [Google Scholar] [CrossRef]
- Haynes, A.B.; Deshpande, V.; Ingkakul, T.; Vagefi, P.A.; Szymonifka, J.; Thayer, S.P.; Ferrone, C.R.; Wargo, J.A.; Warshaw, A.L.; Fernández-del Castillo, C. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: Short-term and long-term patient outcomes. Arch. Surg. 2011, 146, 534–538. [Google Scholar] [CrossRef]
- Ben, Q.; Zhong, J.; Fei, J.; Chen, H.; Yv, L.; Tan, J.; Yuan, Y. Risk Factors for Sporadic Pancreatic Neuroendocrine Tumors: A Case-Control Study. Sci. Rep. 2016, 6, 36073. [Google Scholar] [CrossRef]
- Maharjan, C.K.; Ear, P.H.; Tran, C.G.; Howe, J.R.; Chandrasekharan, C.; Quelle, D.E. Pancreatic Neuroendocrine Tumors: Molecular Mechanisms and Therapeutic Targets. Cancers 2021, 13, 5117. [Google Scholar] [CrossRef]
- Vaghaiwalla, T.; Keutgen, X.M. Surgical Management of Pancreatic Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2020, 29, 243–252. [Google Scholar] [CrossRef]
- Chang, A.; Sherman, S.K.; Howe, J.R.; Sahai, V. Progress in the Management of Pancreatic Neuroendocrine Tumors. Annu. Rev. Med. 2022, 73, 213–229. [Google Scholar] [CrossRef]

| Characteristic | Overall, n = 20,174 1 | Non-Academic Hospitals, n = 4908 1 | Academic/Integrated Hospitals, n = 15,266 1 | p-Value 2 |
|---|---|---|---|---|
| Age at Diagnosis | 62 (52, 70) | 65 (57, 73) | 61 (51, 70) | <0.001 |
| Age > 65 Years | 8730 (43%) | 2585 (53%) | 6145 (40%) | <0.001 |
| Sex | 0.12 | |||
| Female | 9272 (46%) | 2192 (45%) | 7080 (46%) | |
| Male | 10,902 (54%) | 2716 (55%) | 8186 (54%) | |
| Private Insurance | 9409 (47%) | 1987 (40%) | 7422 (49%) | <0.001 |
| Hospital Distance (mi) | <0.001 | |||
| 0 to 12.49 | 6966 (35%) | 2251 (46%) | 4715 (31%) | |
| 12.5 to 249 | 12,624 (63%) | 2617 (53%) | 10,007 (66%) | |
| 250+ | 584 (2.9%) | 40 (0.8%) | 544 (3.6%) | |
| Hospital Volume (tertiles) | <0.001 | |||
| High | 10,841 (54%) | 1088 (22%) | 9753 (64%) | |
| Moderate | 6623 (33%) | 2257 (46%) | 4366 (29%) | |
| Low | 2710 (13%) | 1563 (32%) | 1147 (7.5%) | |
| Charlson/Deyo Score | <0.001 | |||
| 0 | 14,024 (70%) | 3247 (66%) | 10,777 (71%) | |
| 1 | 4036 (20%) | 1106 (23%) | 2930 (19%) | |
| 2 | 1214 (6.0%) | 318 (6.5%) | 896 (5.9%) | |
| 3 | 900 (4.5%) | 237 (4.8%) | 663 (4.3%) | |
| Pathological Stage | <0.001 | |||
| Stage I | 12,962 (64%) | 3258 (66%) | 9704 (64%) | |
| Stage II | 4305 (21%) | 943 (19%) | 3362 (22%) | |
| Stage III | 1171 (5.8%) | 256 (5.2%) | 915 (6.0%) | |
| Stage IV | 1736 (8.6%) | 451 (9.2%) | 1285 (8.4%) | |
| Tumor Size (mm) | 24 (15, 38) | 24 (16, 40) | 24 (15, 37) | <0.001 |
| Univariable Module | Multivariable Module | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR 1 | 95% CI 1 | p-Value | HR 1 | 95% CI 1 | p-Value |
| Hospital Distance (mi) | ||||||
| 0 to 12.49 | — | — | ||||
| 12.5 to 249 | 0.85 | 0.80, 0.91 | <0.001 | |||
| 250+ | 0.52 | 0.42, 0.65 | <0.001 | |||
| Facility Type | ||||||
| Academic | — | — | ||||
| Integrated | 1.24 | 1.14, 1.36 | <0.001 | |||
| Community | 1.76 | 1.64, 1.89 | <0.001 | |||
| Non-Community Hospital Distance | ||||||
| Community (within 250 mi) | — | — | ||||
| Integrated and Academic (beyond 250 mi) | 0.44 | 0.34, 0.55 | <0.001 | |||
| Integrated and Academic (within 250 mi) | 0.62 | 0.58, 0.67 | <0.001 | |||
| Community Hospital Types within 250 mi | ||||||
| Community (within 250 mi) | — | — | ||||
| Comprehensive Community (within 250 mi) | 0.84 | 0.68, 1.03 | 0.090 | |||
| Other facility types | 0.52 | 0.43, 0.64 | <0.001 | |||
| Non-Academic Hospitals < 250 mi | 1.62 | 1.51, 1.74 | <0.001 | 1.21 | 1.12, 1.31 | <0.001 |
| Age > 65 Years | 2.34 | 2.19, 2.49 | <0.001 | 1.70 | 1.57, 1.85 | <0.001 |
| Female Sex | 0.77 | 0.73, 0.83 | <0.001 | 0.84 | 0.79, 0.90 | <0.001 |
| African American (Ref: White) | 1.01 | 0.92, 1.11 | 0.9 | 1.01 | 0.92, 1.12 | 0.8 |
| Private Insurance | 0.45 | 0.42, 0.48 | <0.001 | 0.70 | 0.65, 0.76 | <0.001 |
| Charlson/Deyo Score | ||||||
| 0 | — | — | — | — | ||
| 1 | 1.23 | 1.14, 1.32 | <0.001 | 1.18 | 1.09, 1.27 | <0.001 |
| 2 | 1.58 | 1.41, 1.78 | <0.001 | 1.42 | 1.26, 1.60 | <0.001 |
| 3 | 2.34 | 2.07, 2.65 | <0.001 | 1.93 | 1.71, 2.19 | <0.001 |
| Tumor Size | ||||||
| <1 cm | — | — | — | — | ||
| 1–1.5 cm | 0.84 | 0.71, 0.98 | 0.026 | 0.81 | 0.69, 0.95 | 0.009 |
| 1.6–2 cm | 0.96 | 0.81, 1.13 | 0.6 | 0.99 | 0.84, 1.17 | >0.9 |
| >2 cm | 1.80 | 1.57, 2.06 | <0.001 | 1.71 | 1.49, 1.97 | <0.001 |
| Tumor Grade | ||||||
| G1 | — | — | — | — | ||
| G2 | 1.33 | 1.24, 1.43 | <0.001 | 1.24 | 1.15, 1.33 | <0.001 |
| Pathological Stage | ||||||
| Stage I | — | — | — | — | ||
| Stage II | 0.67 | 0.61, 0.74 | <0.001 | 0.88 | 0.79, 0.97 | 0.012 |
| Stage III | 0.75 | 0.62, 0.90 | 0.002 | 0.98 | 0.81, 1.19 | 0.9 |
| Stage IV | 2.41 | 2.21, 2.62 | <0.001 | 1.90 | 1.74, 2.08 | <0.001 |
| Hospital Volume (tertiles) | ||||||
| High | — | — | — | — | ||
| Moderate | 1.34 | 1.25, 1.43 | <0.001 | 1.20 | 1.12, 1.29 | <0.001 |
| Low | 1.81 | 1.66, 1.97 | <0.001 | 1.25 | 1.14, 1.37 | <0.001 |
| Primary Tumor Resection | 0.31 | 0.29, 0.33 | <0.001 | 0.36 | 0.33, 0.38 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnajar, A.; Collier, A.; Akcin, M.; Lew, J.I.; Vaghaiwalla, T.M. Centralized Surgical Care Improves Survival in Non-Functional Well-Differentiated Pancreatic Neuroendocrine Tumors. Cancers 2025, 17, 3030. https://doi.org/10.3390/cancers17183030
Alnajar A, Collier A, Akcin M, Lew JI, Vaghaiwalla TM. Centralized Surgical Care Improves Survival in Non-Functional Well-Differentiated Pancreatic Neuroendocrine Tumors. Cancers. 2025; 17(18):3030. https://doi.org/10.3390/cancers17183030
Chicago/Turabian StyleAlnajar, Ahmed, Amber Collier, Mehmet Akcin, John I. Lew, and Tanaz M. Vaghaiwalla. 2025. "Centralized Surgical Care Improves Survival in Non-Functional Well-Differentiated Pancreatic Neuroendocrine Tumors" Cancers 17, no. 18: 3030. https://doi.org/10.3390/cancers17183030
APA StyleAlnajar, A., Collier, A., Akcin, M., Lew, J. I., & Vaghaiwalla, T. M. (2025). Centralized Surgical Care Improves Survival in Non-Functional Well-Differentiated Pancreatic Neuroendocrine Tumors. Cancers, 17(18), 3030. https://doi.org/10.3390/cancers17183030






