Artificial Intelligence and Machine Learning in Sexual Health and Dysfunction Across the Cancer Care Continuum: A Systematic Review
Simple Summary
Abstract
1. Introduction
- What are the key applications of AI-ML models in managing sexual health in oncology?
- Which AI-ML algorithms are commonly used to address sexual health and SD throughout every stage of cancer care?
- Which AI-ML models have demonstrated the highest performance in predicting sexual health outcomes?
- What is the overall quality of studies applying AI and ML techniques to sexual health in cancer care, including adherence to TRIPOD+AI guidelines for transparent and accurate model performance reporting and the percentage of risk of bias?
- To what extent are AI and ML effective in improving sexual health and predicting SD among cancer patients?
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Strategy and Screening Process
- Inclusion criteria: Studies eligible to be included in this systematic review had to meet all of these criteria: (1) studies applied AI or ML models in sexual health or sexual dysfunction prediction or management (i.e., either directly or indirectly related to sexual health), (2) studies in the cancer population or in the cancer care continuum (e.g., cancer screening, detection, diagnosis, treatment, or survivorship), (3) full original articles in English, (4) studies involved human subjects, and (5) models were tested for performance.
- Exclusion criteria: Articles were excluded if they met any of the following criteria: (1) the study was beyond the study aim/scope, (2) no AI or ML model was applied, (3) not an original study (i.e., review article, letter, conference abstract, editorial, or response), (4) no sexual health, sexual disease, or dysfunction (biological sex, gender, or sexual minority topics were excluded), (5) not in cancer care or oncology fields, (6) duplicate publication or correction of an original article, and (7) descriptive study that did not apply or test a model.
2.3. Data Synthesis, Collection, and Analysis
2.3.1. Data Extraction
2.3.2. Presentation of Results
2.3.3. Synthesis Methods
2.3.4. Exploring Heterogeneity
2.3.5. Sensitivity Analysis
2.4. Data Quality and Risk of Bias
3. Results
3.1. Search and Screening Results
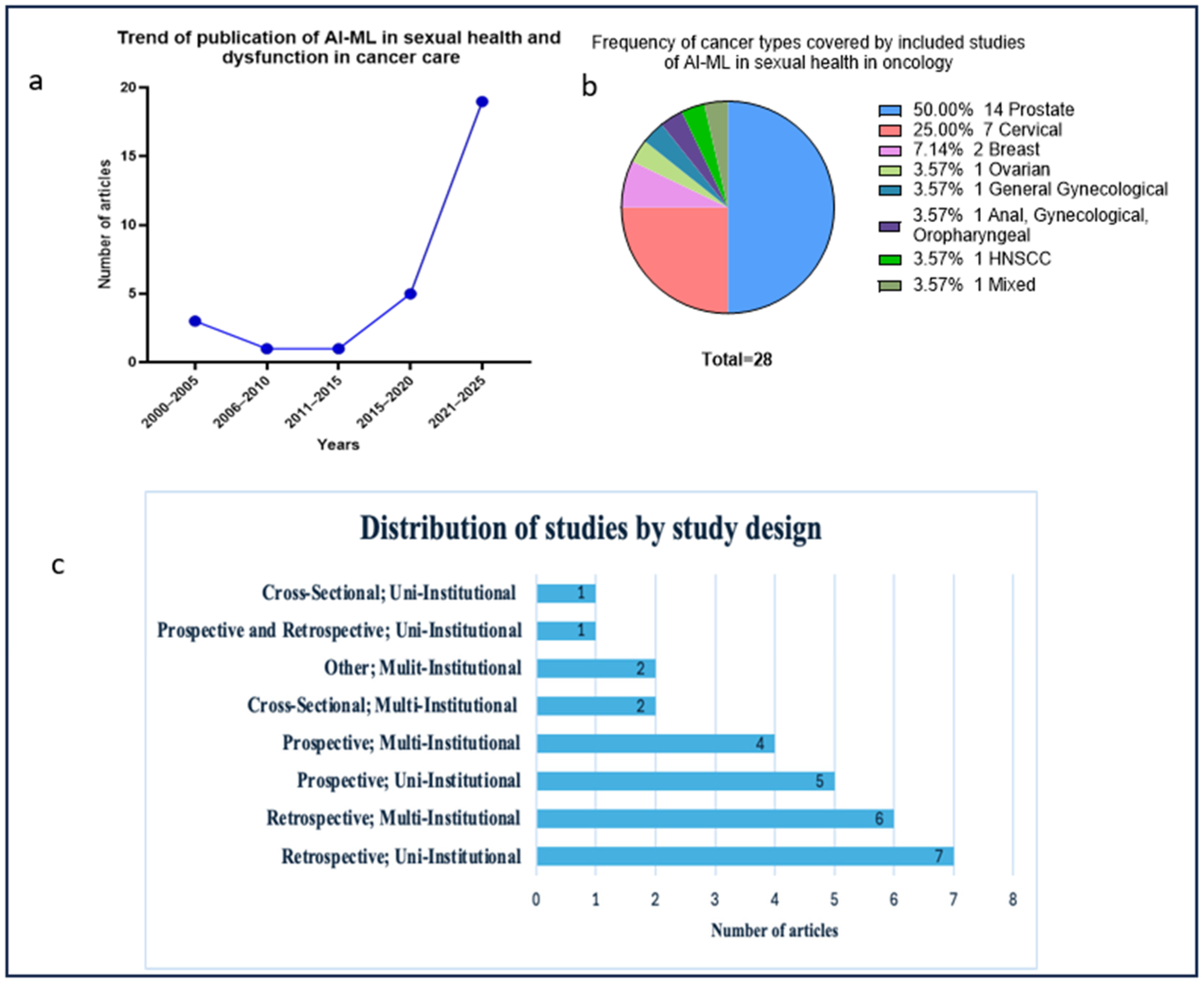
3.2. Publication Trends, Design, and Populations
3.3. Artificial Intelligence and Machine Learning Models Used in Sexual Health Across Cancer Care Continuum
3.3.1. Early Detection, Prevention, and Diagnosis Phases
3.3.2. Automatic Data Extraction
3.3.3. During the Treatment Stage
3.3.4. Post-Treatment and Survivorship Phase
3.3.5. Diagnostic Imaging Analysis
3.3.6. Assessment Tools for Sexual Health in Cancer Care
3.4. Types, Frequency, and Performance of Artificial Intelligence and Machine Learning Models Used in Sexual Health in Oncology
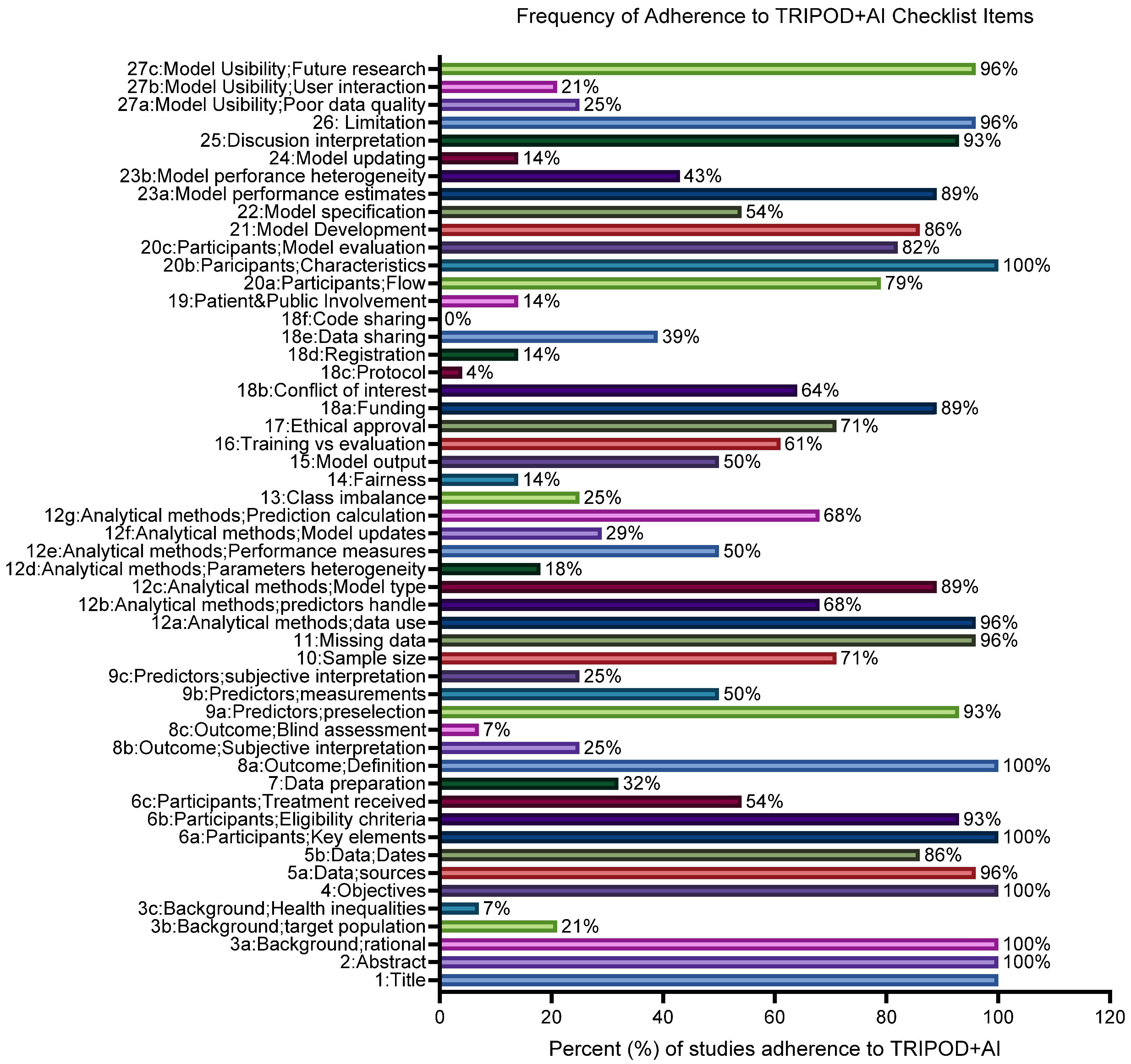
3.5. Quality of Studies Including Adherence to Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis Guidelines and Risk-of-Bias Assessment
4. Discussion
4.1. Future Studies
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QoL | Quality of life |
| SD | Sexual dysfunction |
| PRO | Patient-reported outcome |
| STI | Sexually transmitted infections |
| IUD | Intrauterine device |
| ALND | Axillary lymph node dissection |
| SLNB | Sentinel lymph node biopsy |
| TURP | Prior transurethral resection of the prostate |
| HPV | Human papilloma virus |
| SF-36 | Medical Outcomes Study Short Form–36 Health Status Survey |
| CARES-SF | Cancer Rehabilitation Evaluation System–Short Form |
| UCLA | University of California at Los Angeles |
| HRQoL | Health-related quality of life |
| EPIC-26 | Expanded Prostate Cancer Index Composite-26 |
| SHIM | Sexual Health Inventory of Men |
| EORTC | European Organization for Research and Treatment of Cancer |
| QLQ C-30 | Quality of Life questionnaire |
| CX-24 | Cervical Cancer-24 |
| IIEF-15 | International Index of Erectile Function-15 |
| IIEF-5 | International Index of Erectile Function 5 |
| PROMIS | Patient-Reported Measurement Information System |
| NCBO | National Center for Biomedical Ontology |
| UMLS | Unified Medical Language System |
| ISUP | International Society of Urological Pathology |
| BREAST-Q | Breast-related Quality of life Questionnaire |
| IPSS | International Prostate Symptom Score |
| LR | Logistic regression |
| DT | Decision tree |
| SVM | Support vector machine |
| ANN | Artificial neural network |
| MLP | Multilayer perceptron |
| NLP | Natural language processing |
| GLM | General linear model |
| RF | Random forest |
| kNN | k-Nearest neighbor |
| NB | Naive Bayes |
| XGB-XGBoost | Extreme gradient boosting |
| GBDT | Gradient boosting decision tree |
| GBM | Gradient boosting model |
| SGB | Stochastic gradient boosting |
| MonMLP | Monotone multilayer perceptron neural network |
| SVMRadial | Support vector machine with radial basis function kernel |
| GaussPrRadial | Gaussian process with radial basis function kernel |
| SLDA | Stabilized linear discriminant |
| AdaBoost | AdaBoost classification tree |
| LMT | Logistic model tree |
| TreeBag | Bagged classification and regression tree |
| RgeLogistic | Regularized logistic regression |
| DL | Deep learning |
| NN | Neural network |
| FCN | Topological fully convolutional network |
| LSTM | Long short-term memory |
References
- Izci, F.; Ozdem, G.; Ilgun, A.S.; Agacayak, F.; Duymaz, T.; Erdogan, Z.; Alco, G.; Elbuken, F.; Ozturk, A.; Ordu, C.; et al. Pre-Treatment and Post-Treatment Anxiety, Depression, Sleep and Sexual Function Levels in Patients with Breast Cancer. Eur. J. Breast Health 2020, 16, 219–225. [Google Scholar]
- Soldera, S.V.; Ennis, M.; Lohmann, A.E.; Goodwin, P.J. Sexual health in long-term breast cancer survivors. Breast Cancer Res. Treat. 2018, 172, 159–166. [Google Scholar] [CrossRef]
- Schover, L.R. Sexual quality of life in men and women after cancer. Climacteric 2019, 22, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R. Sexuality and fertility after cancer. Hematol. Am. Soc. Hematol. Educ. Program. 2005, 2005, 523–527. [Google Scholar] [CrossRef]
- Mishra, N.; Singh, N.; Sachdeva, M.; Ghatage, P. Sexual Dysfunction in Cervical Cancer Survivors: A Scoping Review. Women’s Health Rep. 2021, 2, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Iborra, S.; Grimm, D.; Steinsiek, L.; Mahner, S.; Bossart, M.; Woelber, L.; Voss, P.J.; Gitsch, G.; Hasenburg, A. Sexual activity and quality of life in patients after treatment for breast and ovarian cancer. Arch. Gynecol. Obs. 2019, 299, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, W.; Schartau, P.; Kirby, M.; Jenkins, V.; Allen, S.; Payne, H. Sexual Dysfunction in Prostate Cancer Patients According to Disease Stage and Treatment Modality. Clin. Oncol. 2025, 41, 103801. [Google Scholar] [CrossRef]
- Tramacere, F.; Lancellotta, V.; Casa, C.; Fionda, B.; Cornacchione, P.; Mazzarella, C.; De Vincenzo, R.P.; Macchia, G.; Ferioli, M.; Rovirosa, A.; et al. Assessment of Sexual Dysfunction in Cervical Cancer Patients after Different Treatment Modality: A Systematic Review. Medicina 2022, 58, 1223. [Google Scholar] [CrossRef]
- Katz, A. Interventions for sexuality after pelvic radiation therapy and gynecological cancer. Cancer J. 2009, 15, 45–47. [Google Scholar] [CrossRef]
- Barcellini, A.; Dominoni, M.; Dal Mas, F.; Biancuzzi, H.; Venturini, S.C.; Gardella, B.; Orlandi, E.; Bo, K. Sexual Health Dysfunction After Radiotherapy for Gynecological Cancer: Role of Physical Rehabilitation Including Pelvic Floor Muscle Training. Front. Med. 2021, 8, 813352. [Google Scholar] [CrossRef]
- Karacan, Y.; Yildiz, H.; Demircioglu, B.; Ali, R. Evaluation of Sexual Dysfunction in Patients with Hematological Malignancies. Asia Pac. J. Oncol. Nurs. 2021, 8, 51–57. [Google Scholar] [CrossRef]
- Heinzler, J.; Brucker, J.; Bruckner, T.; Dinkic, C.; Hoffmann, J.; Dornhofer, N.; Seitz, S.; Sohn, C.; Rom, J.; Schott, T.C.; et al. Impact of a cervical dysplasia and its treatment on quality of life and sexual function. Arch. Gynecol. Obs. 2018, 298, 737–745. [Google Scholar] [CrossRef]
- Aerts, L.; Enzlin, P.; Verhaeghe, J.; Poppe, W.; Vergote, I.; Amant, F. Sexual functioning in women after surgical treatment for endometrial cancer: A prospective controlled study. J. Sex. Med. 2015, 12, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Guedes, T.S.R.; Guedes, M.B.O.G.; Santana, R.D.; da Silva, J.F.C.; Dantas, A.A.G.; Ochandorena-Acha, M.; Terradas-Monllor, M.; Jerez-Roig, J.; de Souza, D.L.B. Sexual Dysfunction in Women with Cancer: A Systematic Review of Longitudinal Studies. Int. J. Environ. Res. Public Health 2022, 19, 11921. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Ruggiero, M.; Maity, A.; Ko, K.; Greenberger, B.; Donofree, D.; Sherif, K.; Lazar, M.; Jaslow, R.; Richard, S.; et al. Sexual Health Toxicity in Cancer Survivors: Is There a Gender Disparity in Physician Evaluation and Intervention? Int. J. Radiat. Oncol. 2020, 108, S136. [Google Scholar] [CrossRef]
- Sadovsky, R.; Basson, R.; Krychman, M.; Morales, A.M.; Schover, L.; Wang, R.; Incrocci, L. Cancer and sexual problems. J. Sex. Med. 2010, 7 Pt 2, 349–373. [Google Scholar] [CrossRef]
- Eeltink, C.; Embaby, A.; Incrocci, L.; Ket, J.C.F.; Liptrott, S.J.; Verdonck-de Leeuw, I.; Zweegman, S. Sexual problems in patients with hematological diseases: A systematic literature review. Support. Care Cancer 2022, 30, 4603–4616. [Google Scholar] [CrossRef]
- Krouwel, E.M.; Albers, L.F.; Nicolai, M.P.J.; Putter, H.; Osanto, S.; Pelger, R.C.M.; Elzevier, H.W. Discussing Sexual Health in the Medical Oncologist’s Practice: Exploring Current Practice and Challenges. J. Cancer Educ. 2020, 35, 1072–1088. [Google Scholar] [CrossRef]
- Abou Chawareb, E.; Im, B.H.; Lu, S.; Hammad, M.A.M.; Huang, T.R.; Chen, H.; Yafi, F.A. Sexual health in the era of artificial intelligence: A scoping review of the literature. Sex. Med. Rev. 2025, 13, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Himel Mondal, S.M. The Role of Large Language Model Chatbots in Sexual Education: An Unmet Need of Research. J. Psychosex. Health 2025, 7, 120–127. [Google Scholar] [CrossRef]
- Saikali, S.; Reddy, S.; Gokaraju, M.; Goldsztein, N.; Dyer, A.; Gamal, A.; Jaber, A.; Moschovas, M.; Rogers, T.; Vangala, A.; et al. Development and Assessment of an AI-based Machine Learning Model for Predicting Urinary Continence and Erectile Function Recovery after Robotic-Assisted Radical Prostatectomy: Insights from a Prostate Cancer Referral Center. Comput. Methods Programs Biomed. 2025, 259, 108522. [Google Scholar] [CrossRef]
- Balagopal, A.; Dohopolski, M.; Suk Kwon, Y.; Montalvo, S.; Morgan, H.; Bai, T.; Nguyen, D.; Liang, X.; Zhong, X.; Lin, M.H.; et al. Deep learning based automatic segmentation of the Internal Pudendal Artery in definitive radiotherapy treatment planning of localized prostate cancer. Phys. Imaging Radiat. Oncol. 2024, 30, 100577. [Google Scholar] [CrossRef]
- Hanai, A.; Ishikawa, T.; Kawauchi, S.; Iida, Y.; Kawakami, E. Generative artificial intelligence and non-pharmacological bias: An experimental study on cancer patient sexual health communications. BMJ Health Care Inform. 2024, 31, e100924. [Google Scholar] [CrossRef]
- Agochukwu-Mmonu, N.; Murali, A.; Wittmann, D.; Denton, B.; Dunn, R.L.; Montie, J.; Peabody, J.; Miller, D.; Singh, K.; on behalf of the Michigan Urological Surgery Improvement Collaborative. Development and Validation of Dynamic Multivariate Prediction Models of Sexual Function Recovery in Patients with Prostate Cancer Undergoing Radical Prostatectomy: Results from the MUSIC Statewide Collaborative. Eur. Urol. Open Sci. 2022, 40, 1–8. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies: Explanation and Elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; Groupdagger, P. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Dhiman, P.; Andaur Navarro, C.L.; Ma, J.; Hooft, L.; Reitsma, J.B.; Logullo, P.; Beam, A.L.; Peng, L.; Van Calster, B.; et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 2021, 11, e048008. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Br. J. Surg. 2015, 102, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Salama, V.; Godinich, B.; Geng, Y.; Humbert-Vidan, L.; Maule, L.; Wahid, K.A.; Naser, M.A.; He, R.; Mohamed, A.S.R.; Fuller, C.D.; et al. Artificial Intelligence and Machine Learning in Cancer Pain: A Systematic Review. J. Pain Symptom Manag. 2024, 68, e462–e490. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Bacon, C.G.; Giovannucci, E.; Testa, M.; Glass, T.A.; Kawachi, I. The association of treatment-related symptoms with quality-of-life outcomes for localized prostate carcinoma patients. Cancer 2002, 94, 862–871. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Hunt, W.C.; Gilliland, F.D.; Stephenson, R.A.; Potosky, A.L. Patient satisfaction with treatment decisions for clinically localized prostate carcinoma. Results from the Prostate Cancer Outcomes Study. Cancer 2003, 97, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Zhang, H.H.; D’Souza, W.D.; Meyer, R.R.; Gillison, M.L. Moderate predictive value of demographic and behavioral characteristics for a diagnosis of HPV16-positive and HPV16-negative head and neck cancer. Oral Oncol. 2010, 46, 100–104. [Google Scholar] [CrossRef]
- Kumar, S.; Rana, M.L.; Verma, K.; Singh, N.; Sharma, A.K.; Maria, A.K.; Dhaliwal, G.S.; Khaira, H.K.; Saini, S. PrediQt-Cx: Post Treatment Health Related Quality of Life Prediction Model for Cervical Cancer Patients. PLoS ONE 2014, 9, e89851. [Google Scholar] [CrossRef]
- Barocas, D.A.; Alvarez, J.; Resnick, M.J.; Koyama, T.; Hoffman, K.E.; Tyson, M.D.; Conwill, R.; McCollum, D.; Cooperberg, M.R.; Goodman, M.; et al. Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 2017, 317, 1126–1140. [Google Scholar] [CrossRef]
- Hernandez-Boussard, T.; Kourdis, P.D.; Seto, T.; Ferrari, M.; Blayney, D.W.; Rubin, D.; Brooks, J.D. Mining Electronic Health Records to Extract Patient-Centered Outcomes Following Prostate Cancer Treatment. AMIA Annu. Symp. Proc. 2017, 2017, 876–882. [Google Scholar] [PubMed]
- Best, A.L.; Logan, R.G.; Vazquez-Otero, C.; Fung, W.; Chee, V.; Thompson, E.L.; Villalona, S.; Thompson, L.M.A.; Gwede, C.K.; Daley, E.M. Application of a Health Literacy Framework to Explore Patients’ Knowledge of the Link between HPV and Cancer. J. Health Commun. 2018, 23, 695–702. [Google Scholar] [CrossRef]
- Hussain, L.; Ali, A.; Rathore, S.; Saeed, S.; Idris, A.; Usman, M.U.; Iftikhar, M.A.; Suh, D.Y. Applying Bayesian Network Approach to Determine the Association Between Morphological Features Extracted from Prostate Cancer Images. IEEE Access 2019, 7, 1586–1601. [Google Scholar] [CrossRef]
- van Egdom, L.S.E.; Pusic, A.; Verhoef, C.; Hazelzet, J.A.; Koppert, L.B. Machine learning with PROs in breast cancer surgery; caution: Collecting PROs at baseline is crucial. Breast J. 2020, 26, 1213–1215. [Google Scholar] [CrossRef]
- Albers, L.F.; Tillier, C.N.; van Muilekom, E.; van Werkhoven, E.; Elzevier, H.W.; van Rhijn, B.W.G.; van der Poel, H.G.; Hendricksen, K. Sexual Satisfaction in Men Suffering From Erectile Dysfunction After Robot-Assisted Radical Prostatectomy for Prostate Cancer: An Observational Study. J. Sex. Med. 2021, 18, 339–346. [Google Scholar] [CrossRef]
- Bagshaw, H.P.; Martinez, A.; Heidari, N.; Scheinker, D.; Pollack, A.; Stoyanova, R.; Horwitz, E.; Morton, G.; Kishan, A.U.; Buyyounouski, M.K. A personalized decision aid for prostate cancer shared decision making. BMC Med. Inform. Decis. Mak. 2021, 21, 374. [Google Scholar] [CrossRef]
- Charoenkwan, P.; Shoombuatong, W.; Nantasupha, C.; Muangmool, T.; Suprasert, P.; Charoenkwan, K. iPMI: Machine Learning-Aided Identification of Parametrial Invasion in Women with Early-Stage Cervical Cancer. Diagnostics 2021, 11, 1454. [Google Scholar] [CrossRef]
- Chan, M.; Olson, R.; Lapointe, V.; Hamm, J.; Bachand, F.; Holloway, C.; Parsons, C.; Lim, P. Using a Weekly Patient-Reported Outcome Questionnaire to Track Acute Toxicity in Patients Undergoing Pelvic Radiotherapy for Gynecologic Cancers. Curr. Oncol. 2022, 29, 3306–3317. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Wang, S.; Lang, J.; Leng, J.; Fan, Q. The application of risk models based on machine learning to predict endometriosis-associated ovarian cancer in patients with endometriosis. Acta Obstet. Gynecol. Scand. 2022, 101, 1440–1449. [Google Scholar] [CrossRef]
- Gentile, F.; La Civita, E.; Della Ventura, B.; Ferro, M.; Cennamo, M.; Bruzzese, D.; Crocetto, F.; Velotta, R.; Terracciano, D. A Combinatorial Neural Network Analysis Reveals a Synergistic Behaviour of Multiparametric Magnetic Resonance and Prostate Health Index in the Identification of Clinically Significant Prostate Cancer. Clin. Genitourin. Cancer 2022, 20, E406–E410. [Google Scholar] [CrossRef]
- Sun, L.; Yang, L.; Liu, X.; Tang, L.; Zeng, Q.; Gao, Y.; Chen, Q.; Liu, Z.; Peng, B. Optimization of Cervical Cancer Screening: A Stacking-Integrated Machine Learning Algorithm Based on Demographic, Behavioral, and Clinical Factors. Front. Oncol. 2022, 12, 821453. [Google Scholar] [CrossRef]
- Deng, L.; Wang, T.; Chen, Y.; Tang, X.; Xiang, D. A predictive model for residual lesions after LEEP surgery in CIN III patients. Front. Med. 2023, 10, 1326833. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, R.; Navamani, T.M.; Rote, T.R.; Chauhan, I. Design and Development of an Efficient Risk Prediction Model for Cervical Cancer. IEEE Access 2023, 11, 74290–74300. [Google Scholar] [CrossRef]
- Hasannejadasl, H.; Roumen, C.; van der Poel, H.; Vanneste, B.; van Roermund, J.; Aben, K.; Kalendralis, P.; Osong, B.; Kiemeney, L.; Van Oort, I.; et al. Development and external validation of multivariate prediction models for erectile dysfunction in men with localized prostate cancer. PLoS ONE 2023, 18, e0276815. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, T.H.; Roper, J.; Tian, S.B.; Patel, P.; Bradley, J.D.; Jani, A.B.; Liu, T.; Yang, X.F. Automatic segmentation of neurovascular bundle on mri using deep learning based topological modulated network. Med. Phys. 2023, 50, 5479–5488. [Google Scholar] [CrossRef]
- Sibert, N.T.; Kurth, T.; Breidenbach, C.; Wesselmann, S.; Feick, G.; Carl, E.G.; Dieng, S.; Albarghouth, M.H.; Aziz, A.; Baltes, S.; et al. Prediction models of incontinence and sexual function one year after radical prostatectomy based on data from 20 164 prostate cancer patients. PLoS ONE 2023, 18, e0295179. [Google Scholar] [CrossRef]
- Xu, C.; Pfob, A.; Mehrara, B.J.; Yin, P.; Nelson, J.A.; Pusic, A.L.; Sidey-Gibbons, C. Enhanced Surgical Decision-Making Tools in Breast Cancer: Predicting 2-Year Postoperative Physical, Sexual, and Psychosocial Well-Being following Mastectomy and Breast Reconstruction (INSPiRED 004). Ann. Surg. Oncol. 2023, 30, 7046–7059. [Google Scholar] [CrossRef]
- Chauhan, R.; Goel, A.; Alankar, B.; Kaur, H. Predictive modeling and web-based tool for cervical cancer risk assessment: A comparative study of machine learning models. MethodsX 2024, 12, 102653. [Google Scholar] [CrossRef]
- Devi, S.; Gangarde, R.; Deokar, S.; Muqeemuddin, S.F.; Awasthi, S.R.; Shekhar, S.; Sonchhatra, R.; Joshi, S. Public health nurse perspectives on predicting nonattendance for cervical cancer screening through classification, ensemble, and deep learning models. Public Health Nurs. 2024, 41, 781–797. [Google Scholar] [CrossRef]
- Choi, M.S.; Chang, J.S.; Kim, K.; Kim, J.H.; Kim, T.H.; Kim, S.; Cha, H.; Cho, O.; Choi, J.H.; Kim, M.; et al. Assessment of deep learning-based auto-contouring on interobserver consistency in target volume and organs-at-risk delineation for breast cancer: Implications for RTQA program in a multi-institutional study. Breast 2024, 73, 103599. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Lu, W.T.; Zhang, D.; Tan, M.Y.; Qin, X. Development and validation of a prediction model for ED using machine learning: According to NHANES 2001–2004. Sci. Rep. 2024, 14, 27279. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Machado, N.; Bonfill-Cosp, X.; Quintana, M.J.; Santero, M.; Bartolo, A.; Olid, A.S. Sexual dysfunction in women with breast cancer: A systematic review. Support. Care Cancer 2025, 33, 332. [Google Scholar] [CrossRef] [PubMed]



| Author et al., Year | Journal | Type of Cancer | Number of Population/ Participant | AI-ML Type | Input Variables | Output Outcome | Conclusion | Sexual Health Assessment Tools | Application in Cancer Care |
|---|---|---|---|---|---|---|---|---|---|
| Bacon et al., 2002 [31] | Cancer An international Interdisciplinary Journal of the American Cancer Society | Prostate | 783 | Least square regression models. LR models | Sexual function, urinary function, bowel function, covariates: age, marital status, waist circumference, physical activity, smoking status, alcohol intake, comorbid conditions | Quality of life: general health-related and cancer-specific. Symptom bother: UCLA bother scales and SF-36 scales: impact of sexual, urinary, and bowel symptoms on overall and cancer-specific quality of life of prostate cancer patients. | Prostate cancer patients reported significantly greater bother and effect on QOL from sexual, urinary, and bowel symptoms. Sexual symptoms were strongly associated with worse QoL. | SF-36 CARES-SF UCLA Prostate Cancer Index (to assess sexual symptoms related to prostate cancer therapy) | Cancer survivorship |
| Hoffman et al., 2003 [32] | Cancer | Prostate | 2365 | LR | Age, race/ethnicity, treatment type (active vs. conservative), perception of being cancer-free, urinary and bowel functions, erectile function, general health status, and social support measures | Patient satisfaction with treatment decision two years after diagnosis with prostate cancer. | Most men were satisfied with their treatment selection for clinically localized prostate carcinoma. Men tended to minimize loss of urinary, bowel, or sexual functions by not perceiving dysfunction as a problem. | None reported | Post-treatment survivorship |
| D’Souza et al., 2010 [33] | Oral Oncol | Head and Neck (HNSCC) | 255 | DT SVM | Demographics: age, gender, race/ethnicity. Behavioral: tobacco use, alcohol use, sexual behavior (# of oral sex partners), income, education. Biomarkers: HPV16 L1 E6/E7, HPV16 DNA | Prediction of tumor HPV16 status (positive vs. negative) in HNSCC as determined by in situ hybridization | Models used only demographics (tobacco use, age, gender, race) with moderate predictive values: HNSCC: PPV = 75%, NPV = 68%; Oropharyngeal cancers only: PPV = 55%, NPV = 66%. Addition of HPV biomarkers improved predictions | None reported | Cancer prediction |
| Kumar, et al., 2014 [34] | PLoS ONE | Cervical | 198 | SVM with both linear and RBF kernels, multilayer perceptron (ANN), and regularized LR" | 13 variables: age, marital status, vaginal bleeding, vaginal discharge, dyspareunia, abdominal pain, weight loss, parity, bowel or bladder control difficulty, cervical cancer stage, treatment modality, lymphoedema, and peripheral neuropathy. | Three post-treatment HRQoL outcomes (including sexual function), at 6 months post-treatment in cervical cancers. | The prediction model (PrediQt-Cx), based on support vector machine (SVM) for predicting post-treatment HRQoL in cervical cancer patients was developed and internally cross validated. The performance of SVM (linear) exceeded other models in most domains (mean AUC of 0.90). Patients experienced substantial decrease in sexual activity post-treatment. | EORTC QLQ C-30 and CX-24, (HRQoL) questionnaires, | Post-treatment survivorship |
| Barocas et al., 2017 [35] | JAMA | Prostate | 2550 | Longitudinal regression model | Baseline domain scores (EPIC-26), age, race/ethnicity, comorbidity index, prostate cancer risk stratum (D’Amico classification), physical function (SF-36), social support, depression score, medical decision-making style, treatment type (radical prostatectomy, EBRT, active surveillance) | Patient-reported functional outcomes (EPIC-26 domains: sexual, urinary incontinence, urinary irritative, bowel, hormonal); health-related QoL (SF-36); disease-specific and overall survival after prostate cancer treatment. | Radical prostatectomy was associated with worse sexual function and urinary incontinence at 3 years compared to EBRT and active surveillance. Radical prostatectomy was associated with improved urinary irritative symptoms. No meaningful long-term differences in bowel or hormonal function. No significant differences in disease-specific survival (≥99.7%) | EPIC-26 | Post-treatment survivorship |
| Hernandez-Boussard, T., 2017 [36] | AMIA Annu Symp Proc | Prostate | 7109 | Natural Language Processing | Patients’ demographics, health care encounters, diagnosis/problem lists, unstructured EHR clinical notes/documents, lab & diagnosis results, ICD codes, CPT codes, medications, patient history, text features (affirmed, negated, risk discussion mentions of urinary incontinence and ED) | Extraction and classification of patient-centered outcomes (urinary incontinence and erectile dysfunction) from clinical notes of EHR. | "NLP pipeline developed and validated for detecting clinical mentions of patient-centered outcomes in prostate cancer patients, including UI and ED. This system showed performance sufficient for health management and treatment decisions." | "ICD-9/ICD-10, billing codes, medications, and vocabularies matched with existing ontologies from the NCBO and UMLS concepts" | Data extraction EHR |
| Best, A. L., 2018 [37] | Journal of Health Communication | Anal, gynecological, and oropharyngeal | 50 | Applied thematic framework and digital application | Sociodemographic of respondents (age, education level, household income, gender, sexual orientation, and marital status). Attitudes toward digital social prescribing, perceived benefits and challenges, technology acceptance metrics | Assessment, understanding, appraising, and applying cancer patients’ HPV information in the context of their cancer diagnosis. Perceptions of digital social prescribing platforms and identification of facilitators and barriers to implementation in mental health services | "The health literacy framework and the digital applications used to explore how patients diagnosed with HPV-associated cancers accessed, understood, appraised, and applied HPV information. | None reported | Data extraction STD diagnosis |
| Hussain et al., 2019 [38] | IEEE Access | Prostate | 682 | Bayesian Networks | MRI prostate cancer images, including morphological features: variables such as area, equidiameter, circulatory 1 and 2, elongatedness, entropy, maximum radius, minimum radius, and eccentricity | The associations between several key morphological features extracted from prostate cancer images. | "A Bayesian network quantified the association between different imaging morphological features of prostate cancers. | None reported | Imaging analysis |
| van Egdom et al., 2020 [39] | Breast J | Breast | 764 | GLM SVM ANN DL | Age, medical status, tumor characteristics, and possible (neo)adjuvant treatment indications/treatment characteristics | Prediction of patient-reported outcomes (PROs) post-surgery, including HRQoL, sexual, physical, and psychosocial function | No significant predictive relationship was found; model accuracy aligned with outcome prevalence, not effective for individual-level prediction. | EORTC QLQ-C30 EORTC QLQ-BR23 BREAST-Q | Post-treatment survivorship |
| Albers et al., 2021 [40] | J Sex Med (The Journal of Sexual Medicine) | Prostate | 884 | Mixed effect model (regression model) Multiple LR | Age, baseline IIEF-5 score, baseline overall satisfaction score, sexual desire score, IPSS, incontinence score, fascia preservation score, QoL | "ED due to robot-assisted radical prostatectomy (RARP). Primary: Overall satisfaction with sexual life (from IIEF-15 Q13+Q14) Secondary: Factors associated with satisfaction at 24 and 36 months" | The model proved that no increase or decrease in overall satisfaction with sexual life between 6 m and 36 m follow-up after RARP, while a higher overall satisfaction at baseline and high sexual desire were associated with satisfaction at 24 and 26 m follow-up. Erectile function score was not correlated with overall satisfaction. | IIEF-15 IIEF-5 | Post-treatment survivorship |
| Bagshaw et al., 2021 [41] | BMC Medical Informatics and Decision Making | Prostate | 750 | Decision-making template (web-based decision aid) | NCCN risk group, pre-treatment health state (ED, urinary incontinence, nocturia, bowel incontinence), treatment options (AS, SBRT, EBRT ± HDR ± ADT), 5-year biochemical control rate, patient preference thresholds, willingness-to-be-paid values | Prostate cancer patients’ treatment decisions and engagement. [Personalized ranking of treatment alternatives based on individual risk, preferences, side effect tolerances, and value assessment] | Web-based decision aid was successfully built; visual interface allowed real-time updates based on patient preferences. Every treatment could be optimal for different individuals. No single dominant option across all users. | EPIC questionnaire | Cancer treatment |
| Charoenkw et al., 2021 [42] | Diagnostics | Cervical | 1112 | RF-classifier, named iPMI LR DT kNN MLP NB SVM XGBoost | The initial model, iPMI-Econ: basic clinical and pathological features before surgery: demographics, tumor characteristics, and clinical staging. | Presence or absence of parametrial invasion (PMI) was confirmed during surgery. | iPMI-Power was effective and had superior performance compared to other well-known ML classifiers in predicting PMI. | None reported | Cancer treatment |
| Agochukwu-Mmonu et al., 2022 [24] | European Urology Open Science | Prostate | 2653 | GBDT | Clinical and demographic features: age, race, BMI, diabetes, PSA, T stage, Gleason grade, prostate volume, baseline/post-op EPIC-26 sexual domain score, PROMIS satisfaction with sex life, use of erectile aids, nerve-sparing status, surgical volume | Sexual function, sexual activity, and satisfaction with sexual life at 3, 6, 12, and 24 months after radical prostatectomy (RP). EPIC-26 sexual domain score, EPIC-26 dichotomized score ≥73, erection quality at 12 and 24 months | A dynamic ML model (GBM) was developed and validated to predict sexual function before and after RP. GBM achieved high performance for preoperative predictions and even better performance for dynamic predictions | EPIC-26. PROMIS Interest in “Sexual Activity and Global Satisfaction with Sex Life subdomains" | Post-treatment survivorship |
| Chan et al., 2022 [43] | Current Oncology (Curr Oncol) | Gynecologic | 698 | LR | Patient demographics (age, cancer type, concurrent chemotherapy, radiation technique), baseline and weekly patient-reported symptom scores from a 49-item PRO questionnaire covering GI, GU, bowel, urinary, abdominal, gynecological, sexual/vaginal, and general health domains. | Acute toxicity trends, specifically GI and GU toxicity severity, during RT | PRO data can be used to track acute toxicity (including GU and sexual activity) during RT in gynecological cancers. Patients were approximately 6 times less likely to respond to questions about vaginal and sexual health after treatment | "PRO questionnaire. EPIC Bowel/Urinary 2.0, PRO-CTCAE GI, EORTC QLQ CX24, and EuroQol EQ-5D-5L" | Cancer treatment |
| Chao et al., 2022 [44] | Acta Obstetricia et Gynecologica Scandinavica | Ovarian | 6809 | NLP for data extraction from EMR GBDT LR | A total of 94 demographic and clinicopathologic variables for GBM. For LR model: 10 selected features (age, dysmenorrhea, GnRHa before surgery, FH of EC, hist of cystectomy, Mirena, serum CA125, max tumor diameter, leiomyoma, peritoneal endometriosis). | Endometriosis-associated ovarian cancer (EAOC) [which significantly affected sexual health/activity and induced pain] | ML-based risk model was constructed to predict endometriosis EAOC, which had high sensitivity and specificity. The ML model performed significantly better than the LR model | None reported | Cancer prediction and diagnosis |
| Gentile et al., 2022 [45] | Clinical Genitourinary Cancer | Prostate | 135 | NN (DL) | Prostate Health Index (PHI), PI-RADS score from multiparametric MRI (mpMRI), Gleason score from pathology | Identify high-grade prostate cancer. Binary classification: clinically significant prostate cancer (csPCa) vs. indolent PCa (based on Gleason score ≥7 or 7 with pattern 4) | Deep learning combining mpMRI and PHI may help to better estimate the risk category of PCa at initial diagnosis | None reported | Cancer diagnosis |
| Sun et al., 2022 [46] | Frontiers in Oncology | Cervical | 858 | Stacking integrated ML algorithm that combined multiple base models, including RF, SGB, TreeBag, XGBoost, MonMLP, SVMRadial, KNN, GaussPrRadial, RgeLogistic, SLDA, LMT | Hormonal contraceptives (years), number of pregnancies, smoking (years), number of cigarette packets smoked annually, number of sexual partners, the use of an intrauterine device (IUD) (years), number of sexually transmitted diseases (STDs), human immunodeficiency virus (HIV), and age. | Accurate identification of women at high risk of cervical cancer | A stacking integrated model that incorporated multiple algorithms was developed to improve prediction accuracy of women at high risk for cervical cancer. The stacking integrated model with TreeBag, XGBoost, and MonMLP as base classifiers and LMT as the result classifier showed the best performance. | None reported | Cancer prediction/prevention |
| Deng et al., 2023 [47] | Frontiers in Medicine | CIN3 (cervical) | 436 | Multivariate LR analysis for independent risk factors RF for prediction | Preoperative ECC pathology, LEEP margin status, post-LEEP follow-up HPV, post-LEEP follow-up Thin Prep Cytology Test (TCT), and gland involvement | Presence or absence of residual lesions confirmed by histopathology after total hysterectomy performed within three months post-LEEP. | The post-LEEP follow-up HPV, post-LEEP follow-up TCT, and gland involvement are independent risk factors related to residual tissue after LEEP surgery in CIN3. The constructed RF-based nomogram model effectively predicted the presence of residual tissue after LEEP surgery in CIN3 | None reported | Cancer treatment |
| Hariprasad et al., 2024 [48] | IEEE Access | Cervical | 858 | GBM XGBoost RF SVM MLP KNN LR | Age, number of sexual partners, age at first sexual intercourse, smoking status, hormonal contraceptive use, number of pregnancies, history of STIs, IUD, and other gynecological factors | Risk of cervical cancer | The gradient boosting model was effective in associating risk factors with cervical cancer prediction, with an accuracy of 98.9% | None reported | Cancer prediction/prevention |
| Hasannejadasl et al., 2023 [49] | PLoS ONE | Prostate | 964 | Logistic regression algorithm coupled with recursive feature elimination (RFE). | (i) At diagnosis: PROMs data from the EPIC26 questionnaire; (ii) tumor characteristics, such as tumor staging, PSA at diagnosis, and ISUP Gleason grade group; (iii) patient characteristics: age, height, weight, smoking, comorbidities, and treatment | Erectile dysfunction (the frequency of erections) at 1 year and 2 years post-diagnosis with prostate cancer. | Two models generated using an LR algorithm coupled with RFE revealed high AUC (0.84 and 0.81 for 1 year and 2 years, respectively) for prediction of ED post-diagnosis of prostate cancer. | EPIC-26 questionnaire | Cancer survivorship |
| Lei et al., 2023 [50] | Med Phys | Prostate | 60 | DL-based topological modulated network | MRI images | Automatic segmentation of the left and right neurovascular bundles (NVBs) on MRI. | The topological modulated network achieved strong performance in segmenting both left and right NVBs when compared to expert-drawn contours | None reported | Imaging analysis |
| Sibert et al., 2023 [51] | PLoS ONE | Prostate | 20164 | Lasso regression, kNN | Baseline and follow-up EPIC-26 sexual function scores, age, baseline function, hormone therapy use, dosimetric parameters, comorbidities | Urinary incontinence and sexual function scores at one year after radical prostatectomy (RP), as assessed by EPIC-26. | Lasso regression model was developed and validated to predict functional outcomes (sexual function and incontinence) 1 y after RP. Models showed appropriate predictive properties | EPIC-26 questionnaire | Post-treatment survivorship |
| Xu et al., 2023 [52] | Ann Surg Oncol | Breast | 1454 | LR with elastic net XGBoost NN | BMI, preoperative BREAST-Q scores for physical well-being, sexual well-being, and psychosocial well-being; reconstruction technique, ALND, SLNB, socioeconomic status; race; education level; working status | Predict changes in physical, sexual, and psychosocial well-being two years following PMBR | ML models are capable of accurately predicting long-term PRO after postmastectomy breast reconstruction | BREAST-Q | Post-treatment survivorship |
| Balagopal et al., 2024 [22] | Physics & Imaging in Radiation Oncology | Prostate | 86 | DL | Imaging modality (CT and MRI), segmentation metrics (DSC, ASD, and HD95), dose-volume parameters (Dmean, V20), observer contour quality scores | Internal pudendal artery (IPA) segmentation quality for preserving sexual potency, dosimetric similarity, and improvement in physician contouring efficiency and quality | A DL model for good-quality IPA contours with DSC 62.2%, which improved uniformity of segmentation and facilitating standardized IPA segmentation in clinical trials and practice | None reported | Imaging analysis |
| Chauhan et al., 2024 [53] | MethodsX | Cervical | 858 | CHAMP (cervical health assessment using machine learning for prediction) included multiple ML, including XGBoost SVM NB AdaBoost DT KNN | Age, HPV results, cytology outcome, number of sexual partners, smoking, hormonal contraceptive use, IUD use, STDs, etc. | Prediction of cervical cancer risk (binary classification) | The study tested several ML models to predict cervical cancer risk. XGBoost model performed the best. It showed high accuracy and reliability compared to other models like SV and NB | None reported | Cancer prediction/prevention |
| Devi et al., 2024 [54] | Public Health Nursing | Cervical | 1046 | Classification Models: NB LR Ensemble Models: RF, Bagging, Soft Voting, Weighted Averaging DL Models: MLP, NN, Long Short-Term Memory LSTM | 22 predictors | Prediction of nonattendance to cervical cancer screening | Employing ensemble and DL models proved most effective in predicting barriers to nonattendance in cervical screening | None reported | Cancer screening/prevention |
| Hanai et al., 2024 [23] | BMJ Health & Care Informatics | Mixed | 100 | Generative AI (GPT) | Epidemiological surveys data regarding sexual difficulties among cancer survivors. [The prompt ‘I am a cancer survivor. Please create a question about a problem that is hard to consult’ generated 100 questions by DocsBot that had learnt a survey on sexual problems among cancer survivors.]" | Generated questions categorized into 7 topics based on the symptom categories specified in the clinical guidelines: sexual response, body image, intimacy, sexual functioning, vasomotor symptoms, genital symptoms, and others. Distribution and content of AI-generated answers regarding sexual health in cancer survivors | "Generative AI can serve to provide health information on sensitive topics such as sexual health" | None reported | Data Extraction/Cancer survivorship |
| Saikali et al., 2025 [21] | Computer Methods and Programs in Biomedicine | Prostate | 8524 | Neural Network (ANN), SVM, XG Boost | Urinary continence: patient age, BMI, prostate size, and baseline urinary symptom severity as measured by IPSS. Clinical factors: prior (TURP), tumor stage, prostate volume, and the Charlson Comorbidity Index. Erectile function: age, BMI, Gleason score, diabetes, baseline SHIM scores | Prediction of urinary control and erectile function following nerve-sparing robotic radical prostatectomy RARP (12 ms post-op) | AI-based models (ANN) show potential in predicting postoperative functional outcomes (potency (sexual function) and continence) following RARP | Sexual Health Inventory of Men (SHIM) | Post-treatment survivorship |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, V.; Godinich, B.M.; Lilly, P.M.; Pifer, P.M.; Duckworth, A.L.; Hall, S.J.; Alabboodi, M.; Siochi, R.A.; Clump, D.A.; Emadi, A. Artificial Intelligence and Machine Learning in Sexual Health and Dysfunction Across the Cancer Care Continuum: A Systematic Review. Cancers 2025, 17, 3025. https://doi.org/10.3390/cancers17183025
Salama V, Godinich BM, Lilly PM, Pifer PM, Duckworth AL, Hall SJ, Alabboodi M, Siochi RA, Clump DA, Emadi A. Artificial Intelligence and Machine Learning in Sexual Health and Dysfunction Across the Cancer Care Continuum: A Systematic Review. Cancers. 2025; 17(18):3025. https://doi.org/10.3390/cancers17183025
Chicago/Turabian StyleSalama, Vivian, Brandon M. Godinich, Peyton M. Lilly, Phillip M. Pifer, Adrienne L. Duckworth, Samantha J. Hall, Maher Alabboodi, R. Alfredo Siochi, David A. Clump, and Ashkan Emadi. 2025. "Artificial Intelligence and Machine Learning in Sexual Health and Dysfunction Across the Cancer Care Continuum: A Systematic Review" Cancers 17, no. 18: 3025. https://doi.org/10.3390/cancers17183025
APA StyleSalama, V., Godinich, B. M., Lilly, P. M., Pifer, P. M., Duckworth, A. L., Hall, S. J., Alabboodi, M., Siochi, R. A., Clump, D. A., & Emadi, A. (2025). Artificial Intelligence and Machine Learning in Sexual Health and Dysfunction Across the Cancer Care Continuum: A Systematic Review. Cancers, 17(18), 3025. https://doi.org/10.3390/cancers17183025






