Oral Viral DNA Profiling in Obesity, Adenomatous Polyposis, and Colorectal Cancer Identifies Human β-Papillomavirus Types as Potentially Sex-Related and Modifiable Cancer Risk Indicators
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Sample Collection
2.3. DNA Extraction from Oral Rinse and Gargle Samples
2.4. Viral DNA Detection Assay
2.5. Quantification of Plasma Mediators
2.6. Statistical Analysis
3. Results
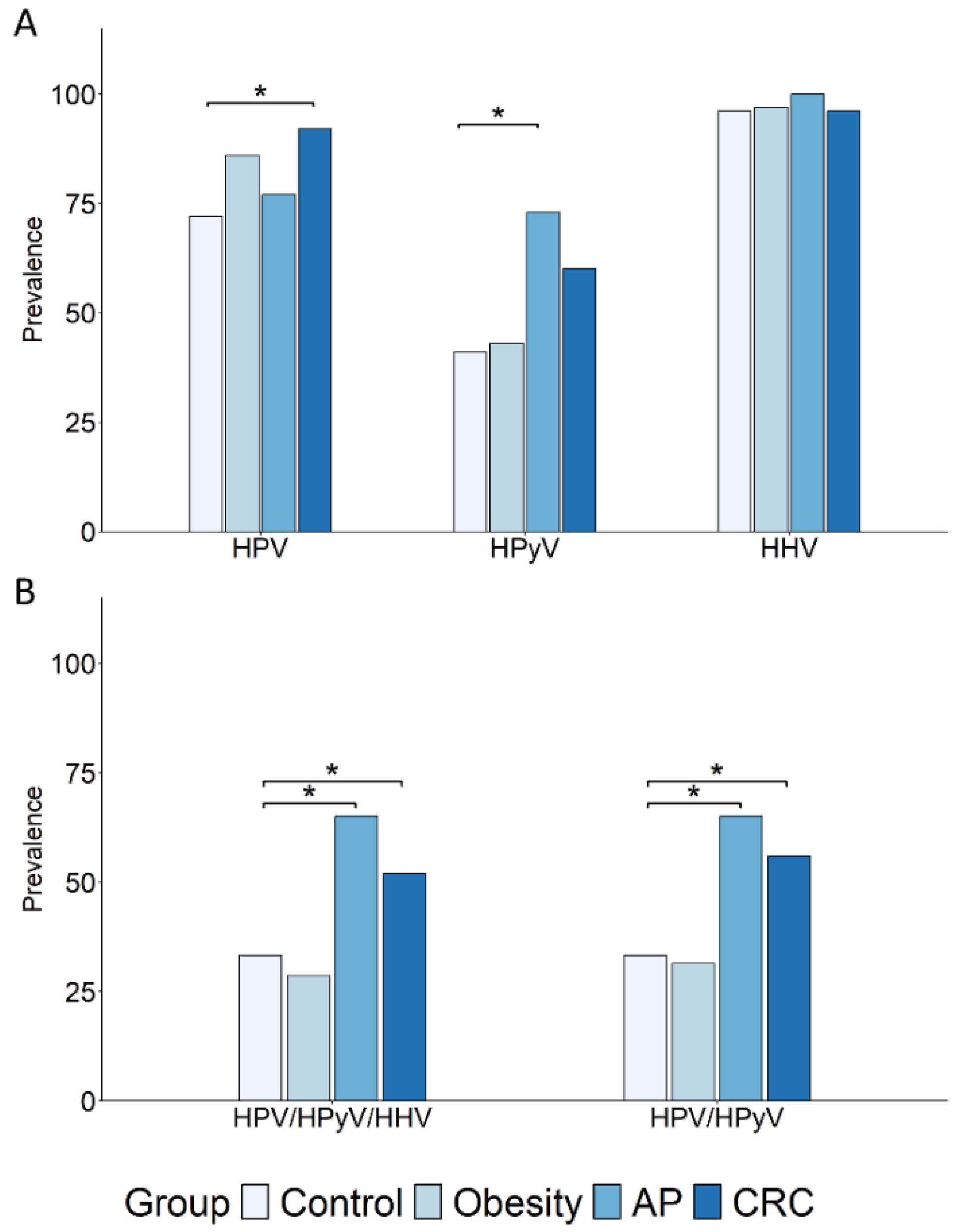
3.1. Comparative Analysis of Oral DNA Virus Profiles in Colorectal Cancer Patients and At-Risk Populations with Obesity or Nonfamilial Adenomatous Polyposis
3.2. The β-HPV Genus Is the Most Frequently Detected and Differentially Distributed Among Subject Groups
3.3. Impact of Tumor Stage and Precancerous Lesion Features on Oral Virus Prevalence
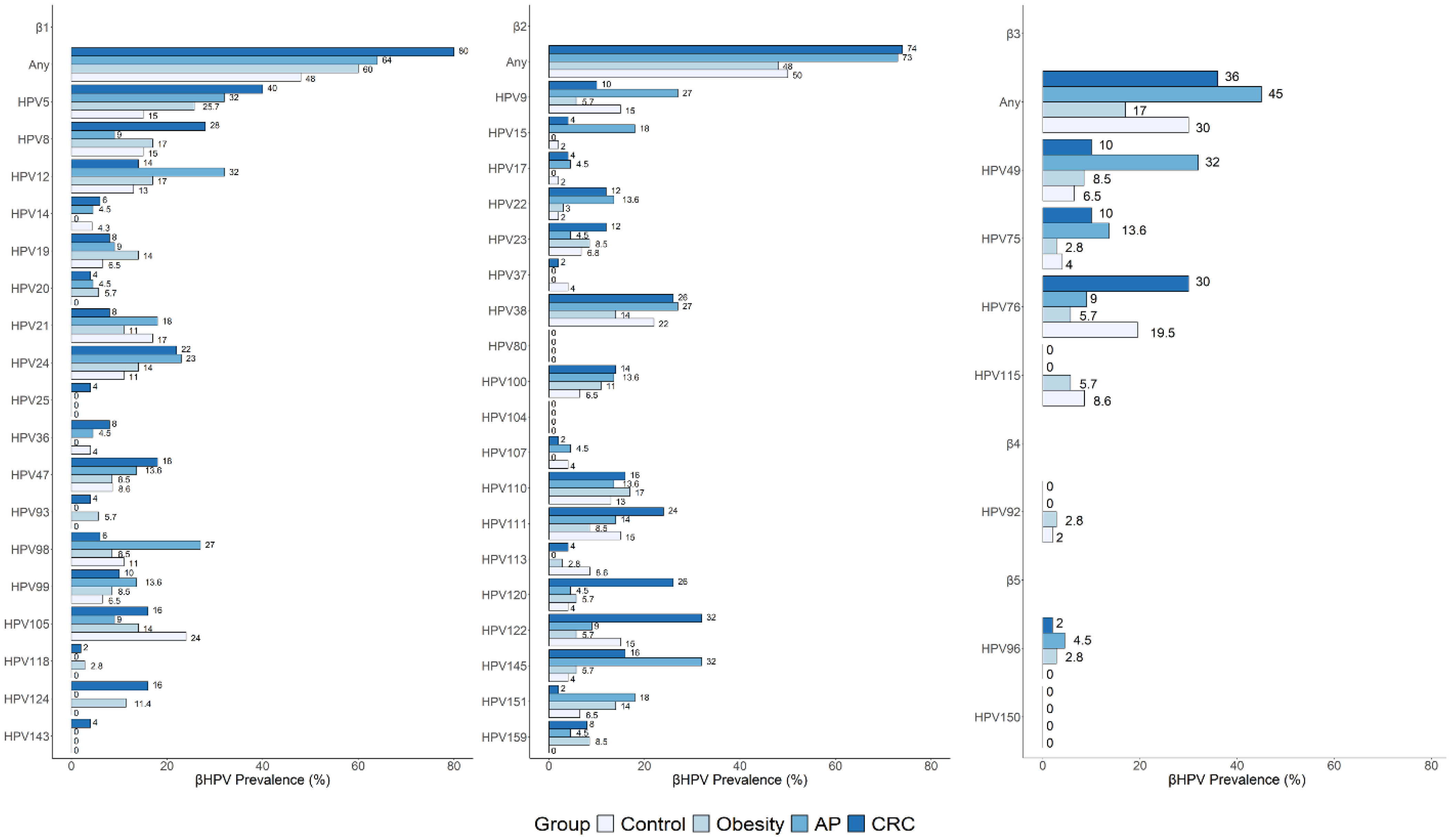
3.4. Distribution and Prevalence of Individual β-HPV Genotypes in Risk and Cancer Groups Compared to Controls
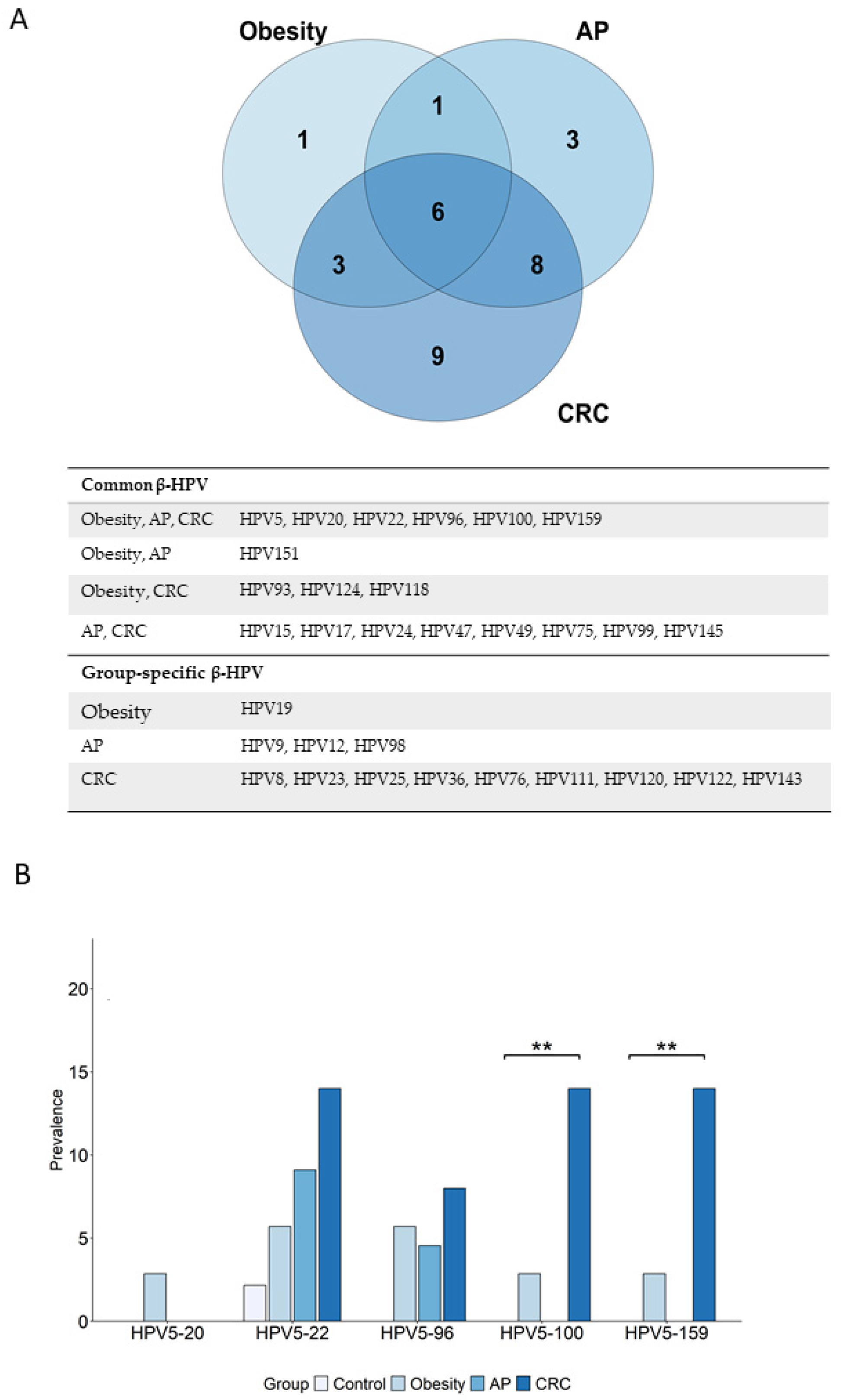
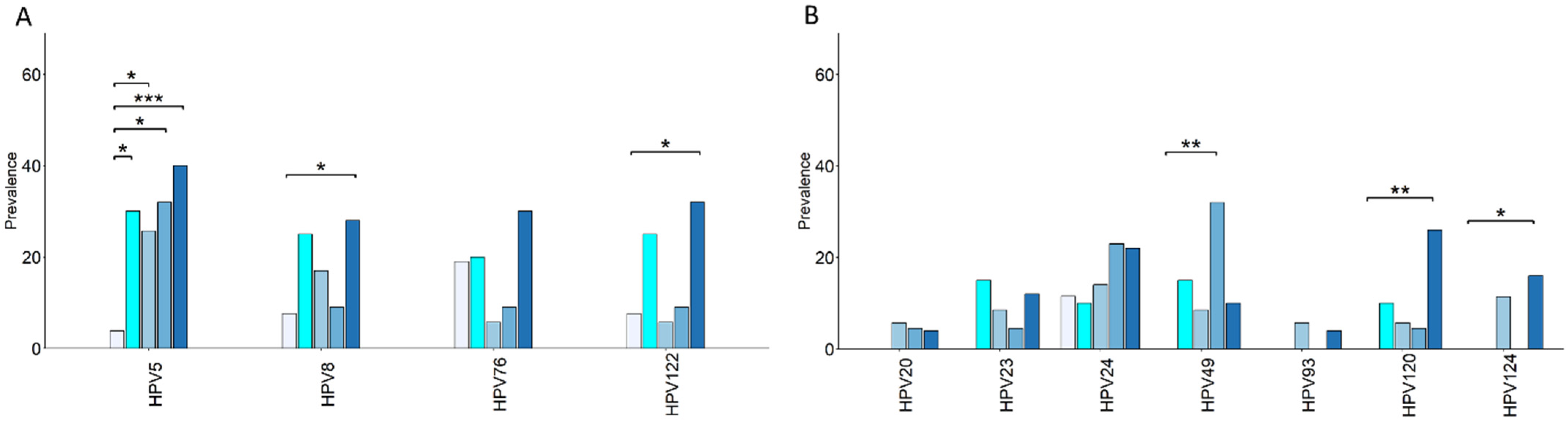
3.5. Overrepresented β-HPV Genotypes in Cancer and Risk Groups Versus Control Group: Unique and Shared Profiles
3.6. Prevalence of Cancer-Relevant β-HPV Genotypes Is Increased in Risk and Cancer Groups, and in Healthy Controls According to BMI
3.7. Bariatric Surgery Shapes Oral Viral Profiles in Individuals with Obesity and Reduces the Prevalence of Cancer-Relevant β-HPV Infections
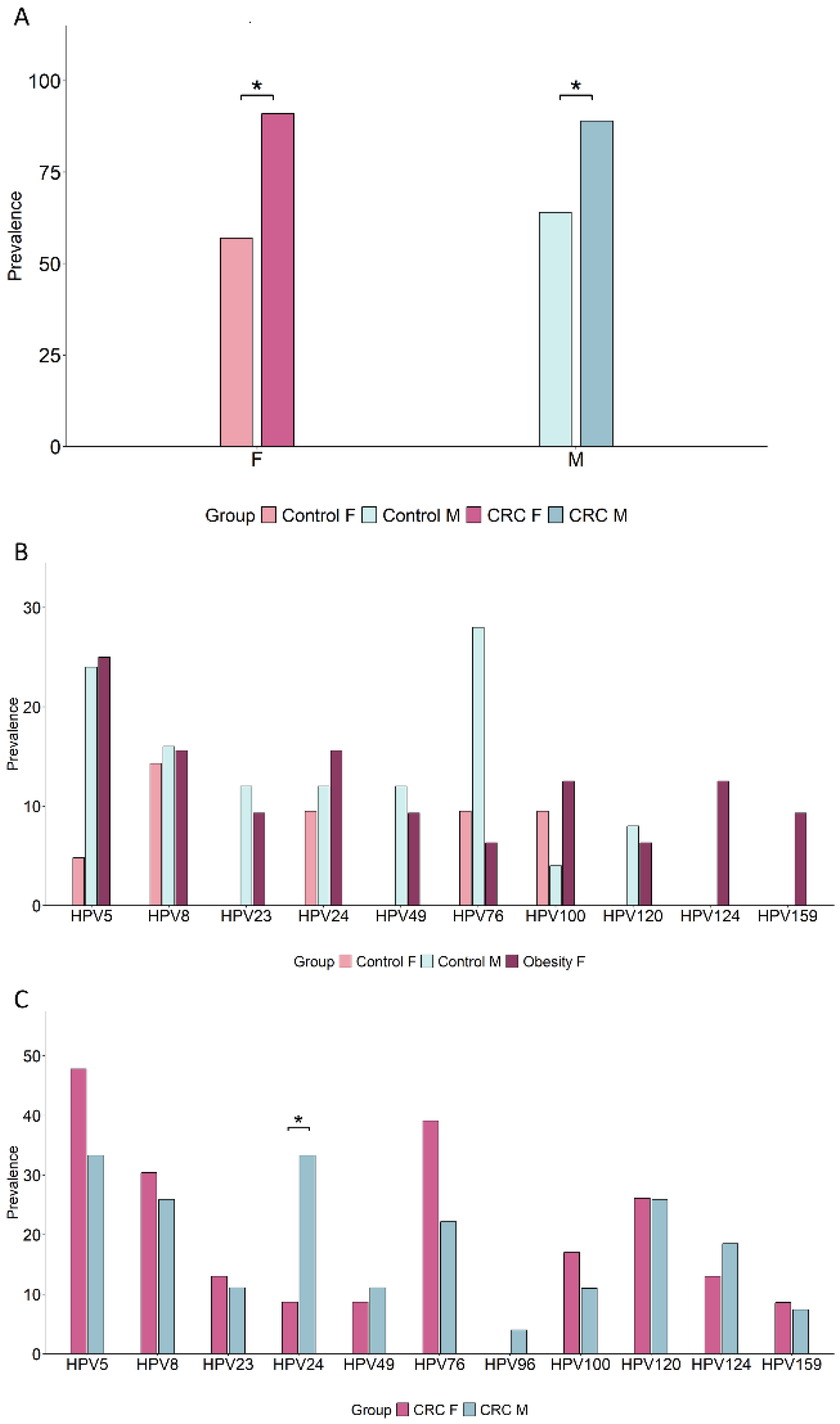
3.8. Sex Differences in Oral β-HPV Genotype Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosure
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Harlid, S.; Gunter, M.J.; Van Guelpen, B. Risk-Predictive and Diagnostic Biomarkers for Colorectal Cancer; a Systematic Review of Studies Using Pre-Diagnostic Blood Samples Collected in Prospective Cohorts and Screening Settings. Cancers 2021, 13, 4406. [Google Scholar] [CrossRef]
- Collaborators, G.A.B. Global, regional, and national prevalence of adult overweight and obesity, 1990-2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, J.; Xu, Z.; Guo, Y.; Zhu, B.; Qian, P. Burden of colorectal cancer attributable to high body-mass index in 204 countries and territories, 1990-2021: Results from the Global Burden of Disease Study 2021. Public Health 2025, 242, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Mandic, M.; Safizadeh, F.; Niedermaier, T.; Hoffmeister, M.; Brenner, H. Association of Overweight, Obesity, and Recent Weight Loss With Colorectal Cancer Risk. JAMA Netw. Open 2023, 6, e239556. [Google Scholar] [CrossRef]
- Morgado-Diaz, J.A. (Ed.) Gastrointestinal Cancers; NBK585999; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Lim, P.W.; Stucky, C.H.; Wasif, N.; Etzioni, D.A.; Harold, K.L.; Madura, J.A.; Ven Fong, Z. Bariatric Surgery and Longitudinal Cancer Risk: A Review. JAMA Surg. 2024, 159, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Legitimo, A.; Failli, A.; Ferrari, P.; Nicolini, A.; Spisni, R.; Miccoli, P.; Consolini, R. Quantification of blood dendritic cells in colorectal cancer patients during the course of disease. Pathol. Oncol. Res. 2014, 20, 267–276. [Google Scholar] [CrossRef]
- Donninelli, G.; Del Corno, M.; Pierdominici, M.; Scazzocchio, B.; Vari, R.; Varano, B.; Pacella, I.; Piconese, S.; Barnaba, V.; D’Archivio, M.; et al. Distinct Blood and Visceral Adipose Tissue Regulatory T Cell and Innate Lymphocyte Profiles Characterize Obesity and Colorectal Cancer. Front. Immunol. 2017, 8, 643. [Google Scholar] [CrossRef]
- Del Cornò, M.; D’Archivio, M.; Conti, L.; Scazzocchio, B.; Varì, R.; Donninelli, G.; Varano, B.; Giammarioli, S.; De Meo, S.; Silecchia, G.; et al. Visceral fat adipocytes from obese and colorectal cancer subjects exhibit distinct secretory and ω6 polyunsaturated fatty acid profiles and deliver immunosuppressive signals to innate immunity cells. Oncotarget 2016, 7, 63093–63105. [Google Scholar] [CrossRef]
- Costanzo, A.E.; Taylor, K.R.; Dutt, S.; Han, P.P.; Fujioka, K.; Jameson, J.M. Obesity impairs γδ T cell homeostasis and antiviral function in humans. PLoS ONE 2015, 10, e0120918. [Google Scholar] [CrossRef]
- Huttunen, R.; Syrjänen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Ghayor, C.; Pérez Dominguez, A.; Weber, F.E. From Influenza Virus to Novel Corona Virus (SARS-CoV-2)-The Contribution of Obesity. Front. Endocrinol. 2020, 11, 556962. [Google Scholar] [CrossRef] [PubMed]
- Damian, D. The Role of Viruses in Cellular Transformation and Cancer. Cancer Rep. 2025, 8, e70150. [Google Scholar] [CrossRef] [PubMed]
- Marônek, M.; Link, R.; Monteleone, G.; Gardlík, R.; Stolfi, C. Viruses in Cancers of the Digestive System: Active Contributors or Idle Bystanders? Int. J. Mol. Sci. 2020, 21, 8133. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, T.; Shimatani, M.; Takeo, M.; Sasaki, K.; Orino, M.; Saito, N.; Matsumoto, H.; Kasai, T.; Kano, M.; Horitani, S.; et al. Viral Infection in Esophageal, Gastric, and Colorectal Cancer. Healthcare 2022, 10, 1626. [Google Scholar] [CrossRef]
- Fernandes, Q.; Gupta, I.; Murshed, K.; Abo Samra, H.; Al-Thawadi, H.; Vranic, S.; Petkar, M.; Babu, G.R.; Al Moustafa, A.E. Coinfection of HPVs Is Associated with Advanced Stage in Colorectal Cancer Patients from Qatar. Pathogens 2023, 12, 424. [Google Scholar] [CrossRef]
- Galati, L.; Gupta, P.; Tufaro, A.; Marinaro, M.; Saponaro, C.; Escobar Marcillo, D.I.; Loisi, D.; Sen, R.; Robitaille, A.; Brancaccio, R.N.; et al. Evaluation of human papillomavirus DNA in colorectal cancer and adjacent mucosal tissue samples. Infect. Agent. Cancer 2023, 18, 71. [Google Scholar] [CrossRef]
- Viarisio, D.; Müller-Decker, K.; Accardi, R.; Robitaille, A.; Dürst, M.; Beer, K.; Jansen, L.; Flechtenmacher, C.; Bozza, M.; Harbottle, R.; et al. Beta HPV38 oncoproteins act with a hit-and-run mechanism in ultraviolet radiation-induced skin carcinogenesis in mice. PLoS Pathog. 2018, 14, e1006783. [Google Scholar] [CrossRef]
- Tommasino, M. The biology of beta human papillomaviruses. Virus Res. 2017, 231, 128–138. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Skelin, J.; Tomaić, V. Comparative Analysis of Alpha and Beta HPV E6 Oncoproteins: Insights into Functional Distinctions and Divergent Mechanisms of Pathogenesis. Viruses 2023, 15, 2253. [Google Scholar] [CrossRef]
- Al-Soneidar, W.A.; Harper, S.; Alli, B.Y.; Nicolau, B. Interaction of HPV16 and Cutaneous HPV in Head and Neck Cancer. Cancers 2022, 14, 5197. [Google Scholar] [CrossRef]
- Guzha, B.T.; Matubu, A.; Nyandoro, G.; Mubata, H.O.; Moyo, E.; Murewanhema, G.; Chirenje, Z.M. The impact of DNA tumor viruses in low-to-middle income countries (LMICS): A literature review. Tumour Virus Res. 2024, 18, 200289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhong, X.; Song, Y.; Shi, J.; Wu, Z.; Guo, Z.; Sun, J.; Wang, Z. Prognostic Biomarkers for Gastric Cancer: An Umbrella Review of the Evidence. Front. Oncol. 2019, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Wiedinger, K.; Bitsaktsis, C.; Chang, S. Reactivation of human polyomaviruses in immunocompromised states. J. Neurovirol. 2014, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.M. Molecular Pathogenesis of Human Immunodeficiency Virus-Associated Disease of Oropharyngeal Mucosal Epithelium. Biomedicines 2023, 11, 1444. [Google Scholar] [CrossRef]
- Sichero, L.; Rollison, D.E.; Amorrortu, R.P.; Tommasino, M. Beta Human Papillomavirus and Associated Diseases. Acta Cytol. 2019, 63, 100–108. [Google Scholar] [CrossRef]
- Torres, M.; Gheit, T.; McKay-Chopin, S.; Rodríguez, C.; Romero, J.D.; Filotico, R.; Doná, M.G.; Ortiz, M.; Tommasino, M. Prevalence of beta and gamma human papillomaviruses in the anal canal of men who have sex with men is influenced by HIV status. J. Clin. Virol. 2015, 67, 47–51. [Google Scholar] [CrossRef]
- Nunes, E.M.; Sudenga, S.L.; Gheit, T.; Tommasino, M.; Baggio, M.L.; Ferreira, S.; Galan, L.; Silva, R.C.; Pierce Campbell, C.M.; Lazcano-Ponce, E.; et al. Diversity of beta-papillomavirus at anogenital and oral anatomic sites of men: The HIM Study. Virology 2016, 495, 33–41. [Google Scholar] [CrossRef]
- Smelov, V.; Muwonge, R.; Sokolova, O.; McKay-Chopin, S.; Eklund, C.; Komyakov, B.; Gheit, T. Beta and gamma human papillomaviruses in anal and genital sites among men: Prevalence and determinants. Sci. Rep. 2018, 8, 8241. [Google Scholar] [CrossRef]
- Hampras, S.S.; Tommasino, M.; Zhao, Y.; Messina, J.L.; Giuliano, A.R.; Fenske, N.A.; Cherpelis, B.; Hesterberg, R.S.; Akuffo, A.A.; Amorrortu, R.P.; et al. Cross-sectional associations between cutaneous viral infections and regulatory T lymphocytes in circulation. Br. J. Dermatol. 2019, 180, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Gheit, T.; Feng, Q.; Stern, J.E.; Lin, J.; Cherne, S.; Tommasino, M. Prevalence and Correlates of β- and γ-Human Papillomavirus Detection in Oral Samples From Mid-Adult Women. J. Infect. Dis. 2019, 219, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Al-Soneidar, W.A.; Harper, S.; Coutlée, F.; Gheit, T.; Tommasino, M.; Nicolau, B. Prevalence of Alpha, Beta, and Gamma Human Papillomaviruses in Patients With Head and Neck Cancer and Noncancer Controls and Relation to Behavioral Factors. J. Infect. Dis. 2024, 229, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Al-Soneidar, W.A.; Harper, S.; Madathil, S.A.; Schlecht, N.F.; Nicolau, B. Do cutaneous human papillomavirus genotypes affect head and neck cancer? Evidence and bias-correction from a case-control study. Cancer Epidemiol. 2022, 79, 102205. [Google Scholar] [CrossRef]
- Agalliu, I.; Chen, Z.; Wang, T.; Hayes, R.B.; Freedman, N.D.; Gapstur, S.M.; Burk, R.D. Oral Alpha, Beta, and Gamma HPV Types and Risk of Incident Esophageal Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1168–1175. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Schlecht, N.F.; Chen, Z.; Bottalico, D.; McKinney, S.; Ostoloza, J.; Dunne, A.; Burk, R.D. Oral human papillomavirus detection in older adults who have human immunodeficiency virus infection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 505–514. [Google Scholar] [CrossRef]
- Bottalico, D.; Chen, Z.; Dunne, A.; Ostoloza, J.; McKinney, S.; Sun, C.; Schlecht, N.F.; Fatahzadeh, M.; Herrero, R.; Schiffman, M.; et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J. Infect. Dis. 2011, 204, 787–792. [Google Scholar] [CrossRef]
- Gheit, T.; Rollo, F.; Brancaccio, R.N.; Robitaille, A.; Galati, L.; Giuliani, M.; Latini, A.; Pichi, B.; Benevolo, M.; Cuenin, C.; et al. Oral Infection by Mucosal and Cutaneous Human Papillomaviruses in the Men Who Have Sex with Men from the OHMAR Study. Viruses 2020, 12, 899. [Google Scholar] [CrossRef]
- Sabol, I.; Smahelova, J.; Klozar, J.; Mravak-Stipetic, M.; Gheit, T.; Tommasino, M.; Grce, M.; Tachezy, R. Beta-HPV types in patients with head and neck pathology and in healthy subjects. J. Clin. Virol. 2016, 82, 159–165. [Google Scholar] [CrossRef]
- Antonsson, A.; Karanfilovska, S.; Lindqvist, P.G.; Hansson, B.G. General acquisition of human papillomavirus infections of skin occurs in early infancy. J. Clin. Microbiol. 2003, 41, 2509–2514. [Google Scholar] [CrossRef]
- Liu, F.; Su, D.; Zhang, H.; Lin, H.C.; Zhou, Q.; Cao, B.; Ren, D.L. Clinical implications of the oral-gut microbiome axis and its association with colorectal cancer (Review). Oncol. Rep. 2022, 48, 192. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Negrut, R.L.; Cote, A.; Maghiar, A.M. Exploring the Potential of Oral Microbiome Biomarkers for Colorectal Cancer Diagnosis and Prognosis: A Systematic Review. Microorganisms 2023, 11, 1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, K.; Liu, Y.; Huang, C.; Wu, M. Dynamic impact of virome on colitis and colorectal cancer: Immunity, inflammation, prevention and treatment. Semin. Cancer Biol. 2022, 86, 943–954. [Google Scholar] [CrossRef]
- Ho, S.X.; Law, J.H.; Png, C.W.; Alberts, R.; Zhang, Y.; Chu, J.J.H.; Tan, K.K. Alterations in colorectal cancer virome and its persistence after surgery. Sci. Rep. 2024, 14, 2819. [Google Scholar] [CrossRef]
- Shabani, E.; Hasanzadi, A.; Allela, O.Q.B.; Kareem, R.A.; Abed, R.E.; Al-Nuaimi, A.M.A.; Athab, Z.H.; Khodarahmi, S. The role of lipids and lipids lowering drugs in human papillomavirus (HPV) and HPV-associated cancers. Infect. Agent. Cancer 2025, 20, 4. [Google Scholar] [CrossRef]
- Castañeda-Avila, M.A.; Pérez, C.M.; Vivaldi, J.; Díaz-Toro, E.C.; Ramos-Cartagena, J.M.; Andriankaja, O.M.; Ortiz, A.P. Oral Inflammation and Human Papilloma Virus Association among Hispanics. Int. J. Dent. 2023, 2023, 7247976. [Google Scholar] [CrossRef]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesæth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Obesity Management Task Force of the European Associations for the Study of Obesity Released “Practical Recommendations for the Post-Bariatric Surgery Medical Management”. Obes. Surg. 2018, 28, 2117–2121. [Google Scholar] [CrossRef]
- Palleschi, S.; Guglielmi, V.; Nisticò, L.; Ferreri, C.; Tabolacci, C.; Facchiano, F.; Iorio, E.; Giuliani, A.; Brescianini, S.; Medda, E.; et al. A multi-marker integrative analysis reveals benefits and risks of bariatric surgery. Sci. Rep. 2022, 12, 18877. [Google Scholar] [CrossRef]
- Schmitt, M.; Dondog, B.; Waterboer, T.; Pawlita, M.; Tommasino, M.; Gheit, T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J. Clin. Microbiol. 2010, 48, 143–149. [Google Scholar] [CrossRef]
- Schmitt, M.; Bravo, I.G.; Snijders, P.J.; Gissmann, L.; Pawlita, M.; Waterboer, T. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol. 2006, 44, 504–512. [Google Scholar] [CrossRef]
- Corbex, M.; Bouzbid, S.; Traverse-Glehen, A.; Aouras, H.; McKay-Chopin, S.; Carreira, C.; Lankar, A.; Tommasino, M.; Gheit, T. Prevalence of papillomaviruses, polyomaviruses, and herpesviruses in triple-negative and inflammatory breast tumors from algeria compared with other types of breast cancer tumors. PLoS ONE 2014, 9, e114559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J. Worldwide burden and cross-regional health inequalities of high BMI-attributable colorectal cancer by gender from 1990 to 2021, with predictions through 2041. BMC Gastroenterol. 2025, 25, 386. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Ryan, O.K.; Ryan, É.; Donlon, N.E.; Reynolds, I.S.; Fearon, N.M.; Martin, S.T.; Heneghan, H.M. The Impact of Bariatric Surgery on the Incidence of Colorectal Cancer in Patients with Obesity-a Systematic Review and Meta-analysis of Registry Data. Obes. Surg. 2023, 33, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- González-Flores, E.; Garcia-Carbonero, R.; Élez, E.; Redondo-Cerezo, E.; Safont, M.J.; Vera García, R. Gender and sex differences in colorectal cancer screening, diagnosis and treatment. Clin. Transl. Oncol. 2025, 27, 2825–2837. [Google Scholar] [CrossRef]
- Paolini, F.; Rizzo, C.; Sperduti, I.; Pichi, B.; Mafera, B.; Rahimi, S.S.; Vigili, M.G.; Venuti, A. Both mucosal and cutaneous papillomaviruses are in the oral cavity but only alpha genus seems to be associated with cancer. J. Clin. Virol. 2013, 56, 72–76. [Google Scholar] [CrossRef]
- Karimi, A.; Mohebbi, E.; Mckay-Chopin, S.; Rashidian, H.; Hadji, M.; Peyghambari, V.; Marzban, M.; Naghibzadeh-Tahami, A.; Gholipour, M.; Kamangar, F.; et al. Human Papillomavirus and Risk of Head and Neck Squamous Cell Carcinoma in Iran. Microbiol. Spectr. 2022, 10, e0011722. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Vlantis, A.C.; Liang, M.; Wong, P.Y.; Ho, W.C.S.; Boon, S.S.; Sze, R.K.H.; Leung, C.; Chan, P.K.S.; Chen, Z. Prevalence and Epidemiologic Profile of Oral Infection with Alpha, Beta, and Gamma Papillomaviruses in an Asian Chinese Population. J. Infect. Dis. 2018, 218, 388–397. [Google Scholar] [CrossRef]
- Senapati, R.; Nayak, B.; Kar, S.K.; Dwibedi, B. HPV genotypes co-infections associated with cervical carcinoma: Special focus on phylogenetically related and non-vaccine targeted genotypes. PLoS ONE 2017, 12, e0187844. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein-Barr virus in the pathogenesis of oral cancers. Oral. Dis. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Vlantis, A.C.; Liang, M.; Wong, P.Y.; Ho, W.C.S.; Boon, S.S.; Leung, C.; Chan, P.K.S.; Chen, Z. Persistence and clearance of oral human papillomavirus infections: A prospective population-based cohort study. J. Med. Virol. 2020, 92, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, V.; Colangeli, L.; D’Adamo, M.; Sbraccia, P. Susceptibility and Severity of Viral Infections in Obesity: Lessons from Influenza to COVID-19. Does Leptin Play a Role? Int. J. Mol. Sci. 2021, 22, 3183. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Fukuda, D.; Sata, M. Emerging roles of Toll-like receptor 9 in cardiometabolic disorders. Inflamm. Regen. 2020, 40, 18. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.R.; Bandopadhyay, P.; Sarkar, J.; Khanna, S.; Chaudhuri, T.; Tantia, O.; Chakrabarti, P.; Ganguly, D. Mitochondrial sourcing of interferogenic ligands and an autoantigen in human obesity-associated metaflammation. Obesity 2023, 31, 2229–2234. [Google Scholar] [CrossRef]
- Escobar Marcillo, D.I.; Guglielmi, V.; Privitera, G.F.; Signore, M.; Simonelli, V.; Manganello, F.; Dell’Orso, A.; Laterza, S.; Parlanti, E.; Pulvirenti, A.; et al. The dual nature of DNA damage response in obesity and bariatric surgery-induced weight loss. Cell Death Dis. 2024, 15, 664. [Google Scholar] [CrossRef]
- Smith, J.A.; Gaikwad, A.A.; Mathew, L.; Rech, B.; Faro, J.P.; Lucci, J.A.; Bai, Y.; Olsen, R.J.; Byrd, T.T. AHCC. Front. Oncol. 2022, 12, 881902. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, J.B.; Lu, L.X.; Jia, Y.J.; Zheng, M.Q.; Debelius, J.W.; He, Y.Q.; Wang, T.M.; Deng, C.M.; Tong, X.T.; et al. Oral Microbiota Alteration and Roles in Epstein-Barr Virus Reactivation in Nasopharyngeal Carcinoma. Microbiol. Spectr. 2023, 11, e0344822. [Google Scholar] [CrossRef]
- Hatta, M.N.A.; Mohamad Hanif, E.A.; Chin, S.F.; Neoh, H.M. Pathogens and Carcinogenesis: A Review. Biology 2021, 10, 533. [Google Scholar] [CrossRef]
- DeDecker, L.; Coppedge, B.; Avelar-Barragan, J.; Karnes, W.; Whiteson, K. Microbiome distinctions between the CRC carcinogenic pathways. Gut Microbes 2021, 13, 1854641. [Google Scholar] [CrossRef]
- Ballini, A.; Scacco, S.; Boccellino, M.; Santacroce, L.; Arrigoni, R. Microbiota and Obesity: Where Are We Now? Biology 2020, 9, 415. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Qian, Y.; Xie, Y.H.; Jiang, S.S.; Kang, Z.R.; Chen, Y.X.; Chen, Z.F.; Fang, J.Y. Alterations in the oral and gut microbiome of colorectal cancer patients and association with host clinical factors. Int. J. Cancer 2021, 149, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Perry, W.A.; Klein, S.L. Mechanisms and consequences of sex differences in immune responses. Nat. Rev. Nephrol. 2024, 20, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Cornet, I.; Bouvard, V.; Campo, M.S.; Thomas, M.; Banks, L.; Gissmann, L.; Lamartine, J.; Sylla, B.S.; Accardi, R.; Tommasino, M. Comparative analysis of transforming properties of E6 and E7 from different beta human papillomavirus types. J. Virol. 2012, 86, 2366–2370. [Google Scholar] [CrossRef]
- Viarisio, D.; Müller-Decker, K.; Zanna, P.; Kloz, U.; Aengeneyndt, B.; Accardi, R.; Flechtenmacher, C.; Gissmann, L.; Tommasino, M. Novel ß-HPV49 Transgenic Mouse Model of Upper Digestive Tract Cancer. Cancer Res. 2016, 76, 4216–4225. [Google Scholar] [CrossRef]
- Etemadi, A.; O’Doherty, M.G.; Freedman, N.D.; Hollenbeck, A.R.; Dawsey, S.M.; Abnet, C.C. A prospective cohort study of body size and risk of head and neck cancers in the NIH-AARP diet and health study. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2422–2429. [Google Scholar] [CrossRef]
- Berrington de González, A.; Masten, S.A.; Bhatti, P.; Fortner, R.T.; Peters, S.; Santonen, T.; Yakubovskaya, M.G.; Barouki, R.; Barros, S.B.M.; Barupal, D.; et al. Advisory Group recommendations on priorities for the IARC Monographs. Lancet Oncol. 2024, 25, 546–548. [Google Scholar] [CrossRef]



| Variables | Control (N = 46) | Obesity (N = 35) | AP (N = 22) | CRC (N = 50) |
|---|---|---|---|---|
| Age, years, mean (SD) | 51.7 (12.44) | 52.6 (10.84) | 65.4 (11.6) | 70.6 (8.98) |
| Sex, n (%) | ||||
| Female | 21 (45.7) | 32 (91.4) | 5 (22.7) | 23 (46) |
| Male | 25 (54.3) | 3 (8.6) | 17 (77.3) | 27 (54) |
| BMI, kg/m2, mean (SD) | 24.68 (2.7) | 44.75 (6.49) | 26.15 (3.46) | 25.55 (3.6) |
| Tumor stage, n (%) | ||||
| 0–I | - | - | - | 12 (24) |
| II | - | - | - | 25 (50) |
| III–IV | - | - | - | 13 (26) |
| Polyp features, n (%) | ||||
| Number | ||||
| Single | - | - | 14 (63.6) | - |
| Multiple (2–4) | - | - | 8 (36.4) | - |
| Size | ||||
| ≤10 mm | - | 5 (23) | ||
| >10 mm | - | - | 17 (77) | - |
| Villous histology | - | 7 (31.8) | ||
| Dysplasia degree | ||||
| Low grade | - | - | 20 (91) | - |
| High grade | - | - | 2 (9) | - |
| Smoker, n (%) | ||||
| Never | 29 (66) | 20 (57) | 13 (59) | 21 (42) |
| Former | 7 (16) | 9 (25.7) | 4 (18) | 21 (42) |
| Current | 8 (18) | 6 (17) | 5 (23) | 8 (16) |
| Family | Genus/Genotype | Control | Obesity | AP | CRC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | OR (95% CI) | p | % | OR (95% CI) | p | % | OR (95% CI) | p | ||
| HPV | Any | 72 | 86 | 2.34 (0.68–9.41) | 0.18 | 77 | 1.33 (0.36–5.60) | 0.77 | 92 | 4.46 (1.23–20.47) | 0.01 |
| α | 9 | 8 | 0.97 (0.13–6.27) | 1 | 0 | 0 (0–3.15) | 0.30 | 10 | 1.16 (0.23–6.28) | 1 | |
| β | 61 | 77 | 2.15 (0.74–6.73) | 0.15 | 73 | 1.7 (0.51–6.34) | 0.42 | 90 | 5.68 (1.77–21.82) | 0.001 | |
| γ | 41 | 31 | 0.65 (0.23–1.80) | 0.49 | 45 | 1.18 (0.37–3.71) | 0.80 | 44 | 1.11 (0.46–2.71) | 0.84 | |
| HPyV | Any | 41 | 43 | 1.06 (0.40–2.84) | 1 | 73 | 3.71 (1.12–13.85) | 0.02 | 60 | 2.11 (0.87–5.22) | 0.10 |
| HPyV6 | 4.34 | 9.37 | 2.25 (0.24–28.50) | 0.40 | 22.70 | 6.27 (0.92–71.93) | 0.03 | 4 | 0.92 (0.06–13.15) | 1 | |
| MCPyV | 41.30 | 40.62 | 0.97 (0.35–2.67) | 1 | 54.50 | 1.69 (0.54–5.39) | 0.43 | 62 | 1.95 (0.81–4.79) | 0.15 | |
| HHV | Any | 96 | 97 | 1.54 (0.08–93.77) | 1 | 100 | Inf (0.09-Inf) | 1 | 96 | 1.09 (0.08–15.63) | 1 |
| EBV1 | 34.78 | 40.62 | 1.28 (0.45–3.59) | 0.64 | 40.90 | 1.29 (0.39–4.14) | 0.79 | 64 | 3.29 (1.33–8.40) | 0.007 | |
| EBV2 | 4.34 | 3.12 | 0.71 (0.01–14.25) | 1 | 13.63 | 3.40 (0.36–43.83) | 0.32 | 0 | 0 (0–4.88) | 0.23 | |
| HSV1 | 6.52 | 18.75 | 3.25 (0.63–21.84) | 0.15 | 13.63 | 2.23 (0.27–18.25) | 0.38 | 10 | 0 (0–4.88) | 0.23 | |
| CMV | 2.17 | 9.37 | 4.56 (0.35–249.33) | 0.30 | 4.54 | 2.12 (0.03–3.78) | 0.37 | 6 | 1.86 (0.09–112.96) | 1 | |
| HHV6B | 41.30 | 62.62 | 2.45 (0.89–7.06) | 0.07 | 68.18 | 2.74 (0.85–9.58) | 0.07 | 46 | 1.02 (0.42–2.47) | 1 | |
| HHV7 | 95.65 | 87.50 | 0.44 (0.03–4.13) | 0.40 | 90.90 | 0.46 (0.03–6.77) | 0.59 | 88 | 0.41 (0.04–2.68) | 0.44 | |
| β-HPV Type | NW | OW | Obesity | AP | CRC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | OR (95% CI) | p | % | OR (95% CI) | p | % | OR (95% CI) | p | % | OR (95% CI) | p | |
| HPV5 | 3.8 | 30 | 10.2 (1.07–10.48) | 0.03 | 25.7 | 8.41 (1.03–393.37) | 0.03 | 32 | 11.11 (1.24–544.58) | 0.02 | 40 | 16.21 (2.27–715.30) | 0.0008 |
| HPV8 | 7.6 | 25 | 3.88 (0.55–5.65) | 0.21 | 17 | 2.4 (0.39–26.98) | 0.45 | 9 | 1.19 (0.08–7.88) | 1 | 28 | 4.59 (0.92–45.23) | 0.04 |
| HPV20 | 0 | 0 | 0 (/) | 1 | 5.7 | / | 0.50 | 4.5 | / | 0.46 | 4 | / | 0.54 |
| HPV23 | 0 | 15 | / | 0.07 | 8.5 | / | 0.25 | 6.5 | / | 0.46 | 12 | / | 0.09 |
| HPV24 | 11.5 | 10 | 0.85 (0.06–8.31) | 1 | 14 | 1.27 (0.22–9.05) | 1 | 11 | 2.16 (0.37–6.28) | 0.44 | 22 | 2.14 (0.49–13.19) | 0.36 |
| HPV49 | 0 | 15 | 0 (/) | 0.07 | 8.5 | / (/) | 0.25 | 6.5 | / | 0.002 | 10 | 0.49 (/) | 0.16 |
| HPV76 | 19 | 20 | 1.05 (0.18–5.78) | 1 | 5.7 | 0.26 (0.02–1.77) | 0.12 | 9 | 0.43 (0.04–2.99) | 0.43 | 30 | 1.79 (0.52–7.22) | 0.41 |
| HPV93 | 0 | 0 | 0 (/) | 1 | 5.7 | / | 0.50 | 0 | / | 1 | 4 | / | 0.54 |
| HPV120 | 0 | 10 | / | 0.18 | 5.7 | / | 0.50 | 4.5 | / | 0.46 | 26 | / | 0.003 |
| HPV122 | 7.6 | 25 | 3.88 (0.55–5.65) | 0.21 | 5.7 | 0.73 (0.05–0.75) | 1 | 9 | 1.19 (0.08–17.88) | 1 | 32 | 5.54 (1.13–54.12) | 0.02 |
| HPV124 | 0 | 0 | 0 (/) | 1 | 11.4 | / | 0.1 | 0 | / | 1 | 16 | / | 0.04 |
| Virus | % | T6 vs. T0 | T12 vs. T0 | T12 vs. T6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T6 | T12 | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| HPyV | 50 | 35.7 | 32 | 0.56 (0.16–1.84) | 0.42 | 0.48 (0.14–1.59) | 0.28 | 0.85 (0.24–2.97) | 1 |
| HPyV6 | 9.1 | 3 | 6.9 | 0.31 (0.005–4.21) | 0.61 | 0.65 (0.05–6.14) | 1 | 2.05 (0.10–126.89) | 1 |
| MCPyV | 42.4 | 39.4 | 31 | 0.88 (0.28–2.69) | 1 | 0.70 (0.21–2.26) | 0.59 | 0.79 (0.23–2.60) | 0.79 |
| HPV | 89 | 75 | 71 | 0.58 (0.14–2.23) | 0.55 | 0.43 (0.11–1.57) | 0.25 | 0.74 (0.21–2.51) | 0.78 |
| β-HPV | 78.5 | 67.8 | 60.7 | 0.58 (0.14–2.23) | 0.55 | 0.43 (0.11–1.57) | 0.25 | 0.74 (0.21–2.51) | 0.78 |
| Multiple infections | 53.5 | 53.5 | 46 | 1 (0.31–3.24) | 1 | 0.75 (0.23–2.43) | 0.79 | 0.75 (0.23–2.43) | 0.79 |
| Genotype | % | T6 vs. T0 | T12 vs. T0 | T12 vs. T6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T6 | T12 | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| HPV5 | 28.5 | 21 | 14 | 0.69 (0.16–2.72) | 0.76 | 0.42 (0.08–1.87) | 0.33 | 0.62 (0.11–3) | 0.73 |
| HPV8 | 21 | 7 | 10.7 | 0.29 (0.02–1.82) | 0.25 | 0.45 (0.64–2.39) | 0.47 | 1.55 (0.16–19.99) | 1 |
| HPV19 | 17.8 | 14 | 10.7 | 0.77 (0.13–4.08) | 1 | 0.56 (0.078–3.24) | 0.70 | 0.72 (0.096–4.78) | 1 |
| HPV23 | 7 | 3.5 | 3.5 | 0.49 (0.008–9.90 | 1 | 0.49 (0.008–9.90) | 1 | 1 (0.12–81.32) | 1 |
| HPV24 | 17.8 | 14 | 14 | 0.77 (0.13–4.08) | 1 | 0.77 (0.13–4.08) | 1 | 1 (0.16–6.04) | 1 |
| HPV49 | 10.7 | 10.7 | 7 | 1 (0.12–8.21) | 1 | 0.65 (0.05–6.14) | 1 | 0.65 (0.05–6.14) | 1 |
| HPV76 | 7 | 3.5 | 3.5 | 0.49 (0.008–9.9) | 1 | 0.49 (0.008–9.9) | 1 | 1 (0.12–81.32) | 1 |
| HPV92 | 3.5 | 0 | 0 | 0 (0–39) | 1 | 0 (0–39) | 1 | 0 (/) | 1 |
| HPV96 | 3.5 | 0 | 0 | 0 (0–39) | 1 | 0 (0–39) | 1 | 0 (/) | 1 |
| HPV100 | 14 | 10.7 | 0 | 0.72 (0.095–4.78) | 1 | 0 (0–1.45) | 0.11 | 0 (0–2.37) | 0.24 |
| HPV105 | 14 | 7 | 7 | 0.47 (0.04–3.61) | 0.67 | 0.47 (0.04–3.61) | 0.67 | 1 (0.68–14.76) | 1 |
| HPV113 | 3.5 | 0 | 0 | 0 (0–39) | 1 | 0 (0–39) | 1 | 0 (/) | 1 |
| HPV120 | 7 | 3.5 | 3.5 | 0.48 (0.008–9.90) | 1 | 0.48 (0.008–9.90) | 1 | 1 (0.12–81.32) | 1 |
| HPV124 | 10.7 | 7 | 7 | 0.65 (0.5–6.14) | 1 | 0.65 (0.5–6.14) | 1 | 1 (0.68–14.76) | 1 |
| HPV159 | 10.7 | 3.5 | 3.5 | 0.31 (0.006–4.21) | 0.61 | 0.31 (0.006–4.21) | 0.61 | 1 (0.012–81.32) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fertitta, V.; Escobar Marcillo, D.I.; Privitera, G.F.; Del Cornò, M.; Guglielmi, V.; Agnes, A.; Varano, B.; Colangeli, L.; Ferri, L.; McKay-Chopin, S.; et al. Oral Viral DNA Profiling in Obesity, Adenomatous Polyposis, and Colorectal Cancer Identifies Human β-Papillomavirus Types as Potentially Sex-Related and Modifiable Cancer Risk Indicators. Cancers 2025, 17, 3024. https://doi.org/10.3390/cancers17183024
Fertitta V, Escobar Marcillo DI, Privitera GF, Del Cornò M, Guglielmi V, Agnes A, Varano B, Colangeli L, Ferri L, McKay-Chopin S, et al. Oral Viral DNA Profiling in Obesity, Adenomatous Polyposis, and Colorectal Cancer Identifies Human β-Papillomavirus Types as Potentially Sex-Related and Modifiable Cancer Risk Indicators. Cancers. 2025; 17(18):3024. https://doi.org/10.3390/cancers17183024
Chicago/Turabian StyleFertitta, Veronica, David Israel Escobar Marcillo, Grete Francesca Privitera, Manuela Del Cornò, Valeria Guglielmi, Annamaria Agnes, Barbara Varano, Luca Colangeli, Lorenzo Ferri, Sandrine McKay-Chopin, and et al. 2025. "Oral Viral DNA Profiling in Obesity, Adenomatous Polyposis, and Colorectal Cancer Identifies Human β-Papillomavirus Types as Potentially Sex-Related and Modifiable Cancer Risk Indicators" Cancers 17, no. 18: 3024. https://doi.org/10.3390/cancers17183024
APA StyleFertitta, V., Escobar Marcillo, D. I., Privitera, G. F., Del Cornò, M., Guglielmi, V., Agnes, A., Varano, B., Colangeli, L., Ferri, L., McKay-Chopin, S., Sbraccia, P., Persiani, R., Pulvirenti, A., Herceg, Z., Tommasino, M., Gheit, T., Fortini, P., & Conti, L. (2025). Oral Viral DNA Profiling in Obesity, Adenomatous Polyposis, and Colorectal Cancer Identifies Human β-Papillomavirus Types as Potentially Sex-Related and Modifiable Cancer Risk Indicators. Cancers, 17(18), 3024. https://doi.org/10.3390/cancers17183024








