Artificial Intelligence in Head and Neck Cancer: Towards Precision Medicine
Simple Summary
Abstract
1. Introduction
1.1. Epidemiology and Economic Burden
1.2. Clinical Challenges in HNC and the Role of AI
2. An Introduction to AI in Medicine
3. Utility of AI in Imaging and Diagnostics
3.1. Artificial Intelligence in Radiology
3.2. Artificial Intelligence in Histopathology and Biomarker Assessment
4. AI in the Treatment Course
Drug Discovery
5. AI in Prognosis and Outcome Prediction, Risk Assessment, Patient Monitoring and Follow-Up
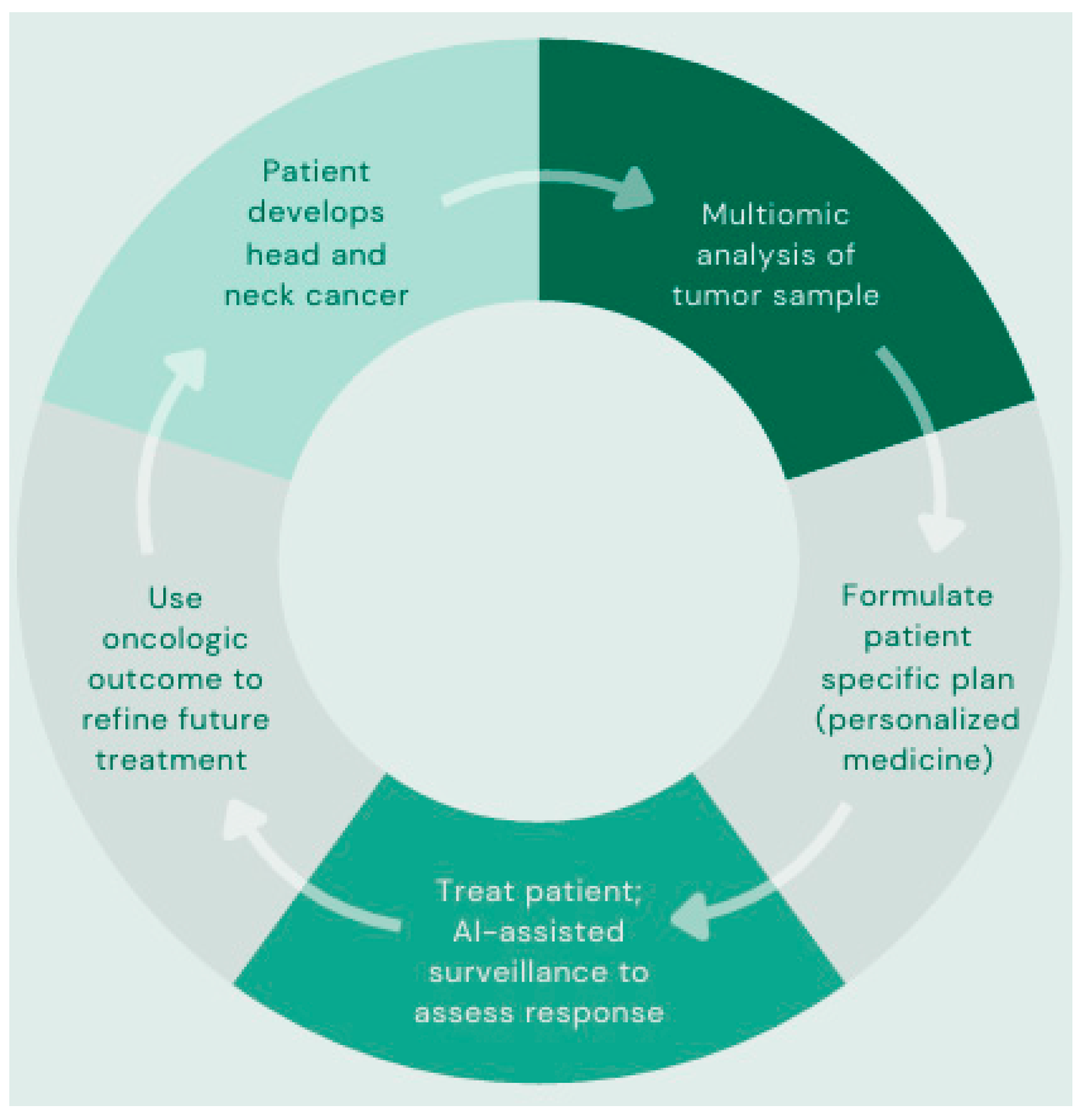
6. AI in Precision Medicine
7. Ethical, Practical and Legal Considerations
8. Emerging Technology and Applications of AI
9. Discussion
9.1. Key Findings
9.2. Challenges
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SEER*Explorer Application. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=80&data_type=1&graph_type=2&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=1#resultsRegion0 (accessed on 30 March 2025).
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Pytynia, K.B.; Dahlstrom, K.R.; Sturgis, E.M. Epidemiology of HPV-Associated Oropharyngeal Cancer. Oral Oncol. 2014, 50, 380–386. [Google Scholar] [CrossRef]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Teh, M.-T.; Chatzopoulou, D.; Holmes, S.; Coulthard, P. Artificial Intelligence in Head and Neck Cancer: Innovations, Applications, and Future Directions. Curr. Oncol. Tor. Ont 2024, 31, 5255–5290. [Google Scholar] [CrossRef]
- Muneer, A.; Waqas, M.; Saad, M.B.; Showkatian, E.; Bandyopadhyay, R.; Xu, H.; Li, W.; Chang, J.Y.; Liao, Z.; Haymaker, C.; et al. From Classical Machine Learning to Emerging Foundation Models: Review on Multimodal Data Integration for Cancer Research. arXiv 2025. [Google Scholar] [CrossRef]
- Klontzas, M.E.; Fanni, S.C.; Febi, M.; Aghakhanyan, G.; Neri, E. Introduction to Artificial Intelligence, 1st ed.; Imaging Informatics for Healthcare Professionals Series; Springer International Publishing AG: Cham, Switzerland, 2023. [Google Scholar]
- Chowdhary, K.R. Fundamentals of Artificial Intelligence; Springer: New Delhi, India, 2020. [Google Scholar]
- Iroju, O.G.; Olaleke, J.O. A Systematic Review of Natural Language Processing in Healthcare. Int. J. Inf. Technol. Comput. Sci. 2015, 7, 44–50. [Google Scholar] [CrossRef]
- Ma, L.; Lu, G.; Wang, D.; Qin, X.; Chen, Z.G.; Fei, B. Adaptive Deep Learning for Head and Neck Cancer Detection Using Hyperspectral Imaging. Vis. Comput. Ind. Biomed. Art 2019, 2, 18. [Google Scholar] [CrossRef]
- Stephens, H.; Li, X.; Sheng, Y.; Wu, Q.; Ge, Y.; Wu, Q.J. A Reinforcement Learning Agent for Head and Neck Intensity-Modulated Radiation Therapy. Front. Phys. 2024, 12, 1331849. [Google Scholar] [CrossRef]
- Meneghetti, A.R.; Hernández, M.L.; Kuehn, J.-P.; Löck, S.; Carrero, Z.I.; Perez-Lopez, R.; Bressem, K.; Brinker, T.K.; Pearson, A.T.; Truhn, D.; et al. End-to-End Prediction of Clinical Outcomes in Head and Neck Squamous Cell Carcinoma with Foundation Model-Based Multiple Instance Learning. MedRxiv Serv. Health Sci. 2025; preprint. [Google Scholar] [CrossRef] [PubMed]
- Illimoottil, M.; Ginat, D. Recent Advances in Deep Learning and Medical Imaging for Head and Neck Cancer Treatment: MRI, CT, and PET Scans. Cancers 2023, 15, 3267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-B.; Liu, C.; Ye, J.; Chang, L.-F.; Xu, Q.; Shi, B.-W.; Liu, L.-L.; Yin, Y.-L.; Shi, B.-B. A Comparison between Deep Learning Convolutional Neural Networks and Radiologists in the Differentiation of Benign and Malignant Thyroid Nodules on CT Images. Endokrynol. Pol. 2021, 72, 217–225. [Google Scholar] [CrossRef]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-Cell Carcinoma Using a Radiomic Approach. Anticancer Res. 2020, 40, 271–280. [Google Scholar] [CrossRef]
- Kann, B.H.; Aneja, S.; Loganadane, G.V.; Kelly, J.R.; Smith, S.M.; Decker, R.H.; Yu, J.B.; Park, H.S.; Yarbrough, W.G.; Malhotra, A.; et al. Pretreatment Identification of Head and Neck Cancer Nodal Metastasis and Extranodal Extension Using Deep Learning Neural Networks. Sci. Rep. 2018, 8, 14036. [Google Scholar] [CrossRef]
- Fujima, N.; Kamagata, K.; Ueda, D.; Fujita, S.; Fushimi, Y.; Yanagawa, M.; Ito, R.; Tsuboyama, T.; Kawamura, M.; Nakaura, T.; et al. Current State of Artificial Intelligence in Clinical Applications for Head and Neck MR Imaging. Magn. Reson. Med. Sci. 2023, 22, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Nakamichi, R.; Ichikawa, K.; Kawamura, M.; Kawai, H.; Yoshida, T.; Sone, M. MR Imaging of Endolymphatic Hydrops: Utility of iHYDROPS-Mi2 Combined with Deep Learning Reconstruction Denoising. Magn. Reson. Med. Sci. 2020, 20, 272–279. [Google Scholar] [CrossRef]
- Fujima, N.; Shimizu, Y.; Ikebe, Y.; Kameda, H.; Harada, T.; Tsushima, N.; Kano, S.; Homma, A.; Kwon, J.; Yoneyama, M.; et al. Dual-Type Deep Learning-Based Image Reconstruction for Advanced Denoising and Super-Resolution Processing in Head and Neck T2-Weighted Imaging. Jpn. J. Radiol. 2025, 43, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Safarian, A.; Mirshahvalad, S.A.; Farbod, A.; Nasrollahi, H.; Pirich, C.; Beheshti, M. Artificial Intelligence for Tumor [18F] FDG-PET Imaging: Advancement and Future Trends—Part I. Semin. Nucl. Med. 2025, 55, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.P.; Zeevi, T.; Baumeister, P.; Reichel, C.; Sharaf, K.; Forghani, R.; Kann, B.H.; Judson, B.L.; Prasad, M.L.; Burtness, B.; et al. Potential Added Value of PET/CT Radiomics for Survival Prognostication beyond AJCC 8th Edition Staging in Oropharyngeal Squamous Cell Carcinoma. Cancers 2020, 12, 1778. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, P.; Jannatdoust, P.; Pahlevan-Fallahy, M.-T.; Hassankhani, A.; Amoukhteh, M.; Bagherieh, S.; Ghadimi, D.J.; Gholamrezanezhad, A. Diagnostic Accuracy of Radiomics and Artificial Intelligence Models in Diagnosing Lymph Node Metastasis in Head and Neck Cancers: A Systematic Review and Meta-Analysis. Neuroradiology 2025, 67, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Frood, R.; Brown, P.; Nelstrop, H.; Prestwich, R.; McDermott, G.; Currie, S.; Vaidyanathan, S.; Scarsbrook, A.F. Machine Learning-Based FDG PET-CT Radiomics for Outcome Prediction in Larynx and Hypopharynx Squamous Cell Carcinoma. Clin. Radiol. 2021, 76, e9–e78. [Google Scholar] [CrossRef]
- Alnafisah, K.H.; Ranjan, A.; Sahu, S.P.; Chen, J.; Alhejji, S.M.; Noël, A.; Gartia, M.R.; Mukhopadhyay, S. Machine Learning for Automated Classification of Lung Collagen in a Urethane-Induced Lung Injury Mouse Model. Biomed. Opt. Express 2024, 15, 5980–5998. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.P.; Liu, Q.; Prasad, A.; Hasan, S.M.A.; Liu, Q.; Rodriguez, M.X.B.; Mukhopadhyay, O.; Burk, D.; Francis, J.; Mukhopadhyay, S.; et al. Characterization of Fibrillar Collagen Isoforms in Infarcted Mouse Hearts Using Second Harmonic Generation Imaging. Biomed. Opt. Express 2021, 12, 604–618. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Abouzid, M.; Kaczmarek, E. Deep Learning Approaches in Histopathology. Cancers 2022, 14, 5264. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Wang, Y.; Ping, B.; Li, D.; Du, J.; Qin, Y.; Lu, H.; Wan, X.; Xiang, J. Deep Convolutional Neural Network VGG-16 Model for Differential Diagnosing of Papillary Thyroid Carcinomas in Cytological Images: A Pilot Study. J. Cancer 2019, 10, 4876–4882. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, Q.; Lao, I.; Wang, L.; Wu, Y.; Li, D.; Ji, Q.; Wang, Y.; Zhu, Y.; Lu, H.; et al. Using Deep Convolutional Neural Networks for Multi-Classification of Thyroid Tumor by Histopathology: A Large-Scale Pilot Study. Ann. Transl. Med. 2019, 7, 468. [Google Scholar] [CrossRef]
- Fati, S.M.; Senan, E.M.; Javed, Y. Early Diagnosis of Oral Squamous Cell Carcinoma Based on Histopathological Images Using Deep and Hybrid Learning Approaches. Diagnostics 2022, 12, 1899. [Google Scholar] [CrossRef]
- Wang, W.; Ruan, S.; Xie, Y.; Fang, S.; Yang, J.; Li, X.; Zhang, Y. Development and Validation of a Pathomics Model Using Machine Learning to Predict CXCL8 Expression and Prognosis in Head and Neck Cancer. Clin. Exp. Otorhinolaryngol. 2024, 17, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C.; Li, T.; Luo, J. Prognostic Value of CDKN2A in Head and Neck Squamous Cell Carcinoma via Pathomics and Machine Learning. J. Cell. Mol. Med. 2024, 28, e18394. [Google Scholar] [CrossRef] [PubMed]
- Aye, L.; Bryan, M.E.; Das, D.; Hirayama, S.; Al-Inaya, Y.; Mendel, J.; Naegele, S.; Fisch, A.S.; Faquin, W.C.; Sadow, P. Multi-Feature next-Generation Liquid Biopsy for Diagnosis and Prognosis in HPV-Associated Head and Neck Cancer. J. Clin. Oncol. 2024, 42 (Suppl. S16), 6064. [Google Scholar] [CrossRef]
- Das, D.; Hirayama, S.; Aye, L.; Bryan, M.E.; Naegele, S.; Zhao, B.; Efthymiou, V.; Mendel, J.; Fisch, A.S.; Kröller, L.; et al. Blood-Based Screening for HPV-Associated Cancers. medRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Kawamura, M.; Kamomae, T.; Yanagawa, M.; Kamagata, K.; Fujita, S.; Ueda, D.; Matsui, Y.; Fushimi, Y.; Fujioka, T.; Nozaki, T.; et al. Revolutionizing Radiation Therapy: The Role of AI in Clinical Practice. J. Radiat. Res. 2024, 65, 1–9. [Google Scholar] [CrossRef]
- Lucido, J.J.; DeWees, T.A.; Leavitt, T.R.; Anand, A.; Beltran, C.J.; Brooke, M.D.; Buroker, J.R.; Foote, R.L.; Foss, O.R.; Gleason, A.M.; et al. Validation of Clinical Acceptability of Deep-Learning-Based Automated Segmentation of Organs-at-Risk for Head-and-Neck Radiotherapy Treatment Planning. Front. Oncol. 2023, 13, 1137803. [Google Scholar] [CrossRef] [PubMed]
- Koktzoglou, I.; Huang, R.; Ankenbrandt, W.J.; Walker, M.T.; Edelman, R.R. Super-Resolution Head and Neck MRA Using Deep Machine Learning. Magn. Reson. Med. 2021, 86, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Pertzborn, D.; Nguyen, H.-N.; Hüttmann, K.; Prengel, J.; Ernst, G.; Guntinas-Lichius, O.; von Eggeling, F.; Hoffmann, F. Intraoperative Assessment of Tumor Margins in Tissue Sections with Hyperspectral Imaging and Machine Learning. Cancers 2022, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Halicek, M.; Dormer, J.D.; Little, J.V.; Chen, A.Y.; Myers, L.; Sumer, B.D.; Fei, B. Hyperspectral Imaging of Head and Neck Squamous Cell Carcinoma for Cancer Margin Detection in Surgical Specimens from 102 Patients Using Deep Learning. Cancers 2019, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Little, J.V.; Wang, X.; Zhang, H.; Patel, M.R.; Griffith, C.C.; El-Deiry, M.W.; Chen, A.Y.; Fei, B. Detection of Head and Neck Cancer in Surgical Specimens Using Quantitative Hyperspectral Imaging. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5426–5436. [Google Scholar] [CrossRef]
- Kok Wah, J.N. AI-Driven Robotic Surgery in Oncology: Advancing Precision, Personalization, and Patient Outcomes. J. Robot. Surg. 2025, 19, 382. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Lin, L.; Huang, W.; Xiao, C.; Liu, J.; Chai, G. Intelligent Electromagnetic Navigation System for Robot-Assisted Intraoral Osteotomy in Mandibular Tumor Resection: A Model Experiment. Front. Immunol. 2024, 15, 1436276. [Google Scholar] [CrossRef]
- Pedrett, R.; Mascagni, P.; Beldi, G.; Padoy, N.; Lavanchy, J.L. Technical Skill Assessment in Minimally Invasive Surgery Using Artificial Intelligence: A Systematic Review. Surg. Endosc. 2023, 37, 7412–7424. [Google Scholar] [CrossRef] [PubMed]
- Home-GEO-NCBI. Available online: https://www.ncbi.nlm.nih.gov/geo/ (accessed on 26 April 2025).
- The Cancer Genome Atlas Program (TCGA)—NCI. Available online: https://www.cancer.gov/ccg/research/genome-sequencing/tcga (accessed on 26 April 2025).
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial Intelligence to Deep Learning: Machine Intelligence Approach for Drug Discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial Intelligence in Drug Discovery and Development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Bess, A.; Berglind, F.; Mukhopadhyay, S.; Brylinski, M.; Griggs, N.; Cho, T.; Galliano, C.; Wasan, K.M. Artificial Intelligence for the Discovery of Novel Antimicrobial Agents for Emerging Infectious Diseases. Drug Discov. Today 2022, 27, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Naderi, M.; Liu, T.; Wu, H.-C.; Mukhopadhyay, S.; Brylinski, M. eToxPred: A Machine Learning-Based Approach to Estimate the Toxicity of Drug Candidates. BMC Pharmacol. Toxicol. 2019, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Naderi, M.; Alvin, C.; Mukhopadhyay, S.; Brylinski, M. Break Down in Order To Build Up: Decomposing Small Molecules for Fragment-Based Drug Design with eMolFrag. J. Chem. Inf. Model. 2017, 57, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Alvin, C.; Ding, Y.; Mukhopadhyay, S.; Brylinski, M. A Graph-Based Approach to Construct Target-Focused Libraries for Virtual Screening. J. Cheminform. 2016, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Bess, A.; Berglind, F.; Mukhopadhyay, S.; Brylinski, M.; Alvin, C.; Fattah, F.; Wasan, K.M. Identification of Oral Therapeutics Using an AI Platform against the Virus Responsible for COVID-19, SARS-CoV-2. Front. Pharmacol. 2023, 14, 1297924. [Google Scholar] [CrossRef]
- Ranjan, A.; Bess, A.; Alvin, C.; Mukhopadhyay, S. MDF-DTA: A Multi-Dimensional Fusion Approach for Drug-Target Binding Affinity Prediction. J. Chem. Inf. Model. 2024, 64, 4980–4990. [Google Scholar] [CrossRef]
- Lewis, J.S.; Ali, S.; Luo, J.; Thorstad, W.L.; Madabhushi, A. A Quantitative Histomorphometric Classifier (QuHbIC) Identifies Aggressive Versus Indolent P16-Positive Oropharyngeal Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2014, 38, 128–137. [Google Scholar] [CrossRef]
- Choi, N.; Kim, J.; Yi, H.; Kim, H.; Kim, T.H.; Chung, M.J.; Ji, M.; Kim, Z.; Son, Y.-I. The Use of Artificial Intelligence Models to Predict Survival in Patients with Laryngeal Squamous Cell Carcinoma. Sci. Rep. 2023, 13, 9734. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Shen, Y.-J.; Huang, Q.; Wu, C.-P.; Zhou, L.; Ren, H.-L. Predicting Survival of Advanced Laryngeal Squamous Cell Carcinoma: Comparison of Machine Learning Models and Cox Regression Models. Sci. Rep. 2023, 13, 18498. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, L.; Yu, F.; Guo, R.; Zhou, X.; Zhang, F.; Zhang, H.; Zhang, J.; Li, T. Development of a Pathomics-Based Model for the Prediction of Malignant Transformation in Oral Leukoplakia. Lab. Investig. 2023, 103, 100173. [Google Scholar] [CrossRef]
- Bera, K.; Braman, N.; Gupta, A.; Velcheti, V.; Madabhushi, A. Predicting Cancer Outcomes with Radiomics and Artificial Intelligence in Radiology. Nat. Rev. Clin. Oncol. 2022, 19, 132–146. [Google Scholar] [CrossRef]
- OuYang, P.-Y.; He, Y.; Guo, J.-G.; Liu, J.-N.; Wang, Z.-L.; Li, A.; Li, J.; Yang, S.-S.; Zhang, X.; Fan, W. Artificial Intelligence Aided Precise Detection of Local Recurrence on MRI for Nasopharyngeal Carcinoma: A Multicenter Cohort Study. EClinicalMedicine 2023, 63, 102202. [Google Scholar] [CrossRef] [PubMed]
- Fatapour, Y.; Abiri, A.; Kuan, E.C.; Brody, J.P. Development of a Machine Learning Model to Predict Recurrence of Oral Tongue Squamous Cell Carcinoma. Cancers 2023, 15, 2769. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, X.A.; Matamala, F.; Venegas, B.; Rivera, C. Machine-Learning Applications in Oral Cancer: A Systematic Review. Appl. Sci. 2022, 12, 5715. [Google Scholar] [CrossRef]
- Villanueva-Bueno, C.; Collado-Borrell, R.; Escudero-Vilaplana, V.; Revuelta-Herrero, J.L.; Marzal-Alfaro, M.B.; González-Haba, E.; Arranz-Arija, J.Á.; Osorio, S.; Herranz-Alonso, A.; Sanjurjo-Saez, M. A Smartphone App to Improve the Safety of Patients Undergoing Treatment with Oral Antineoplastic Agents: 4 Years of Experience in a University Hospital. Front. Public Health 2022, 10, 978783. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Linder, L.A.; Kanokvimankul, P.; Fowler, B.; Parsons, B.G.; Macpherson, C.F.; Johnson, R.H. Use of a Smartphone Application for Prompting Oral Medication Adherence Among Adolescents and Young Adults With Cancer. Oncol. Nurs. Forum 2018, 45, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Wei, W.-Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, D. Precision Medicine 2.0: How Digital Health and AI Are Changing the Game. J. Pers. Med. 2023, 13, 1057. [Google Scholar] [CrossRef]
- Bibault, J.-E.; Giraud, P.; Housset, M.; Durdux, C.; Taieb, J.; Berger, A.; Coriat, R.; Chaussade, S.; Dousset, B.; Nordlinger, B. Deep Learning and Radiomics Predict Complete Response after Neo-Adjuvant Chemoradiation for Locally Advanced Rectal Cancer. Sci. Rep. 2018, 8, 12611. [Google Scholar] [CrossRef]
- Edemekong, P.F.; Annamaraju, P.; Afzal, M.; Haydel, M.J. Health Insurance Portability and Accountability Act (HIPAA) Compliance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Richardson, J.P.; Smith, C.; Curtis, S.; Watson, S.; Zhu, X.; Barry, B.; Sharp, R.R. Patient Apprehensions about the Use of Artificial Intelligence in Healthcare. Npj Digit. Med. 2021, 4, 140. [Google Scholar] [CrossRef]
- Istasy, P.; Lee, W.S.; Iansavichene, A.; Upshur, R.; Gyawali, B.; Burkell, J.; Sadikovic, B.; Lazo-Langner, A.; Chin-Yee, B. The Impact of Artificial Intelligence on Health Equity in Oncology: Scoping Review. J. Med. Internet Res. 2022, 24, e39748. [Google Scholar] [CrossRef]
- Panch, T.; Mattie, H.; Atun, R. Artificial Intelligence and Algorithmic Bias: Implications for Health Systems. J. Glob. Health 2019, 9, 010318. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key Challenges for Delivering Clinical Impact with Artificial Intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, C.F.; Frederick, M.J.; Thompson, L.D.R.; Corredor, G.; Khalighi, S.; Zhang, Z.; Song, B.; Lu, C.; Nag, R.; Sankar Viswanathan, V. Machine Learning Driven Index of Tumor Multinucleation Correlates with Survival and Suppressed Anti-Tumor Immunity in Head and Neck Squamous Cell Carcinoma Patients. Oral Oncol. 2023, 143, 106459. [Google Scholar] [CrossRef]
- Khoury, Z.H.; Ferguson, A.; Price, J.B.; Sultan, A.S.; Wang, R. Responsible Artificial Intelligence for Addressing Equity in Oral Healthcare. Front. Oral Health 2024, 5, 1408867. [Google Scholar] [CrossRef]
- Adeoye, J.; Hui, L.; Su, Y.-X. Data-Centric Artificial Intelligence in Oncology: A Systematic Review Assessing Data Quality in Machine Learning Models for Head and Neck Cancer. J. Big Data 2023, 10, 28. [Google Scholar] [CrossRef]
- Shevtsova, D.; Ahmed, A.; Boot, I.W.A.; Sanges, C.; Hudecek, M.; Jacobs, J.J.L.; Hort, S.; Vrijhoef, H.J.M. Trust in and Acceptance of Artificial Intelligence Applications in Medicine: Mixed Methods Study. JMIR Hum. Factors 2024, 11, e47031. [Google Scholar] [CrossRef] [PubMed]
- Wachter, R.M.; Brynjolfsson, E. Will Generative Artificial Intelligence Deliver on Its Promise in Health Care? JAMA 2024, 331, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, E.; Muneer, A.; Zhang, J.; Xia, Y.; Li, X.; Zhou, C.; Heymach, J.V.; Wu, J.; Le, X. Progress and Challenges of Artificial Intelligence in Lung Cancer Clinical Translation. NPJ Precis. Oncol. 2025, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Virdee, P.S.; Tonner, S.; Oke, J.L.; Perera, R.; Riahi, K.; Luan, Y.; Hiom, S.; Kumar, H.; Nandani, H. Validity and Timeliness of Cancer Diagnosis Data Collected during a Prospective Cohort Study and Reported by the English and Welsh Cancer Registries: A Retrospective, Comparative Analysis. Lancet Oncol. 2024, 25, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Broggi, G.; Maniaci, A.; Lentini, M.; Palicelli, A.; Zanelli, M.; Zizzo, M.; Koufopoulos, N.; Salzano, S.; Mazzucchelli, M.; Caltabiano, R. Artificial Intelligence in Head and Neck Cancer Diagnosis: A Comprehensive Review with Emphasis on Radiomics, Histopathological, and Molecular Applications. Cancers 2024, 16, 3623. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Nicholas, K.; Flynn, J.; Silva, N.; Panageas, K.; Mao, J.J.; Gazit, L.; Gorenshteyn, D.; Sokolowski, S.; Newman, T. Analysis of a Remote Monitoring Program for Symptoms Among Adults With Cancer Receiving Antineoplastic Therapy. JAMA Netw. Open 2022, 5, e221078. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Coppieters, M.W.; Smalbrugge, M.; Jones, C.; Byrnes, J.; Todorovic, M.; Moyle, W. Implementing PainChek and PARO to Support Pain Assessment and Management in Residents with Dementia: A Qualitative Study. Pain Manag. Nurs. 2023, 24, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Tempus|AI-Enabled Precision Medicine. Tempus. Available online: https://www.tempus.com/ (accessed on 7 April 2025).
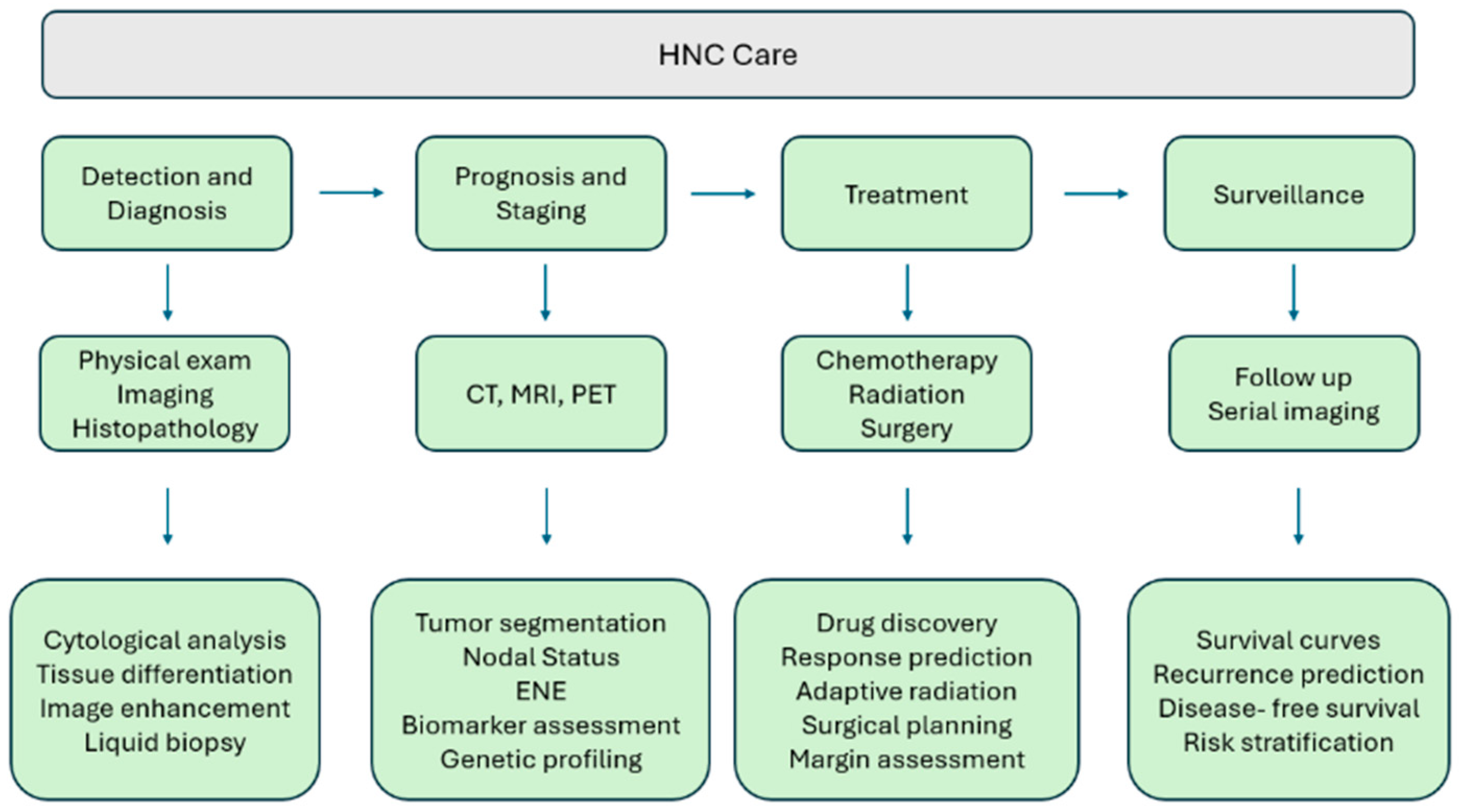


| Type | Summary | Example | Reference |
|---|---|---|---|
| Supervised Learning | Trains models using labeled data, where each input is paired with a known output, to make predictions or classifications on new data | Integrated imaging and clinical data to predict PD-L1 expression using PET/CT, in NSCLC, potentially achieving AUC of 0.82–0.89 for guiding immunotherapy. | [6] |
| Unsupervised Learning | Discovers patterns and structures in unlabeled data without predefined outputs | Auto-encoder networks were used to extract deep features from hyperspectral images to identify tumor margins in head and neck cancer patients, achieving sensitivity of 92.32% and specificity of 91.31%. | [10] |
| Reinforcement Learning | Learns optimal actions through trial-and-error interactions with an environment, guided by rewards and penalties | Has been used to automate intensity-modulated radiation therapy planning by adjusting objective functions to balance target coverage and organ-at-risk sparing in radiotherapy for HNC. | [11] |
| Foundation Models | Large-scale, pre-trained deep learning models that can be fine-tuned for various tasks, often handling multimodal data integration for generalizable insights | In HNSCC, Foundation model-based multiple instance learning predicted 2-year overall survival from routine imaging across external cohorts with an AUC of 0.75–0.84. | [12] |
| Algorithms | Type | Summary | Uses in HNC |
|---|---|---|---|
| Decision Tree | Supervised ML | Uses a branching, treelike structure to make binary decisions | Tissue classification, outcome prediction |
| Naïve Bayes | Supervised ML | Uses probability and Bayes’ theorem to classify data | Biomarker based classification, histopathology categorization |
| K-Nearest Neighbor (KNN) | Supervised ML | Classifies a sample based on the majority class of its closest neighbors in the dataset | Image classification, histopathology slide analysis |
| Support Vector Machine (SVM) | Supervised ML | Finds boundaries (hyperplanes) that separates data into categories | Distinguishing malignant vs. benign lesions, radiomic diagnosis |
| Random Forest | Ensemble ML | Builds many decision trees and combines results to improve accuracy and reduce bias | Prognostication, risk stratification, treatment response prediction |
| Gradient Boosting Machine (GBM) | Ensemble ML | Builds many decision trees sequentially, each tree correcting the errors of the previous | Survival prediction, recurrence risk assessment, treatment planning |
| Artificial Neural Network (ANN) | DL | Mimics brain neurons with layers of interconnected nodes to learn complex patterns | Treatment outcome prediction, biomarker discovery, risk modeling |
| Deep Convolutional Neural Network (CNN/DCNN) | DL | Specialized neural network for image analysis; employs convolutional layers for feature detection | Radiology and histopathology image interpretation, tumor detection, precision diagnostics |
| 3D U-Net | DL | Specialized CNN that captures 3D spatial features | Tumor delineation, radiotherapy planning, organ-at-risk segmentation |
| Generative Adversarial Network (GAN) | DL | Uses a generator and discriminator neural network to generate synthetic data or enhance images | Improving image resolution, generating synthetic pathology/radiology images |
| Reference | Algorithm | Model | Performance |
|---|---|---|---|
| [14] | CNN | ResNet50 | Differentiated benign vs. malignant thyroid tissue with an accuracy of 0.874 |
| [16] | ANN | DualNet | Predicted nodal status and ENE of HNSCC with an AUC of 0.89 |
| [27] | CNN | VGG-16 | Diagnosed PTC based on cytology with an accuracy of 97.66% |
| [28] | CNN | VGG-19 | Diagnosed and differentiated thyroid neoplasms based on cytology with an accuracy of 97.34% |
| [29] | ANN | AlexNet/ResNet-18 hybrid | Diagnosed OSCC based on cytology with an accuracy of 99.1% |
| [54] | RF | QuHbIC | Predicted outcomes of patients with p16 + OSCC based on cytology with an accuracy of 87.5% |
| [57] | RF | * | Predicted malignant transformation or oral leukoplakia based on cytology with an AUC of 0.84 |
| [60] | GBM | * | Predicted 5- and 10-year recurrence rates for OSCC based on cytology with accuracies of 81.8% and 80%, respectively |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, J.; Hornung, L.; Barham, W.; Mukhopadhyay, S.; Bess, A.; Contrera, K.; Basu, D.; Sandulache, V.; Spielmann, G.; Kansara, S. Artificial Intelligence in Head and Neck Cancer: Towards Precision Medicine. Cancers 2025, 17, 3023. https://doi.org/10.3390/cancers17183023
Hagen J, Hornung L, Barham W, Mukhopadhyay S, Bess A, Contrera K, Basu D, Sandulache V, Spielmann G, Kansara S. Artificial Intelligence in Head and Neck Cancer: Towards Precision Medicine. Cancers. 2025; 17(18):3023. https://doi.org/10.3390/cancers17183023
Chicago/Turabian StyleHagen, Jacob, Logan Hornung, William Barham, Supratik Mukhopadhyay, Adam Bess, Kevin Contrera, Devraj Basu, Vlad Sandulache, Guillaume Spielmann, and Sagar Kansara. 2025. "Artificial Intelligence in Head and Neck Cancer: Towards Precision Medicine" Cancers 17, no. 18: 3023. https://doi.org/10.3390/cancers17183023
APA StyleHagen, J., Hornung, L., Barham, W., Mukhopadhyay, S., Bess, A., Contrera, K., Basu, D., Sandulache, V., Spielmann, G., & Kansara, S. (2025). Artificial Intelligence in Head and Neck Cancer: Towards Precision Medicine. Cancers, 17(18), 3023. https://doi.org/10.3390/cancers17183023






