Peritumoral Invasion and Survival in Patients with Oral Squamous Cell Carcinoma—The Role of Perineural and Lymphovascular Invasion
Simple Summary
Abstract
1. Introduction
- To identify the factors associated with PnI and LVI.
- To assess the association of PnI and LVI with regional neck metastasis.
- To evaluate the association of PnI and LVI with survival outcomes.
- To investigate the effect of postoperative radiotherapy in the different subgroups of patients.
2. Methods
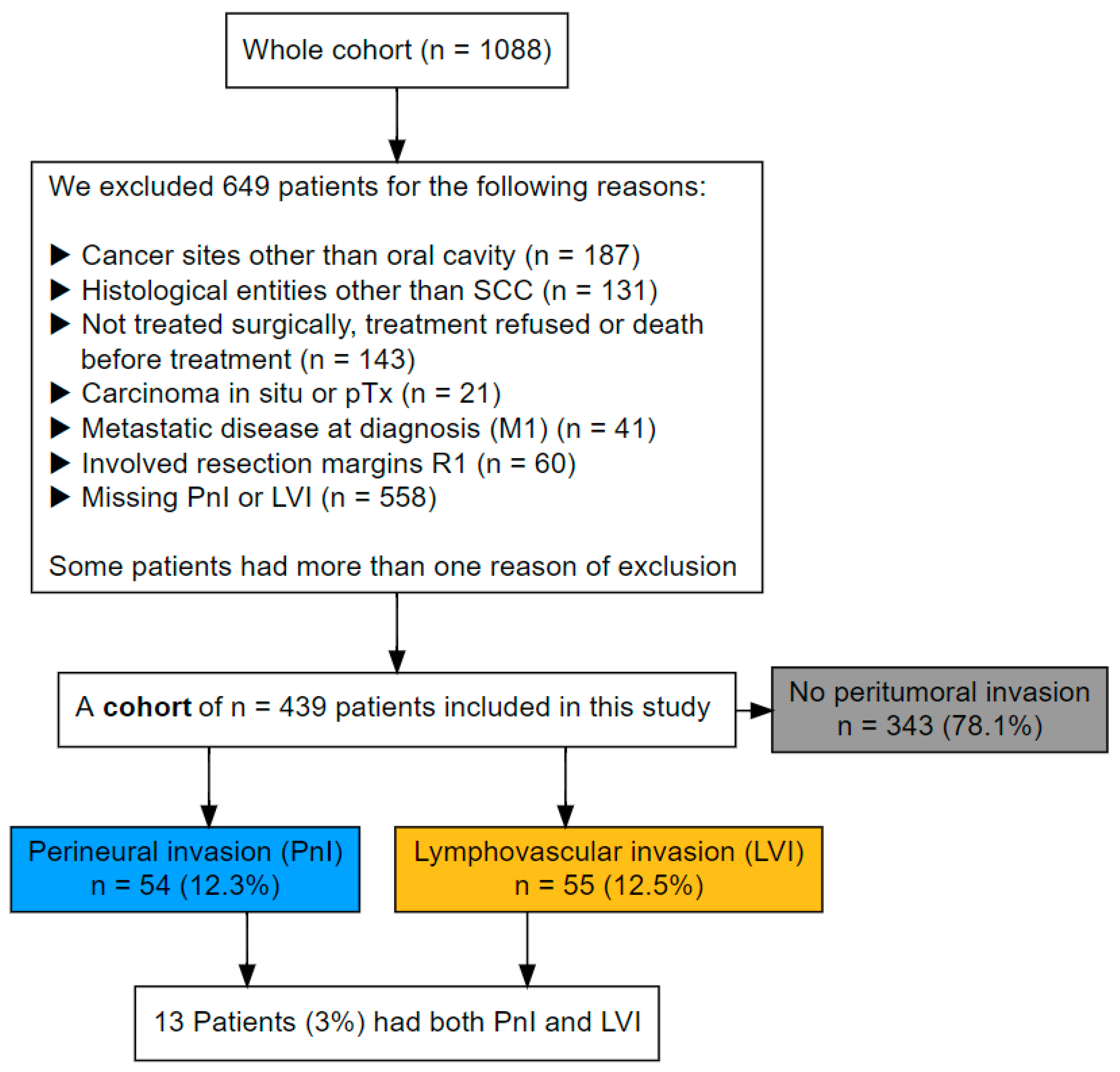
2.1. Study Population and Data Acquisition
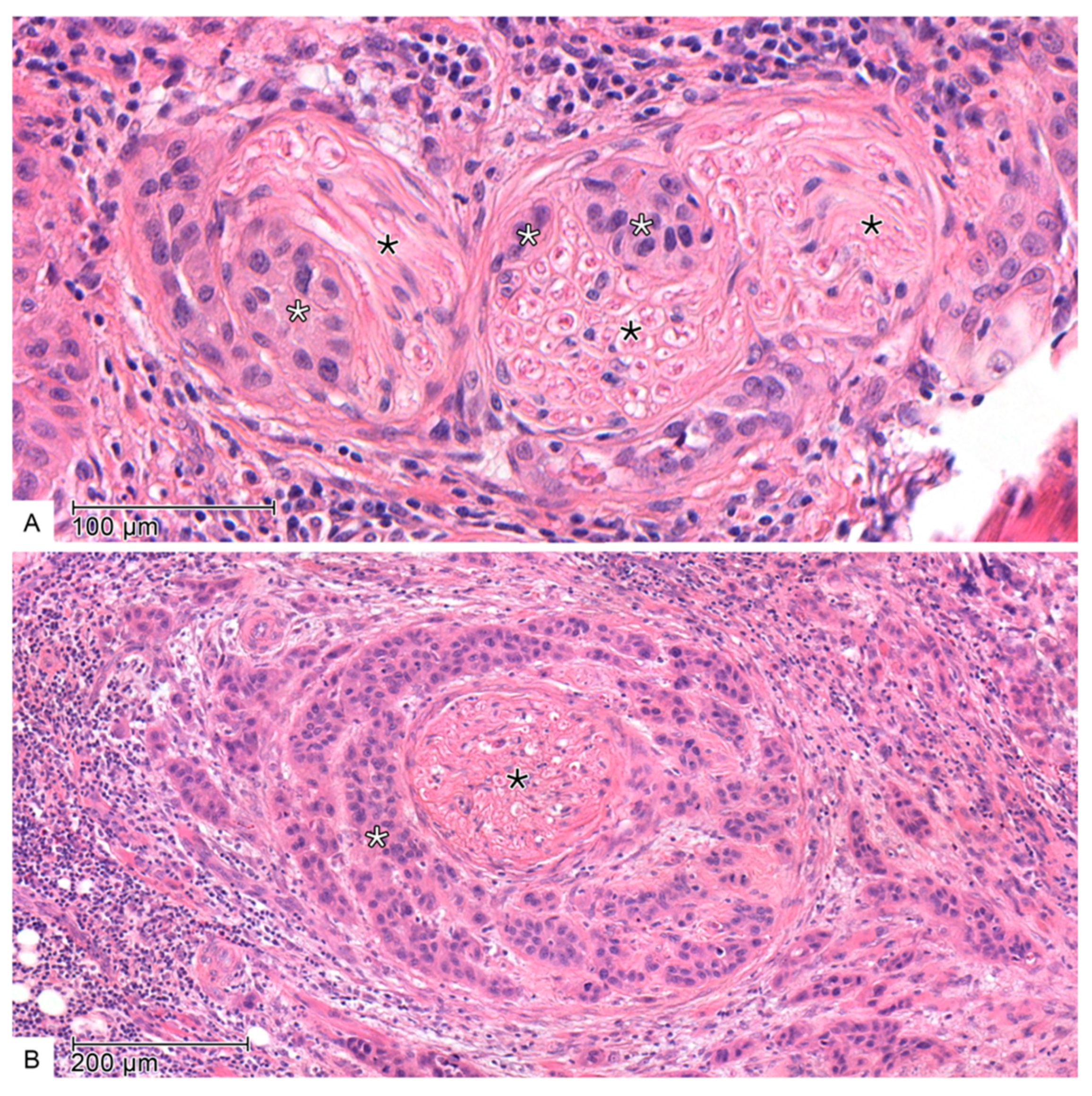
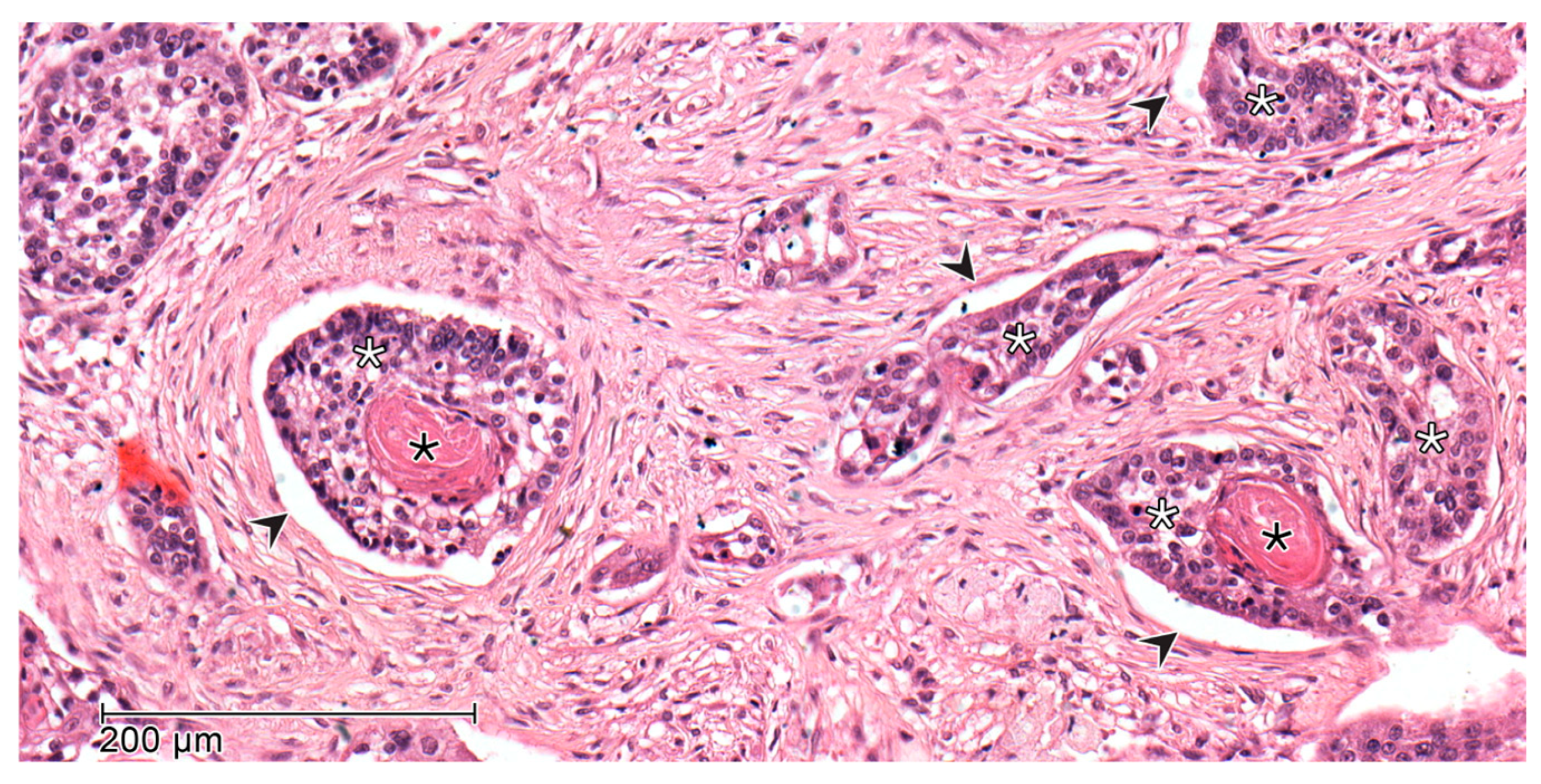
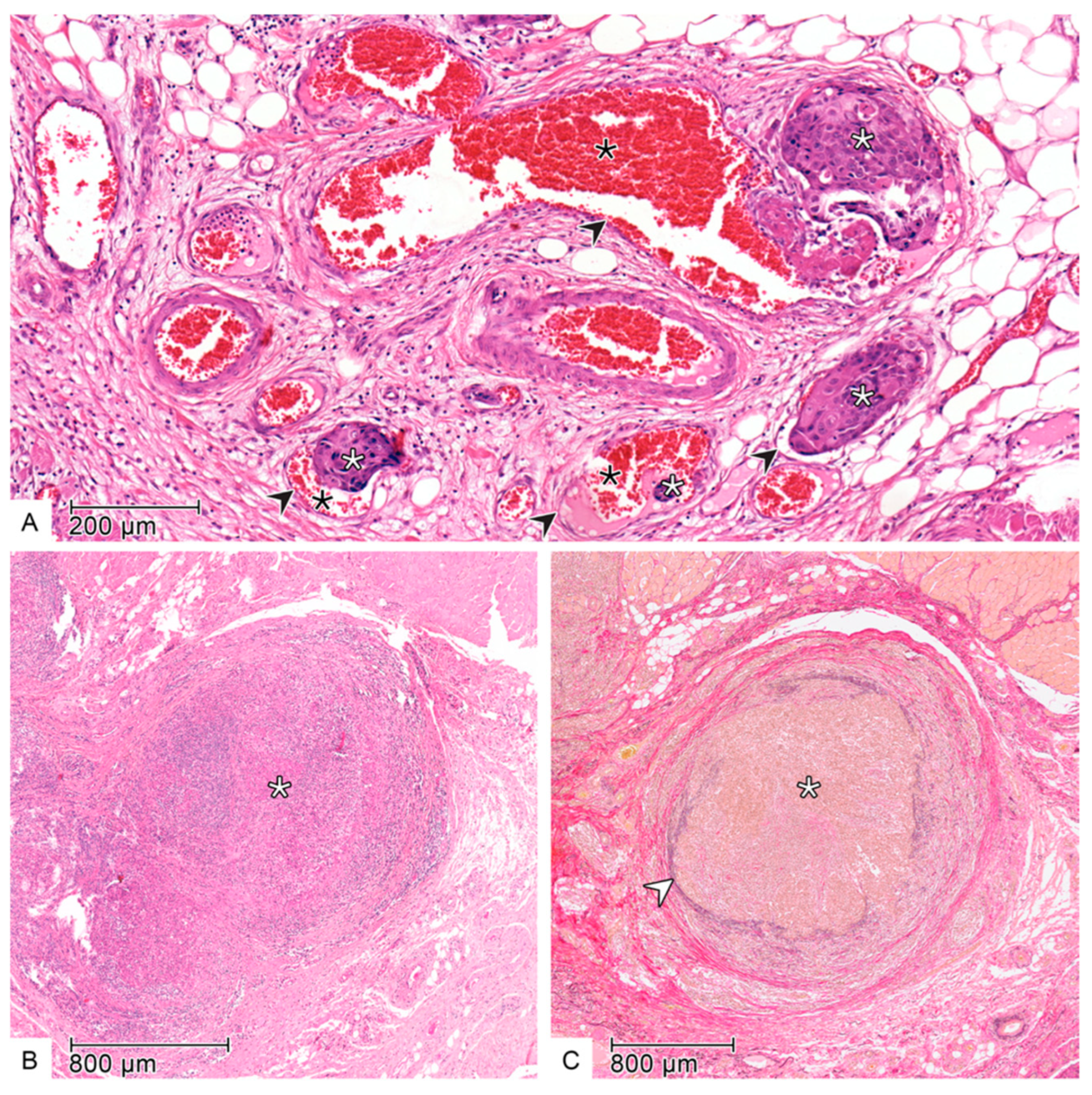
2.2. Histopathology
2.3. Follow-Up and Survival Endpoints
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Patients’ Characteristics
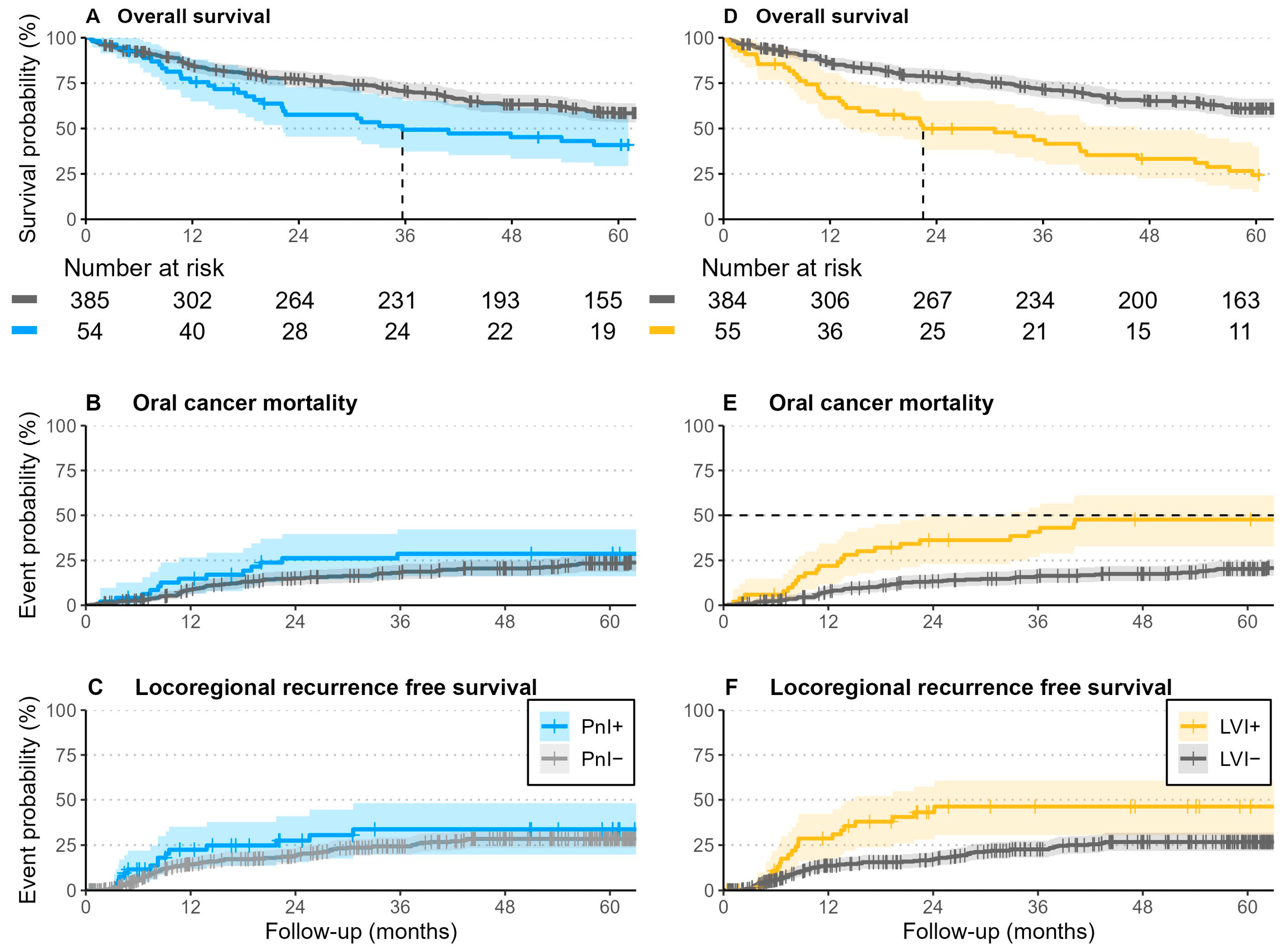
3.2. Survival Outcomes
3.3. Association of PnI and LVI with Initial Presence of Lymph Nodes Metastasis
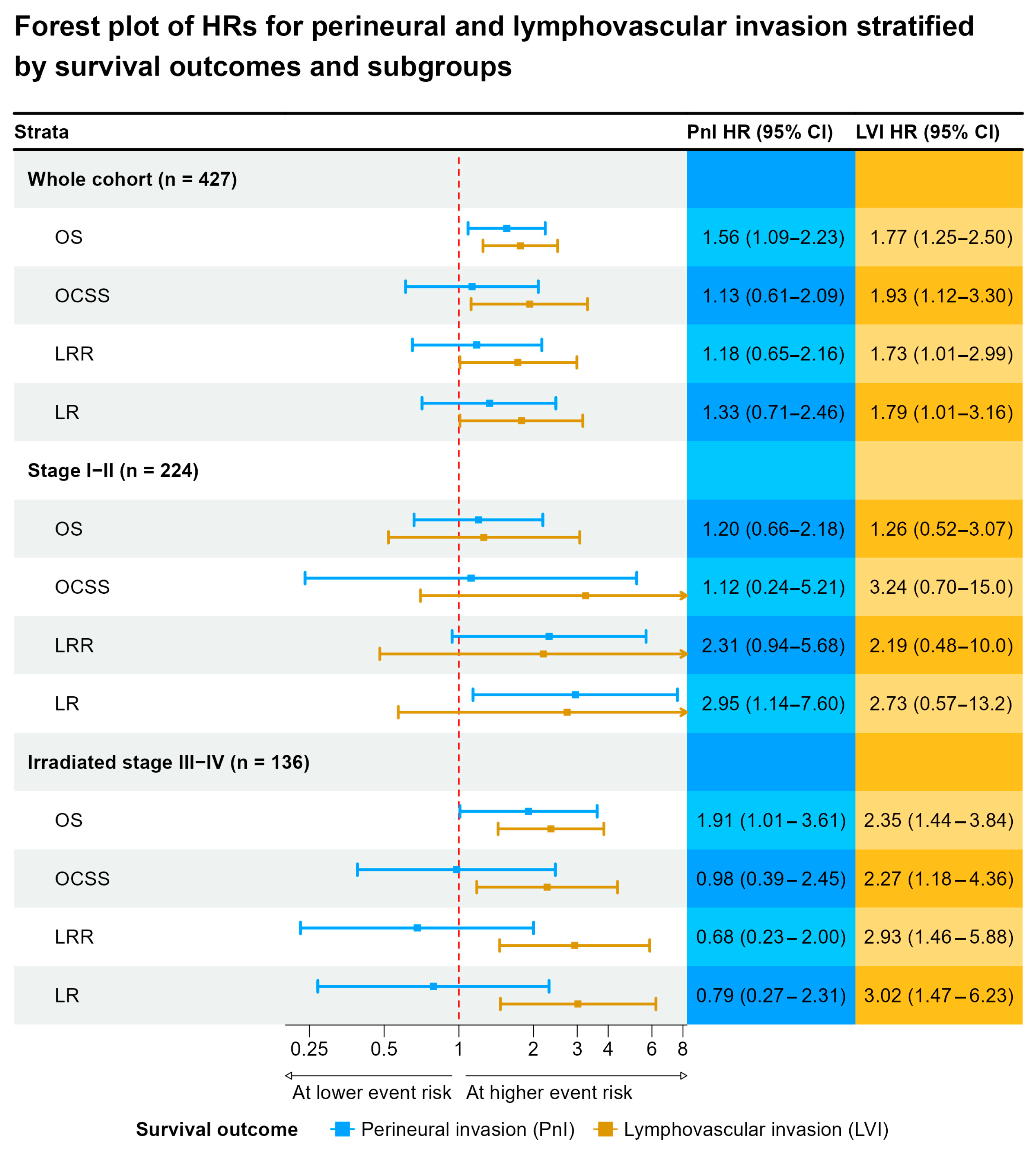
3.4. Survival Analysis
4. Discussion
4.1. PnI and LVI as Histologic Markers and Prognostic Risk Factors
4.2. Effect of Postoperative Radio(Chemo)Therapy on Survival in Patients with PnI and LVI
4.3. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonifazi, M.; Malvezzi, M.; Bertuccio, P.; Edefonti, V.; Garavello, W.; Levi, F.; La Vecchia, C.; Negri, E. Age-period-cohort analysis of oral cancer mortality in Europe: The end of an epidemic? Oral Oncol. 2011, 47, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.R.D.; Compton, K.; Xu, R.; Mishra, R.; Drangsholt, M.T.; Antunes, J.L.F.; Kerr, A.R.; Acheson, A.R.; Lu, D.; Wallace, L.E.; et al. The Global, Regional, and National Burden of Adult Lip, Oral, and Pharyngeal Cancer in 204 Countries and Territories: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2023, 9, 1401–1416. [Google Scholar] [PubMed]
- Hakim, S.G.; Alsharif, U.; Falougy, M.; Tharun, L.; Rades, D.; Jensen, J. The impact of tumor budding and single-cell invasion on survival in patients with stage III/IV locally advanced oral squamous cell carcinoma- results from a prospective cohort study. Front. Oncol. 2024, 14, 1404361. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Wolff, K.-D.; Follmann, M.; Nast, A. The Diagnosis and Treatment of Oral Cavity Cancer. Dtsch. Aerzteblatt Online 2012, 109, 829–835. [Google Scholar] [CrossRef]
- Ettinger, K.S.; Ganry, L.; Fernandes, R.P. Oral Cavity Cancer. Oral Maxillofac Surg. Clin. N. Am. 2019, 31, 13–29. [Google Scholar] [CrossRef]
- Rautava, J.; Syrjänen, S. Biology of Human Papillomavirus Infections in Head and Neck Carcinogenesis. Head Neck Pathol. 2012, 6, 3–15. [Google Scholar] [CrossRef]
- Larson, A.R.; Kemmer, J.; Formeister, E.; El-Sayed, I.; Ha, P.; George, J.; Ryan, W.; Chan, E.; Heaton, C. Beyond Depth of Invasion: Adverse Pathologic Tumor Features in Early Oral Tongue Squamous Cell Carcinoma. Laryngoscope 2019, 130, 1715–1720. [Google Scholar] [CrossRef]
- Tai, S.-K.; Li, W.-Y.; Yang, M.-H.; Chu, P.-Y.; Wang, Y.-F. Perineural Invasion in T1 Oral Squamous Cell Carcinoma Indicates the Need for Aggressive Elective Neck Dissection. Am. J. Surg. Pathol. 2013, 37, 1164–1172. [Google Scholar] [CrossRef]
- Nair, D.; Mair, M.; Singhvi, H.; Mishra, A.; Nair, S.; Agrawal, J.; Chaturvedi, P. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck 2018, 40, 1780–1787. [Google Scholar] [CrossRef]
- Vonk, J.; Smit, K.A.; Roodenburg, J.L.N.; van der Vegt, B.; Halmos, G.B.; Hoek, J.G.M.V.D.; Dijkstra, P.U.; Witjes, M.J.H. Effect of adjuvant radiotherapy on the local recurrence of oral squamous cell carcinoma with perineural invasion: A systematic review. Clin. Otolaryngol. 2018, 44, 131–137. [Google Scholar] [CrossRef]
- Hasmat, S.; Ebrahimi, A.; Gao, K.; Low, T.H.; Palme, C.; Gupta, R.; Clark, J. Multifocal perineural invasion is a better prognosticator than depth of invasion in oral squamous cell carcinoma. Head Neck 2019, 41, 3992–3999. [Google Scholar] [CrossRef]
- Spoerl, S.; Spoerl, S.; Reil, S.; Gerken, M.; Ludwig, N.; Taxis, J.; Fischer, R.; Ettl, T.; Reichert, T.E.; Spanier, G. Prognostic Value of Perineural Invasion on Survival and Recurrence in Oral Squamous Cell Carcinoma. Diagnostics 2022, 12, 1062. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Togni, L.; Caponio, V.; Zhurakivska, K.; Bizzoca, M.; Contaldo, M.; Serpico, R.; Muzio, L.L.; Santarelli, A. Lymphovascular invasion as a prognostic tool for oral squamous cell carcinoma: A comprehensive review. Int. J. Oral Maxillofac. Surg. 2022, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Close, L.G.; Burns, D.K.; Reisch, J.; Schaefer, S.D. Microvascular Invasion in Cancer of the Oral Cavity and Oropharynx. Arch. Otolaryngol. Neck Surg. 1987, 113, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Fang, Y.; Wato, M.; Hata, S.; Tanaka, A. Immunohistochemical co-localization of lymphatics and blood vessels in oral squamous cell carcinomas. J. Oral Pathol. Med. 2005, 34, 334–339. [Google Scholar] [CrossRef]
- Sahoo, A.; Panda, S.; Mohanty, N.; Jena, D.; Mishra, N.; Surabhi; Baisakh, M.R. Perinerural, lymphovascular and depths of invasion in extrapolating nodal metastasis in oral cancer. Clin. Oral Investig. 2019, 24, 747–755. [Google Scholar] [CrossRef]
- Adel, M.; Kao, H.-K.; Hsu, C.-L.; Huang, J.-J.; Lee, L.-Y.; Huang, Y.; Browne, T.; Tsang, N.-M.; Chang, Y.-L.; Chang, K.-P. Evaluation of Lymphatic and Vascular Invasion in Relation to Clinicopathological Factors and Treatment Outcome in Oral Cavity Squamous Cell Carcinoma. Medicine 2015, 94, e1510. [Google Scholar] [CrossRef]
- Caponio, V.C.A.; Troiano, G.; Togni, L.; Zhurakivska, K.; Santarelli, A.; Laino, L.; Rubini, C.; Muzio, L.L.; Mascitti, M. Pattern and localization of perineural invasion predict poor survival in oral tongue carcinoma. Oral Dis. 2021, 29, 411–422. [Google Scholar] [CrossRef]
- Yang, X.; Tian, X.; Wu, K.; Liu, W.; Li, S.; Zhang, Z.; Zhang, C. Prognostic impact of perineural invasion in early stage oral tongue squamous cell carcinoma: Results from a prospective randomized trial. Surg. Oncol. 2018, 27, 123–128. [Google Scholar] [CrossRef]
- Lee, L.; De Paz, D.; Lin, C.; Fan, K.; Wang, H.; Hsieh, C.; Lee, L.; Yen, T.; Liao, C.; Yeh, C.; et al. Prognostic impact of extratumoral perineural invasion in patients with oral cavity squamous cell carcinoma. Cancer Med. 2019, 8, 6185–6194. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Tai, S.-K.; Li, W.-Y.; Yang, M.-H.; Chu, P.-Y.; Wang, Y.-F.; Chang, P.M.-H. Perineural Invasion as a Major Determinant for the Aggressiveness Associated with Increased Tumor Thickness in T1–2 Oral Tongue and Buccal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2013, 20, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Sauter, B.; Foedinger, D.; Sterniczky, B.; Wolff, K.; Rappersberger, K. Immunoelectron Microscopic Characterization of Human Dermal Lymphatic Microvascular Endothelial Cells: Differential Expression of CD31, CD34, and Type IV Collagen with Lymphatic Endothelial Cells vs Blood Capillary Endothelial Cells in Normal Human Skin, Lymphangioma, and Hemangioma In Situ. J. Histochem. Cytochem. 1998, 46, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 2005, 46, 396–402. [Google Scholar] [CrossRef]
- Muñoz-Guerra, M.F.; Marazuela, E.G.; Fernández-Contreras, M.E.; Gamallo, C. P-cadherin expression reduced in squamous cell carcinoma of the oral cavity: An indicatior of poor prognosis. Cancer 2005, 103, 960–969. [Google Scholar] [CrossRef]
- O’DOnnell, R.K.; Kupferman, M.; Wei, S.J.; Singhal, S.; Weber, R.; O’MAlley, B.; Cheng, Y.; Putt, M.; Feldman, M.; Ziober, B.; et al. Gene expression signature predicts lymphatic metastasis in squamous cell carcinoma of the oral cavity. Oncogene 2004, 24, 1244–1251. [Google Scholar] [CrossRef]
- Miyahara, M.; Tanuma, J.; Sugihara, K.; Semba, I. Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer 2007, 110, 1287–1294. [Google Scholar] [CrossRef]
- Muñoz-Guerra, M.F.; Marazuela, E.G.; Martín-Villar, E.; Quintanilla, M.; Gamallo, C. Prognostic significance of intratumoral lymphangiogenesis in squamous cell carcinoma of the oral cavity. Cancer 2003, 100, 553–560. [Google Scholar] [CrossRef]
- Zhuang, Z.; Jian, P.; Longjiang, L.; Bo, H.; Wenlin, X. Altered phenotype of lymphatic endothelial cells induced by highly metastatic OTSCC cells contributed to the lymphatic metastasis of OTSCC cells. Cancer Sci. 2010, 101, 686–692. [Google Scholar] [CrossRef]
- Chatzistefanou, I.; Lubek, J.; Markou, K.; Ord, R.A. The role of perineural invasion in treatment decisions for oral cancer patients: A review of the literature. J. Cranio-Maxillofac. Surg. 2017, 45, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, N.; Balasubramanian, D.; Murthy, S.; Limbachiya, S.; Thankappan, K.; Iyer, S. Adverse pathologic features in early oral squamous cell carcinoma and the role of postoperative radiotherapy—A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Chang, C.-F.; Li, Y.-H.; Yang, C.-Y.; Su, R.-Y.; Lin, C.-K.; Chen, Y.-W. A histopathological evaluation and potential prognostic implications of oral squamous cell carcinoma with adverse features. Oral Oncol. 2019, 95, 65–73. [Google Scholar] [CrossRef]
- Oliver, J.R.; Wu, S.P.; Chang, C.M.; Roden, D.F.; Wang, B.; Hu, K.S.; Schreiber, D.; Givi, B. Survival of oral tongue squamous cell carcinoma in young adults. Head Neck 2019, 41, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Wang, C.-P.; Ko, J.-Y.; Yang, T.-L.; Hsu, C.-W.; Yeh, K.-A.; Chang, Y.-L.; Lou, P.-J. The Impact of Perineural Invasion and/or Lymphovascular Invasion on the Survival of Early-Stage Oral Squamous Cell Carcinoma Patients. Ann. Surg. Oncol. 2013, 20, 2388–2395. [Google Scholar] [CrossRef]
- Spoerl, S.; Gerken, M.; Fischer, R.; Mamilos, A.; Spoerl, S.; Wolf, S.; Pohl, F.; Klingelhöffer, C.; Ettl, T.; Reichert, T.E.; et al. Lymphatic and vascular invasion in oral squamous cell carcinoma: Implications for recurrence and survival in a population-based cohort study. Oral Oncol. 2020, 111, 105009. [Google Scholar] [CrossRef]
- Poleksic, S.; Kalwaic, H.J. Prognostic Value of Vascular Invasion in Squamous Cell Carcinoma of the Head And Neck. Plast. Reconstr. Surg. 1978, 61, 234–240. [Google Scholar] [CrossRef]
- López-Graniel, C.M.; De León, D.T.; Meneses-García, A.; Gómez-Ruiz, C.; Frias-Mendivil, M.; Granados-García, M.; Barrera-Franco, J.L. Tumor angiogenesis as a prognostic factor in oral cavity carcinomas. J. Exp. Clin. Cancer Res. 2001, 20, 463–468. [Google Scholar]
- Tae, K.; El-Naggar, A.K.; Yoo, E.; Feng, L.; Lee, J.J.; Hong, W.K.; Hittelman, W.N.; Shin, D.M. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clin. Cancer Res. 2000, 6, 2821–2828. [Google Scholar]
- Salven, P.; Heikkilä, P.; Anttonen, A.; Kajanti, M.; Joensuu, H. Vascular endothelial growth factor in squamous cell head and neck carcinoma: Expression and prognostic significance. Mod. Pathol. 1997, 10, 1128–1133. [Google Scholar]
- Tian, Q.; Jiang, L.; Dai, D.; Liu, L.; Shi, X.; Guo, Y.; Wu, D.; Yang, J.; Xu, J.; Cai, Z.; et al. Impact of Postoperative Radiotherapy on the Prognosis of Early-Stage (pT1-2N0M0) Oral Tongue Squamous Cell Carcinoma. J. Clin. Oncol. 2024, 42, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Sreeram, M.P.; de Bree, R.; Mendenhall, W.M.; Strojan, P.; Stenman, G.; Mäkitie, A.; Nadal, A.; Rodrigo, J.P.; Ng, S.P.; et al. The Oncological Outcome of Postoperative Radiotherapy in Patients with Node-Negative Early-Stage (T1/T2/N0) Oral Squamous Cell Carcinoma and Perineural Invasion: A Meta-Analysis. Cancers 2025, 17, 862. [Google Scholar] [CrossRef]
- Holcomb, A.J.; Farrokhian, N.; Tolan, C.; Whiteford, E.; Villwock, M.; Kakarala, K.; Shnayder, Y.; Sykes, K.; Lominska, C.; Gan, G.; et al. Adjuvant radiotherapy mitigates impact of perineural invasion on oncologic outcomes in early-stage oral cavity squamous cell carcinoma. A multi-institutional analysis of 557 patients. Oral Oncol. 2023, 142, 106420. [Google Scholar] [CrossRef]
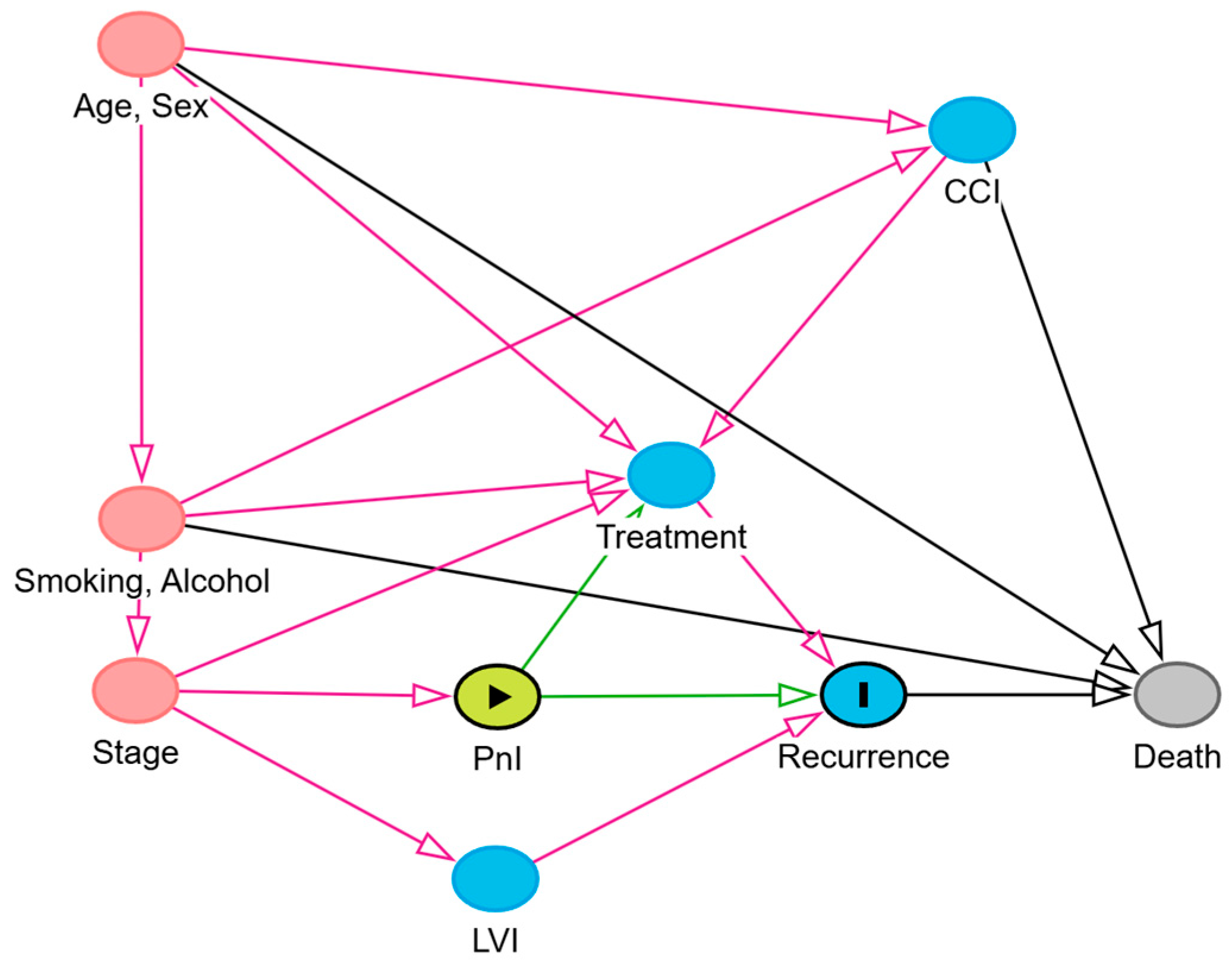
| Stratified by PnI | Stratified by LVI | Overall N = 439 (100%) 1 | |||
|---|---|---|---|---|---|
| Variable | PnI− N = 385 (88%) 1 | PnI+ N = 54 (12%) 1 | LVI− N = 384 (87%) 1 | LVI+ N = 55 (13%) 1 | |
| Age | 62 (54–71) | 64 (51–72) | 63 (54–72) | 61 (53–67) | 62 (54–71) |
| Gender | |||||
| Male | 236 (61%) | 28 (52%) | 222 (58%) | 42 (76%) | 264 (60%) |
| Female | 149 (39%) | 26 (48%) | 162 (42%) | 13 (24%) | 175 (40%) |
| CCI score | |||||
| 0 | 261 (68%) | 33 (61%) | 256 (67%) | 38 (69%) | 294 (67%) |
| 1≤ | 124 (32%) | 21 (39%) | 128 (33%) | 17 (31%) | 145 (33%) |
| Smoking | |||||
| Never | 82 (22%) | 11 (20%) | 89 (24%) | 4 (7.3%) | 93 (22%) |
| Former or current | 291 (78%) | 43 (80%) | 283 (76%) | 51 (93%) | 334 (78%) |
| Missing | 12 | 0 | 12 | 0 | 12 |
| Alcohol consumption | |||||
| None or moderate | 164 (45%) | 15 (29%) | 163 (45%) | 16 (31%) | 179 (43%) |
| Excessive | 198 (55%) | 37 (71%) | 199 (55%) | 36 (69%) | 235 (57%) |
| Missing | 23 | 2 | 22 | 3 | 25 |
| Tumor subsite | |||||
| Floor of mouth | 131 (34%) | 18 (33%) | 123 (32%) | 26 (47%) | 149 (34%) |
| Anterior tongue | 104 (27%) | 20 (37%) | 112 (29%) | 12 (22%) | 124 (28%) |
| Gum | 84 (22%) | 8 (15%) | 77 (20%) | 15 (27%) | 92 (21%) |
| Cheek, Vestibule, retromolar | 34 (8.8%) | 6 (11%) | 39 (10%) | 1 (1.8%) | 40 (9.1%) |
| Palate | 32 (8.3%) | 2 (3.7%) | 33 (8.6%) | 1 (1.8%) | 34 (7.7%) |
| Tumor size | |||||
| T1 | 163 (42%) | 12 (22%) | 171 (45%) | 4 (7.3%) | 175 (40%) |
| T2 | 121 (31%) | 22 (41%) | 123 (32%) | 20 (36%) | 143 (33%) |
| T3 | 40 (10%) | 7 (13%) | 36 (9.4%) | 11 (20%) | 47 (11%) |
| T4 | 61 (16%) | 13 (24%) | 54 (14%) | 20 (36%) | 74 (17%) |
| Nodal disease | |||||
| N0 | 267 (69%) | 27 (50%) | 275 (72%) | 19 (35%) | 294 (67%) |
| N1 | 51 (13%) | 8 (15%) | 50 (13%) | 9 (16%) | 59 (13%) |
| N2a/b | 45 (12%) | 9 (17%) | 41 (11%) | 13 (24%) | 54 (12%) |
| N2c/3 | 22 (5.7%) | 10 (19%) | 18 (4.7%) | 14 (25%) | 32 (7.3%) |
| UICC stage | |||||
| I | 147 (38%) | 7 (13%) | 153 (40%) | 1 (1.8%) | 154 (35%) |
| II | 64 (17%) | 13 (24%) | 70 (18%) | 7 (13%) | 77 (18%) |
| III | 65 (17%) | 9 (17%) | 64 (17%) | 10 (18%) | 74 (17%) |
| IV | 109 (28%) | 25 (46%) | 97 (25%) | 37 (67%) | 134 (31%) |
| Grade of differentiation | |||||
| Well | 36 (9.4%) | 2 (3.7%) | 38 (9.9%) | 0 (0%) | 38 (8.7%) |
| Moderate | 276 (72%) | 39 (72%) | 281 (73%) | 34 (62%) | 315 (72%) |
| Poor | 72 (19%) | 13 (24%) | 64 (17%) | 21 (38%) | 85 (19%) |
| Missing | 1 | 0 | 1 | 0 | 1 |
| Resection margins | |||||
| R0 | 152 (39%) | 13 (24%) | 157 (41%) | 8 (15%) | 165 (38%) |
| R0cm | 122 (32%) | 22 (41%) | 125 (33%) | 19 (35%) | 144 (33%) |
| R0hr | 111 (29%) | 19 (35%) | 102 (27%) | 28 (51%) | 130 (30%) |
| Overall Survival | Death from Oral Cancer | Locoregional Recurrence | Local Recurrence | |
|---|---|---|---|---|
| Variable | At 2 Years | |||
| Overall | 75% (71–79%) | 14% (11–18%) | 20% (16–24%) | 18% (14–22%) |
| Perineural invasion | ||||
| PnI− | 77% (73–81%) | 13% (10–17%) | 19% (15–23%) | 16% (12–20%) |
| PnI+ | 58% (46–73%) | 23% (13–35%) | 28% (15–41%) | 28% (15–41%) |
| Lymphovascular invasion | ||||
| LVI− | 78% (74–83%) | 12% (8.6–15%) | 17% (13–21%) | 14% (11–18%) |
| LVI+ | 50% (38–65%) | 33% (21–46%) | 43% (28–57%) | 41% (26–55%) |
| At 5 years | ||||
| Overall | 56% (51–61%) | 21% (17–25%) | 29% (24–34%) | 26% (22–31%) |
| Perineural invasion | ||||
| PnI− | 58% (53–64%) | 21% (16–25%) | 28% (23–34%) | 25% (21–31%) |
| PnI+ | 41% (29–57%) | 25% (14–38%) | 34% (20–48%) | 34% (20–48%) |
| Lymphovascular invasion | ||||
| LVI− | 61% (56–66%) | 18% (14–22%) | 27% (22–32%) | 24% (19–29%) |
| LVI+ | 24% (15–40%) | 44% (30–57%) | 46% (30–61%) | 44% (28–58%) |
| Perineural Invasion (PnI) | Lymphovascular Invasion (LVI) | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Age | 0.98 | 0.95–1.01 | 0.2 | 1.00 | 0.96–1.03 | 0.8 |
| Gender | ||||||
| Male | — | — | — | — | ||
| Female | 2.02 | 1.08–3.81 | 0.028 | 0.69 | 0.32–1.41 | 0.3 |
| CCI score | ||||||
| 0 | — | — | — | — | ||
| 1≤ | 1.81 | 0.93–3.53 | 0.080 | 1.12 | 0.54–2.27 | 0.8 |
| Smoking | ||||||
| Never | — | — | — | — | ||
| Former | 1.14 | 0.40–3.22 | 0.8 | 2.93 | 0.81–12.4 | 0.12 |
| Current | 0.91 | 0.37–2.34 | 0.8 | 3.52 | 1.10–14.0 | 0.049 |
| Tumor subsite | ||||||
| Floor of mouth | — | — | — | — | ||
| Anterior tongue | 2.02 | 0.92–4.52 | 0.082 | 1.13 | 0.48–2.64 | 0.8 |
| Gum | 0.65 | 0.23–1.68 | 0.4 | 1.21 | 0.50–2.90 | 0.7 |
| Cheek, Vestibule, retromolar | 1.69 | 0.54–4.85 | 0.3 | 0.18 | 0.01–1.01 | 0.11 |
| Palate | 0.50 | 0.07–1.97 | 0.4 | 0.21 | 0.01–1.20 | 0.2 |
| UICC stage | ||||||
| I | — | — | — | — | ||
| II | 4.71 | 1.77–13.6 | 0.003 | 12.2 | 2.04–232 | 0.022 |
| III | 3.39 | 1.16–10.4 | 0.026 | 18.5 | 3.31–347 | 0.006 |
| IV | 7.71 | 3.13–21.4 | <0.001 | 47.3 | 9.60–858 | <0.001 |
| Grade of differentiation | ||||||
| Well/Moderate | — | — | — | — | ||
| Poor | 1.19 | 0.56–2.37 | 0.6 | 2.82 | 1.40–5.68 | 0.003 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Gender | ||||||
| Male | — | — | — | — | ||
| Female | 0.68 | 0.45–1.02 | 0.066 | 0.80 | 0.51–1.27 | 0.4 |
| Tumor subsite | ||||||
| Floor of mouth | — | — | ||||
| Anterior tongue | 0.84 | 0.50–1.38 | 0.5 | |||
| Gum | 1.29 | 0.76–2.20 | 0.3 | |||
| Cheek, Vestibule, retromolar | 0.59 | 0.26–1.26 | 0.2 | |||
| Palate | 0.73 | 0.31–1.61 | 0.5 | |||
| Smoking | ||||||
| Never | — | — | — | — | ||
| Former | 1.51 | 0.77–2.96 | 0.2 | 1.20 | 0.58–2.48 | 0.6 |
| Current | 1.58 | 0.95–2.70 | 0.086 | 1.13 | 0.64–2.01 | 0.7 |
| Tumor size | ||||||
| T1 | — | — | — | — | ||
| T2 | 2.91 | 1.77–4.84 | <0.001 | 2.13 | 1.25–3.65 | 0.005 |
| T3 | 4.71 | 2.39–9.43 | <0.001 | 3.09 | 1.48–6.47 | 0.003 |
| T4 | 3.72 | 2.07–6.76 | <0.001 | 2.66 | 1.42–5.00 | 0.002 |
| Grade of differentiation | ||||||
| Well/Moderate | — | — | — | — | ||
| Poor | 1.80 | 1.11–2.91 | 0.017 | 1.45 | 0.85–2.47 | 0.2 |
| Perineural invasion | ||||||
| None | — | — | — | — | ||
| Evident | 2.24 | 1.26–4.00 | 0.006 | 1.85 | 1.00–3.43 | 0.051 |
| Lymphovascular invasion | ||||||
| None | — | — | — | — | ||
| Evident | 4.32 | 2.41–7.99 | <0.001 | 2.72 | 1.45–5.24 | 0.002 |
| OS | OCSS | LRRFS | LRFS | DMFS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | 1.04 | 1.02–1.05 | <0.001 | ||||||||||||
| CCI score | |||||||||||||||
| 0 | — | — | |||||||||||||
| 1≤ | 1.54 | 1.17–2.03 | 0.002 | ||||||||||||
| Gender | |||||||||||||||
| Male | — | — | — | — | — | — | — | — | — | — | |||||
| Female | 0.75 | 0.56–0.99 | 0.045 | 0.92 | 0.54–1.55 | 0.8 | 1.01 | 0.66–1.55 | >0.9 | 0.98 | 0.62–1.54 | >0.9 | 0.82 | 0.42–1.62 | 0.6 |
| Smoking | |||||||||||||||
| Never | — | — | — | — | — | — | — | — | — | — | |||||
| Former | 1.61 | 1.00–2.59 | 0.049 | 1.47 | 0.65–3.33 | 0.4 | 1.06 | 0.55–2.03 | 0.9 | 1.06 | 0.53–2.09 | 0.9 | 1.31 | 0.48–3.54 | 0.6 |
| Current | 2.23 | 1.49–3.34 | <0.001 | 1.49 | 0.76–2.93 | 0.3 | 0.94 | 0.56–1.60 | 0.8 | 0.90 | 0.52–1.57 | 0.7 | 1.35 | 0.59–3.14 | 0.5 |
| Alcohol consumption | |||||||||||||||
| None or moderate | — | — | — | — | — | — | — | — | — | — | |||||
| Excessive | 1.08 | 0.80–1.45 | 0.6 | 1.03 | 0.61–1.74 | >0.9 | 0.87 | 0.55–1.39 | 0.6 | 0.89 | 0.55–1.44 | 0.6 | 1.01 | 0.50–2.03 | >0.9 |
| UICC stage | |||||||||||||||
| I | — | — | — | — | — | — | — | — | — | — | |||||
| II | 1.00 | 0.67–1.49 | >0.9 | 1.62 | 0.67–3.90 | 0.3 | 0.98 | 0.49–1.95 | >0.9 | 1.04 | 0.50–2.13 | >0.9 | 0.98 | 0.30–3.23 | >0.9 |
| III | 1.89 | 1.30–2.77 | <0.001 | 3.77 | 1.78–7.99 | <0.001 | 1.69 | 0.90–3.18 | 0.10 | 1.57 | 0.80–3.07 | 0.2 | 3.22 | 1.27–8.16 | 0.014 |
| IV | 1.98 | 1.42–2.76 | <0.001 | 4.65 | 2.37–9.10 | <0.001 | 2.74 | 1.67–4.50 | <0.001 | 2.73 | 1.62–4.61 | <0.001 | 5.49 | 2.47–12.2 | <0.001 |
| Perineural invasion | |||||||||||||||
| PnI- | — | — | — | — | — | — | — | — | — | — | |||||
| PnI+ | 1.50 | 1.04–2.17 | 0.031 | 1.05 | 0.55–2.00 | 0.9 | 1.22 | 0.67–2.24 | 0.5 | 1.34 | 0.73–2.48 | 0.4 | 1.02 | 0.46–2.30 | >0.9 |
| OS | OCSS | LRRFS | LRFS | DMFS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | 1.04 | 1.02–1.05 | <0.001 | ||||||||||||
| CCI score | |||||||||||||||
| 0 | — | — | |||||||||||||
| 1≤ | 1.54 | 1.17–2.02 | 0.002 | ||||||||||||
| Gender | |||||||||||||||
| Male | — | — | — | — | — | — | — | — | — | — | |||||
| Female | 0.80 | 0.60–1.06 | 0.12 | 1.00 | 0.59–1.72 | >0.9 | 1.05 | 0.68–1.61 | 0.8 | 0.99 | 0.63–1.57 | >0.9 | 0.87 | 0.44–1.72 | 0.7 |
| Smoking | |||||||||||||||
| Never | — | — | — | — | — | — | — | — | — | — | |||||
| Former | 1.54 | 0.96–2.47 | 0.074 | 1.35 | 0.61–3.02 | 0.5 | 0.98 | 0.51–1.88 | >0.9 | 1.03 | 0.52–2.04 | >0.9 | 1.22 | 0.45–3.27 | 0.7 |
| Current | 2.07 | 1.38–3.10 | <0.001 | 1.31 | 0.68–2.55 | 0.4 | 0.87 | 0.52–1.47 | 0.6 | 0.87 | 0.50–1.52 | 0.6 | 1.22 | 0.53–2.80 | 0.6 |
| Alcohol consumption | |||||||||||||||
| None or moderate | — | — | — | — | — | — | — | — | — | — | |||||
| Excessive | 1.14 | 0.85–1.53 | 0.4 | 1.06 | 0.64–1.76 | 0.8 | 0.87 | 0.55–1.38 | 0.6 | 0.90 | 0.56–1.44 | 0.7 | 0.98 | 0.50–1.96 | >0.9 |
| UICC stage | |||||||||||||||
| I | — | — | — | — | — | — | — | — | — | — | |||||
| II | 1.01 | 0.68–1.50 | >0.9 | 1.53 | 0.64–3.63 | 0.3 | 0.96 | 0.48–1.92 | >0.9 | 1.02 | 0.49–2.12 | >0.9 | 0.92 | 0.28–2.96 | 0.9 |
| III | 1.69 | 1.15–2.49 | 0.008 | 3.35 | 1.57–7.13 | 0.002 | 1.56 | 0.81–2.98 | 0.2 | 1.57 | 0.78–3.14 | 0.2 | 2.75 | 1.06–7.18 | 0.038 |
| IV | 1.85 | 1.32–2.59 | <0.001 | 3.68 | 1.83–7.39 | <0.001 | 2.41 | 1.44–4.03 | <0.001 | 2.71 | 1.57–4.67 | <0.001 | 4.37 | 1.92–9.94 | <0.001 |
| Lymphovascular invasion | |||||||||||||||
| LVI- | — | — | — | — | — | — | — | — | — | — | |||||
| LVI+ | 1.88 | 1.32–2.69 | <0.001 | 2.19 | 1.27–3.78 | 0.005 | 1.88 | 1.08–3.26 | 0.024 | 1.92 | 1.08–3.42 | 0.026 | 2.38 | 1.23–4.58 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, S.G.; Alsharif, U.; Falougy, M.; Tharun, L.; Rades, D.; Kümpers, C.; Jensen, J. Peritumoral Invasion and Survival in Patients with Oral Squamous Cell Carcinoma—The Role of Perineural and Lymphovascular Invasion. Cancers 2025, 17, 2812. https://doi.org/10.3390/cancers17172812
Hakim SG, Alsharif U, Falougy M, Tharun L, Rades D, Kümpers C, Jensen J. Peritumoral Invasion and Survival in Patients with Oral Squamous Cell Carcinoma—The Role of Perineural and Lymphovascular Invasion. Cancers. 2025; 17(17):2812. https://doi.org/10.3390/cancers17172812
Chicago/Turabian StyleHakim, Samer George, Ubai Alsharif, Mohamed Falougy, Lars Tharun, Dirk Rades, Christiane Kümpers, and Justus Jensen. 2025. "Peritumoral Invasion and Survival in Patients with Oral Squamous Cell Carcinoma—The Role of Perineural and Lymphovascular Invasion" Cancers 17, no. 17: 2812. https://doi.org/10.3390/cancers17172812
APA StyleHakim, S. G., Alsharif, U., Falougy, M., Tharun, L., Rades, D., Kümpers, C., & Jensen, J. (2025). Peritumoral Invasion and Survival in Patients with Oral Squamous Cell Carcinoma—The Role of Perineural and Lymphovascular Invasion. Cancers, 17(17), 2812. https://doi.org/10.3390/cancers17172812






