Nanoformulated Terpenoids in Cancer: A Review of Therapeutic Applications, Mechanisms, and Challenges
Simple Summary
Abstract
1. Introduction
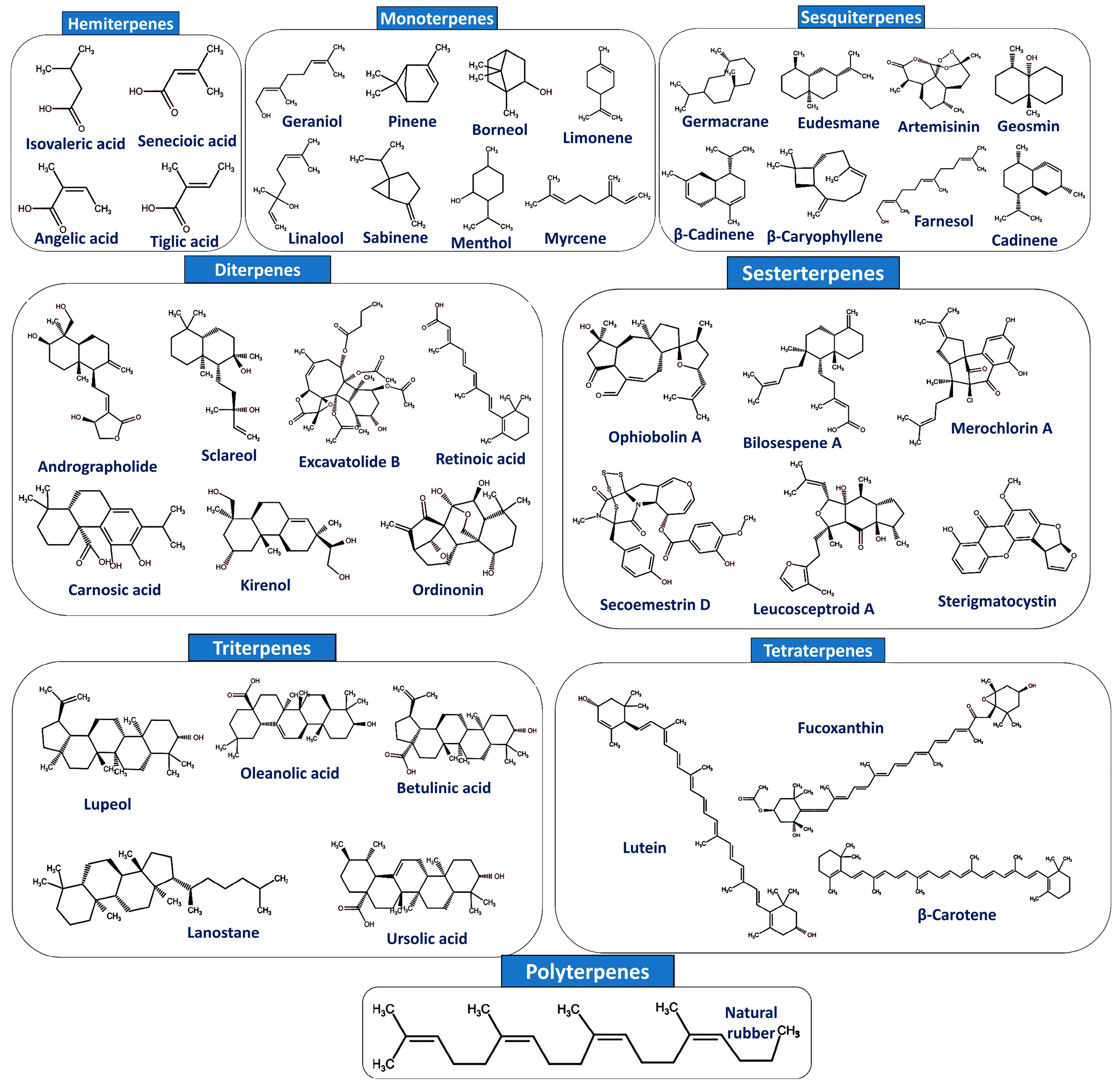
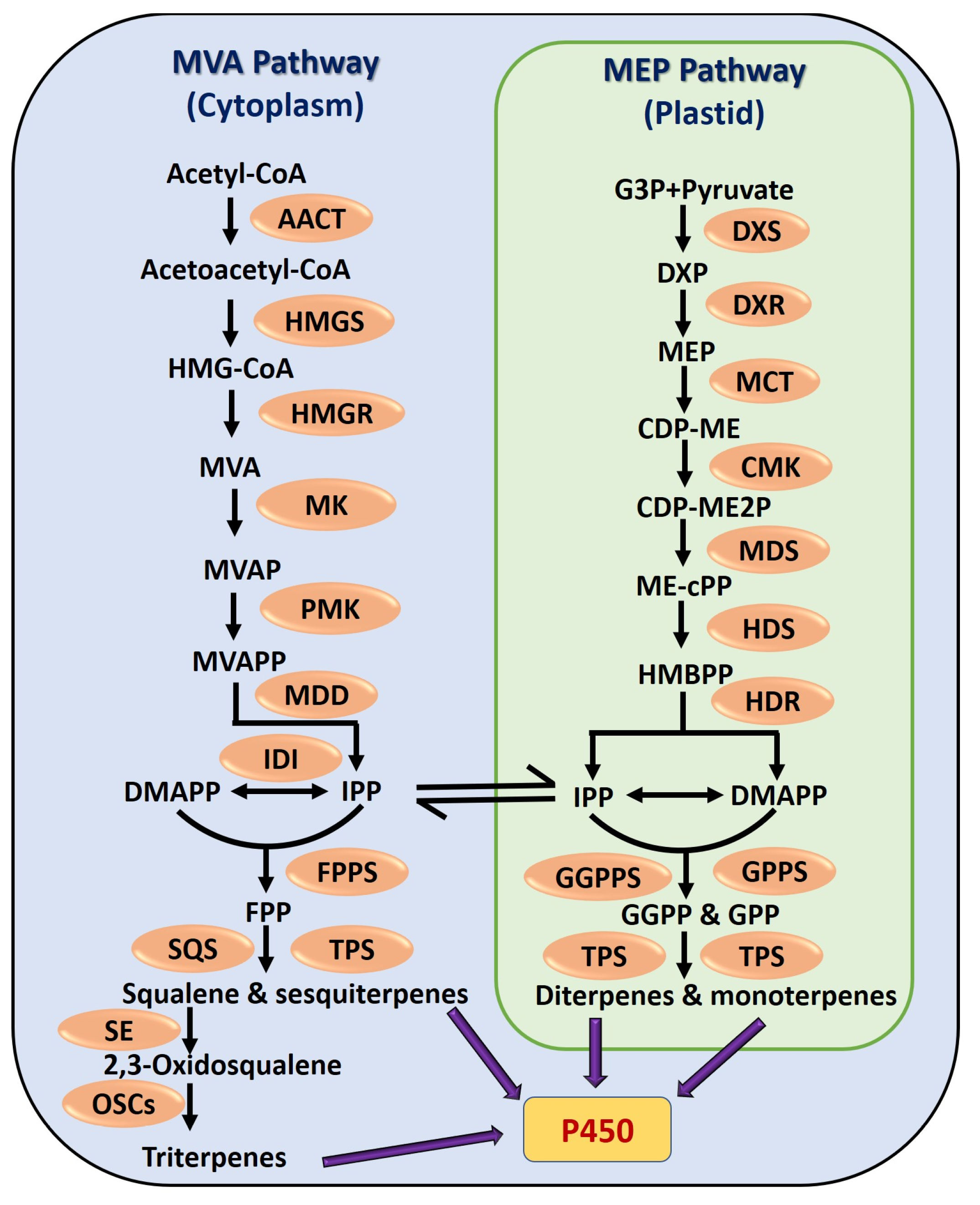
2. Classification and Biosynthesis of Terpenoids
| Class | No. of C Atoms | No. of Isoprene Units (C5H8)n | Name of Compounds | Biological Activities | Ref. |
|---|---|---|---|---|---|
| Hemiterpenes | C5 | 1 | Isovaleric acid, senecioic acid, angelic acid, and tiglic acid | Constituents in medicines, rubber, potential biofuels, and flavors | [62] |
| Monoterpenes | C10 | 2 | Geraniol, pinene, linalool, sabinene, borneol, menthol, myrcene, and limonene | Constituents in cosmetics, food, and pharmaceuticals. It shows antimicrobial, anti-inflammatory, antitumor, and cardioprotective properties | [63] |
| Sesquiterpenes | C15 | 3 | Germacrane, eudesmane, β-cadinene, β-caryophyllene, artemisinin, farnesol, geosmin, and cadinene | Possess antiviral, antibacterial, antidiabetic, antiobesity, and anti-inflammatory properties | [55,64] |
| Diterpenes | C20 | 4 | Andrographolide, sclareol, excavatolide B, kirenol, carnosic acid, phytol, retinoic acid, and oridonin | Possess antioxidant, anti-inflammatory, immune-modulatory, and antirheumatoid arthritis action | [53] |
| Sesterterpenes | C25 | 5 | Ophiobolin A, bilosespene A, merochlorin A, secoemestrin D, sterigmatocystin, leucosceptroid A, and colquhounoid A | Exhibits anticancer, antifeedant, and antifungal activities | [51,65] |
| Triterpenes | C30 | 6 | Squalene, lupeol, lanostane, oleanolic acid, betulinic acid, ursolic acid, dammarane, and ginsenoside | Possess antibacterial, antifungal, anti-inflammatory, antioxidant, anticancer, antiviral, and chemopreventive properties | [66] |
| Tetraterpenes | C40 | 8 | Carotenoid, lutein, astaxanthin, fucoxanthin, lycopene, phytoene, and β-carotene | Immunomodulatory, cardioprotective, and anticancer activities | [67] |
| Polyterpenes | >C40 | >8 | Natural rubber and resins | Used as sealants, hot-melt adhesives, and pressure-sensitive adhesives, chewing gum contains certain polyterpene resins | [68] |
3. Mechanistic Insights into the Anticancer Properties of Terpenoids
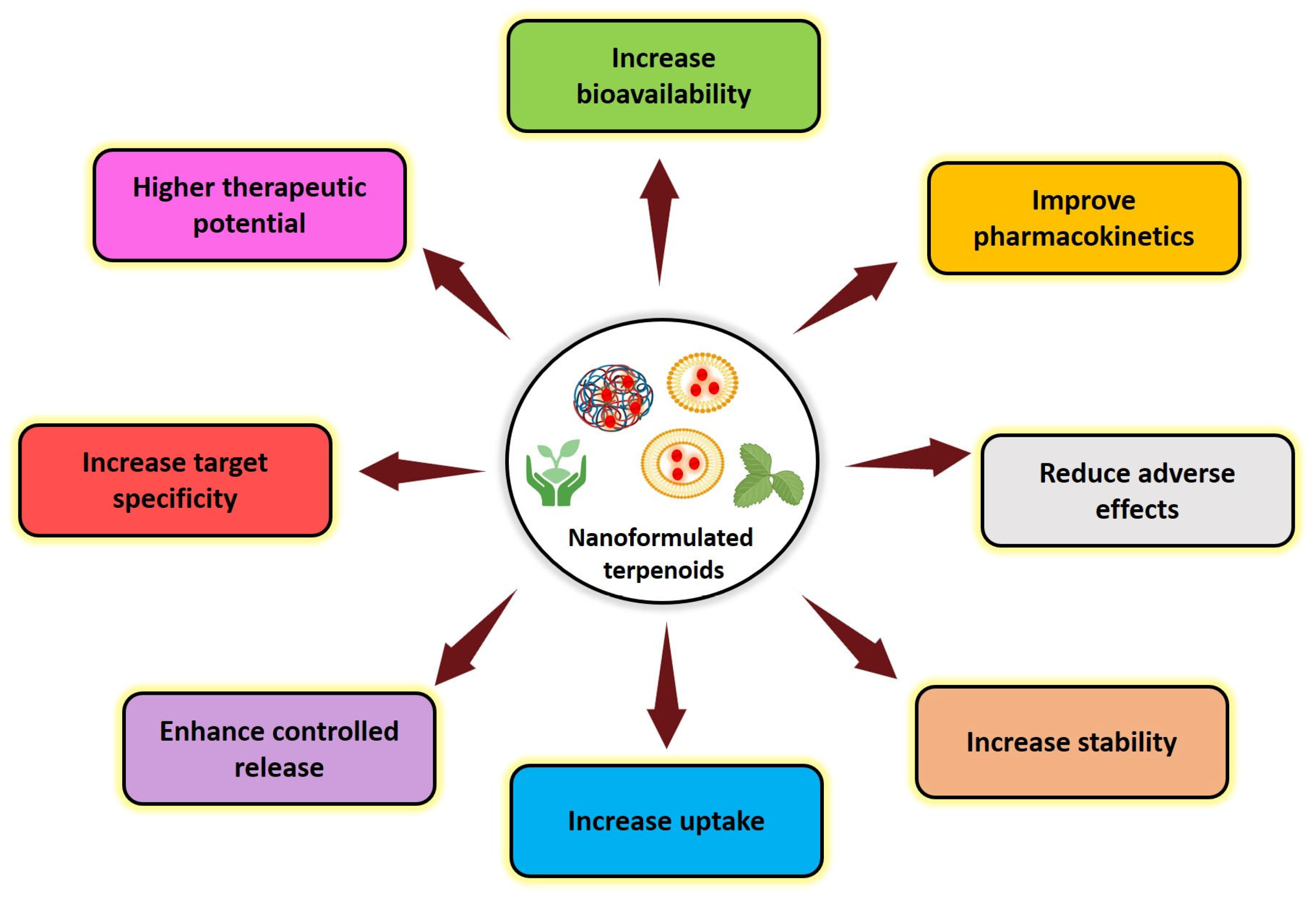
4. Challenges with Natural Terpenoids and Advantage of Nanoformulations
5. Various Processes of Preparing Nanoformulations Containing Terpenoids
6. Mechanistic Insights and Anticancer Applications of Nanoformulated Terpenoids
6.1. Monoterpenoids
6.2. Sesquiterpenoids
6.3. Diterpenoids
6.4. Triterpenoids
6.5. Tetraterpenoids
| Type of Terpenoid | Compound | Type of Nanocarriers | Targeting Mediator/ Ligand | (Size, Shape and ζ Potential) | Cancer Type and Cells | IC50 | Anticancer Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Monoterpenoids | Geraniol | Nanostructured lipid nanocarriers | None | 110 nm, ζ potential = −10 mV, shape = NR | Lung cancer (A549) | 1.5 mM | ↓Cell viability | ↑MMP loss | [147] |
| Hyaluronic acid-based polymeric nanoconjugate | Hyaluronic acid | 110 nm, Spherical, ζ potential = NR | Liver cancer (HepG2 and Huh7) | 80 and 100 μM | ↓Cell growth; ↑apoptosis; ↓cell proliferation | ↑Bax; caspase-3; ↑caspase-9; ↓Bcl-2, ↓PARP | [148] | ||
| Zinc–tin oxide/dextran/geraniol nanocomposites | None | 197.40 nm, Agglomerated, ζ potential = NR | Colon cancer (HCT-116) | 10 μg/mL | ↑Cytotoxicity; ↑apoptosis | ↑Caspase-3, ↑caspase-8; ↑caspase-9 | [149] | ||
| α-Pinene | Nanoemlusion | None | 190 ± 8 nm, ζ potential = −10.4 ± 8 mV, shape = NR | Melanoma (A-375) and breast cancer (MCF-7) | 106.19 and 168.02 μg/mL | ↑Cytotoxicity; ↑apoptosis | ↑Bax/Bcl-2 ratio | [150] | |
| Chitosan nanoparticles | None | 102 ± 6 nm, ζ potential = 41.7 ± 1 mV, shape = NR | Melanoma (A-375) | 76.4 µg/mL | ↑Cytotoxicity | ↑Bax/Bcl-2 ratio | [151] | ||
| Linalool | Gold nanoparticles | CALNN peptide | 5–20 nm, Spherical, ζ potential = NR | Breast cancer (MCF-7) | 10 μg/mL | ↑Cell growth; ↑apoptosis; ↓cell proliferation | ↑ROS; ↓MMP; ↑caspase-8; ↑p53; ↓NF-κB | [152] | |
| Solid lipid nanoparticles | None | 90–130 nm, Spherical, ζ potential = −4.0 mV | Liver (HepG2) and lung cancer (A549) | 2 mM | ↑Cytotoxicity | Not reported | [153] | ||
| Gold nanoparticles | Glutathione + CALNN peptide | 13 nm, shape and ζ potential = NR | Ovarian cancer (SKOV-3) | 10 µg/mL | ↑Cytotoxicity; ↑apoptosis; ↓cell proliferation | ↑ROS; ↓MMP; ↑caspase-8; ↑p53; ↓NF-κB | [154] | ||
| Menthol | Iron oxide nanoparticles | None | 20–60 nm, shape and ζ potential = NR | Gastric cancer (AGS) | 252 μg/mL | ↑Cytotoxicity; ↑apoptosis | ↑Caspase-8 | [155] | |
| Chitosan gum | None | 160 ± 15 nm, ζ potential = 43 ± 4 mV, shape = NR | Melanoma (A-375) | 29 µg/mL | ↑Cytotoxicity | Not reported | [156] | ||
| Limonene | Chitosan nanoparticles | None | 209 ± 13 nm, shape and ζ potential = NR | Melanoma (A-375) and breast cancer (MDA-MB-468) | 30.24 and 650.70 µg/mL | ↓Cell viability | Not reported | [157] | |
| Sesquiterpenoids | β-caryophyllene | Silver nanoparticles | None | 3.2 nm, Agglomerated, ζ potential = NR | Liver cancer (HepG2) | 51.71 µg/mL | ↓Cell growth | Not reported | [158] |
| Silver nanoparticles | None | 29.42 nm, Spherical, ζ potential = NR | Lung cancer (A549) | 9.39 ± 0.08 g/mL | ↓Cell proliferation | Not reported | [159] | ||
| Artemisinin | Chitosan magnetic nanoparticles | None | 349–445 nm, Spherical, ζ potential = −9.34 to −33.3 mV | Breast cancer (MCF-7) | 25.61 ± 13 g/mL | ↑Cytotoxicity; ↑apoptosis | Not reported | [160] | |
| Lipid nanoparticles | None | 70 ± 20 nm, shape and ζ potential = NR | Triple negative breast cancer (MDA-MB-231) | 7 ± 2 μm | ↑Cytotoxicity | ↓HER2; ↓survivin; ↓cyclin D1; ↓EGFR | [161] | ||
| Mesoporous silica nanoparticles-loaded PLGA nanofibers | None | 150–200 nm, shape and ζ potential = NR | Breast cancer (SK-BR-3) | 55 μM | ↑Cytotoxicity; ↑apoptosis | ↑Bax; ↑caspase-3; ↑p53; ↓Bcl-2; ↓hTERT | [162] | ||
| Farnesol | Chitosan-encapsulated nickel oxide, tin dioxide nanoparticles | None | 34.8 nm, Agglomerated hexagonal structure, ζ potential = NR | Breast cancer (MDA-MB-231) | 17.58 μg/mL | ↑Cytotoxicity; ↑apoptosis; ↓cell proliferation | ↓MMP; ↑ROS; ↑G2/M checkpoint arrest | [163] | |
| Diterpenoids | Andrographolide | Solid lipid nanoparticles | None | Not reported | Head and neck squamous cell carcinoma (HN6 and HN30) | 6.087 and 11.74 μg/mL | ↑Cytotoxicity; ↑apoptosis; ↓cell proliferation | Not reported | [164] |
| Albumin nanoparticles | None | 100–200 nm, shape and ζ potential = NR | Cervical cancer (HeLa) | 39.46 μg/mL | ↑Cytotoxicity | Not reported | [165] | ||
| Sclareol | Hyaluronan-coated PLGA nanoparticles | None | 100–150 nm, ζ potential = −30 mV, shape = NR | Breast (MCF-7, MDA-MB468) and colon cancer (CaCo-2) | 50 μM | ↑Cytotoxicity | Not reported | [166] | |
| Solid lipid nanoparticles | None | 88 nm, shape and ζ potential = NR | Lung cancer (A549) | 19 μg/mL | ↓Cell viability; ↑apoptosis | Not reported | [167] | ||
| Carnosic acid | Albumin nanoparticles | None | 97.29–144.26 nm, ζ potential = −21.03 mV, shape = NR | Colon (Caco-2) and breast cancer (MCF-7) | 2.60 and 6.02 μg/mL | ↓Cell viability; ↑apoptosis | ↑G2/M checkpoint arrest; ↓COX-2; ↓Bcl-2; ↑GCLC; ↑p53 | [168] | |
| Retinoic acid | Chitosan nanoparticles | None | 313.23 ± 1.75 nm, ζ potential = 2.42 ± 0.04 mV, shape = NR | Breast cancer (MCF-7) | 2.28 ± 0.02 µg/mL | ↑Cytotoxicity; ↑apoptosis | ↓Bcl-2; ↑caspase-3; ↑Bax; ↑cleaved PARP; ↑8-oxo-dG | [169] | |
| Glutenin Nanoparticles | Folic acid | ~185 nm, Spherical, ζ potential = −3 mV | Breast cancer (MCF-7) | 55.93 µg/mL | ↑Cytotoxicity; ↑apoptosis; ↓cell proliferation | ↓MMP; ↑ROS | [170] | ||
| Solid lipid nanoparticles | None | 140–150 nm, Spherical shape, ζ potential = −13 and −19 mV | Prostate cancer (LNCap) | 200 μg/mL | ↑Cytotoxicity; ↑apoptosis | Not reported | [171] | ||
| Triterpenoids | Squalene | Cisplatin-nanoprecipitated particles | None | 128–160 nm, Spherical, ζ potential = NR | Colon cancer (HT-29 and KM-12) | 8 μmol/L | ↑Cytotoxicity; ↑apoptosis; ↓cell proliferation; | Not reported | [172] |
| PLGA nanoparticles | None | Not reported | Colon cancer (Caco-2) | 140 µg/mL | ↑Cytotoxicity; ↑apoptosis | ↑ROS generation | [173] | ||
| Lupeol | Chitosan nanoparticles with cellulose acetate membrane | None | 12 nm, shape and ζ potential = NR | Skin cancer (A431) | 42.2 μg/mL | ↑Cytotoxicity | Not reported | [174] | |
| Galactosylated liposomes | None | 100 nm, shape and ζ potential = NR | Liver cancer (HepG2) | 30 µM | ↑Cytotoxicity; ↑apoptosis | ↓p-Akt308; ↓p-Akt473 levels | [175] | ||
| Oleanolic acid | Albumin nanoparticles | Cetuximab | 171 ± 4.8 nm to 180 ± 3.7 nm, ζ potential = − 33.3 ± 3.4 mV, shape = NR | Lung cancer (A549) | 4.34 ± 1.90 μg/mL | ↑Cytotoxicity; ↑apoptosis; ↓cell proliferation | ↑ROS generation; G0/G1 checkpoint arrest | [176] | |
| Ursolic acid | Gold PLGA nanoparticles | None | 80 nm, Spherical, ζ potential = NR | Cervical cancer (SiHa, CaSki, and HeLa cells) | 100 μM | ↓Cell viability; ↑apoptosis; ↓cell proliferation; ↓cell migration ↓cell invasion | ↑Bax; ↓Bcl-2; ↑caspase-3, ↑caspase-8; ↑capase-9; ↓procaspase-3; ↓procaspase-8; ↓procaspase-9; ↑p53; ↑fas; ↓cIAP | [177] | |
| Chitosan-coated PLGA nanoparticles | None | 250 nm, Spherical, ζ potential = NR | Breast cancer (MCF-7 and MDA-MB-231) | 26.74 and 40.67 μM | ↑Cytotoxicity | Not reported | [178] | ||
| PLGA-PEG nanoparticles | None | 133.6 ± 0.7 nm, ζ potential = −22.6 ± 2.8 mV, shape = NR | Pancreatic ductal adenocarcinoma (AsPC-1 and BxPC-3) | 11.7 ± 0.6 and 14.1 ± 2.2 μM | ↑Cytotoxicity | Not reported | [179] | ||
| Oridonin | Solid lipid nanoparticles | None | 108.53 ± 10.92 nm, ζ potential = −37.97 ± 3.78 mV, shape = NR | Breast (MCF-7), lung (A549) and liver cancer (HepG2) | 22.6, 25.3 and 30.1 μM | ↓Cell viability; ↑apoptosis; ↓cell proliferation | ↑G2/M checkpoint arrest; ↓G1/G0 checkpoint arrest; | [180] | |
| Ginsenoside | Liposomes combined with paclitaxel | None | 77.71 ± 3.22 nm, ζ potential = −39.21 ± 1.03 mV, shape = NR | Gastric cancer (BGC-823) | 0.04 μg/mL | ↑Cytotoxicity; ↑apoptosis | Not reported | [181] | |
| Tetraterpenoids | Lycopene | Eudragit RL 100 polymeric nanoparticles | None | 62.10 ± 3.7 nm, Spherical | Prostate cancer (LNCaP and PC-3) | 25.43 and 10.03 μg/mL | ↑Cytotoxicity | Not reported | [182] |
| Gold nanoemulsion | None | 25.0 ± 4.2 nm, ζ potential = −32.2 ± 1.8 mV | Colon cancer (HT-29) | 0.1 µM | ↑Cytotoxicity; ↑apoptosis; ↓cell migration | ↑Bax; ↓Bcl-2; ↓procaspase-8; ↓procaspase-3; ↓procaspase-9; ↑E-cadherin; ↓Akt; ↓NF-κB; ↓MMP-2; ↓MMP-9 | [183] | ||
| Carotenoid | Nanoemulsion | None | 15.1 nm, shape and ζ potential = NR | Colon cancer (HT-29) | 4.9 μg/mL | ↑Cytotoxicity; ↑apoptosis; ↓cell proliferation | ↑G2/M checkpoint arrest; ↑p53; ↑p21; ↓CDK1; ↓CDK2; ↓cyclin A; ↓cyclin B | [184] | |
| Lutein | Chitosan/alginate iron oxide nanoparticles | None | 264 ± 6 nm, ζ potential = −13.3 ± 1.6 mV, shape = NR | Breast cancer (MCF-7) | 4.12 ± 0.4 μg/mL | ↓Cell viability | Not reported | [185] | |
| Fucoxanthin | Polyvinylpyrrolidone nanoparticles | None | <50 nm, shape and ζ potential = NR | Colon cancer (Caco-2) | 20 μM | ↓Cell viability; ↓cell migration | ↑Pro-oxidative effect | [186] | |
| Astaxanthin | Chitosan nanoparticles | None | <400 nm, shape and ζ potential = NR | Melanoma (B16F10) | 20 µg/mL | ↑Antioxidant activity; ↓cell proliferation; ↓cell migration | Not reported | [187] | |
| Gold nanoparticles | None | 60–120 nm, Spherical, ζ potential = NR | Breast cancer (MDA-MB-231) | 50 μg/mL | ↑Cytotoxicity; ↑apoptosis; ↓cell proliferation | Not reported | [188] | ||
| β-Carotene | Solid lipid nanoparticles | None | 203 ± 7.23 nm, ζ potential = −7.21 ± 0.82 mV, shape = NR | Breast cancer (MCF-7) | 40 μg/mL | ↑Antioxidant activity; ↑cytotoxicity | Not reported | [189] | |
| Solid lipid nanoparticles | None | 111.78 nm, Spherical, ζ potential = −26.3 ± 1.3 mV, shape = NR | Breast cancer (MCF-7) | 14.89 ± 0.02 μg/mL | ↑Cytotoxic effect | Not reported | [190] |
| Type of Terpenoid | Compound | Type of Nanocarriers | Targeting Mediator/ Ligand | Size, Shape and ζ Potential | Cancer Type (Model) | Dosage and Route of Administration | Antitumor Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Monoterpenoids | Geraniol | Hyaluronic acid-based polymeric nanoconjugate | Hyaluronic acid | 110 nm, ζ potential = −10 mV, shape = NR | Liver cancer (H22 cells-injected mice) | Intravenous injection, 1.0 mg geraniol/kg | ↓Tumor size; ↓tumor weight; ↓tumor volume | ↓Ki-67 | [148] |
| Zinc–tin oxide/dextran/geraniol nanocomposites | None | 197.40 nm, Agglomerated, ζ potential = NR | Colon cancer (DMH-induced rats) | 20 and 40 mg/kg | ↓Tumor volume; ↓tumor size; ↓tumor incidence | ↓COX-2 | [149] | ||
| Diterpenoids | Andrographolide | PLGA nanocapsulation | None | 163 nm, Nanospheres, ζ potential = −57.85 mV | Cervical cancer (HeLa cells-injected mice) | 10mg/kg | ↓Tumor size | Not reported | [191] |
| Carnosic acid | Liposomes | Transferrin | 97.06 ± 3.389 nm, ζ potential = 2.55 ± 1.26 mV, shape = NR | Liver cancer (HepG2- and SMMC-7721-transplanted BALB/c nude mice) | Intraperitoneal injection, 2 mg/kg | ↓Tumor growth; ↓tumor volume | ↑Caspase-3; ↑caspase-9; ↑Bax; ↑Bad; ↑PARP; ↓Bcl-2 | [192] | |
| Oridonin | PEG-PLGA nanoparticles | None | 100 nm, ζ potential = −5 mV, shape = NR | Breast cancer (MCF-7 cells-injected BALB/c nude mice) | Intraperitoneal injection, 10 mg/kg | ↓Tumor size; ↓tumor volume; ↓angiogenesis | NR | [193] | |
| Triterpenoids | Ursolic acid | Chitosan nanoparticles | Folate | 160nm, ζ potential = 39.3mV, shape = NR | Breast cancer (MCF-7 cells-injected BALB/c mice) | Intraperitoneal injection, 12.5mg/kg | ↓Tumor size; ↓tumor weight | NR | [194] |
| Gold PLGA nanoparticles | None | 80 nm, Spherical, ζ potential = NR | Cervical cancer (SiHa and HeLa cells-injected nude mice) | Intraperitoneal injection, 20 mg/kg | ↓Tumor size; ↓tumor weight | NR | [177] | ||
| Tetraterpenoids | Lycopene | N-isopropylacrylamide with N-vinyl2-pyrrolidone poly(ethyleneglycol)monoacrylate copolymeric nanoparticles | None | <100 nm, Spherical, ζ potential = NR | Melanoma (TPA-induced Swiss albino mice) | Topical treatment, 1 μg/mL | NR | ↓COX-2, ↓Bcl-2, ↑Bax | [195] |
| Astaxanthin | Ethylcellulose nanoparticles | None | 185 nm, Spherical, ζ potential = NR | Oral cancer (DMBA- induced Syrian hamsters) | Oral treatment, 15 mg/kg | ↓Tumor size; ↓tumor growth | Cyclin D1; ↓Bcl-2, ↑Bax; ↑caspase-3; ↑caspase-9 | [196] | |
| β-Carotene | Thiolated chitosan -lithocholic acid nanomicelles | None | <300 nm, ζ potential = +27.0 mV, shape = NR | Skin cancer (DMBA- induced BALB/c mice) | Topical treatment, 1 mg/mL | ↓Tumor size; ↓tumor weight | NR | [197] |
7. Challenges and Emerging Trends in Nanoformulated Terpenoids
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cao, W.; Qin, K.; Li, F.; Chen, W. Comparative Study of Cancer Profiles Between 2020 and 2022 Using Global Cancer Statistics (GLOBOCAN). J. Natl. Cancer Cent. 2024, 4, 128–134. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. Ca 2025, 75, 10. [Google Scholar] [CrossRef]
- Jena, D.; Padhi, B.K.; Zahiruddin, Q.S.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Kumar, M.R.; Rustagi, S.; Gaidhane, A.M.; et al. Estimation of Burden of Cancer Incidence and Mortality in India: Based on Global Burden of Disease Study 1990–2021. BMC Cancer 2024, 24, 1278. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent Progress in Nanomedicine for Enhanced Cancer Chemotherapy. Theranostics 2021, 11, 6370. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Khan, M.S.; Gowda, B.J.; Hasan, N.; Gupta, G.; Singh, T.; Md, S.; Kesharwani, P. Carbon Nanotube-Mediated Platinum-Based Drug Delivery for the Treatment of Cancer: Advancements and Future Perspectives. Eur. Polym. J. 2024, 206, 112800. [Google Scholar] [CrossRef]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.-H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for Cancer Prevention and Therapy: Current Progress and Future Perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef]
- Khan, H.; Saeedi, M.; Nabavi, S.M.; Mubarak, M.S.; Bishayee, A. Glycosides from Medicinal Plants as Potential Anticancer Agents: Emerging Trends towards Future Drugs. Curr. Med. Chem. 2019, 26, 2389–2406. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in Cancer Treatment: Current Progress and Future Prospects. J. Pathophysiol. 2021, 28, 250–272. [Google Scholar]
- Zheng, H.; Wang, Y.; Ren, Y.; Wang, X.; Sui, L.; Xu, H.; Zheng, C. Design, Synthesis and Biological Evaluation of Sulfur-Containing Tetrahydroxanthones as Potential Anti-Tumor Agents. Bioorg. Med. Chem. Lett. 2025, 121, 130154. [Google Scholar] [CrossRef]
- Sati, P.; Sharma, E.; Dhyani, P.; Attri, D.C.; Rana, R.; Kiyekbayeva, L.; Büsselberg, D.; Samuel, S.M.; Sharifi-Rad, J. Paclitaxel and Its Semi-Synthetic Derivatives: Comprehensive Insights into Chemical Structure, Mechanisms of Action, and Anticancer Properties. Eur. J. Med. Res. 2024, 29, 90. [Google Scholar] [CrossRef]
- Bakhshi, S.; Shoari, A.; Alibolandi, P.; Ganji, M.; Ghazy, E.; Rahdar, A.; Fathi-karkan, S.; Pandey, S. Emerging Innovations in Vincristine-Encapsulated Nanoparticles: Pioneering a New Era in Oncological Therapeutics. J. Drug Deliv. Sci. Technol. 2024, 91, 105270. [Google Scholar]
- Kamle, M.; Pandhi, S.; Mishra, S.; Barua, S.; Kurian, A.; Mahato, D.K.; Rasane, P.; Büsselberg, D.; Kumar, P.; Calina, D. Camptothecin and Its Derivatives: Advancements, Mechanisms and Clinical Potential in Cancer Therapy. Med. Oncol. 2024, 41, 263. [Google Scholar] [PubMed]
- Jang, J.Y.; Kim, D.; Im, E.; Kim, N.D. Etoposide as a Key Therapeutic Agent in Lung Cancer: Mechanisms, Efficacy, and Emerging Strategies. Int. J. Mol. Sci. 2025, 26, 796. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Oluokun, O.O. Food Bioactives: The Food Image behind the Curtain of Health Promotion and Prevention against Several Degenerative Diseases. Stud. Nat. Prod. Chem. 2022, 72, 391–421. [Google Scholar]
- Siddiqui, T.; Sharma, V.; Khan, M.U.; Gupta, K. Terpenoids in Essential Oils: Chemistry, Classification, and Potential Impact on Human Health and Industry. Phytomed. Plus 2024, 4, 100549. [Google Scholar]
- Li, Y.; Wang, J.; Li, L.; Song, W.; Li, M.; Hua, X.; Wang, Y.; Yuan, J.; Xue, Z. Natural Products of Pentacyclic Triterpenoids: From Discovery to Heterologous Biosynthesis. Nat. Prod. Rep. 2023, 40, 1303–1353. [Google Scholar]
- Gozari, M.; Alborz, M.; El-Seedi, H.R.; Jassbi, A.R. Chemistry, Biosynthesis and Biological Activity of Terpenoids and Meroterpenoids in Bacteria and Fungi Isolated from Different Marine Habitats. Eur. J. Med. Chem. 2021, 210, 112957. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Li, W.-X.; Gao, K.; Jin, X.-J.; Yao, X.-J. Absolute Structures of Monoterpenoids with a δ-Lactone-Containing Skeleton from Ligularia hodgsonii. J. Nat. Prod. 2012, 75, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Huang, S.; Jiang, T.; Wang, C.; Tao, Z.; He, C.; Tang, Q.; Li, P. Volatile DMNT Directly Protects Plants against Plutella Xylostella by Disrupting the Peritrophic Matrix Barrier in Insect Midgut. eLife 2021, 10, e63938. [Google Scholar] [CrossRef]
- Silva Santos, A.; Antunes, A.; D’Avila, L.; Bizzo, H.; Souza-Santos, L. The Use of Essential Oils and Terpenes/Terpeneoids in Cosmetic and Perfumery. Perf. Flavorist 2005, 30, 50–55. [Google Scholar]
- Fan, W.; Xu, Y.; Qian, M. Current Practice and Future Trends of Aroma and Flavor Research in Chinese Baijiu. In ACS Symposium Series; Guthrie, B., Beauchamp, J.D., Buettner, A., Toth, S., Qian, M.C., Eds.; American Chemical Society: Washington, DC, USA, 2019; Volume 1321, pp. 145–175. ISBN 978-0-8412-3467-3. [Google Scholar]
- Pang, B.; Li, J.; Eiben, C.B.; Oksen, E.; Barcelos, C.; Chen, R.; Englund, E.; Sundstrom, E.; Keasling, J.D. Lepidopteran Mevalonate Pathway Optimization in Escherichia coli Efficiently Produces Isoprenol Analogs for Next-Generation Biofuels. Metab. Eng. 2021, 68, 210–219. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Mahizan, N.A.; Yang, S.-K.; Moo, C.-L.; Song, A.A.-L.; Chong, C.-M.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.E.; Lai, K.-S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I. Potential Antioxidant Activity of Terpenes. In Terpenes and Terpenoids-Recent Advances; IntechOpen: London, UK, 2021; pp. 53–62. [Google Scholar]
- Graßmann, J. Terpenoids as Plant Antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar]
- Mohandas, G.G.; Kumaraswamy, M. Antioxidant Activities of Terpenoids from Thuidium tamariscellum (C. Muell.) Bosch. and Sande-Lac. a Moss. Pharmacogn. J. 2018, 10, 645–649. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.; Bamrah, P.K.; Goyal, R.; Choudhary, M.; Chopra, H. Insights on the Emerging Therapeutic Potential of Terpenoids as Anti-Inflammatory Agents: A Scoping Review. J. Bio-X Res. 2024, 7, 0006. [Google Scholar] [CrossRef]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural Terpenoids with Anti-Inflammatory Activities: Potential Leads for Anti-Inflammatory Drug Discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, A.; Majie, A.; Karmakar, V.; Das, S.; Dinda, S.C.; Bose, A.; Gorain, B. Terpenoids as Potential Phytoconstituent in the Treatment of Diabetes: From Preclinical to Clinical Advancement. Phytomedicine 2024, 129, 155638. [Google Scholar] [CrossRef]
- Peana, A.T.; Paolo, S.D.; Chessa, M.L.; Moretti, M.D.; Serra, G.; Pippia, P. (−)-Linalool Produces Antinociception in Two Experimental Models of Pain. Eur. J. Pharmacol. 2003, 460, 37–41. [Google Scholar] [CrossRef]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ Anti-Cancer Effects: Focus on Autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef]
- He, H. Potential and Challenges of Terpenoids in Cancer Therapy: Mechanistic Review and Future Perspectives. Theor. Nat. Sci. 2025, 75, 13–19. [Google Scholar] [CrossRef]
- Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for Delivery of Pentacyclic Triterpenoids in Anticancer Therapies. Molecules 2021, 26, 1764. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Attri, K.; Sharda, D.; Chudasama, B.; Mahajan, R.L.; Choudhury, D. A Review on Terpenes for Treatment of Gastric Cancer: Current Status and Nanotechnology-Enabled Future. RSC Sustain. 2023, 1, 1109–1124. [Google Scholar] [CrossRef]
- Elsori, D.; Pandey, P.; Obaidur Rab, S.; Alharbi, A.; Sahoo, S.; Pandey, S.; Srivastava, M.; Lakhanpal, S.; Saeed, M.; Khan, F. How Effective Are Key Phytocompound Carrying Polysaccharide Nanocarriers as Anti-Breast Cancer Therapy? A Comprehensive Review of the Literature. Int. J. Nanomed. 2025, 20, 8393–8413. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of Nanotechnology in Improving Bioavailability and Bioactivity of Diet-Derived Phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural Product-Based Nanoformulations for Cancer Therapy: Opportunities and Challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in Phytochemical Delivery Systems for Improved Anticancer Activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.Z.; Faraji, F.; Farhadi, T.; Hesami, O.; Iranpanah, A.; Webber, K.; Bishayee, A. Current Advances in Nanoformulations of Therapeutic Agents Targeting Tumor Microenvironment to Overcome Drug Resistance. Cancer Metastasis Rev. 2023, 42, 959–1020. [Google Scholar] [CrossRef]
- Nahata, A.; Seng, L.S. Terpenes and Terpene Based Nanoformulations: A New Prospect for Arthritis Management. Int. J. Med. Pharm. Health Sci. 2024, 1, 196–207. [Google Scholar]
- Pandey, P.; Verma, M.; Sanghvi, G.; Roopashree, R.; Joshi, K.K.; Kavitha, V.; Ray, S.; Ramniwas, S.; Singh, A.; Lakhanpal, S.; et al. Plant-Derived Terpenoids Modulating Cancer Cell Metabolism and Cross-Linked Signaling Pathways: An Updated Reviews. Naunyn-Schmiedeb. Arch. Pharmacol. 2025, 398, 8313–8333. [Google Scholar] [CrossRef]
- Nayila, I.; Sharif, S.; Lodhi, M.S.; Ullah, R.; Alotaibi, A.; Maqbool, T.; Hameed, S. Formulation, Characterization and Evaluation of Anti-Breast Cancer Activity of 2-Carene Nanoemulsion; in Silico, in Vitro and in Vivo Study. Arab. J. Chem. 2024, 17, 105937. [Google Scholar] [CrossRef]
- Negi, K.; Singh, S.; Gahlot, M.S.; Tyagi, S.; Gupta, A. Terpenoids from Medicinal Plants Beneficial for Human Health Care. Int. J. Botany Stud. 2020, 5, 135–138. [Google Scholar]
- Evidente, A.; Kornienko, A.; Lefranc, F.; Cimmino, A.; Dasari, R.; Evidente, M.; Mathieu, V.; Kiss, R. Sesterterpenoids with Anticancer Activity. Curr. Med. Chem. 2015, 22, 3502–3522. [Google Scholar] [CrossRef]
- Mabou, F.D.; Yossa, I.B.N. TERPENES: Structural Classification and Biological Activities. IOSR J. Pharm. Biol. Sci. 2021, 16, 25–40. [Google Scholar]
- Torequl Islam, M.; Quispe, C.; Herrera-Bravo, J.; Rahaman, M.M.; Hossain, R.; Sarkar, C.; Raihan, M.A.; Chowdhury, M.M.; Uddin, S.J.; Shilpi, J.A.; et al. Activities and Molecular Mechanisms of Diterpenes, Diterpenoids, and Their Derivatives in Rheumatoid Arthritis. Evid. Based Complement. Alternat. Med. 2022, 2022, 4787643. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y. Targeting Cancer with Sesterterpenoids: The New Potential Antitumor Drugs. J. Nat. Med. 2015, 69, 255–266. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Chapter 7—Bioactive Phytocomponents and Their Analysis. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–328. ISBN 978-0-12-813374-3. [Google Scholar]
- Huang, Y.; Xie, F.-J.; Cao, X.; Li, M.-Y. Research Progress in Biosynthesis and Regulation of Plant Terpenoids. Biotechnol. Biotechnol. Equip. 2021, 35, 1799–1808. [Google Scholar] [CrossRef]
- Abdallah, I.I.; Quax, W.J. A Glimpse into the Biosynthesis of Terpenoids. In International Conference on Natural Resources and Life Sciences (NRLS-2016); KnE Life Sciences: Dubai, United Arab Emirates, 2017; pp. 81–98. [Google Scholar]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Germany, 2015; Volume 148, pp. 63–106. ISBN 978-3-319-20106-1. [Google Scholar]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant Volatile Terpenoid Metabolism: Biosynthetic Genes, Transcriptional Regulation and Subcellular Compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Powder-George, Y.L.; Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Chapter 10—Terpenoids. In Pharmacognosy, 2nd ed.; McCreath, S.B., Clement, Y.N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 253–294. ISBN 978-0-443-18657-8. [Google Scholar]
- Liu, Y.; Ma, X.; Liang, H.; Stephanopoulos, G.; Zhou, K. Monoterpenoid Biosynthesis by Engineered Microbes. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab065. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; Jiang, Z.; Zafar, S.; Xie, Q.; Yang, Y.; Liu, Y.; Yuan, H.; Jian, Y.; Wang, W. Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus. Molecules 2021, 26, 3038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, J.; Ma, Y.; Yuan, W.; Zhang, P.; Wang, G. Occurrence and Biosynthesis of Plant Sesterterpenes (C25), a New Addition to Terpene Diversity. Plant Commun. 2021, 2, 100184. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Fink, J.K. Chapter 12—Terpene Resins. In Reactive Polymers Fundamentals and Applications, 2nd ed.; Fink, J.K., Ed.; Plastics Design Library; William Andrew Publishing: Oxford, UK, 2013; pp. 303–315. ISBN 978-1-4557-3149-7. [Google Scholar]
- Singh, A.; Debnath, R.; Sharma, A.; Saini, A.; Seni, K.; Chawla, V.; Chawla, P.A. Exploring the Potential of Terpenoids as a Possible Treatment for Cancer: Structure-Activity Relationship and Mechanistic Studies. Curr. Med. Chem. 2024, 31, 1–27. [Google Scholar] [CrossRef]
- Tu, S.; Hu, F.; Zhang, J.; Cai, H.; Yang, J. Research Progress on the Signaling Pathway Mechanism of Terpenoids against Breast Cancer. Discov. Oncol. 2025, 16, 433. [Google Scholar] [CrossRef]
- Guo, J.; Huang, M.; Hou, S.; Yuan, J.; Chang, X.; Gao, S.; Zhang, Z.; Wu, Z.; Li, J. Therapeutic Potential of Terpenoids in Cancer Treatment: Targeting Mitochondrial Pathways. Cancer Rep. 2024, 7, e70006. [Google Scholar] [CrossRef]
- Andreani, T.; Cheng, R.; Elbadri, K.; Ferro, C.; Menezes, T.; Dos Santos, M.R.; Pereira, C.M.; Santos, H.A. Natural Compounds-Based Nanomedicines for Cancer Treatment: Future Directions and Challenges. Drug Deliv. Transl. Res. 2024, 14, 2845–2916. [Google Scholar] [CrossRef]
- Mohapatra, P.; Singh, P.; Singh, D.; Sahoo, S.; Sahoo, S.K. Phytochemical Based Nanomedicine: A Panacea for Cancer Treatment, Present Status and Future Prospective. OpenNano 2022, 7, 100055. [Google Scholar] [CrossRef]
- Sanna, V.; Chamcheu, J.C.; Pala, N.; Mukhtar, H.; Sechi, M.; Siddiqui, I.A. Nanoencapsulation of Natural Triterpenoid Celastrol for Prostate Cancer Treatment. Int. J. Nanomed. 2015, 10, 6835–6846. [Google Scholar] [CrossRef] [PubMed]
- Rahat, I.; Rizwanullah, M.; Gilani, S.J.; Bin-Jummah, M.N.; Imam, S.S.; Kala, C.; Asif, M.; Alshehri, S.; Sharma, S.K. Thymoquinone Loaded Chitosan-Solid Lipid Nanoparticles: Formulation Optimization to Oral Bioavailability Study. J. Drug Deliv. Sci. Technol. 2021, 64, 102565. [Google Scholar]
- Lathwal, R. Development and Characterization of Ursolic Acid-Loaded Liposomes for Enhanced Bioavailability and Sustained Anticancer Drug Delivery. Int. J. Sci. Info. 2024, 1, 103–110. [Google Scholar]
- Kumar, P.; Singh, A.K.; Raj, V.; Rai, A.; Keshari, A.K.; Kumar, D.; Maity, B.; Prakash, A.; Maiti, S.; Saha, S. Poly(Lactic-Co-Glycolic Acid)-Loaded Nanoparticles of Betulinic Acid for Improved Treatment of Hepatic Cancer: Characterization, in Vitro and in Vivo Evaluations. Int. J. Nanomed. 2018, 13, 975–990. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Li, X.; Lu, J.; Wang, M. Recent Advances in Drug Delivery of Celastrol for Enhancing Efficiency and Reducing the Toxicity. Front. Pharmacol. 2024, 15, 1137289. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef]
- Kaushik, N.; Borkar, S.B.; Nandanwar, S.K.; Panda, P.K.; Choi, E.H.; Kaushik, N.K. Nanocarrier Cancer Therapeutics with Functional Stimuli-Responsive Mechanisms. J. Nanobiotechnol. 2022, 20, 152. [Google Scholar] [CrossRef] [PubMed]
- Atriya, A.; Majee, C.; Mazumder, R.; Choudhary, A.N.; Salahuddin; Mazumder, A.; Dahiya, A.; Priya, N. Insight into the Various Approaches for the Enhancement of Bioavailabilityand Pharmacological Potency of Terpenoids: A Review. Curr. Pharm. Biotechnol. 2023, 24, 1228–1244. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, M. Herbal Nanomedicine Interactions to Enhance Pharmacokinetics, Pharmacodynamics, and Therapeutic Index for Better Bioavailability and Biocompatibility of Herbal Formulations. Mater. NanoSci. 2018, 5, 35–60. [Google Scholar]
- Lôbo, G.C.; Paiva, K.L.; Silva, A.L.G.; Simões, M.M.; Radicchi, M.A.; Báo, S.N. Nanocarriers Used in Drug Delivery to Enhance Immune System in Cancer Therapy. Pharmaceutics 2021, 13, 1167. [Google Scholar] [CrossRef]
- Hadkar, V.M.; Mohanty, C.; Selvaraj, C.I. Biopolymeric Nanocarriers in Cancer Therapy: Unleashing the Potency of Bioactive Anticancer Compounds for Enhancing Drug Delivery. RSC Adv. 2024, 14, 25149–25173. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Wong, S.W.; Yeo, H.L.; Zhao, Y. Smart Nanocarriers for Cancer Treatment: Clinical Impact and Safety. NanoImpact 2020, 20, 100253. [Google Scholar] [CrossRef]
- Lasoń, E. Topical Administration of Terpenes Encapsulated in Nanostructured Lipid-Based Systems. Molecules 2020, 25, 5758. [Google Scholar] [CrossRef]
- Milan, A.; Mioc, A.; Prodea, A.; Mioc, M.; Buzatu, R.; Ghiulai, R.; Racoviceanu, R.; Caruntu, F.; Şoica, C. The Optimized Delivery of Triterpenes by Liposomal Nanoformulations: Overcoming the Challenges. Int. Mol. Sci. 2022, 23, 1140. [Google Scholar] [CrossRef]
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef]
- Nigade, P.M.; Patil, S.L.; Tiwari, S.S. Self Emulsifying Drug Delivery System (SEDDS): A Review. Int. J. Pharm. Biol. Sci. 2012, 2, 42–52. [Google Scholar]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Sundar, S.; Kumar Prajapati, V. Drug Targeting to Infectious Diseases by Nanoparticles Surface Functionalized with Special Biomolecules. Curr. Med. Chem. 2012, 19, 3196–3202. [Google Scholar] [CrossRef] [PubMed]
- Adeola, H.A.; Sabiu, S.; Adekiya, T.A.; Aruleba, R.T.; Aruwa, C.E.; Oyinloye, B.E. Prospects of Nanodentistry for the Diagnosis and Treatment of Maxillofacial Pathologies and Cancers. Heliyon 2020, 6, e04890. [Google Scholar] [CrossRef] [PubMed]
- Elsori, D.; Pandey, P.; Ramniwas, S.; Kumar, R.; Lakhanpal, S.; Rab, S.O.; Siddiqui, S.; Singh, A.; Saeed, M.; Khan, F. Naringenin as Potent Anticancer Phytocompound in Breast Carcinoma: From Mechanistic Approach to Nanoformulations Based Therapeutics. Front. Pharmacol. 2024, 15, 1406619. [Google Scholar] [CrossRef]
- Wahi, A.; Bishnoi, M.; Raina, N.; Singh, M.A.; Verma, P.; Gupta, P.K.; Kaur, G.; Tuli, H.S.; Gupta, M. Recent Updates on Nano-Phyto-Formulations Based Therapeutic Intervention for Cancer Treatment. Oncol. Res. 2023, 32, 19. [Google Scholar] [CrossRef]
- Das, C.A.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Kumar, C.V. Nanomaterials in Anticancer Applications and Their Mechanism of Action-A Review. Nanomed. Nanotechnol. Biol. Med. 2023, 47, 102613. [Google Scholar] [CrossRef]
- Silva, B.I.; Nascimento, E.A.; Silva, C.J.; Silva, T.G.; Aguiar, J.S. Anticancer Activity of Monoterpenes: A Systematic Review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef]
- Batista, F.A.; Fontele, S.B.C.; Santos, L.K.B.; Filgueiras, L.A.; Nascimento, S.Q.; de Castro e Sousa, J.M.; Gonçalves, J.C.R.; Mendes, A.N. Synthesis, Characterization of α-Terpineol-Loaded PMMA Nanoparticles as Proposed of Therapy for Melanoma. Mater. Today Commun. 2020, 22, 100762. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. In Vitro and in Vivo Antitumor Potential of Carvacrol Nanoemulsion against Human Lung Adenocarcinoma A549 Cells via Mitochondrial Mediated Apoptosis. Sci. Rep. 2018, 8, 144. [Google Scholar] [CrossRef]
- Khan, I.; Bhardwaj, M.; Shukla, S.; Lee, H.; Oh, M.-H.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Carvacrol Encapsulated Nanocarrier/Nanoemulsion Abrogates Angiogenesis by Downregulating COX-2, VEGF and CD31 in Vitro and in Vivo in a Lung Adenocarcinoma Model. Colloids Surf. B Biointerfaces 2019, 181, 612–622. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Mohamad, N.E.; Abu, N.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model. Molecules 2020, 25, 2670. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Almehmady, A.M.; Alharbi, W.S.; Alshehri, A.A.; Almughem, F.A.; Altamimi, R.M.; Alshabibi, M.A.; Omar, A.M.; El-Say, K.M. Incorporation of Perillyl Alcohol into Lipid-Based Nanocarriers Enhances the Antiproliferative Activity in Malignant Glioma Cells. Biomedicines 2023, 11, 2771. [Google Scholar] [CrossRef]
- Yu, H.; Ning, N.; He, F.; Xu, J.; Zhao, H.; Duan, S.; Zhao, Y. Targeted Delivery of Geraniol via Hyaluronic Acid-Conjugation Enhances Its Anti-Tumor Activity Against Prostate Cancer. Int. J. Nanomed. 2024, 19, 155–169. [Google Scholar] [CrossRef]
- Han, H.D.; Cho, Y.-J.; Cho, S.K.; Byeon, Y.; Jeon, H.N.; Kim, H.-S.; Kim, B.-G.; Bae, D.-S.; Lopez-Berestein, G.; Sood, A.K. Linalool-Incorporated Nanoparticles as a Novel Anticancer Agent for Epithelial Ovarian Carcinoma. Mol. Cancer Ther. 2016, 15, 618–627. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Wang, S.; Liu, J.; Zhang, T.; Wei, Y.; Zhu, L.; Bai, W.; Ye, T.; Wang, S. Menthol Nanoliposomes Enhanced Anti-Tumor Immunotherapy by Increasing Lymph Node Homing of Dendritic Cell Vaccines. Clin. Immunol. 2022, 244, 109119. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R. Sesquiterpenes and Their Derivatives-Natural Anticancer Compounds: An Update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Karole, A.; Mudavath, S.L. Fabrication, Physicochemical Characterization and In Vitro Anticancer Activity of Nerolidol Encapsulated Solid Lipid Nanoparticles in Human Colorectal Cell Line. Colloids Surf. B Biointerfaces 2022, 215, 112520. [Google Scholar] [CrossRef] [PubMed]
- Rasedee, A.; Abdul, A.B.; Zeenathul, N.A.; Rahman, H.; Yeap, S.; Wan Ghani, W.N.H.; Othman, H.H.; How, C.W. Zerumbone-Loaded Nanostructured Lipid Carrier Induces G2/M Cell Cycle Arrest and Apoptosis via Mitochondrial Pathway in a Human Lymphoblastic Leukemia Cell Line. Int. J. Nanomed. 2014, 9, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Alamoudi, A.J.; Badr-Eldin, S.M.; Ahmed, O.A.; Fahmy, U.A.; Elbehairi, S.E.I.; Alfaifi, M.Y.; Asfour, H.Z.; Mohamed, G.A.; Ibrahim, S.R.; Abdel-Naim, A.B. Optimized Bilosome-Based Nanoparticles Enhance Cytotoxic and pro-Apoptotic Activity of Costunolide in LS174T Colon Cancer Cells. Biomed. Pharmacother. 2023, 168, 115757. [Google Scholar] [CrossRef]
- Kordi, S.; Zarghami, N.; Akbarzadeh, A.; Rahmati, Y.M.; Ghasemali, S.; Barkhordari, A.; Tozihi, M. A Comparison of the Inhibitory Effect of Nano-Encapsulated Helenalin and Free Helenalin on Telomerase Gene Expression in the Breast Cancer Cell Line, by Real-Time PCR. Artif. Cells Nanomed. Biotechnol. 2016, 44, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Firouzi Amandi, A.; Jokar, E.; Eslami, M.; Dadashpour, M.; Rezaie, M.; Yazdani, Y.; Nejati, B. Enhanced Anti-Cancer Effect of Artemisinin- and Curcumin-Loaded Niosomal Nanoparticles against Human Colon Cancer Cells. Med. Oncol. 2023, 40, 170. [Google Scholar] [CrossRef]
- Lalami, Z.A.; Tafvizi, F.; Naseh, V.; Salehipour, M. Characterization and Optimization of Co-Delivery Farnesol-Gingerol Niosomal Formulation to Enhance Anticancer Activities against Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 72, 103371. [Google Scholar] [CrossRef]
- Indumathi, T.; Suriyaprakash, J.; Alarfaj, A.A.; Hirad, A.H.; Jaganathan, R.; Mathanmohun, M. Synergistic Effects of CuO/TiO2 -chitosan-farnesol Nanocomposites: Synthesis, Characterization, Antimicrobial, and Anticancer Activities on Melanoma Cells SK-MEL-3. J. Basic Microbiol. 2024, 64, 2300505. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Khuda-Bukhsh, A.R. Cisplatin and Farnesol Co-Encapsulated PLGA Nano-Particles Demonstrate Enhanced Anti-Cancer Potential against Hepatocellular Carcinoma Cells in Vitro. Mol. Biol. Rep. 2020, 47, 3615–3628. [Google Scholar] [CrossRef]
- Pirali-Hamedani, Z.; Abbasi, A.; Hassan, Z.M. Synthesis of Artemether-Loaded Albumin Nanoparticles and Measurement of Their Anti-Cancer Effects. Biomedicines 2022, 10, 2713. [Google Scholar] [CrossRef]
- Ma, C.; Gao, L.; Song, K.; Gu, B.; Wang, B.; Pu, W.; Chen, H. Exploring the Therapeutic Potential of Diterpenes in Gastric Cancer: Mechanisms, Efficacy, and Clinical Prospects. Biomol. Biomed. 2025, 25, 1–15. [Google Scholar] [CrossRef]
- Zheng, Y.; Kong, F.; Liu, S.; Liu, X.; Pei, D.; Miao, X. Membrane Protein-Chimeric Liposome-Mediated Delivery of Triptolide for Targeted Hepatocellular Carcinoma Therapy. Drug Deliv. 2021, 28, 2033–2043. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Li, J.; Yao, L.; Sun, L.; Shi, Y.; Zhang, W.; Lin, J.; Liang, D.; Li, Y. Antitumor Activity of Celastrol Nanoparticles in a Xenograft Retinoblastoma Tumor Model. Int. J. Nanomed. 2012, 7, 2389. [Google Scholar] [CrossRef]
- Velhal, K.; Sah, P.M.; Naik, H.S.; Raut, R.; Patil, S.; Yamgar, R.; Lakkakula, J.; Uddin, I. Synergistic Nanoformulation: Streamlined One-Pot Synthesis Enhances Paclitaxel Functionalization Gold Nanoparticles for Potent Anticancer Activity. Cell Biochem. Biophy. 2025, 83, 3187–3203. [Google Scholar] [CrossRef]
- Borges, G.S.M.; Prazeres, P.H.D.M.; de Souza, Â.M.; Yoshida, M.I.; Vilela, J.M.C.; e Silva, A.T.M.; Oliveira, M.S.; Gomes, D.A.; Andrade, M.S.; de Souza-Fagundes, E.M. Nanostructured Lipid Carriers as a Novel Tool to Deliver Sclareol: Physicochemical Characterisation and Evaluation in Human Cancer Cell Lines. Braz. J. Pharm. Sci. 2021, 57, e18497. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, P.; Dutta, S.; Karak, S.; Mahalanobis, S.; Ghosh, N.; Dewanjee, S.; Sil, P.C. Formulation of Carnosic-Acid-Loaded Polymeric Nanoparticles: An Attempt to Endorse the Bioavailability and Anticancer Efficacy of Carnosic Acid against Triple-Negative Breast Cancer. ACS Appl. Bio Mater. 2024, 7, 1656–1670. [Google Scholar] [CrossRef] [PubMed]
- Kiruthiga, C.; Balan, D.J.; Prasath, N.H.; Manikandakrishnan, M.; Jafni, S.; Prabhu, N.M.; Pandian, S.K.; Devi, K.P. Synergistic Induction of Apoptosis in Lung Cancer Cells through Co-Delivery of PLGA Phytol/α-Bisabolol Nanoparticles. Naunyn-Schmiedeb. Arch. Pharmacol. 2024, 397, 5131–5144. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Sun, J.; Huang, L.; Li, Q. Targeting Lung Cancer Initiating Cells by All-Trans Retinoic Acid-Loaded Lipid-PLGA Nanoparticles with CD133 Aptamers. Exp. Ther. Med. 2018, 16, 4639–4649. [Google Scholar] [CrossRef]
- Roy, P.; Das, S.; Mondal, A.; Chatterji, U.; Mukherjee, A. Nanoparticle Engineering Enhances Anticancer Efficacy of Andrographolide in MCF-7 Cells and Mice Bearing EAC. Curr. Pharmaceut. Biotechnol. 2012, 13, 2669–2681. [Google Scholar] [CrossRef]
- Balakarthikeyan, J.; Mayakrishnan, V.; Kannappan, P.; Al-Ghamdi, S.; Alrudian, N.A.; Alqahtani, M.S.; El-Bidawy, M.H.; Albasheer, K.; Gamil, S.; Alsanousi, N. Cetuximab-Conjugated Andrographolide Loaded Chitosan-Pectin Composite Nanoparticles for Colorectal Cancer. J. King Saud Univ. Sci. 2024, 36, 103261. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, H. Oridonin-Loaded Lipid-Coated Calcium Phosphate Nanoparticles: Preparation, Characterization, and Application in A549 Lung Cancer. Pharm. Dev. Technol. 2022, 27, 598–605. [Google Scholar] [CrossRef]
- Parikh, N.R.; Mandal, A.; Bhatia, D.; Siveen, K.S.; Sethi, G.; Bishayee, A. Oleanane Triterpenoids in the Prevention and Therapy of Breast Cancer: Current Evidence and Future Perspectives. Phytochem. Rev. 2014, 13, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Kumbham, S.; Paul, M.; Itoo, A.; Ghosh, B.; Biswas, S. Oleanolic Acid-Conjugated Human Serum Albumin Nanoparticles Encapsulating Doxorubicin as Synergistic Combination Chemotherapy in Oropharyngeal Carcinoma and Melanoma. Int. J. Pharm. 2022, 614, 121479. [Google Scholar] [CrossRef]
- Man, D.K.W.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F.; Sze, S.C.W.; Leung, G.P.H.; Lam, J.K.W.; Kwok, P.C.L. Oleanolic Acid Loaded PEGylated PLA and PLGA Nanoparticles with Enhanced Cytotoxic Activity against Cancer Cells. Mol. Pharm. 2015, 12, 2112–2125. [Google Scholar] [CrossRef]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M.L.; García, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef]
- Lee, K.-J.; Lee, J.M.; Shin, J.-S.; Kim, D.-H.; An, J.H. Cytotoxic Effect of Oleanolic Acid-Coated Magnetic Nanoparticles on Reversal of Multidrug Resistance in A549 Lung Cancer Cells. J. Nanosci. Nanotechnol. 2016, 16, 11031–11034. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of Ursolic Acid (UA) in Polymeric Nanoparticles Effectively Promotes the Apoptosis of Gastric Cancer Cells through Enhanced Inhibition of Cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yi, Y.; Liu, L.; Lin, Y.; Li, J.; Ruan, J.; Zhong, Z. Polymeric Micelles Loading with Ursolic Acid Enhancing Anti-Tumor Effect on Hepatocellular Carcinoma. J. Cancer 2019, 10, 5820. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Alemi, A.; Taheri-Kafrani, A.; Hosseini, S.A.; Soltaninejad, H.; Hamidieh, A.A.; Haghi Karamallah, M.; Farrokhifar, M.; Farrokhifar, M. Assessment of a New Ginsenoside Rh2 Nanoniosomal Formulation for Enhanced Antitumor Efficacy on Prostate Cancer: An In Vitro Study. Drug Des. Dev. Ther. 2020, 14, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Beg, S.; Alharbi, K.S.; Alruwaili, N.K.; Alotaibi, N.H.; Alzarea, A.I.; Almalki, W.H.; Alenezi, S.K.; Altowayan, W.M.; Alshammari, M.S. Implications of Solid Lipid Nanoparticles of Ganoderic Acid for the Treatment and Management of Hepatocellular Carcinoma. J. Pharm. Innov. 2021, 16, 359–370. [Google Scholar] [CrossRef]
- Rahman, M.; Al-Ghamdi, S.A.; Alharbi, K.S.; Beg, S.; Sharma, K.; Anwar, F.; Al-Abbasi, F.A.; Kumar, V. Ganoderic Acid Loaded Nano-Lipidic Carriers Improvise Treatment of Hepatocellular Carcinoma. Drug Deliv. 2019, 26, 782–793. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, A.; Zhang, F.; Linhardt, R.J.; Zhu, Z.; Yang, Y.; Zhang, T.; Lin, Z.; Zhang, S.; Zhao, H. Ganoderenic Acid D-Loaded Functionalized Graphene Oxide-Based Carrier for Active Targeting Therapy of Cervical Carcinoma. Biomed. Pharmacother. 2023, 164, 114947. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Garg, M. Phytochemistry and Pharmacological Update on Tetraterpenoids. Nat. Prod. J. 2021, 11, 617–628. [Google Scholar] [CrossRef]
- Zare, M.; Norouzi Roshan, Z.; Assadpour, E.; Jafari, S.M. Improving the Cancer Prevention/Treatment Role of Carotenoids through Various Nano-Delivery Systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 522–534. [Google Scholar] [CrossRef]
- Shejawal, K.P.; Randive, D.S.; Bhinge, S.D.; Bhutkar, M.A.; Todkar, S.S.; Mulla, A.S.; Jadhav, N.R. Green Synthesis of Silver, Iron and Gold Nanoparticles of Lycopene Extracted from Tomato: Their Characterization and Cytotoxicity against COLO320DM, HT29 and Hella Cell. J. Mater. Sci. Mater. Med. 2021, 32, 19. [Google Scholar] [CrossRef]
- Tian, B.; Li, J.; Pang, R.; Dai, S.; Li, T.; Weng, Y.; Jin, Y.; Hua, Y. Gold Nanoparticles Biosynthesized and Functionalized Using a Hydroxylated Tetraterpenoid Trigger Gene Expression Changes and Apoptosis in Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 37353–37363. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Gote, V.; Singh, M.; Yellepeddi, V.K.; Patel, M.; Pal, D.; Gong, X.; Sambalingam, D.; Renukuntla, J. Preparation and Characterization of Lutein Loaded Folate Conjugated Polymeric Nanoparticles. J. Microencapsul. 2020, 37, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Shanmugham, V.; Subban, R. Capsanthin-Loaded Micelles: Preparation, Characterization and in Vitro Evaluation of Cytotoxicity Using MDA-MB-231 Breast Cancer Cell Line. Food Technol. Biotechnol. 2022, 60, 350–360. [Google Scholar] [CrossRef]
- Ravi, H.; Kurrey, N.; Manabe, Y.; Sugawara, T.; Baskaran, V. Polymeric Chitosan-Glycolipid Nanocarriers for an Effective Delivery of Marine Carotenoid Fucoxanthin for Induction of Apoptosis in Human Colon Cancer Cells (Caco-2 Cells). Mater. Sci. Eng. C 2018, 91, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Noviendri, D.; Irwandi Jaswir, M.T.; Mohammad, F. Analysis of Apoptosis-Inducing Effect of Free Fucoxanthin and Fucoxanthin-Loaded PLGA Microsphere on Human Lung Cancer H1299 Cell Lines. Int. J. Agric. Biol. 2024, 31, 174–182. [Google Scholar]
- Bharathiraja, S.; Manivasagan, P.; Oh, Y.-O.; Moorthy, M.S.; Seo, H.; Bui, N.Q.; Oh, J. Astaxanthin Conjugated Polypyrrole Nanoparticles as a Multimodal Agent for Photo-Based Therapy and Imaging. Int. J. Pharm. 2017, 517, 216–225. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Gambaro, R.; Cisneros, J.S.; Huck-Iriart, C.; Padula, G.; Castro, G.R.; Chain, C.Y.; Islan, G.A. Enhanced Anticancer Activity of Encapsulated Geraniol into Biocompatible Lipid Nanoparticles against A549 Human Lung Cancer Cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104159. [Google Scholar] [CrossRef]
- Duan, S.; Xia, Y.; Tian, X.; Cui, J.; Zhang, X.; Yang, Q.; Zhao, T.; Lin, Y.; Zhang, F.; Zhang, X. A Multi-Bioresponsive Self-Assembled Nano Drug Delivery System Based on Hyaluronic Acid and Geraniol against Liver Cancer. Carbohydr. Polym. 2023, 310, 120695. [Google Scholar] [CrossRef]
- Han, W.; Shi, W.; Yao, Q.; Wu, H.; Lei, X.; Guo, G. Anticancer Activity of Zinc-Tin Oxide/Dextran/Geraniol Nanocomposites Against 1, 2-Dimethylhydrazine-Induced Colon Cancer in Rats: In Vitro and In Vivo Study. J. Drug Deliv. Sci. Technol. 2024, 98, 105819. [Google Scholar] [CrossRef]
- Ghanbariasad, A.; Osanloo, M.; Hatami, S.; Khaksar, S.; Zarenezhad, E.; Ranjbar, R.; Alipanah, H. Synthesis, Characterization, and Development of Alpha-Pinene Nanoemulsion as an Apoptotic Inducer with Cytotoxicity Activity on Human Melanoma and Breast Cancer. Chem. Pap. 2024, 78, 1181–1191. [Google Scholar] [CrossRef]
- Rahmani, H.; Ghanbariasad, A.; Meshkibaf, M.H.; Molazade, A.; Heiran, R.; Safari, M.; Osanloo, M. Chitosan Nanoparticles Containing α-Pinene and Rosmarinus officinalis L. Essential Oil: Effects on Human Melanoma Cells’ Viability and Expression of Apoptosis-Involved Genes. Polym. Bull. 2024, 81, 2505–2523. [Google Scholar] [CrossRef]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I.; Taqi, Z.J.; Al-Shammari, A.M.; Salman, A.S. Novel of Nano Delivery System for Linalool Loaded on Gold Nanoparticles Conjugated with CALNN Peptide for Application in Drug Uptake and Induction of Cell Death on Breast Cancer Cell Line. Mater. Sci. Eng. C. 2019, 94, 949–964. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Islan, G.A.; de Bravo, M.G.; Durán, N.; Castro, G.R. Design, Characterization and in Vitro Evaluation of Linalool-Loaded Solid Lipid Nanoparticles as Potent Tool in Cancer Therapy. Colloids Surf. B Biointerfaces 2017, 154, 123–132. [Google Scholar] [CrossRef]
- Jabir, M.; Sahib, U.I.; Taqi, Z.; Taha, A.; Sulaiman, G.; Albukhaty, S.; Al-Shammari, A.; Alwahibi, M.; Soliman, D.; Dewir, Y.H.; et al. Linalool-Loaded Glutathione-Modified Gold Nanoparticles Conjugated with CALNN Peptide as Apoptosis Inducer and NF-κB Translocation Inhibitor in SKOV-3 Cell Line. Int. J. Nanomed. 2020, 15, 9025–9047. [Google Scholar] [CrossRef]
- Jalali, A.; Bari, I.J.; Salehzadeh, A. Menthol Conjugated Magnetic Iron Oxide Nanoparticles Induce Apoptosis and Increase Caspase-8 Gene Expression in Gastric Cancer Cell Line. BioNanoScience 2024, 14, 5276–5285. [Google Scholar] [CrossRef]
- Osanloo, M.; Sedaghati, F.; Zarenezhad, E.; Amani, A. Novel Nanocarrier for Melanoma Treatment: Chitosan-Gum Arabic Nanoparticles Containing Menthol. BioNanoScience 2024, 14, 2638–2648. [Google Scholar] [CrossRef]
- Alipanah, H.; Farjam, M.; Zarenezhad, E.; Roozitalab, G.; Osanloo, M. Chitosan Nanoparticles Containing Limonene and Limonene-Rich Essential Oils: Potential Phytotherapy Agents for the Treatment of Melanoma and Breast Cancers. BMC Complement. Med. Ther. 2021, 21, 186. [Google Scholar] [CrossRef]
- Swathi, B.; Thangaraj, R.; Gayathri, D.; Subha, V.; Thiyagarajan, P.; Thiruvengadam, M.; Venkidasamy, B. Green Synthesis of β-Caryophyllene-Coated Silver Nanoparticles: Biophysical Characterization and Evaluation of Antibacterial, Cytotoxic, and Larvicidal Activities. BioNanoScience 2025, 15, 102. [Google Scholar] [CrossRef]
- Kamaraj, C.; Balasubramani, G.; Siva, C.; Raja, M.; Balasubramanian, V.; Raja, R.K.; Tamilselvan, S.; Benelli, G.; Perumal, P. Ag Nanoparticles Synthesized Using β-Caryophyllene Isolated from Murraya Koenigii: Antimalarial (Plasmodium Falciparum 3D7) and Anticancer Activity (A549 and HeLa Cell Lines). J. Clust. Sci. 2017, 28, 1667–1684. [Google Scholar] [CrossRef]
- Natesan, S.; Ponnusamy, C.; Sugumaran, A.; Chelladurai, S.; Palaniappan, S.S.; Palanichamy, R. Artemisinin Loaded Chitosan Magnetic Nanoparticles for the Efficient Targeting to the Breast Cancer. Int. J. Biol. Macromol. 2017, 104, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gallis, B.; Taya, M.; Wang, S.; Ho, R.J.; Sasaki, T. pH-Responsive Artemisinin Derivatives and Lipid Nanoparticle Formulations Inhibit Growth of Breast Cancer Cells in Vitro and Induce down-Regulation of HER Family Members. PLoS ONE 2013, 8, e59086. [Google Scholar] [CrossRef]
- Eslami Vaghar, M.; Dadashpour, M.; Derakhshan, E.; Vahedian, V.; Shahrtash, S.A.; Firouzi Amandi, A.; Eslami, M.; Hasannia, M.; Mehrabi, Z.; Roshangar, L. Artemisinin-Loaded Mesoporous Silica Nanoparticles/Electrospun Poly(Lactic-Co-Glycolic Acid) Composite Nanofibers for Enhanced Anticancer Efficiency in Breast Cancer Cells. Cancer Nanotechnol. 2024, 15, 58. [Google Scholar] [CrossRef]
- Mickymaray, S.; Al Aboody, M.S.; Eraqi, M.M.; Alhoqail, W.A.; Alothaim, A.S.; Suresh, K.; Arulselvan, P. Chitosan-Encapsulated Nickel Oxide, Tin Dioxide, and Farnesol Nanoparticles: Antimicrobial and Anticancer Properties in Breast Cancer Cells. Int. J. Biol. Macromol. 2023, 248, 125799. [Google Scholar] [CrossRef]
- Li, H.; Qu, X.; Qian, W.; Song, Y.; Wang, C.; Liu, W. Andrographolide-loaded Solid Lipid Nanoparticles Enhance Anti-cancer Activity against Head and Neck Cancer and Precancerous Cells. Oral Dis. 2022, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, S.K.; Patil, S.; Sivapatham, S. Synthesis and Characterization of Andrographolide: Albumin Nanoparticles and Their Anticancer Activity in HeLa Cancer Cell Line. Int. J. Nutr. Pharmacol. Neurol. Dis. 2024, 14, 137–141. [Google Scholar] [CrossRef]
- Cosco, D.; Mare, R.; Paolino, D.; Salvatici, M.C.; Cilurzo, F.; Fresta, M. Sclareol-Loaded Hyaluronan-Coated PLGA Nanoparticles: Physico-Chemical Properties and in Vitro Anticancer Features. Int. J. Biol. Macromol. 2019, 132, 550–557. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Bahadori, M.B.; Vandghanooni, S.; Eskandani, M.; Nakhlband, A.; Eskandani, M. Preparation, Characterization and Anti-Proliferative Effects of Sclareol-Loaded Solid Lipid Nanoparticles on A549 Human Lung Epithelial Cancer Cells. J. Drug Deliv. Sci. Technol. 2018, 45, 272–280. [Google Scholar] [CrossRef]
- Khella, K.F.; Abd El Maksoud, A.I.; Hassan, A.; Abdel-Ghany, S.E.; Elsanhoty, R.M.; Aladhadh, M.A.; Abdel-Hakeem, M.A. Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules 2022, 27, 4102. [Google Scholar] [CrossRef]
- Dogan, M. Analysis of the Mechanisms Underlying the Anticancer and Biological Activity of Retinoic Acid and Chitosan Nanoparticles Containing Retinoic Acid. Med. Oncol. 2024, 41, 251. [Google Scholar] [CrossRef]
- Rajeshkumar, R.R.; Pavadai, P.; Panneerselvam, T.; Pandian, S.R.K.; Kumar, A.S.K.; Maszczyk, P.; Babkiewicz, E.; Kabilan, S.J.; Kunjiappan, S. Enhanced Delivery of Retinoic Acid to Breast Cancer Cells by Folate Receptor-Targeted Folic Acid-Conjugated Glutenin Nanoparticles for Promising Treatment of Breast Cancer. J. Polym. Environ. 2024, 32, 2120–2139. [Google Scholar] [CrossRef]
- Akanda, M.H.; Rai, R.; Slipper, I.J.; Chowdhry, B.Z.; Lamprou, D.; Getti, G.; Douroumis, D. Delivery of Retinoic Acid to LNCap Human Prostate Cancer Cells Using Solid Lipid Nanoparticles. Int. J. Pharm. 2015, 493, 161–171. [Google Scholar] [CrossRef]
- Kotelevets, L.; Chastre, E.; Caron, J.; Mougin, J.; Bastian, G.; Pineau, A.; Walker, F.; Lehy, T.; Desmaële, D.; Couvreur, P. A Squalene-Based Nanomedicine for Oral Treatment of Colon Cancer. Cancer Res. 2017, 77, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Bidooki, S.H.; Quero, J.; Sánchez-Marco, J.; Herrero-Continente, T.; Marmol, I.; Lasheras, R.; Sebastian, V.; Arruebo, M.; Osada, J.; Rodriguez-Yoldi, M.J. Squalene in Nanoparticles Improves Antiproliferative Effect on Human Colon Carcinoma Cells Through Apoptosis by Disturbances in Redox Balance. Int. J. Mol. Sci. 2024, 25, 13048. [Google Scholar] [CrossRef]
- Ravichandran, S.; Kandaswamy, K.; Muthu, K. Evaluation of Lupeol-Chitosan Nanoparticles Infused Cellulose Acetate Membranes for Enhanced in-Vitro Anticancer and Antidiabetic Activities. Chemosphere 2024, 351, 141149. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Zheng, G.; Yao, H.; Liang, H. In Vitro and in Vivo Antitumor Effects of Lupeol-Loaded Galactosylated Liposomes. Drug Deliv. 2021, 28, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.N.; Mehata, A.K.; Setia, A.; Kumari, P.; Mahto, S.K.; Muthu, M.S.; Mishra, S.K. EGFR Targeted Albumin Nanoparticles of Oleanolic Acid: In Silico Screening of Nanocarrier, Cytotoxicity and Pharmacokinetics for Lung Cancer Therapy. Int. J. Biol. Macromol. 2023, 246, 125719. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Dong, Y. Ursolic Acid Nanoparticles Inhibit Cervical Cancer Growth in Vitro and in Vivo via Apoptosis Induction. Int. J. Oncol. 2017, 50, 1330–1340. [Google Scholar] [CrossRef]
- Payomhom, P.; Panyain, N.; Sakonsinsiri, C.; Wongtrakoongate, P.; Lertsuwan, K.; Pissuwan, D.; Katewongsa, K.P. Chitosan-Coated Poly(Lactic-Co-Glycolic Acid) Nanoparticles Loaded with Ursolic Acid for Breast Cancer Therapy. ACS Appl. Nano Mater. 2024, 7, 5383–5395. [Google Scholar] [CrossRef]
- Markowski, A.; Migdał, P.; Zygmunt, A.; Zaremba-Czogalla, M.; Gubernator, J. Evaluation of the in Vitro Cytotoxic Activity of Ursolic Acid PLGA Nanoparticles against Pancreatic Ductal Adenocarcinoma Cell Lines. Materials 2021, 14, 4917. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, S.; Chen, R.; Wang, Y.; Li, H.; Wang, Y.; Chen, M. Oridonin Loaded Solid Lipid Nanoparticles Enhanced Antitumor Activity in MCF-7 Cells. J. Nanomater. 2014, 2014, 903646. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel Ginsenoside-Based Multifunctional Liposomal Delivery System for Combination Therapy of Gastric Cancer. Theranostics 2019, 9, 4437. [Google Scholar] [CrossRef]
- Goswami, A.; Patel, N.; Bhatt, V.; Raval, M.; Kundariya, M.; Sheth, N. Lycopene Loaded Polymeric Nanoparticles for Prostate Cancer Treatment: Formulation, Optimization Using Box-Behnken Design and Cytotoxicity Studies. J. Drug Deliv. Sci. Technol. 2022, 67, 102930. [Google Scholar] [CrossRef]
- Chen, B.-H.; Huang, R.-F.S.; Wei, Y.-J.; Stephen Inbaraj, B. Inhibition of Colon Cancer Cell Growth by Nanoemulsion Carrying Gold Nanoparticles And Lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar] [CrossRef]
- Hsu, H.J.; Huang, R.F.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Preparation of Carotenoid Extracts and Nanoemulsions from Lycium barbarum L. and Their Effects on Growth of HT-29 Colon Cancer Cells. Nanotechnology 2017, 28, 135103. [Google Scholar] [CrossRef] [PubMed]
- Bulatao, B.P.; Nalinratana, N.; Jantaratana, P.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Lutein-Loaded Chitosan/Alginate-Coated Fe3O4 Nanoparticles as Effective Targeted Carriers for Breast Cancer Treatment. Int. J. Biol. Macromol. 2023, 242, 124673. [Google Scholar] [CrossRef]
- Sui, Y.; Gu, Y.; Lu, Y.; Yu, C.; Zheng, J.; Qi, H. Fucoxanthin@ Polyvinylpyrrolidone Nanoparticles Promoted Oxidative Stress-Induced Cell Death in Caco-2 Human Colon Cancer Cells. Mar. Drugs 2021, 19, 92. [Google Scholar] [CrossRef]
- Hwang, E.J.; Jeong, Y.-I.; Lee, K.-J.; Yu, Y.-B.; Ohk, S.-H.; Lee, S.-Y. Anticancer Activity of Astaxanthin-Incorporated Chitosan Nanoparticles. Molecules 2024, 29, 529. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Manivasagan, P.; Quang Bui, N.; Oh, Y.-O.; Lim, I.G.; Park, S.; Oh, J. Cytotoxic Induction and Photoacoustic Imaging of Breast Cancer Cells Using Astaxanthin-Reduced Gold Nanoparticles. Nanomaterials 2016, 6, 78. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Thakur, K.; Raza, K.; Shivhare, U.S.; Ghoshal, G.; Katare, O.P. Beta-Carotene-Encapsulated Solid Lipid Nanoparticles (BC-SLNs) as Promising Vehicle for Cancer: An Investigative Assessment. AAPS PharmSciTech 2019, 20, 100. [Google Scholar] [CrossRef]
- Dutta, R.S.; Elhassan, G.O.; Devi, T.B.; Bhattacharjee, B.; Singh, M.; Jana, B.K.; Sahu, S.; Mazumder, B.; Sahu, R.K.; Khan, J. Enhanced Efficacy of β-Carotene Loaded Solid Lipid Nanoparticles Optimized and Developed via Central Composite Design on Breast Cancer Cell Lines. Heliyon 2024, 10, e28457. [Google Scholar] [CrossRef]
- Sanati, P.; Chua, L.S.; Nasiri, R.; Hashemi, S.-S. Nanoencapsulation of Andrographolide Rich Extract for the Inhibition of Cervical and Neuroblastoma Cancer Cells. J. Biomed. Nanotechnol. 2020, 16, 1370–1380. [Google Scholar] [CrossRef]
- Liu, X.; Dong, S.; Dong, M.; Li, Y.; Sun, Z.; Zhang, X.; Wang, Y.; Teng, L.; Wang, D. Transferrin-Conjugated Liposomes Loaded with Carnosic Acid Inhibit Liver Cancer Growth by Inducing Mitochondria-Mediated Apoptosis. Int. J. Pharm. 2021, 607, 121034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, W.; Peng, W.; Huang, Q.; Wu, K.; Evans, C.E.; Liu, X.; Jin, H. Oridonin-Loaded Nanoparticles Inhibit Breast Cancer Progression through Regulation of ROS-Related Nrf2 Signaling Pathway. Front. Bioeng. Biotechnol. 2021, 9, 600579. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pi, J.; Yang, F.; Jiang, J.; Wang, X.; Bai, H.; Shao, M.; Huang, L.; Zhu, H.; Yang, P. Folate-Chitosan Nanoparticles Loaded with Ursolic Acid Confer Anti-Breast Cancer Activities In Vitro and In Vivo. Sci. Rep. 2016, 6, 30782. [Google Scholar] [CrossRef]
- Bano, S.; Ahmed, F.; Khan, F.; Chaudhary, S.C.; Samim, M. Targeted Delivery of Thermoresponsive Polymeric Nanoparticle-Encapsulated Lycopene: In Vitro Anticancer Activity and Chemopreventive Effect on Murine Skin Inflammation and Tumorigenesis. RSC Adv. 2020, 10, 16637–16649. [Google Scholar] [CrossRef]
- Kowshik, J.; Nivetha, R.; Ranjani, S.; Venkatesan, P.; Selvamuthukumar, S.; Veeravarmal, V.; Nagini, S. Astaxanthin Inhibits Hallmarks of Cancer by Targeting the PI3K/NF-κΒ/STAT3 Signalling Axis in Oral Squamous Cell Carcinoma Models. IUBMB Life 2019, 71, 1595–1610. [Google Scholar] [CrossRef]
- Azhar, F.; Naureen, H.; Shahnaz, G.; Hamdani, S.D.A.; Kiani, M.H.; Khattak, S.; Manna, M.K.; Babar, M.M.; Rajadas, J.; Rahdar, A. Development of Chitosan Based β-Carotene Mucoadhesive Formulation for Skin Cancer Treatment. Int. J. Biol. Macromol. 2023, 253, 126659. [Google Scholar] [CrossRef]
- Joshi, M.; Pathak, K.; Dhaneshwar, S. Nanotechnology-Based Strategies for Effective Delivery of Phytoconstituents for the Management of Rheumatoid Arthritis. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100061. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Sharma, N. Precision Nanotoxicology in Drug Development: Current Trends and Challenges in Safety and Toxicity Implications of Customized Multifunctional Nanocarriers for Drug-Delivery Applications. Pharmaceutics 2022, 14, 2463. [Google Scholar] [CrossRef]
- Jeevanandam, J.; San Chan, Y.; Danquah, M.K. Nano-Formulations of Drugs: Recent Developments, Impact and Challenges. Biochimie 2016, 128, 99–112. [Google Scholar] [CrossRef]
- Chaurasiya, B.; Mahanty, A.; Roy, D.; Shen, Y.; Tu, J.; Sun, C. Influence of Tumor Microenvironment on the Distribution and Elimination of Nano-Formulations. Curr. Drug Metabol. 2016, 17, 783–798. [Google Scholar] [CrossRef]
- Teja, P.K.; Mithiya, J.; Kate, A.S.; Bairwa, K.; Chauthe, S.K. Herbal Nanomedicines: Recent Advancements, Challenges, Opportunities and Regulatory Overview. Phytomedicine 2022, 96, 153890. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Vandana, K.; Kumar, V.; Rathour, A. Advancements In Herbal-Nano Formulations: A Systematic Review. Educ. Adm. Theory Pract. 2024, 30, 11099–11109. [Google Scholar]
- Shrihastini, V.; Muthuramalingam, P.; Adarshan, S.; Sujitha, M.; Chen, J.-T.; Shin, H.; Ramesh, M. Plant Derived Bioactive Compounds, Their Anti-Cancer Effects and in Silico Approaches as an Alternative Target Treatment Strategy for Breast Cancer: An Updated Overview. Cancers 2021, 13, 6222. [Google Scholar] [CrossRef] [PubMed]
- Kour, S.; Biswas, I.; Sheoran, S.; Arora, S.; Sheela, P.; Duppala, S.K.; Murthy, D.K.; Pawar, S.C.; Singh, H.; Kumar, D. Artificial Intelligence and Nanotechnology for Cervical Cancer Treatment: Current Status and Future Perspectives. J. Drug Deliv. Sci. Technol. 2023, 83, 104392. [Google Scholar] [CrossRef]
- Heydari, S.; Masoumi, N.; Esmaeeli, E.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorpeh, F.; Ahmadi, M. Artificial Intelligence in Nanotechnology for Treatment of Diseases. J. Drug Target. 2024, 32, 1247–1266. [Google Scholar] [CrossRef]


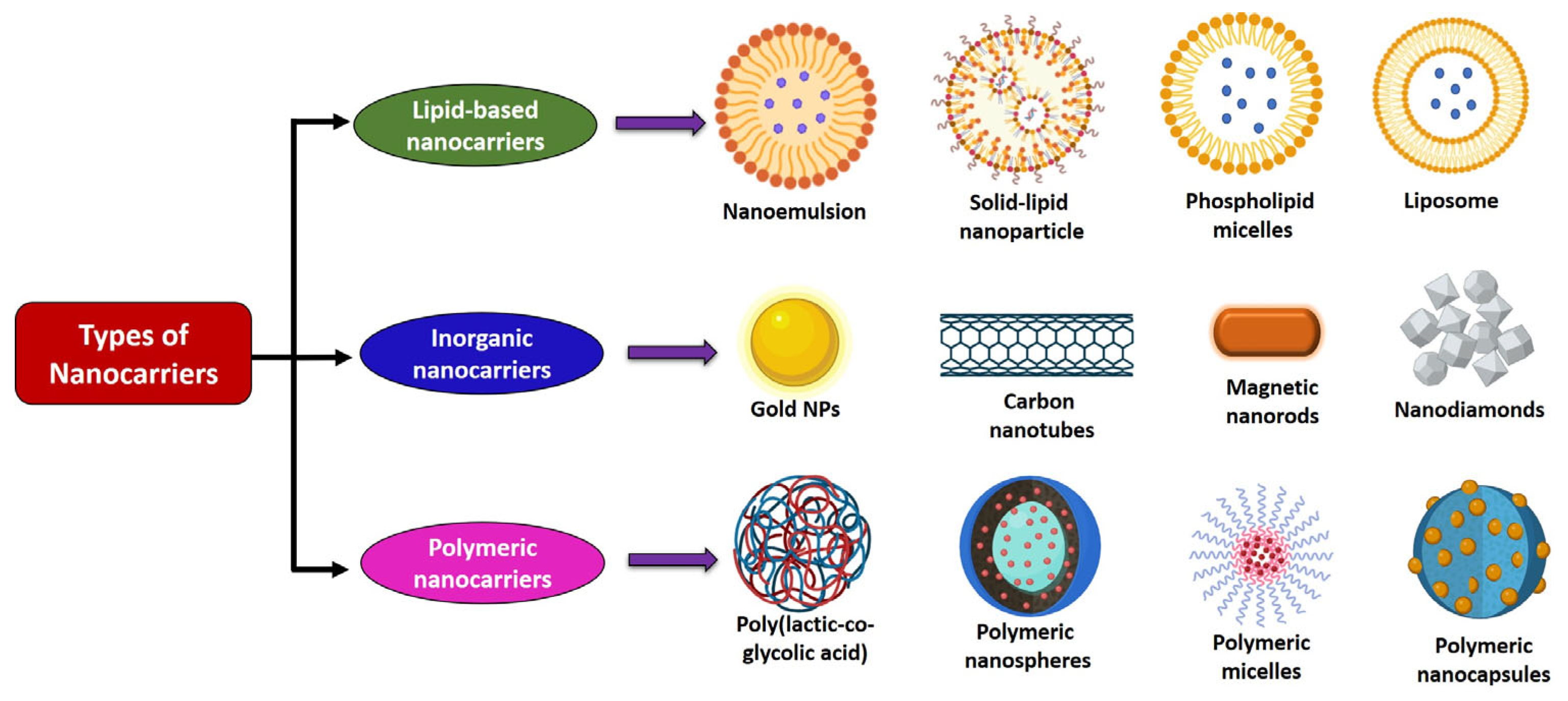

| Type of Nanocarrier | Route of Administration | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Self-emulsifying drug delivery systems | Oral | Enhance solubility and absorption of hydrophobic terpenoids | Limited drug loading and requires high surfactant content | [89] |
| Polymeric nanoparticles | Oral and IV | Sustained/controlled release; biodegradable; and targeted delivery | Complex synthesis; morphology and size dependency; and burst release possible | [90] |
| Pickering emulsions | Oral | Surfactant-free; good physical stability | Scale-up challenges | [91] |
| Liposomes | Oral, IV, and topical | Encapsulate both hydrophilic and lipophilic terpenoids; biocompatible; enhance solubility and systemic availability | Stability issues; drug leakage; and high cost | [87] |
| Solid lipid nanoparticles/nanostructured lipid carriers | Oral and IV | Encapsulate hydrophobic terpenoids; high stability; controlled release; and reduced toxicity | Limited drug loading and risk of drug expulsion | [86] |
| Ethosomes, phytosomes, niosomes, and invasomes | Oral, transdermal, and topical | Enhance skin permeability, absorption, and stability | Limited systemic application and variable reproducibility | [86,87] |
| Inorganic nanoparticles | IV | Easy surface modification and theranostic potential | Long-term toxicity concerns and poor biodegradability | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharmila, A.; Bhadra, P.; Kishore, C.; Selvaraj, C.I.; Kavalakatt, J.; Bishayee, A. Nanoformulated Terpenoids in Cancer: A Review of Therapeutic Applications, Mechanisms, and Challenges. Cancers 2025, 17, 3013. https://doi.org/10.3390/cancers17183013
Sharmila A, Bhadra P, Kishore C, Selvaraj CI, Kavalakatt J, Bishayee A. Nanoformulated Terpenoids in Cancer: A Review of Therapeutic Applications, Mechanisms, and Challenges. Cancers. 2025; 17(18):3013. https://doi.org/10.3390/cancers17183013
Chicago/Turabian StyleSharmila, Arunagiri, Priyanka Bhadra, Chandra Kishore, Chinnadurai Immanuel Selvaraj, Joachim Kavalakatt, and Anupam Bishayee. 2025. "Nanoformulated Terpenoids in Cancer: A Review of Therapeutic Applications, Mechanisms, and Challenges" Cancers 17, no. 18: 3013. https://doi.org/10.3390/cancers17183013
APA StyleSharmila, A., Bhadra, P., Kishore, C., Selvaraj, C. I., Kavalakatt, J., & Bishayee, A. (2025). Nanoformulated Terpenoids in Cancer: A Review of Therapeutic Applications, Mechanisms, and Challenges. Cancers, 17(18), 3013. https://doi.org/10.3390/cancers17183013







