Prognostic Role of Kidney Disease in Newly Diagnosed Acute Myeloid Leukemia Under Venetoclax-Based Low-Intensity Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Administration
2.3. Cytogenetic and Molecular Analysis
2.4. Safety and Efficacy Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Response and Outcome
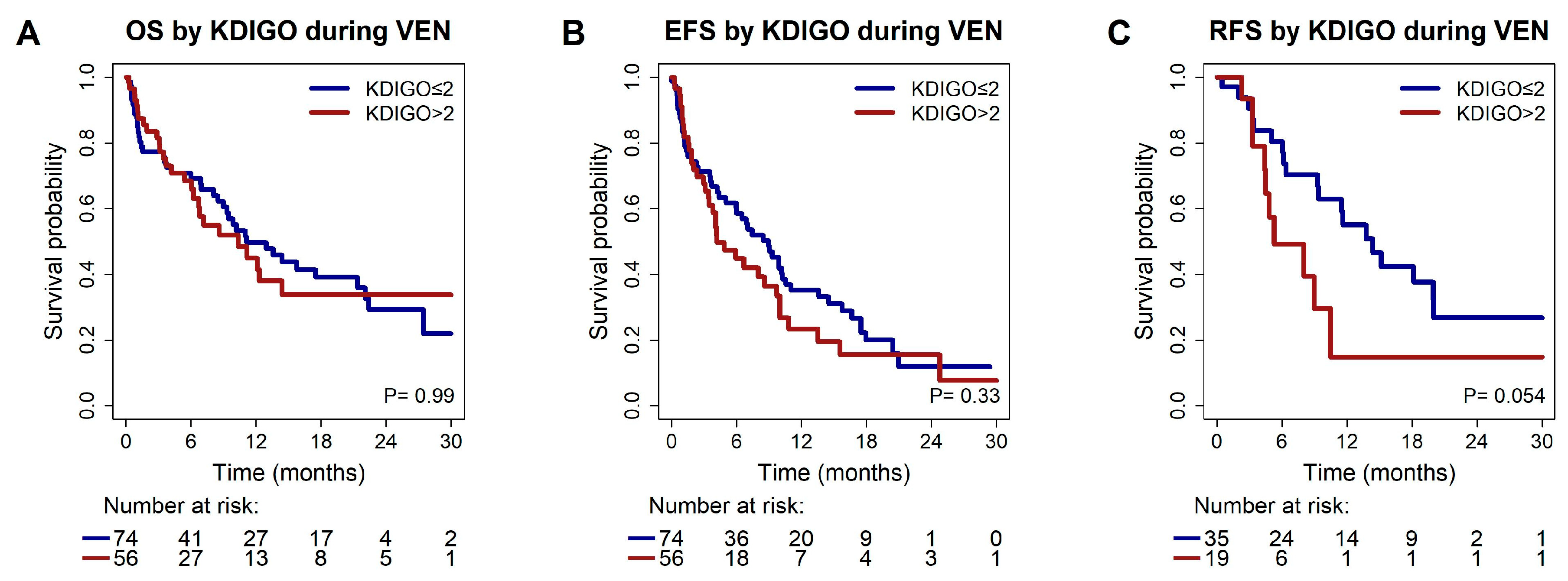
3.3. Association of Pre-Existing Chronic Kidney Disease with Response and Survival
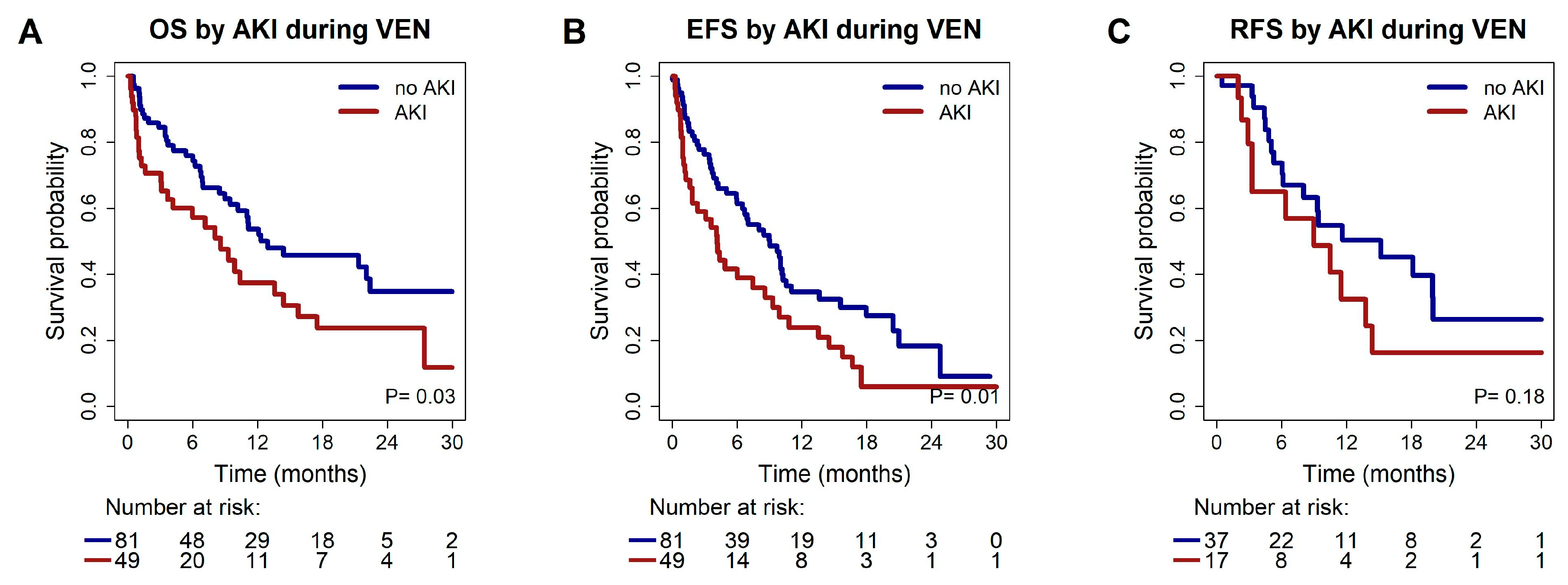
3.4. Risk Factors for Acute Kidney Injury During VEN Treatment
3.5. Association of Acute Kidney Injury During the First 4 Weeks of VEN Treatment with Response and Survival
3.6. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| AKI | Acute kidney injury |
| AML | Acute myeloid leukemia |
| AZA | Azacitidine |
| CART | Classification and regression trees |
| CHIP | Clonal hematopoiesis of indeterminate potential |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| CR | Complete remission |
| CRi | Complete remission with incomplete blood count recovery |
| CTCE | Common Terminology Criteria for Adverse Events |
| CTLS | Clinical tumor lysis syndrome |
| ECOG | Eastern Cooperative Oncology Group |
| EFS | Event-free survival |
| eGFR | Estimated glomerular filtration rate |
| ELN | European LeukemiaNet |
| HMA | Hypomethylating agents |
| HR | Hazard ratio |
| ICC | International consensus classification |
| KDIGO | Kidney disease improving global outcomes |
| KT | karyotype |
| LDAC | Low-dose cytarabine |
| LTLS | Laboratory tumor lysis syndrome |
| MPN | Myeloproliferative neoplasms |
| mPRS | Molecular prognostic risk signature |
| MRCA | Myelodysplasia-related cytogenetic abnoramlities |
| MRGM | Myelodysplasia-related gene mutations |
| mut | mutated |
| MVA | Multivariable analysis |
| OR | Odds ratio |
| ORR | Overall response rate |
| OS | Overall survival |
| p | p-value |
| PK | pharmacokinetics |
| RFS | Relapse-free survival |
| TLS | Tumor lysis syndrome |
| UVA | Univariate analysis |
| VEN | Venetoclax |
| VENreg | Venetoclax registry |
| WBC | White blood cell |
| wt | Wildtype |
References
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Morsia, E.; McCullough, K.; Joshi, M.; Cook, J.; Alkhateeb, H.B.; Al-Kali, A.; Begna, K.; Elliott, M.; Hogan, W.; Litzow, M.; et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am. J. Hematol. 2020, 95, 1511–1521. [Google Scholar] [CrossRef]
- Kwag, D.; Cho, B.-S.; Bang, S.-Y.; Lee, J.H.; Min, G.-J.; Park, S.-S.; Park, S.; Yoon, J.-H.; Lee, S.-E.; Eom, K.-S.; et al. Venetoclax with decitabine versus decitabine monotherapy in elderly acute myeloid leukemia: A propensity score-matched analysis. Blood Cancer J. 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.; Williams, K.; Puto, M.; Waller, A.; McLaughlin, E.M.; Blachly, J.S.; Roddy, J. Real-World Experience of Adults with Acute Myeloid Leukemia on Hypomethylating Agents with or Without Venetoclax at a Comprehensive Cancer Center. World J. Oncol. 2023, 14, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.H.; Perl, A.E.; Luger, S.M.; Loren, A.W.; Gill, S.I.; Porter, D.L.; Babushok, D.V.; Maillard, I.P.; Carroll, M.P.; Frey, N.V.; et al. Real-world effectiveness of CPX-351 vs venetoclax and azacitidine in acute myeloid leukemia. Blood Adv. 2022, 6, 3997–4005. [Google Scholar] [CrossRef]
- Döhner, H.; Pratz, K.W.; DiNardo, C.D.; Wei, A.H.; Jonas, B.A.; Pullarkat, V.A.; Thirman, M.J.; Récher, C.; Schuh, A.C.; Babu, S.; et al. Genetic risk stratification and outcomes among treatment-naive patients with AML treated with venetoclax and azacitidine. Blood 2024, 144, 2211–2222. [Google Scholar] [CrossRef]
- Döhner, H.; DiNardo, C.D.; Appelbaum, F.R.; Craddock, C.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; Larson, R.A.; et al. Genetic risk classification for adults with AML receiving less-intensive therapies: The 2024 ELN recommendations. Blood 2024, 144, 2169–2173. [Google Scholar] [CrossRef]
- Bataller, A.; Bazinet, A.; DiNardo, C.D.; Maiti, A.; Borthakur, G.; Daver, N.G.; Short, N.J.; Jabbour, E.J.; Issa, G.C.; Pemmaraju, N.; et al. Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax. Blood Adv. 2024, 8, 927–935. [Google Scholar] [CrossRef]
- Shimony, S.; Garcia, J.S.; Keating, J.; Chen, E.C.; Luskin, M.R.; Stahl, M.; Neuberg, D.S.; DeAngelo, D.J.; Stone, R.M.; Lindsley, R.C. Molecular ontogeny underlies the benefit of adding venetoclax to hypomethylating agents in newly diagnosed AML patients. Leukemia 2024, 38, 1494–1500. [Google Scholar] [CrossRef]
- Gangat, N.; Karrar, O.; Iftikhar, M.; McCullough, K.; Johnson, I.M.; Abdelmagid, M.; Abdallah, M.; Al-Kali, A.; Alkhateeb, H.B.; Begna, K.H.; et al. Venetoclax and hypomethylating agent combination therapy in newly diagnosed acute myeloid leukemia: Genotype signatures for response and survival among 301 consecutive patients. Am. J. Hematol. 2023, 99, 193–202. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Johnson, I.M.; Karrar, O.; Rana, M.; Iftikhar, M.; Chen, S.; McCullough, K.; Saliba, A.N.; Al-Kali, A.; Alkhateeb, H.; Begna, K.; et al. Cardiac events in newly diagnosed acute myeloid leukaemia during treatment with venetoclax + hypomethylating agents. Br. J. Haematol. 2024, 204, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Michmerhuizen, M.J.; Lao, Y.; Wan, K.; Salem, A.H.; Sawicki, J.; Serby, M.; Vaidyanathan, S.; Wong, S.L.; Agarwal, S.; et al. Metabolism and Disposition of a Novel B-Cell Lymphoma-2 Inhibitor Venetoclax in Humans and Characterization of Its Unusual Metabolites. Drug Metab. Dispos. 2017, 45, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Elston, A.C.; Bayliss, M.K.; Park, G.R. Effect of renal failure on drug metabolism by the liver. Br. J. Anaesth. 1993, 71, 282–290. [Google Scholar] [CrossRef]
- Akagi, Y.; Yamashita, Y.; Kosako, H.; Furuya, Y.; Hosoi, H.; Mushino, T.; Murata, S.; Nishikawa, A.; Tamura, S.; Nakao, T.; et al. Administration of combined venetoclax and azacitidine in a patient with acute myeloid leukemia and multiple comorbidities undergoing dialysis: A case report. eJHaem 2023, 4, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Brackman, D.; Eckert, D.; Menon, R.; Salem, A.H.; Potluri, J.; Smith, B.D.; Wei, A.H.; Hayslip, J.; Miles, D.; Mensing, S.; et al. Venetoclax Exposure-efficacy and Exposure-safety Relationships in Patients with Treatment-naïve Acute Myeloid Leukemia Who Are Ineligible for Intensive Chemotherapy. Hematol. Oncol. 2022, 40, 269–279. [Google Scholar] [CrossRef]
- Noorani, B.; Menon, R.M.; Chen, X.; Marsh, K.C.; Huang, W.; Gupta, S.; Dobkowska, E.; Marbury, T.; Salem, A.H. Venetoclax pharmacokinetics in participants with end-stage renal disease undergoing haemodialysis. Br. J. Clin. Pharmacol. 2023, 90, 748–758. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Preva-lence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Jones, C.R.; Daratha, K.B.; Koyama, A.K.; Nicholas, S.B.; Alicic, R.Z.; Duru, O.K.; Neumiller, J.J.; Norris, K.C.; Burrows, N.R.; et al. Incidence of Chronic Kidney Disease among Adults with Diabetes, 2015–2020. N. Engl. J. Med. 2022, 387, 1430–1431. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Zhao, X.; Cui, H.; Han, M.; Ren, X.; Gang, X.; Wang, G. Obesity and Chronic Kidney Disease. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E24–E41. [Google Scholar] [CrossRef]
- Hoy, W.E.; White, A.V.; Dowling, A.; Sharma, S.K.; Bloomfield, H.; Tipiloura, B.T.; Swanson, C.E.; Mathews, J.D.; McCredie, D.A. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012, 81, 1026–1032. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Cruz, D.; Cerda, J.; Abulfaraj, M.; Algahtani, F.; Koulouridis, I.; Jaber, B. Acute Kidney Injury Ad-visory Group of the American Society of Nephrology. World Incidence of AKI: A Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Vlasschaert, C.; Robinson-Cohen, C.; Chen, J.; Akwo, E.; Parker, A.C.; Silver, S.A.; Bhatraju, P.K.; Poisner, H.; Cao, S.; Jiang, M.; et al. Clonal hematopoiesis of indeterminate potential is associated with acute kidney injury. Nat. Med. 2024, 30, 810–817. [Google Scholar] [CrossRef]
- Cairo, M.S.; Coiffier, B.; Reiter, A.; Younes, A.; TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: An expert TLS panel consensus. Br. J. Haematol. 2010, 149, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Otiker, H.; Rivero, G.; Sidebottom, A.; Hwang, G. Is Renal Function Equally Impacted by Hypomethylating Agents during Acute Myelogenous Leukemia Induction Therapy? Blood 2022, 140, 3297–3298. [Google Scholar] [CrossRef]
- Batty, G.N.; Kantarjian, H.; Issa, J.-P.; Jabbour, E.; Santos, F.P.S.; McCue, D.; Garcia-Manero, G.; Pierce, S.; O’Brien, S.; Cortés, J.E.; et al. Feasibility of Therapy with Hypomethylating Agents in Patients with Renal Insuffi-ciency. Clin. Lymphoma Myeloma Leuk. 2010, 10, 205–210. [Google Scholar] [CrossRef]
- Montesinos, P.; Lorenzo, I.; Martín, G.; Sanz, J.; Pérez-Sirvent, M.L.; Martínez, D.; Ortí, G.; Algarra, L.; Martínez, J.; Moscardó, F.; et al. Tumor lysis syndrome in patients with acute myeloid leukemia: Identification of risk factors and development of a predictive model. Haematologica 2008, 93, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Cairo, M.S.; Bishop, M. Tumour lysis syndrome: New therapeutic strategies and classification. Br. J. Haematol. 2004, 127, 3–11. [Google Scholar] [CrossRef]
- Shahswar, R.; Beutel, G.; Gabdoulline, R.; Koenecke, C.; Markel, D.; Eder, M.; Stadler, M.; Gohring, G.; Schlegelberger, B.; Trummer, A.; et al. Risk of tumor lysis syndrome in patients with acute myeloid leukemia treated with venetoclax-containing regimens without dose ramp-up. Ann. Hematol. 2020, 100, 595–599. [Google Scholar] [CrossRef]
- Apel, A.; Moshe, Y.; Ofran, Y.; Gural, A.; Wolach, O.; Ganzel, C.; Canaani, J.; Zektser, M.; Duek, A.; Stemer, G.; et al. Venetoclax combinations induce high response rates in newly diagnosed acute myeloid leukemia patients ineligible for intensive chemotherapy in routine practice. Am. J. Hematol. 2021, 96, 790–795. [Google Scholar] [CrossRef]
- Arora, S.; Zainaldin, C.; Bathini, S.; Gupta, U.; Worth, S.; Bachiashvili, K.; Bhatia, R.; Godby, K.; Jamy, O.; Rangaraju, S.; et al. Tumor lysis syndrome and infectious complications during treatment with venetoclax combined with azacitidine or decitabine in patients with acute myeloid leukemia. Leuk. Res. 2022, 117, 106844. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Shahswar, R.; Beutel, G.; Klement, P.; Rehberg, A.; Gabdoulline, R.; Koenecke, C.; Markel, D.; Eggers, H.; Eder, M.; Stadler, M.; et al. FLA-IDA salvage chemotherapy combined with a seven-day course of venetoclax (FLAVIDA) in patients with relapsed/refractory acute leukaemia. Br. J. Haematol. 2019, 188, e11–e15. [Google Scholar] [CrossRef]
- Shahswar, R.; Gabdoulline, R.; Krueger, K.; Wichmann, M.; Götze, K.S.; Braitsch, K.; Meggendorfer, M.; Schmalbrock, L.; Bullinger, L.; Modemann, F.; et al. A novel prognostic risk model for patients with refractory/relapsed acute myeloid leukemia receiving venetoclax plus hypomethylating agents. Leukemia 2025, 39, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Gabdoulline, R.; Liebich, A.; Klement, P.; Schiller, J.; Kandziora, C.; Hambach, L.; Stadler, M.; Koenecke, C.; Flintrop, M.; et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018, 132, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Heida, B.; Büttner, K.; Wienecke, C.P.; Teich, K.; Funke, C.; Brandes, M.; Klement, P.; Liebich, A.; Wichmann, M.; et al. Posttransplantation MRD monitoring in patients with AML by next-generation sequencing using DTA and non-DTA mutations. Blood Adv. 2021, 5, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, C.P.; Heida, B.; Venturini, L.; Gabdoulline, R.; Krüger, K.; Teich, K.; Büttner, K.; Wichmann, M.; Puppe, W.; Neziri, B.; et al. Clonal relapse dynamics in acute myeloid leukemia following allogeneic hematopoietic cell transplantation. Blood 2024, 144, 296–307. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0 Published: November 27, 2017. U.S. Department of Health and Human Services. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronicapplications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 10 November 2024).
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network Acute Kidney Injury Network: Report of an Initiative to Improve Outcomes in Acute Kidney Injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Olshen, R.A.; Stone, C.J. Regression Tree. In Classification and Regression Trees, 1st ed.; Chapman & Hall/CRC: New York, NY, USA, 1984. [Google Scholar]
- Dinardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Ballo, O.; Eladly, F.; Büttner, S.; Stratmann, J.A.; Rudolf, S.; Brunnberg, U.; Kreisel, E.-M.; Steffen, B.; Wagner, S.; Finkelmeier, F.; et al. Acute kidney injury adversely affects the clinical course of acute myeloid leukemia patients undergoing induction chemotherapy. Ann. Hematol. 2021, 100, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Lahoti, A.; Kantarjian, H.; Salahudeen, A.K.; Ravandi, F.; Cortes, J.E.; Faderl, S.; O’BRien, S.; Wierda, W.; Mattiuzzi, G.N. Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer 2010, 116, 4063–4068. [Google Scholar] [CrossRef]
- Short, N.J.; Daver, N.; Dinardo, C.D.; Kadia, T.; Nasr, L.F.; Macaron, W.; Yilmaz, M.; Borthakur, G.; Montalban-Bravo, G.; Garcia-Manero, G.; et al. Azacitidine, Venetoclax, and Gilteritinib in Newly Diagnosed and Relapsed or Refractory FLT3-Mutated AML. J. Clin. Oncol. 2024, 42, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Kitchlu, A.; McArthur, E.; Amir, E.; Booth, C.M.; Sutradhar, R.; Majeed, H.; Nash, D.M.; Silver, S.A.; Garg, A.X.; Chan, C.T.; et al. Acute Kidney Injury in Patients Receiving Systemic Treatment for Cancer: A Population-Based Cohort Study. JNCI J. Natl. Cancer Inst. 2018, 111, 727–736. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Acute Kidney Injury: Prevention, Detection and Management (NG148). Available online: https://www.ncbi.nlm.nih.gov/books/NBK552160 (accessed on 6 September 2025).
- Khwaja, A. KDIGO Clinical Practice Guideline for Acute Kidney Injruy. Nephron Clin Pract. 2012, 120, c179-84. [Google Scholar] [CrossRef]
- Lai, T.-S.; Wang, C.-Y.; Pan, S.-C.; Huang, T.-M.; Lin, M.-C.; Lai, C.-F.; Wu, C.-H.; Wu, V.-C.; Chien, K.-L. Risk of developing severe sepsis after acute kidney injury: A population-based cohort study. Crit. Care 2013, 17, R231. [Google Scholar] [CrossRef]
- Griffin, B.R.; You, Z.; Holmen, J.; SooHoo, M.; Gist, K.M.; Colbert, J.F.; Chonchol, M.; Faubel, S.; Jovanovich, A.; Chan, M. Incident infection following acute kidney injury with recovery to baseline creatinine: A propensity score matched analysis. PLoS ONE 2019, 14, e0217935. [Google Scholar] [CrossRef]
- Grigoryev, D.N.; Liu, M.; Hassoun, H.T.; Cheadle, C.; Barnes, K.C.; Rabb, H. The Local and Systemic Inflammatory Transcriptome after Acute Kidney Injury. J. Am. Soc. Nephrol. 2008, 19, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Le, P.; Klenzak, J.; Freedman, S.; Mcmenamin, M.E.; Ikizler, T.A. Impaired monocyte cytokine production in critically ill patients with acute renal failure. Kidney Int. 2004, 66, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Abe, C.; Sung, S.-S.J.; Moscalu, S.; Jankowski, J.; Huang, L.; Ye, H.; Rosin, D.L.; Guyenet, P.G.; Okusa, M.D. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J. Clin. Investig. 2016, 126, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Lucas, D.; Battista, M.; Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008, 452, 442–447. [Google Scholar] [CrossRef]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Döhner, H.; Récher, C.; Fiedler, W.; Yamamoto, K.; Wang, J.; et al. Long-term Follow-up of VIALE-A: Venetoclax and Azacitidine in Chemotherapy-ineligible Untreated Acute Myeloid Leukemia. Am. J. Hematol. 2024, 99, 615–624. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Pratz, K.; Letai, A.; Jonas, B.A.; Wei, A.H.; Pullarkat, V.; Konopleva, M.; Thirman, M.J.; Arellano, M.; Becker, P.S.; et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: Long term follow-up from a phase 1b study. Am. J. Hematol. 2020, 96, 208–217. [Google Scholar] [CrossRef]

| Baseline Characteristics | All Patients (N = 130) |
|---|---|
| Age, years Median Range | 76 27–90 |
| Sex, n (%) Male Female | 87 (67) 43 (33) |
| ECOG performance status, n (%) ≤1 >1 Missing data | 50 (39) 37 (28) 43 (33) |
| ICC 2022 classification, n (%) AML with recurrent genetic abnormality AML with MRGM AML with MRCA AML with mutated TP53 AML not otherwise specified Missing data | 29 (22) 47 (37) 13 (10) 21 (16) 17 (13) 3 (2) |
| Type of AML, n (%) De novo Secondary or therapy related Missing data | 67 (52) 56 (43) 7 (5) |
| mPRS risk group, n (%) Higher benefit Intermediate benefit Lower benefit Missing data | 80 (62) 20 (15) 21 (16 9 (7) |
| ELN 2022 risk group, n (%) Favorable/Intermediate Adverse Missing data | 43 (33) 82 (63) 5 (4) |
| Complex karyotype, n (%) Yes No Missing data | 26 (20) 87 (67) 17 (13) |
| Prior CMML, n (%) Yes No Missing data | 9 (7) 105 (81) 16 (12) |
| Cardiovascular risk factors 1, n (%) Yes No Missing data | 96 (73) 32 (24) 2 (2) |
| Peripheral blood blasts (%) Median Range Missing data | 16 0–99 23 |
| Bone marrow blasts (%) Median Range Missing data | 55 10–99 31 |
| WBC count (×109/L) Median Range Missing data | 4.6 0.2–171 4 |
| Hemoglobin (g/dL) Median Range Missing data | 8.5 4.9–11.7 6 |
| Platelet count (×109/L) Median Range Missing data | 42 5–378 6 |
| Endpoint | Variables in the Model | Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR # | 95% CI | p | HR # | 95% CI | p | |||
| OS | AKI during VEN yes vs. no | 1.68 | 1.0;2.7 | 0.03 | 1.86 | 1.2;3.0 | 0.01 | |
| Complex karyotype yes vs. no | 2.23 | 1.3;3.8 | 0.003 | 1.44 | 0.7;2.9 | 0.29 | ||
| IDH2 mut vs. wt | 0.39 | 0.2;1.0 | 0.05 | 0.37 | 0.2;0.9 | 0.03 | ||
| TP53 mut vs. wt | 2.23 | 1.2;3.8 | 0.007 | 2.19 | 1.3;3.8 | 0.006 | ||
| EFS | AKI during VEN yes vs. no | 1.72 | 1.1;2.6 | 0.01 | 1.81 | 1.2;2.8 | 0.007 | |
| Complex karyotype yes vs. no | 1.51 | 1.0;1.5 | 0.08 | 1.20 | 0.7;2.2 | 0.56 | ||
| ECOG > 1 vs. ≤1 | 1.43 | 0.9;2.2 | 0.10 | 1.49 | 1.0;2.3 | 0.06 | ||
| TP53 mut vs. wt | 1.78 | 1.1;3.0 | 0.03 | 1.91 | 1.1;3.2 | 0.02 | ||
| RFS | ECOG > 1 vs. ≤1 | 1.77 | 0.9;3.7 | 0.13 | 1.78 | 0.9;3.7 | 0.12 | |
| KDIGO > 2 vs. ≤2 | 2.16 | 0.97;4.83 | 0.06 | 1.93 | 0.8;4.7 | 0.15 | ||
| TP53 mut vs. wt | 2.91 | 1.2;7.0 | 0.02 | 2.91 | 1.2;7.0 | 0.02 | ||
| OR ## | 95% CI | p | OR # | 95% CI | p | |||
| ORR | AKI during VEN yes vs. no | 0.53 | 0.25;1.15 | 0.11 | 0.57 | 0.26;1.26 | 0.16 | |
| KDIGO > 2 vs. ≤2 | 0.49 | 0.23;1.05 | 0.07 | 0.49 | 0.23;1.05 | 0.07 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, K.; Gabdoulline, R.; Wichmann, M.; Schmidt, B.M.W.; Götze, K.; Braitsch, K.; Schmalbrock, L.; Bullinger, L.; Westendorf, F.; Fiedler, W.; et al. Prognostic Role of Kidney Disease in Newly Diagnosed Acute Myeloid Leukemia Under Venetoclax-Based Low-Intensity Therapy. Cancers 2025, 17, 2993. https://doi.org/10.3390/cancers17182993
Krüger K, Gabdoulline R, Wichmann M, Schmidt BMW, Götze K, Braitsch K, Schmalbrock L, Bullinger L, Westendorf F, Fiedler W, et al. Prognostic Role of Kidney Disease in Newly Diagnosed Acute Myeloid Leukemia Under Venetoclax-Based Low-Intensity Therapy. Cancers. 2025; 17(18):2993. https://doi.org/10.3390/cancers17182993
Chicago/Turabian StyleKrüger, Katja, Razif Gabdoulline, Martin Wichmann, Bernhard M. W. Schmidt, Katharina Götze, Krischan Braitsch, Laura Schmalbrock, Lars Bullinger, Franziska Westendorf, Walter Fiedler, and et al. 2025. "Prognostic Role of Kidney Disease in Newly Diagnosed Acute Myeloid Leukemia Under Venetoclax-Based Low-Intensity Therapy" Cancers 17, no. 18: 2993. https://doi.org/10.3390/cancers17182993
APA StyleKrüger, K., Gabdoulline, R., Wichmann, M., Schmidt, B. M. W., Götze, K., Braitsch, K., Schmalbrock, L., Bullinger, L., Westendorf, F., Fiedler, W., Bergmann, A. K., Krauter, J., Kaun, S., Voß, A., Koller, E., Germing, U., Wille, K., Grießhammer, M., Braess, J., ... Shahswar, R. (2025). Prognostic Role of Kidney Disease in Newly Diagnosed Acute Myeloid Leukemia Under Venetoclax-Based Low-Intensity Therapy. Cancers, 17(18), 2993. https://doi.org/10.3390/cancers17182993







