Development and Validation of a Centrosome Amplification-Related Prognostic Model in Pancreatic Cancer: Multi-Omics Guided Risk Stratification and Tumor Microenvironment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Methods
2.2.1. Differential Gene Expression Analysis Between PAAD and Non-Tumor Tissues and Identification of Centrosome Amplification-Related Differential Genes
2.2.2. Construction and Validation of a Prognostic Signature Based on Centrosome Amplification-Related Genes
2.2.3. Identification of Differentially Expressed Genes Between High- and Low-Risk Groups and Functional Enrichment Analysis
2.2.4. Tumor Microenvironment and Immune Infiltration Analysis
2.2.5. Mutation Analysis
2.2.6. Drug Sensitivity Evaluation
2.2.7. Single-Cell RNA Sequencing Analysis
2.2.8. Cell–Cell Communication
2.2.9. Spatial Transcriptomics Analysis
2.2.10. Quantitative Real-Time PCR Analysis and HPA Immunohistochemistry Validation
2.3. Statistical Analysis
3. Results
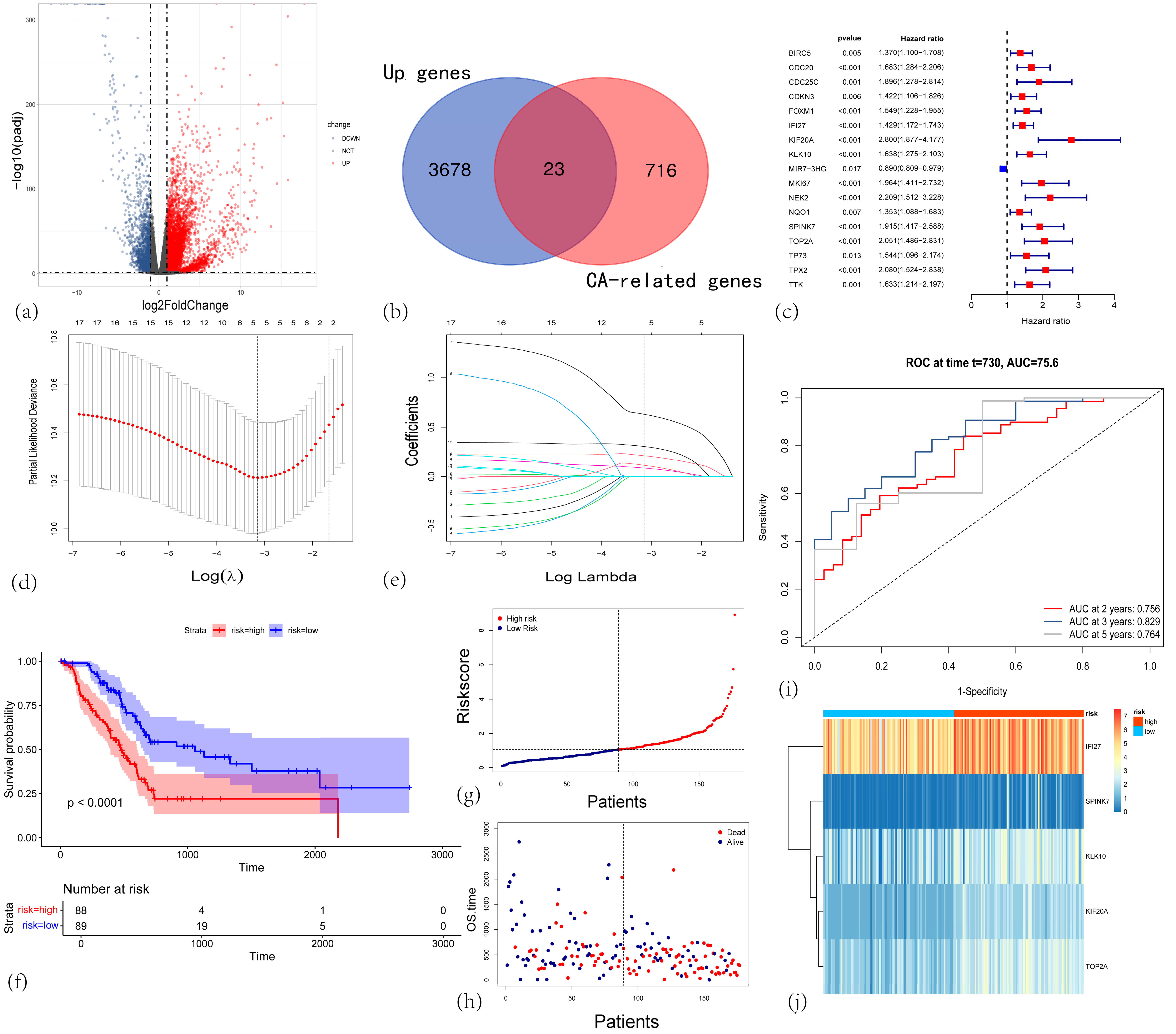
3.1. Identification of Differentially Expressed Genes in Pancreatic Cancer
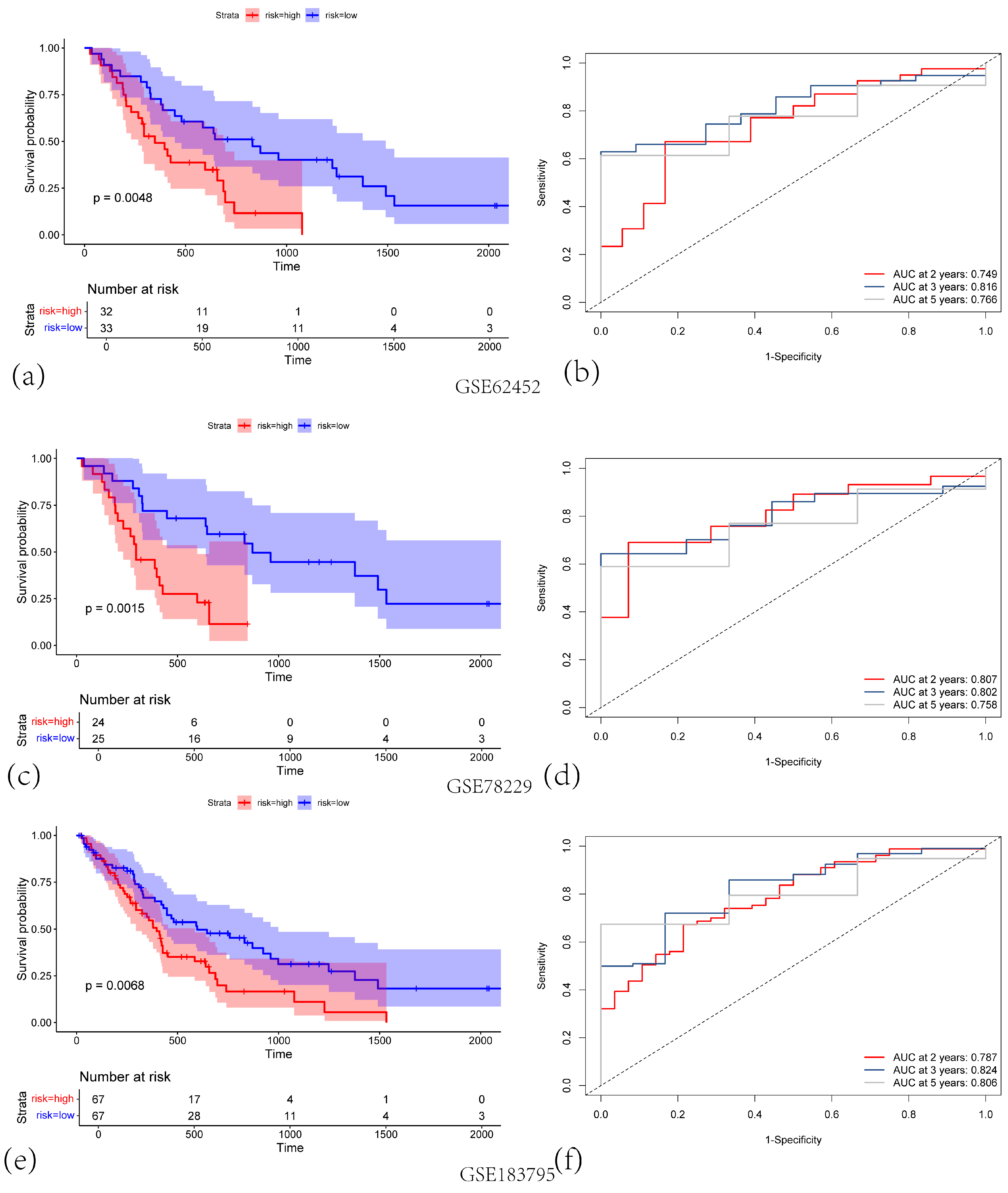
3.2. Construction and Validation of a Centrosome Amplification-Related Prognostic Gene Model in PAAD
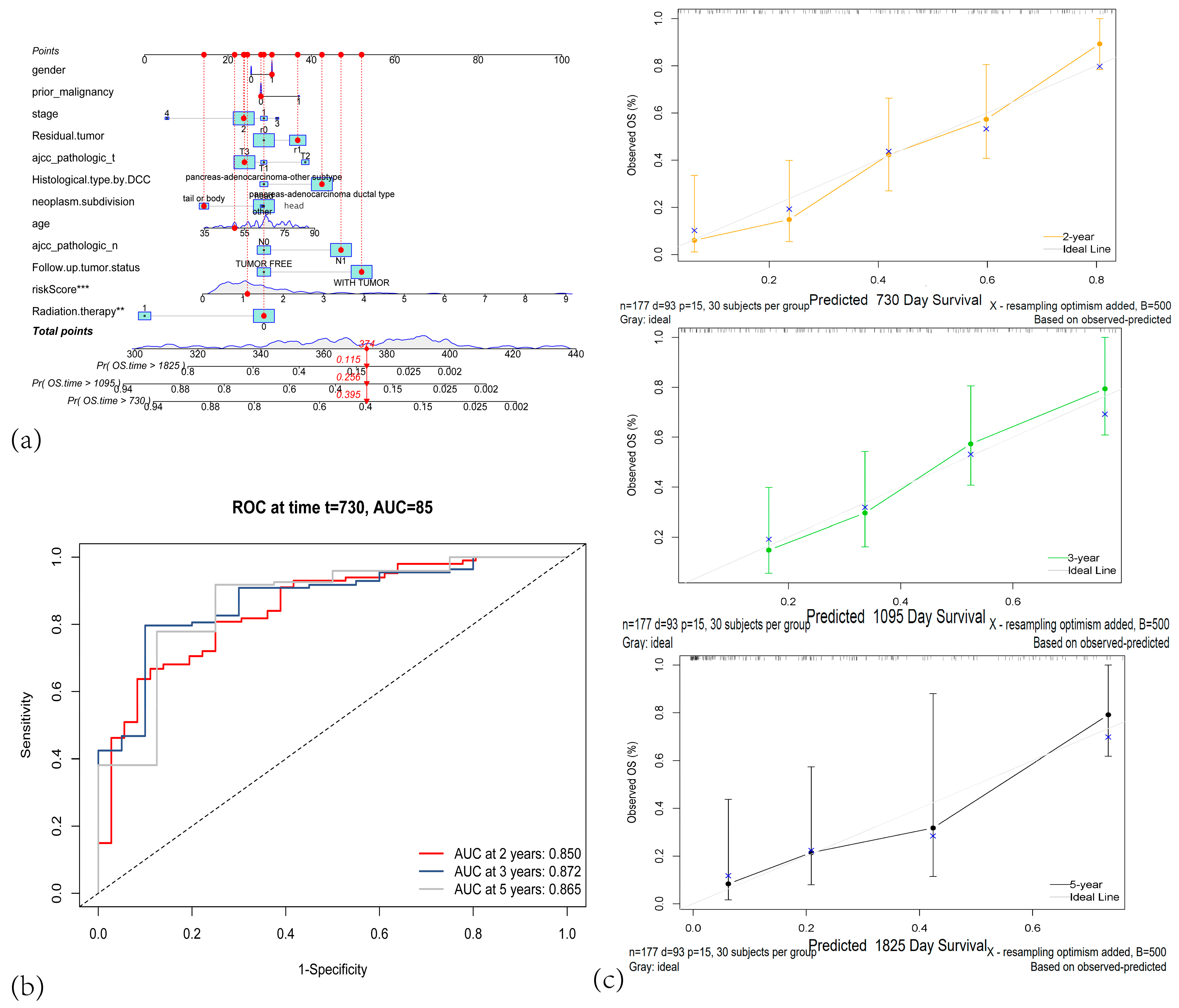
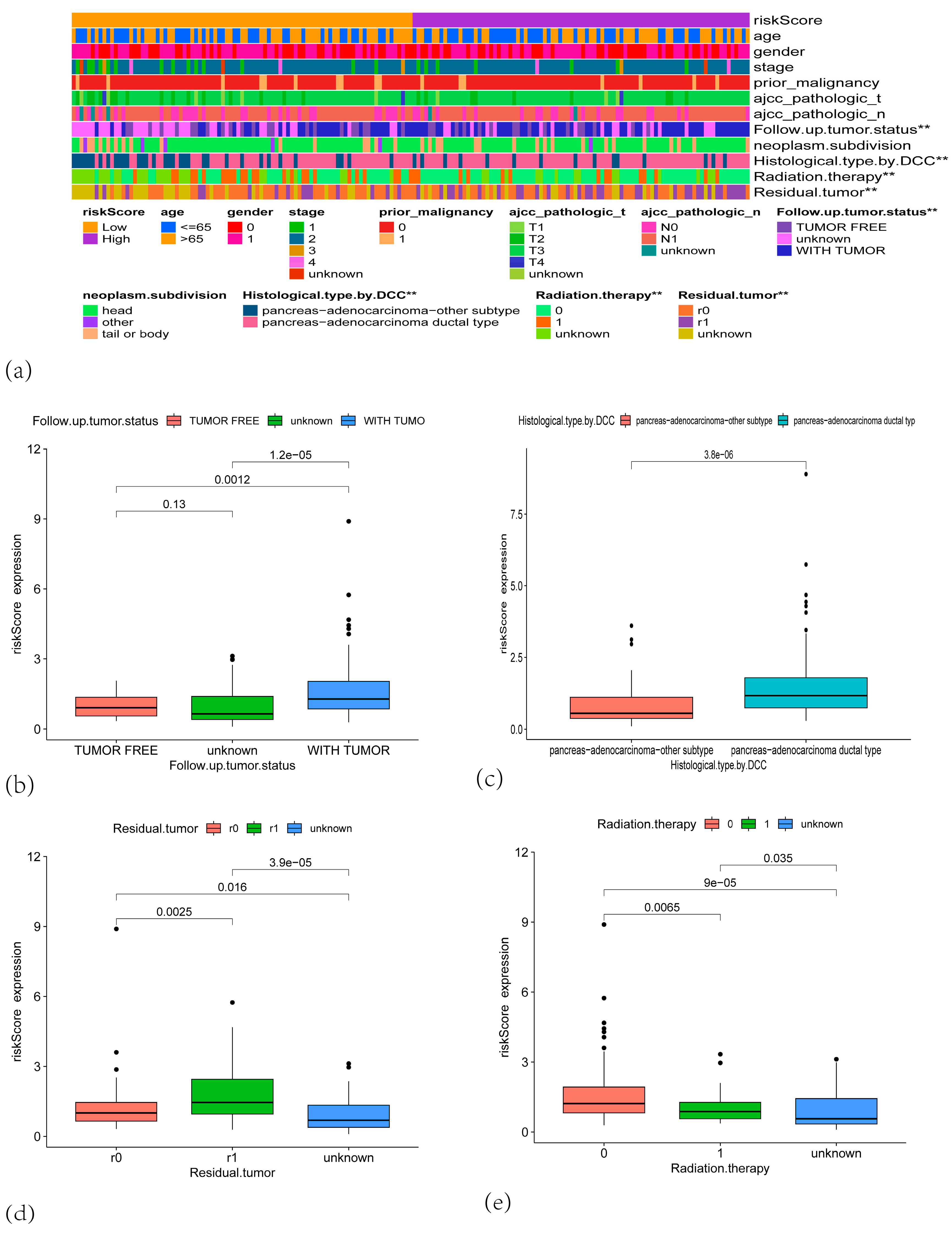
3.3. The Centrosome Amplification-Related Prognostic Signature Is an Independent Prognostic Factor in PAAD
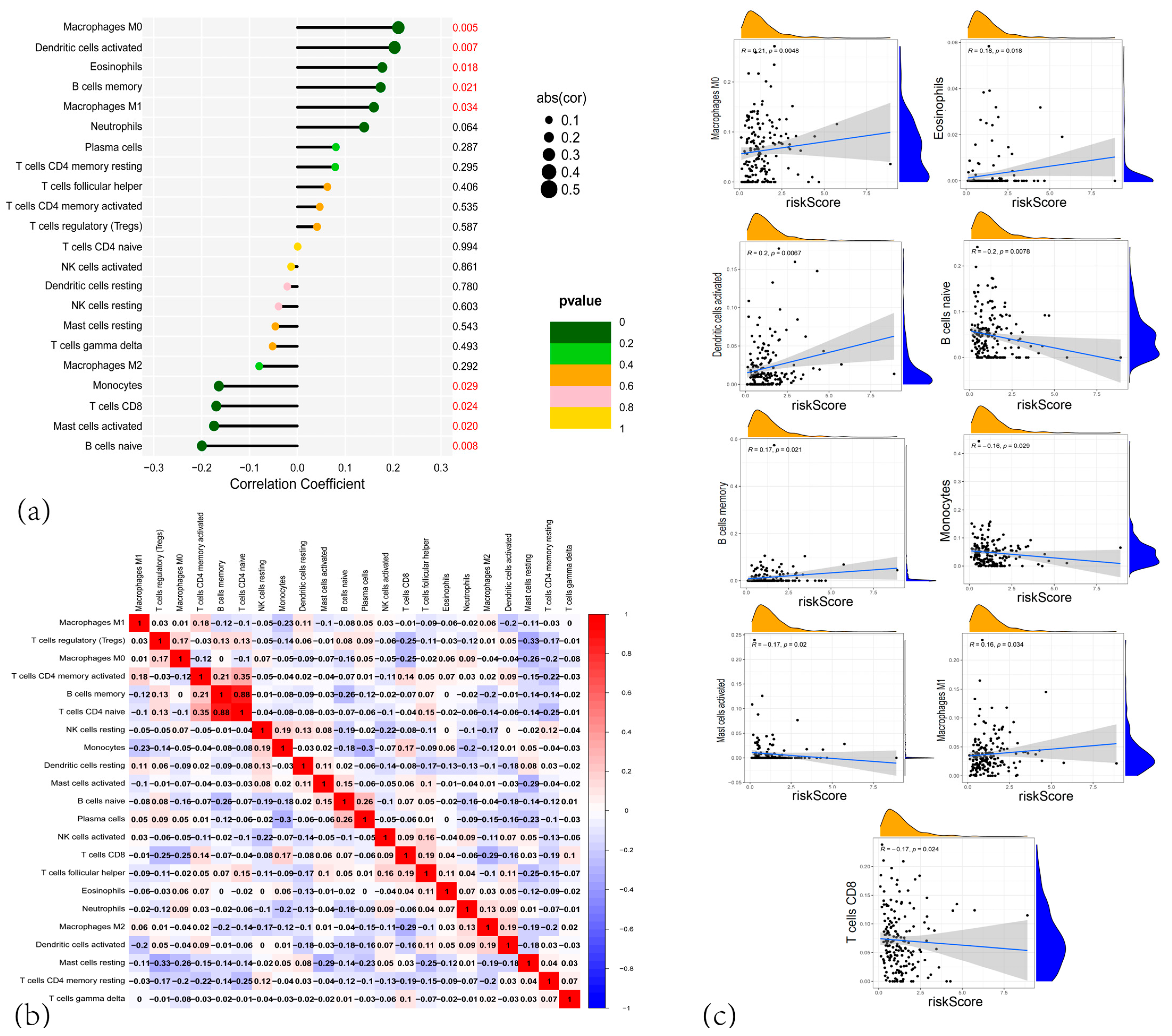
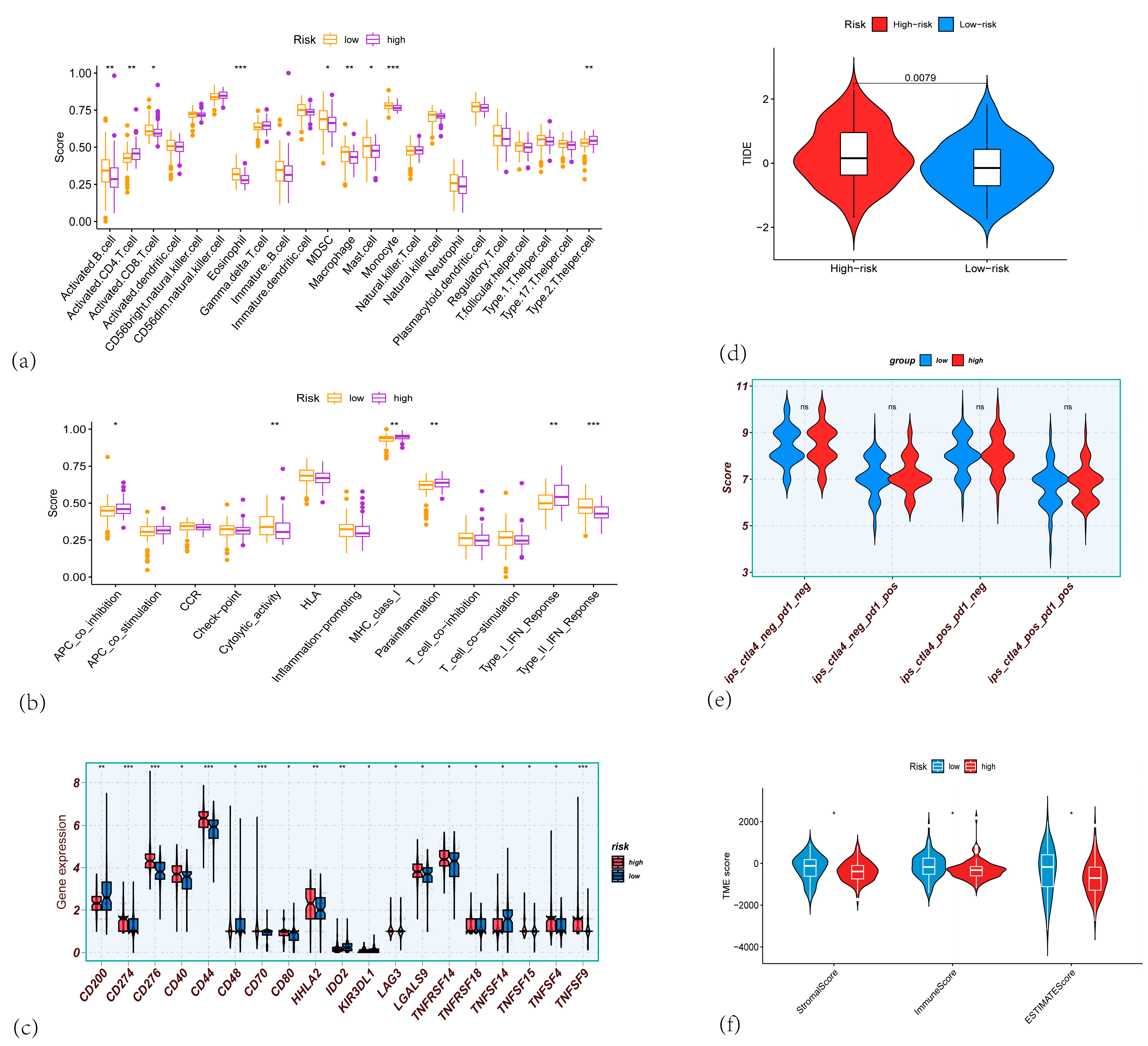
3.4. Correlation Analysis of the CARG Signature with Immune Infiltration and the Tumor Microenvironment
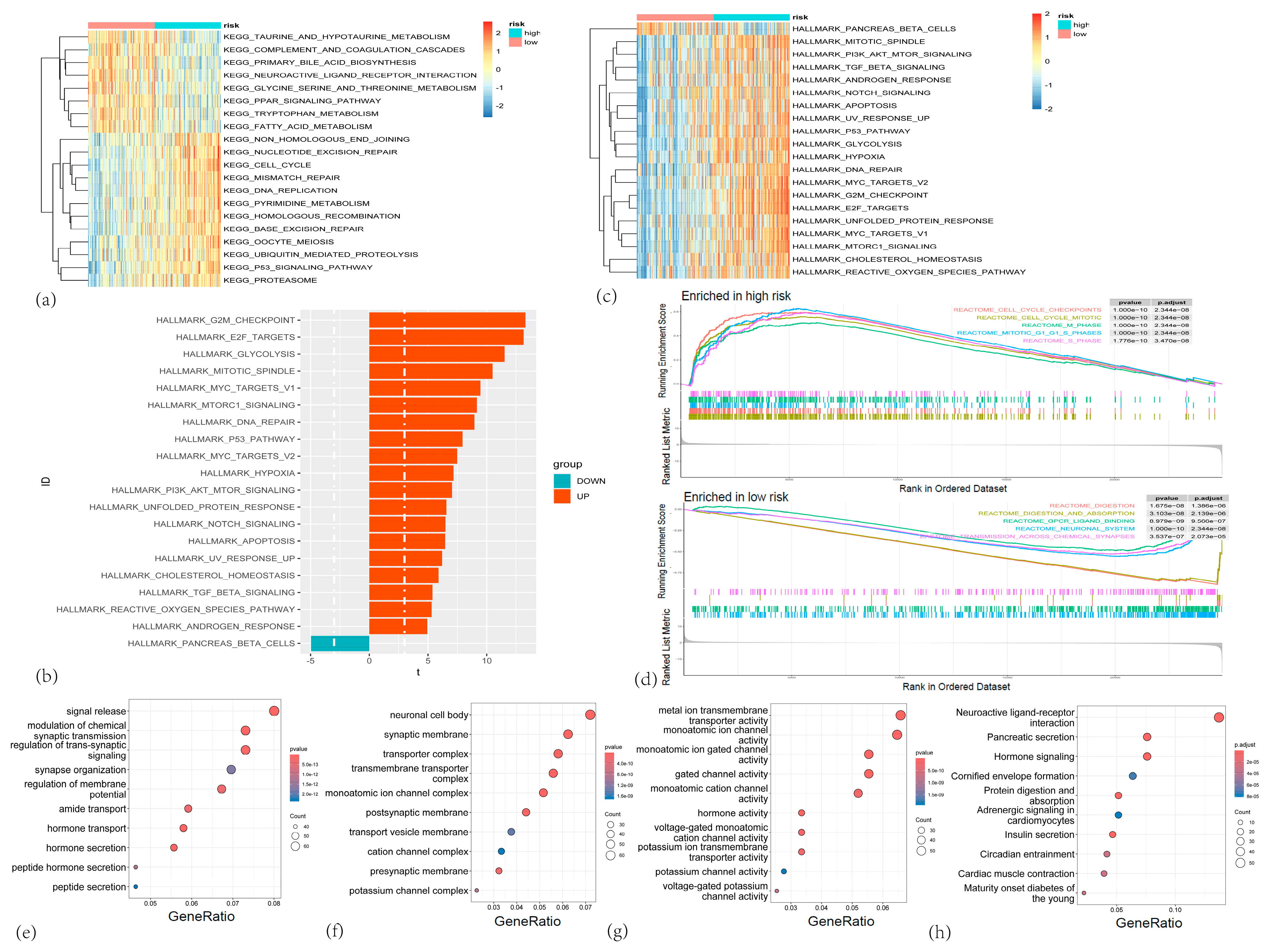
3.5. Functional Analysis of the CARG Gene Set
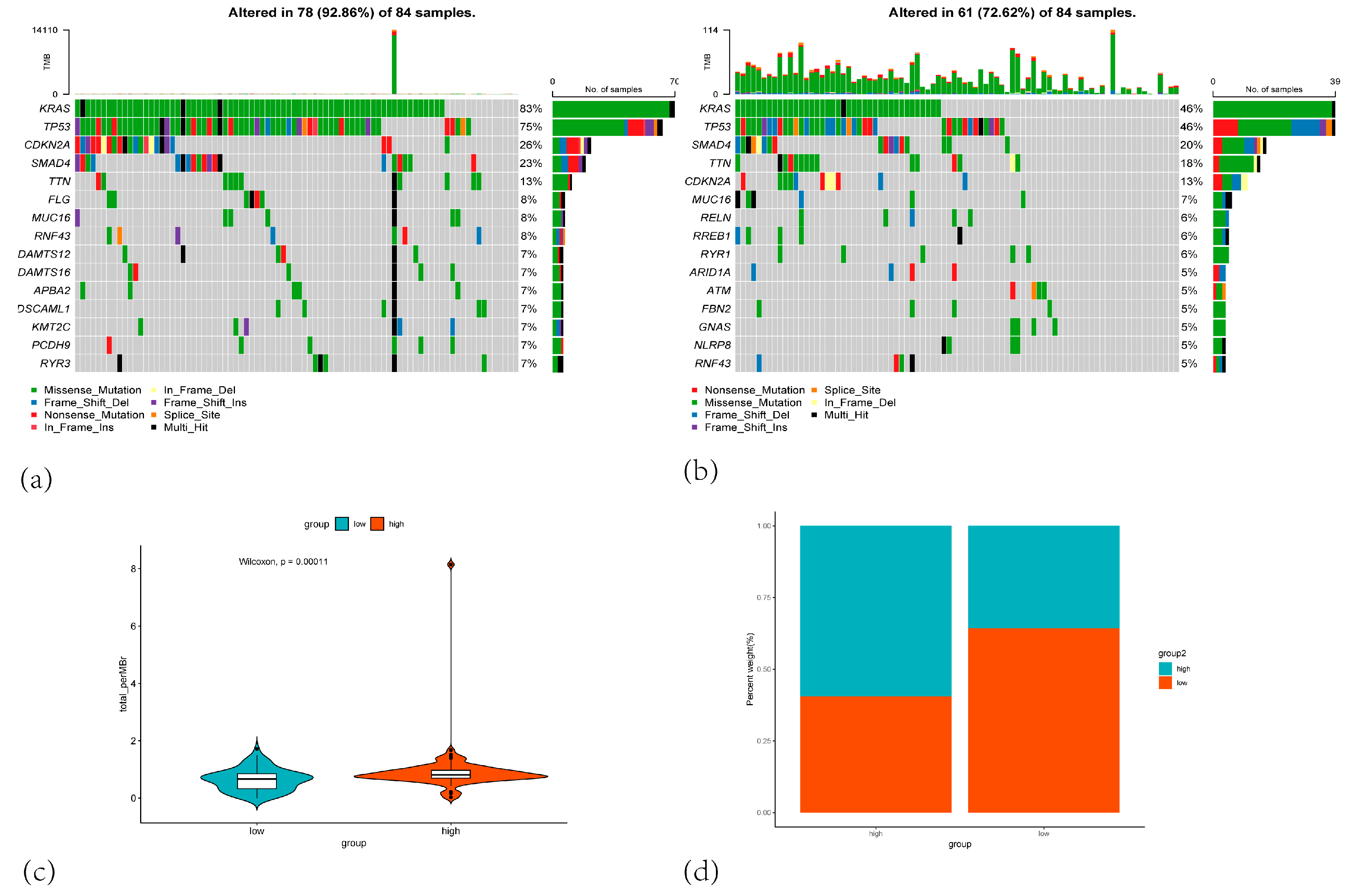
3.6. Mutation Analysis Result
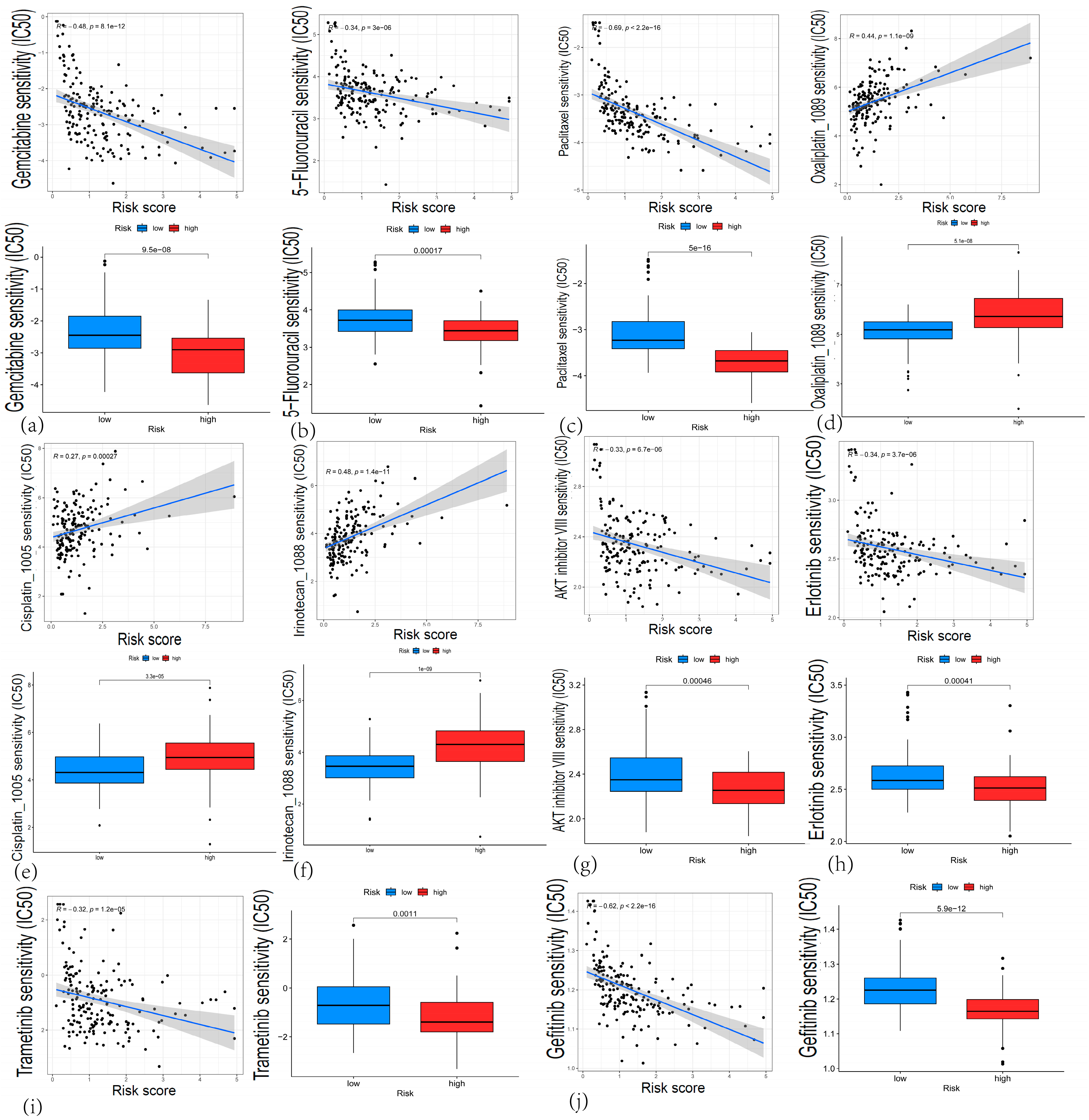
3.7. Chemotherapy Response Prediction and the CARG-Based Risk Score
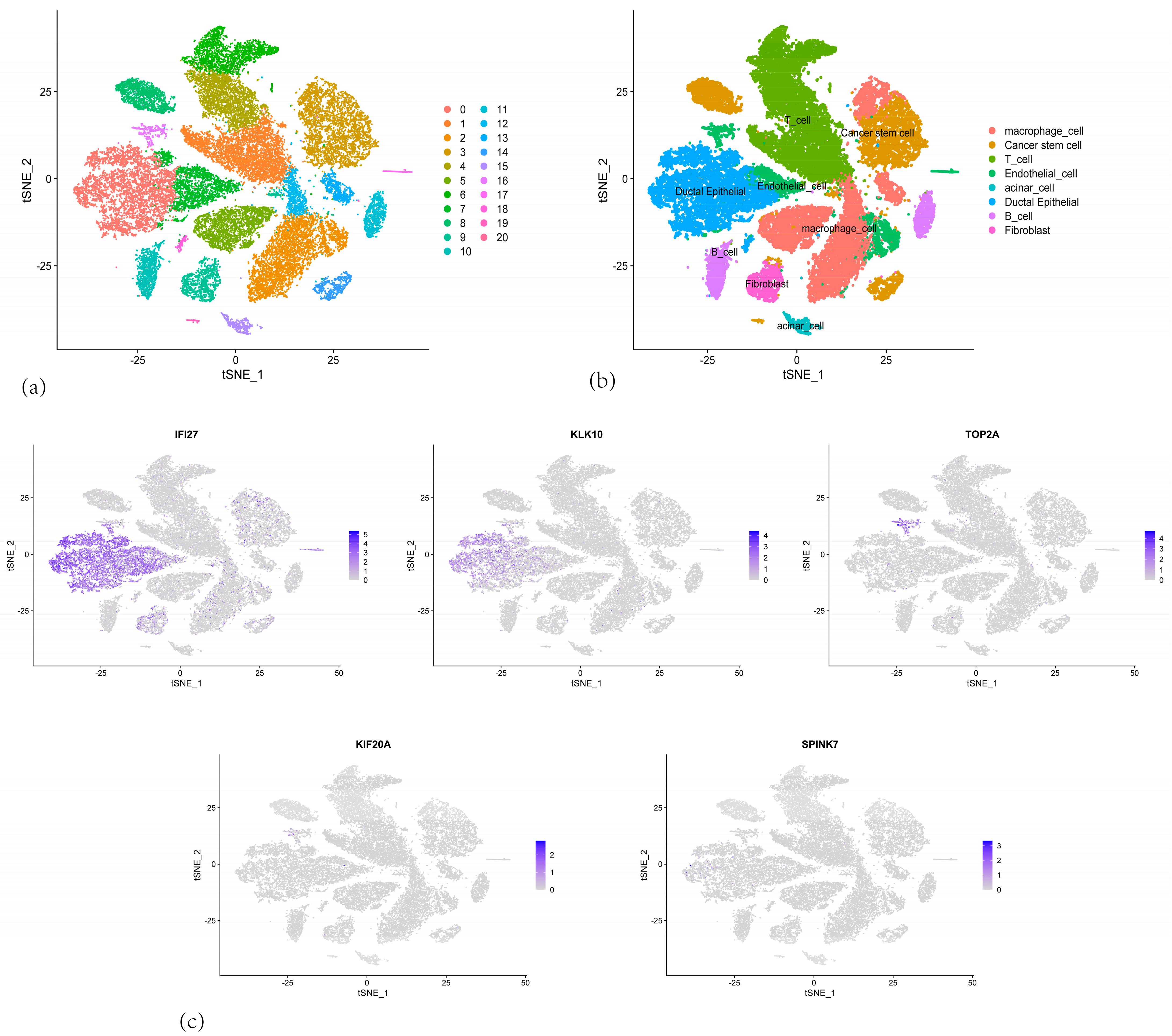
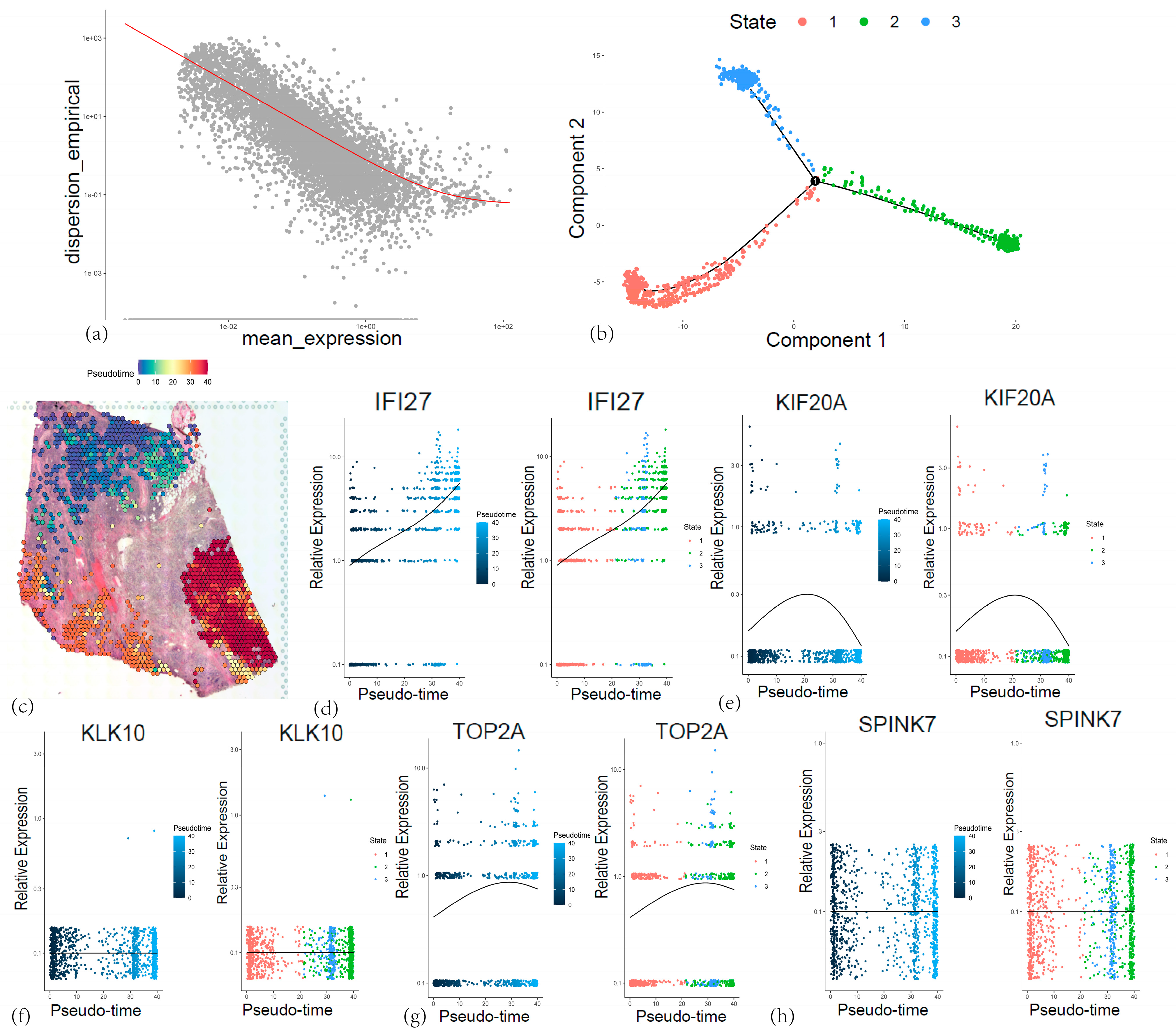
3.8. Validation of the Five-Gene Signature Using Single-Cell Sequencing
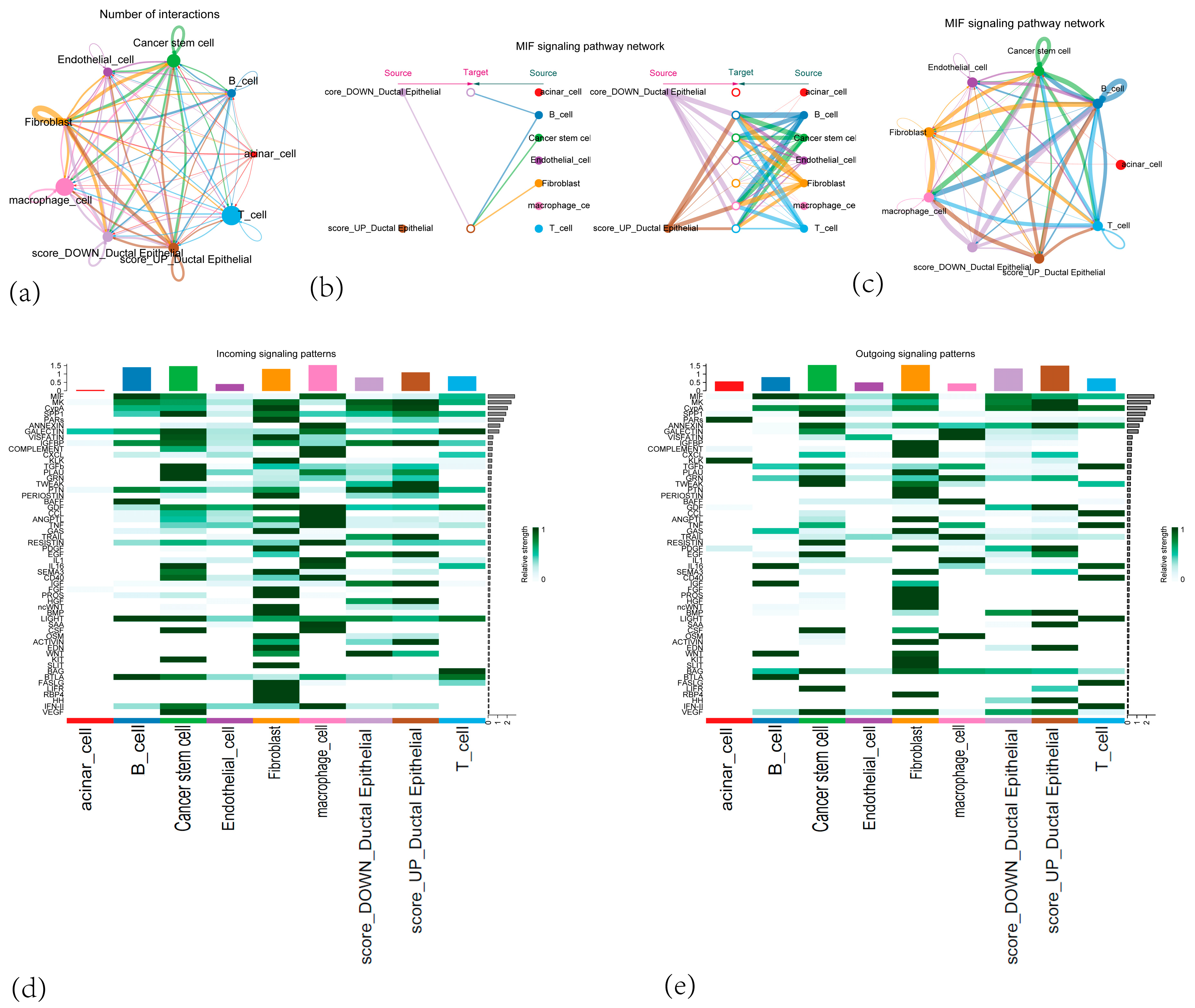
3.9. Cell–Cell Communication Result
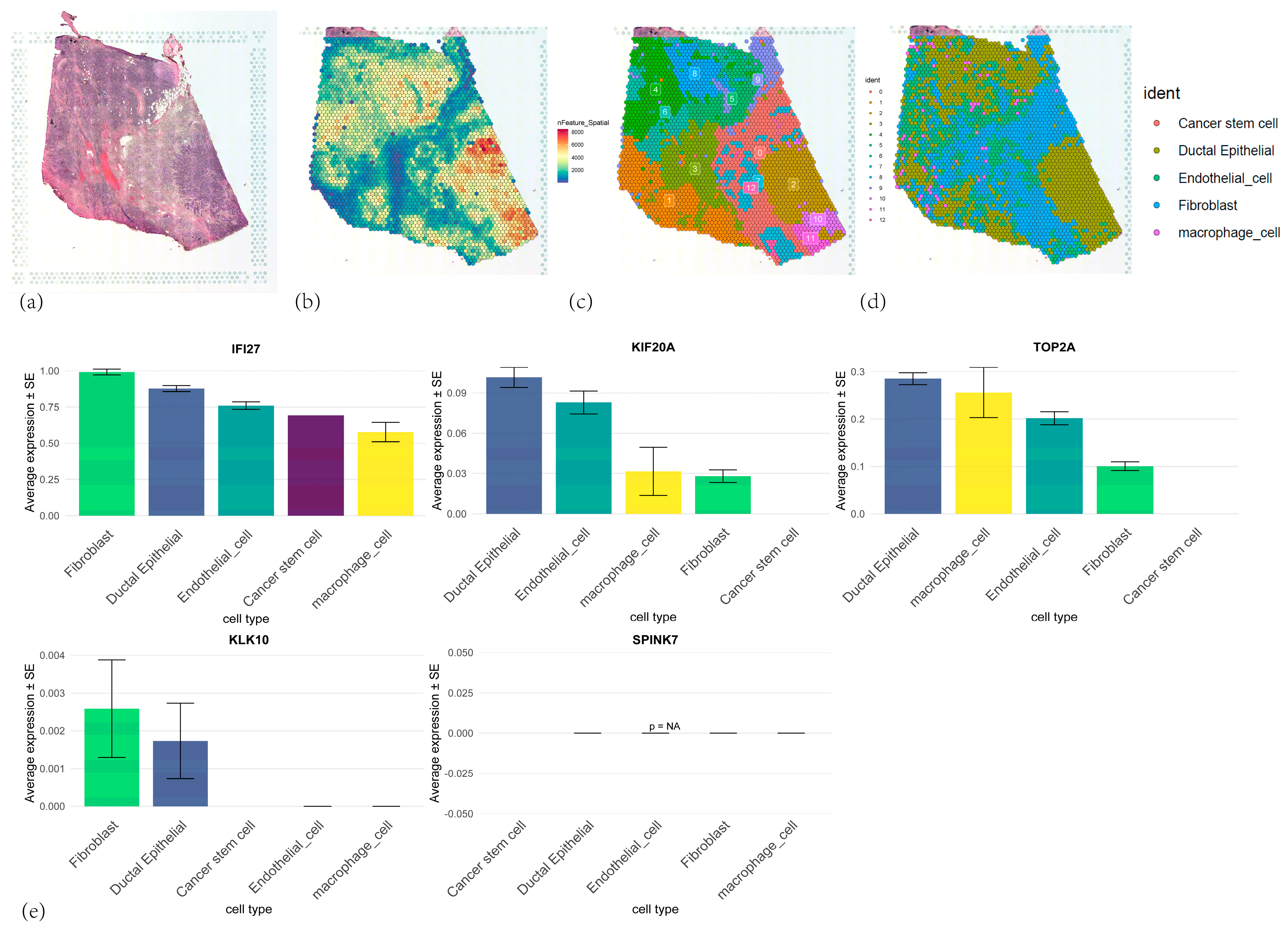
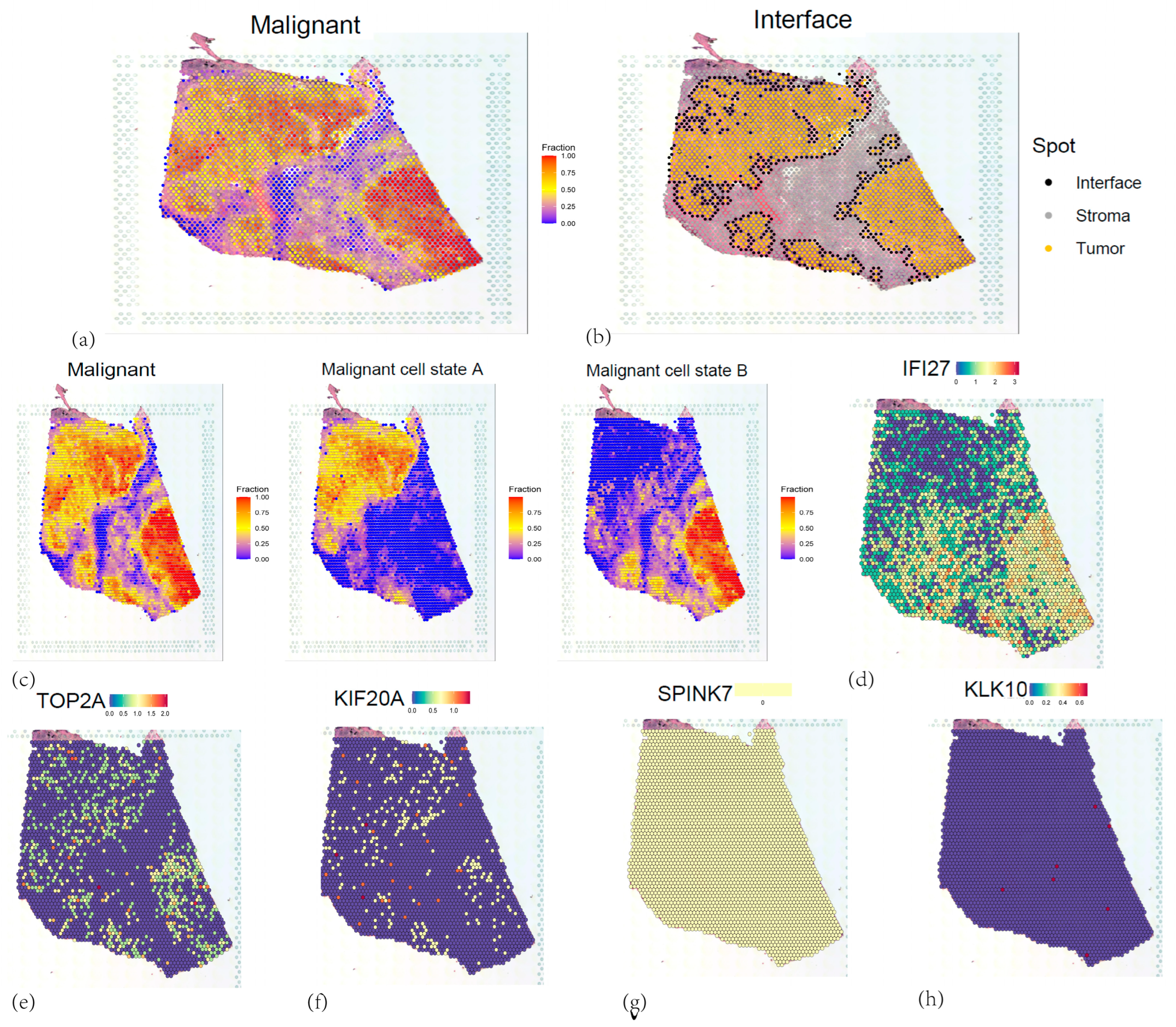
3.10. Spatial Transcriptomic Analyses of Five Core Genes
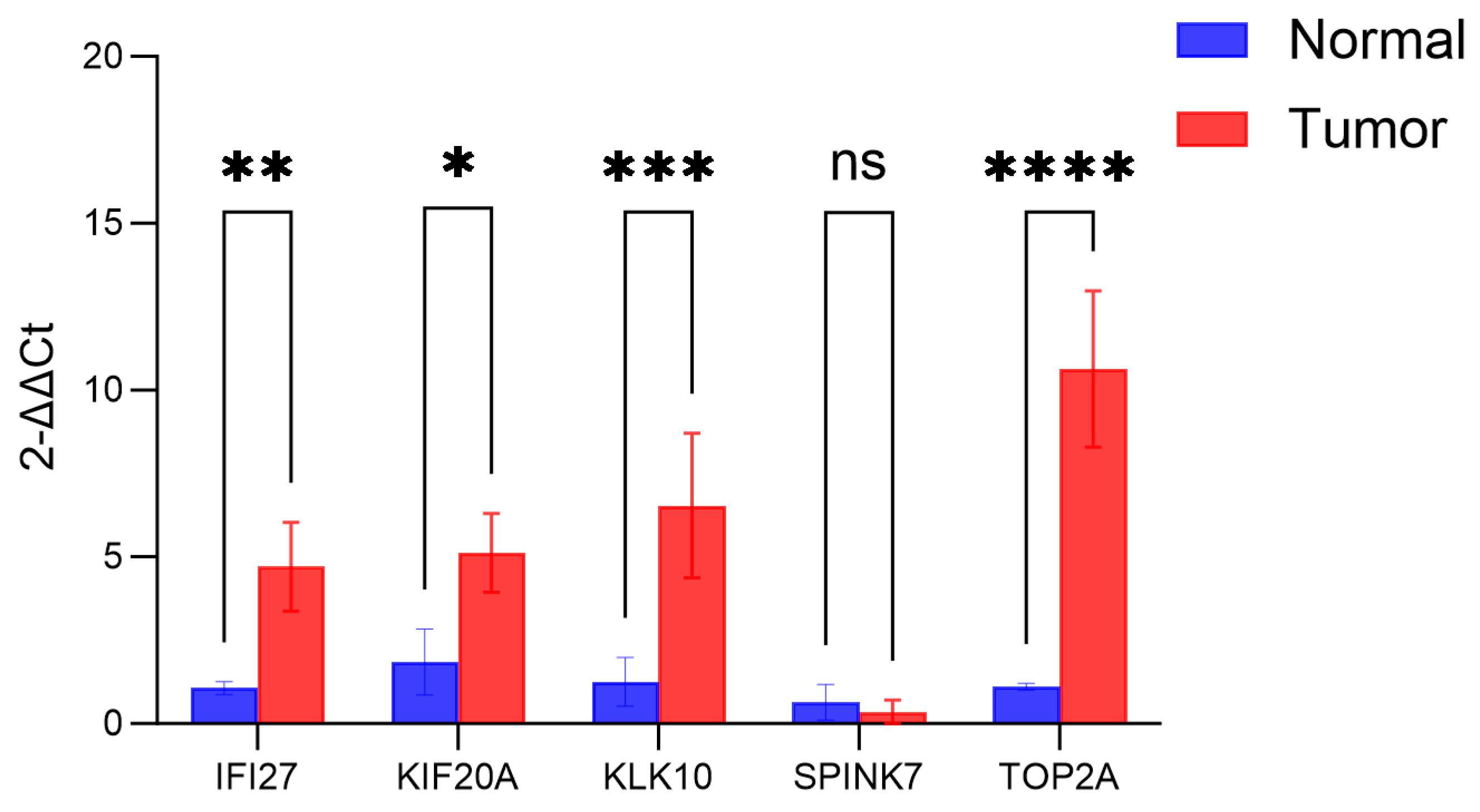
3.11. In PCR Validation of CARG-Related Prognostic Genes and HPA Immunohistochemistry in PAAD
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Padrón, L.J.; Maurer, D.M.; O’hAra, M.H.; O’rEilly, E.M.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: Clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat. Med. 2022, 28, 1167–1177. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Bai, Y.; Wang, Z.; Chen, Z.; Xu, R.; Xu, J.; Zhang, H.; Chen, J.; Yuan, Y.; et al. Nimotuzumab Plus Gemcitabine for K-Ras Wild-Type Locally Advanced or Metastatic Pancreatic Cancer. J. Clin. Oncol. 2023, 41, 5163–5173. [Google Scholar] [CrossRef]
- Arnandis, T.; Monteiro, P.; Adams, S.D.; Bridgeman, V.L.; Rajeeve, V.; Gadaleta, E.; Marzec, J.; Chelala, C.; Malanchi, I.; Cutillas, P.R.; et al. Oxidative Stress in Cells with Extra Centrosomes Drives Non-Cell-Autonomous Invasion. Dev. Cell 2018, 47, 409–424.e9. [Google Scholar] [CrossRef]
- Mittal, K.; Kaur, J.; Sharma, S.; Sharma, N.; Wei, G.; Choudhary, I.; Imhansi-Jacob, P.; Maganti, N.; Pawar, S.; Rida, P.; et al. Hypoxia Drives Centrosome Amplification in Cancer Cells via HIF1α-dependent Induction of Polo-Like Kinase 4. Mol. Cancer Res. 2022, 20, 596–606. [Google Scholar] [CrossRef]
- Nagao, S.; Onishi, H.; Kawamoto, M.; Masuda, S.; Na, L.; Morisaki, S.; Iwamoto, N.; Yamada, Y.; Koga, S.; Ichimiya, S.; et al. C4orf47 contributes to the dormancy of pancreatic cancer under hypoxic conditions. J. Cancer 2023, 14, 306–317. [Google Scholar] [CrossRef]
- Stoop, T.F.; Theijse, R.T.; Seelen, L.W.F.; Koerkamp, B.G.; van Eijck, C.H.J.; Wolfgang, C.L.; van Tienhoven, G.; van Santvoort, H.C.; Molenaar, I.Q.; Wilmink, J.W.; et al. Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 101–124. [Google Scholar] [CrossRef]
- Song, W.; Hu, H.; Yuan, Z.; Yao, H. A prognostic model for anoikis-related genes in pancreatic cancer. Sci. Rep. 2024, 14, 15200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; Lv, L.; Ma, P.; Zhang, Y.; Deng, J.; Zhang, Y. Identification of an Autophagy-Related Pair Signature for Predicting Prognoses and Immune Activity in Pancreatic Adenocarcinoma. Front. Immunol. 2021, 12, 743938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Hu, C.; Yang, Z.; Yang, M.; Fang, J.; Zhou, X. Bioinformatic Analysis of Prognostic and Immune-Related Genes in Pancreatic Cancer. Comput. Math. Methods Med. 2021, 2021, 5549298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, S.; Zhao, J.; Song, J.; Li, Y.; Zuo, R.; Sa, Y.; Ma, Z.; OuYang, H. Interferon alpha-inducible protein 27 (IFI27) is a prognostic marker for pancreatic cancer based on comprehensive bioinformatics analysis. Bioengineered 2021, 12, 8515–8528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qazi, S.; Trieu, V. TGFB2 mRNA Levels Prognostically Interact with Interferon-Alpha Receptor Activation of IRF9 and IFI27, and an Immune Checkpoint LGALS9 to Impact Overall Survival in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2024, 25, 11221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirokawa, N.; Noda, Y.; Okada, Y. Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr. Opin. Cell Biol. 1998, 10, 60–73. [Google Scholar] [CrossRef]
- Aniuchi, K.; Nakagawa, H.; Nakamura, T.; Eguchi, H.; Ohigashi, H.; Ishikawa, O.; Katagiri, T.; Nakamura, Y. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res. 2005, 65, 105–112. [Google Scholar] [CrossRef]
- Morita, H.; Matsuoka, A.; Kida, J.-I.; Tabata, H.; Tohyama, K.; Tohyama, Y. KIF20A, highly expressed in immature hematopoietic cells, supports the growth of HL60 cell line. Int. J. Hematol. 2018, 108, 607–614. [Google Scholar] [CrossRef]
- Rückert, F.; Hennig, M.; Petraki, C.D.; Wehrum, D.; Distler, M.; Denz, A.; Schröder, M.; Dawelbait, G.; Kalthoff, H.; Saeger, H.-D.; et al. Co-expression of KLK6 and KLK10 as prognostic factors for survival in pancreatic ductal adenocarcinoma. Br. J. Cancer 2008, 99, 1484–1492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, X.-Y.; Zhang, X.-X.; Yang, M.-W.; Hu, L.-P.; Jiang, S.-H.; Tian, G.-A.; Zhu, L.-L.; Li, Q.; Sun, Y.-W.; Zhang, Z.-G. Aberrant upregulation of KLK10 promotes metastasis via enhancement of EMT and FAK/SRC/ERK axis in PDAC. Biochem. Biophys. Res. Commun. 2018, 499, 584–593, Erratum in Biochem. Biophys. Res. Commun. 2024, 696, 149426. https://doi.org/10.1016/j.bbrc.2023.149426. [Google Scholar] [CrossRef] [PubMed]
- Yusenko, M.V.; Kovacs, G. Identifying CD82 (KAI1) as a Marker for Human Chromophobe Renal Cell Carcinoma. Histopathology 2009, 55, 687–695. [Google Scholar] [CrossRef]
- Telford, W.G. Multiparametric Analysis of Apoptosis by Flow Cytometry. Methods Mol. Biol. 2024, 2779, 217–257. [Google Scholar] [CrossRef]
- Liu, S.-L.; Cai, C.; Yang, Z.-Y.; Wu, Z.-Y.; Wu, X.-S.; Wang, X.-F.; Dong, P.; Gong, W. DGCR5 is activated by PAX5 and promotes pancreatic cancer via targeting miR-3163/TOP2A and activating Wnt/β-catenin pathway. Int. J. Biol. Sci. 2021, 17, 498–513. [Google Scholar] [CrossRef]
- Sharma, S.S.; Ma, L.; Bagui, T.K.; Forinash, K.D.; Pledger, W.J. A p27Kip1 mutant that does not inhibit CDK activity promotes centrosome amplification and micronucleation. Oncogene 2012, 31, 3989–3998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pandit, B.; Royzen, M. Recent Development of Prodrugs of Gemcitabine. Genes 2022, 13, 466. [Google Scholar] [CrossRef]
- Kang, L.; Tian, Y.; Xu, S.; Chen, H. Oxaliplatin-induced peripheral neuropathy: Clinical features, mechanisms, prevention and treatment. J. Neurol. 2021, 268, 3269–3282. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- LaCourse, K.D.; Zepeda-Rivera, M.; Kempchinsky, A.G.; Baryiames, A.; Minot, S.S.; Johnston, C.D.; Bullman, S. The cancer chemotherapeutic 5-fluorouracil is a potent Fusobacterium nucleatum inhibitor and its activity is modified by intratumoral microbiota. Cell Rep. 2022, 41, 111625. [Google Scholar] [CrossRef]
- Alagoz, M.; Gilbert, D.C.; El-Khamisy, S.; Chalmers, A.J. DNA repair and resistance to topoisomerase I inhibitors: Mechanisms, biomarkers and therapeutic targets. Curr. Med. Chem. 2012, 19, 3874–3885. [Google Scholar] [CrossRef]
- Wang, X.-S.; Bai, Y.-F.; Verma, V.; Yu, R.-L.; Tian, W.; Ao, R.; Deng, Y.; Zhu, X.-Q.; Liu, H.; Pan, H.-X.; et al. Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer. J. Natl. Cancer Inst. 2023, 115, 742–748, Erratum in J. Natl. Cancer Inst. 2023, 115, 773. https://doi.org/10.1093/jnci/djad084. [Google Scholar] [CrossRef]
- Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Shaikh, F.Y.; Harrison, M.K.; Adon, A.M.; Trimboli, A.J.; A Carroll, K.; Sharma, N.; Timmers, C.; A Chodosh, L.; Leone, G.; et al. The Ras oncogene signals centrosome amplification in mammary epithelial cells through cyclin D1/Cdk4 and Nek2. Oncogene 2010, 29, 5103–5112. [Google Scholar] [CrossRef]
- Lopes, C.A.; Mesquita, M.; Cunha, A.I.; Cardoso, J.; Carapeta, S.; Laranjeira, C.; Pinto, A.E.; Pereira-Leal, J.B.; Dias-Pereira, A.; Bettencourt-Dias, M.; et al. Centrosome amplification arises before neoplasia and increases upon p53 loss in tumorigenesis. J. Cell Biol. 2018, 217, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Raimondo, S.; Urzì, O.; Moschetti, M.; Di Bella, M.A.; Conigliaro, A.; Caccamo, N.; La Manna, M.P.; Fontana, S.; Alessandro, R. Tumor-Derived Small Extracellular Vesicles Induce Pro-Inflammatory Cytokine Expression and PD-L1 Regulation in M0 Macrophages via IL-6/STAT3 and TLR4 Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 12118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barriga, F.M.; Tsanov, K.M.; Ho, Y.-J.; Sohail, N.; Zhang, A.; Baslan, T.; Wuest, A.N.; Del Priore, I.; Meškauskaitė, B.; Livshits, G.; et al. MACHETE identifies interferon-encompassing chromosome 9p21.3 deletions as mediators of immune evasion and metastasis. Nat. Cancer 2022, 3, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 2005, 230, 6–19. [Google Scholar] [CrossRef]
- Tripathi, R.; Modur, V.; Senovilla, L.; Kroemer, G.; Komurov, K. Suppression of tumor antigen presentation during aneuploid tumor evolution contributes to immune evasion. Oncoimmunology 2019, 8, 1657374. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Beatty, G.L. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 123–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Lazarus, J.; Steele, N.G.; Yan, W.; Lee, H.-J.; Nwosu, Z.C.; Halbrook, C.J.; Menjivar, R.E.; Kemp, S.B.; Sirihorachai, V.R.; et al. Regulatory T-cell Depletion Alters the Tumor Microenvironment and Accelerates Pancreatic Carcinogenesis. Cancer Discov. 2020, 10, 422–439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halbrook, C.J.; Pontious, C.; Kovalenko, I.; Lapienyte, L.; Dreyer, S.; Lee, H.-J.; Thurston, G.; Zhang, Y.; Lazarus, J.; Sajjakulnukit, P.; et al. Macrophage-Released Pyrimidines Inhibit Gemcitabine Therapy in Pancreatic Cancer. Cell Metab. 2019, 29, 1390–1399.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Hu, T.; Li, Y.; Li, M. Development and Validation of a Centrosome Amplification-Related Prognostic Model in Pancreatic Cancer: Multi-Omics Guided Risk Stratification and Tumor Microenvironment. Cancers 2025, 17, 2983. https://doi.org/10.3390/cancers17182983
Sun Y, Hu T, Li Y, Li M. Development and Validation of a Centrosome Amplification-Related Prognostic Model in Pancreatic Cancer: Multi-Omics Guided Risk Stratification and Tumor Microenvironment. Cancers. 2025; 17(18):2983. https://doi.org/10.3390/cancers17182983
Chicago/Turabian StyleSun, Yuan, Tao Hu, Yan Li, and Ming Li. 2025. "Development and Validation of a Centrosome Amplification-Related Prognostic Model in Pancreatic Cancer: Multi-Omics Guided Risk Stratification and Tumor Microenvironment" Cancers 17, no. 18: 2983. https://doi.org/10.3390/cancers17182983
APA StyleSun, Y., Hu, T., Li, Y., & Li, M. (2025). Development and Validation of a Centrosome Amplification-Related Prognostic Model in Pancreatic Cancer: Multi-Omics Guided Risk Stratification and Tumor Microenvironment. Cancers, 17(18), 2983. https://doi.org/10.3390/cancers17182983





