18-Hour Planar Scintigraphy Versus SPECT/CT for Sentinel Lymph Node Detection in Early-Stage Endometrial Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Inclusion and Exclusion Criteria
2.3. Sentinel Lymph Node Mapping and Imaging
- Early planar scintigraphy—performed 30 min after injection (~113 MBq, acquisition time 600 s) using a Mediso, Budapest, Hungary, AnyScan SC gamma camera, field of view (FoV): 530 × 390 mm2, patient surface-to-detector distance: 3 cm.
- SPECT/CT—performed 1 h after injection (~107 MBq, acquisition time 600 s) using the Mediso AnyScan SPECT/CT system; the additional radiation dose from the CT component was estimated at 1–4 mSv [20], field of view (FoV): 530 × 390 mm2, patient surface-to-detector distance: 3 cm, attenuation correction: on.
- Delayed planar scintigraphy was performed 18 h after injection (~15 MBq, acquisition time 600 s), on the day of surgery, field of view (FoV): 530 × 390 mm2, patient surface-to-detector distance: 3cm. The median time from the 18-h planar acquisition to skin incision was 1.5 h (IQR: 1.0–2.0).
2.4. Histopathological Analysis
2.5. Quantitative Metrics and Statistical Analysis
2.6. SLN Evaluation Parameters
- Detection sensitivity: The percentage of patients with at least one SLN identified and confirmed intraoperatively and histologically. This metric was based on delayed (18-h) scintigraphy, which was used for surgical planning.
- Bilateral detection: The proportion of cases with at least one SLN identified on each side of the pelvis.
- Positive predictive value (PPV) and negative predictive value (NPV): Calculated based on intraoperative and histopathological verification of SLNs identified on 18-h scintigraphic images.
- Quantitative metrics: Mean values and standard deviations of the signal-to-noise ratio (SNR) and contrast factor (C-factor), analyzed independently for each imaging protocol.
2.7. Ethical Approval and Informed Consent
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Backes, F. The role of sentinel lymph nodes in endometrial and cervical cancer. J. Surg. Oncol. 2015, 112, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Frati, A.; Ballester, M.; Dubernard, G.; Bats, A.S.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; et al. Contribution of Lymphoscintigraphy for Sentinel Lymph Node Biopsy in Women with Early Stage Endometrial Cancer: Results of the SENTI-ENDO Study. Ann. Surg. Oncol. 2014, 22, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Mar, M.V.; Miller, S.A.; Kim, E.E.; Macapinlac, H.A. Evaluation and localization of lymphatic drainage and sentinel lymph nodes in patients with head and neck melanomas by hybrid SPECT/CT lymphoscintigraphic imaging. J. Nucl. Med. Technol. 2007, 35, 10–16; quiz 17–20. [Google Scholar]
- Ballinger, J.R. Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals. Molecules 2022, 27, 8596. [Google Scholar] [CrossRef]
- Szatkowski, W.; Słonina, D.; Ryś, J.; Blecharz, P.; Banaś, T.; Nowak-Jastrząb, M. The role of technetium-99m isotope in sentinel lymph node identification in gynecological cancers. Rep. Pract. Oncol. Radiother. 2025, 30, 257–268. [Google Scholar] [CrossRef]
- Bats, A.-S.; Lavoué, V.; Rouzier, R.; Coutant, C.; Kerrou, K.; Daraï, E. Limits of Day-Before Lymphoscintigraphy to Localize Sentinel Nodes in Women with Cervical Cancer. Ann. Surg. Oncol. 2008, 15, 2173–2179. [Google Scholar] [CrossRef]
- Papadia, A.; Zapardiel, I.; Bussi, B.; Ghezzi, F.; Ceccaroni, M.; De Ponti, E.; Elisei, F.; Imboden, S.; de la Noval, B.D.; Gasparri, M.L.; et al. Sentinel lymph node mapping in patients with stage I endometrial carcinoma: A focus on bilateral mapping identification by comparing radiotracer Tc99m with blue dye versus indocyanine green fluorescent dye. J. Cancer Res. Clin. Oncol. 2016, 143, 475–480. [Google Scholar] [CrossRef]
- Szatkowski, W.; Pniewska, K.; Janeczek, M.; Ryś, J.; Banaś, T.; Muzykiewicz, K.; Iwańska, E.; Jakubowicz, J.; Karolewski, K.; Szadurska, A.; et al. The Assessment of Sentinel Lymph Node Mapping Methods in Endometrial Cancer. J. Clin. Med. 2025, 14, 676. [Google Scholar] [CrossRef]
- Tew, K.; Farlow, D. SPECT/CT in Melanoma Lymphoscintigraphy. Clin. Nucl. Med. 2016, 41, 961–963. [Google Scholar] [CrossRef]
- Even-Sapir, E.; Lerman, H.; Lievshitz, G.; Khafif, A.; Fliss, D.M.; Schwartz, A.; Gur, E.; Skornick, Y.; Schneebaum, S. Lymphoscintigraphy for sentinel node mapping using a hybrid SPECT/CT system. J. Nucl. Med. 2003, 44, 1413–1420. [Google Scholar]
- Togami, S.; Kawamura, T.; Yanazume, S.; Kamio, M.; Kobayashi, H. Comparison of lymphoscintigraphy and single photon emission computed tomography with computed tomography (SPECT/CT) for sentinel lymph node detection in endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.-S.; Angeles, M.A.; Migliorelli, F.; Illac, C.; Martínez-Gómez, C.; Leray, H.; Betrian, S.; Chantalat, E.; Le Gac, Y.T.; Motton, S.; et al. Comparison of SPECT-CT with intraoperative mapping in cervical and uterine malignancies. Int. J. Gynecol. Cancer 2021, 31, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Buda, A.; Elisei, F.; Arosio, M.; Dolci, C.; Signorelli, M.; Perego, P.; Giuliani, D.; Recalcati, D.; Cattoretti, G.; Milani, R.; et al. Integration of hybrid single-photon emission computed tomography/computed tomography in the preoperative assessment of sentinel node in patients with cervical and endometrial cancer: Our experience and literature review. Int. J. Gynecol. Cancer 2012, 22, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Zerdoud, S.; Mery, E.; Bouissou, E.; Ferron, G.; Querleu, D. Hybrid imaging by SPECT/CT for sentinel lymph node detection in patients with cancer of the uterine cervix. Gynecol. Oncol. 2010, 119, 431–435. [Google Scholar] [CrossRef]
- Belhocine, T.Z.; Prefontaine, M.; Lanvin, D.; Bertrand, M.; Rachinsky, I.; Ettler, H.; Zabel, P.; Stitt, L.W.; Sugimoto, A.; Urbain, J.-L. Added-value of SPECT/CT to lymphatic mapping and sentinel lymphadenectomy in gynaecological cancers. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 182–193. [Google Scholar]
- Szatkowski, W.; Popieluch, J.; Blecharz, P.; Kisielewicz, K. Numerical Analysis of Sentinel Lymph Node Detection Using Technetium-99m: A Step Toward Objective Scintigraphy Evaluation in Oncology. Bio-Algorithms Med-Syst. 2025, 21, 7–12. [Google Scholar] [CrossRef]
- Ballester, M.; Rouzier, R.; Coutant, C.; Kerrou, K.; Daraï, E. Limits of lymphoscintigraphy for sentinel node biopsy in women with endometrial cancer. Gynecol. Oncol. 2009, 112, 348–352. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Female Genital Tumours. In WHO Classification of Tumours, 5th ed.; World Health Organization: Geneva, Switzerland, 2020; Volume 4. [Google Scholar]
- Buck, A.K.; Nekolla, S. SPECT/CT. J. Nucl. Med. 2008, 49, 1305–1319. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, Z.; Jiang, B.; Zhao, P.; Lin, C. Advances and Perspectives in Nanoprobes for Noninvasive Lymph Node Mapping. Nanomedicine 2015, 10, 1019–1036. [Google Scholar] [CrossRef]
- Ballester, M.; Dubernard, G.; Lécuru, F.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011, 12, 469–476. [Google Scholar] [CrossRef]
- Ogawa, S.; Kobayashi, H.; Amada, S.; Yahata, H.; Sonoda, K.; Abe, K.; Baba, S.; Sasaki, M.; Kaku, T.; Wake, N. Sentinel node detection with 99mTc phytate alone is satisfactory for cervical cancer patients undergoing radical hysterectomy and pelvic lymphadenectomy. Int. J. Clin. Oncol. 2010, 15, 52–58. [Google Scholar] [CrossRef]
- Frumovitz, M.; Coleman, R.L.; Gayed, I.W.; Ramirez, P.T.; Wolf, J.K.; Gershenson, D.M.; Levenback, C.F. Usefulness of preoperative lymphoscintigraphy in patients who undergo radical hysterectomy and pelvic lymphadenectomy for cervical cancer. Am. J. Obstet. Gynecol. 2006, 194, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; Gemignani, M.L.; Lyall, A.; Larson, S.M.; Barakat, R.R.; Abu Rustum, N.R. Single photon emission computed tomography SPECT-CT improves sentinel node detection and localization in cervical and uterine malignancy. Gynecol. Oncol. 2010, 117, 59–64. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Zheng, R.; Wu, L.-Y.; Li, X.-G.; Li, B.; Chen, S.-Z. [Clinical application of sentinel lymph node detection to early stage cervical cancer]. Ai Zheng 2006, 25, 224–228. [Google Scholar] [PubMed]
- Kraft, O.; Havel, M. Detection of Sentinel Lymph Nodes in Gynecologic Tumours by Planar Scintigraphy and SPECT/CT. Mol. Imaging Radionucl. Ther. 2012, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- van der Ploeg, I.M.; Valdés Olmos, R.A. The additional value of SPECT/CT in lymphatic mapping in breast cancer and melanoma. J. Nucl. Med. 2007, 48, 1756–1760. [Google Scholar] [CrossRef]
- Dampali, R.; Nikolettos, K.; Psilopatis, I.; Kostaki, E.-G.; Papadopoulos, A.J.; Attard-Montalto, S.; Devaja, O. The Impact of Body Mass Index on Sentinel Lymph Node Identification in Endometrial Cancer. Anticancer Res. 2025, 45, 1575–1581. [Google Scholar] [CrossRef]
- Law, M.; Ma, W.-H.; Leung, R.; Li, S.; Wong, K.-K.; Ho, W.-Y.; Kwong, A. Evaluation of patient effective dose from sentinel lymph node lymphoscintigraphy in breast cancer: A phantom study with SPECT/CT and ICRP-103 recommendations. Eur. J. Radiol. 2012, 81, e717–e720. [Google Scholar] [CrossRef]
- Le, T.D.; Shitiri, N.C.; Jung, S.-H.; Kwon, S.-Y.; Lee, C. Image Synthesis in Nuclear Medicine Imaging with Deep Learning: A Review. Sensors 2024, 24, 8068. [Google Scholar] [CrossRef]
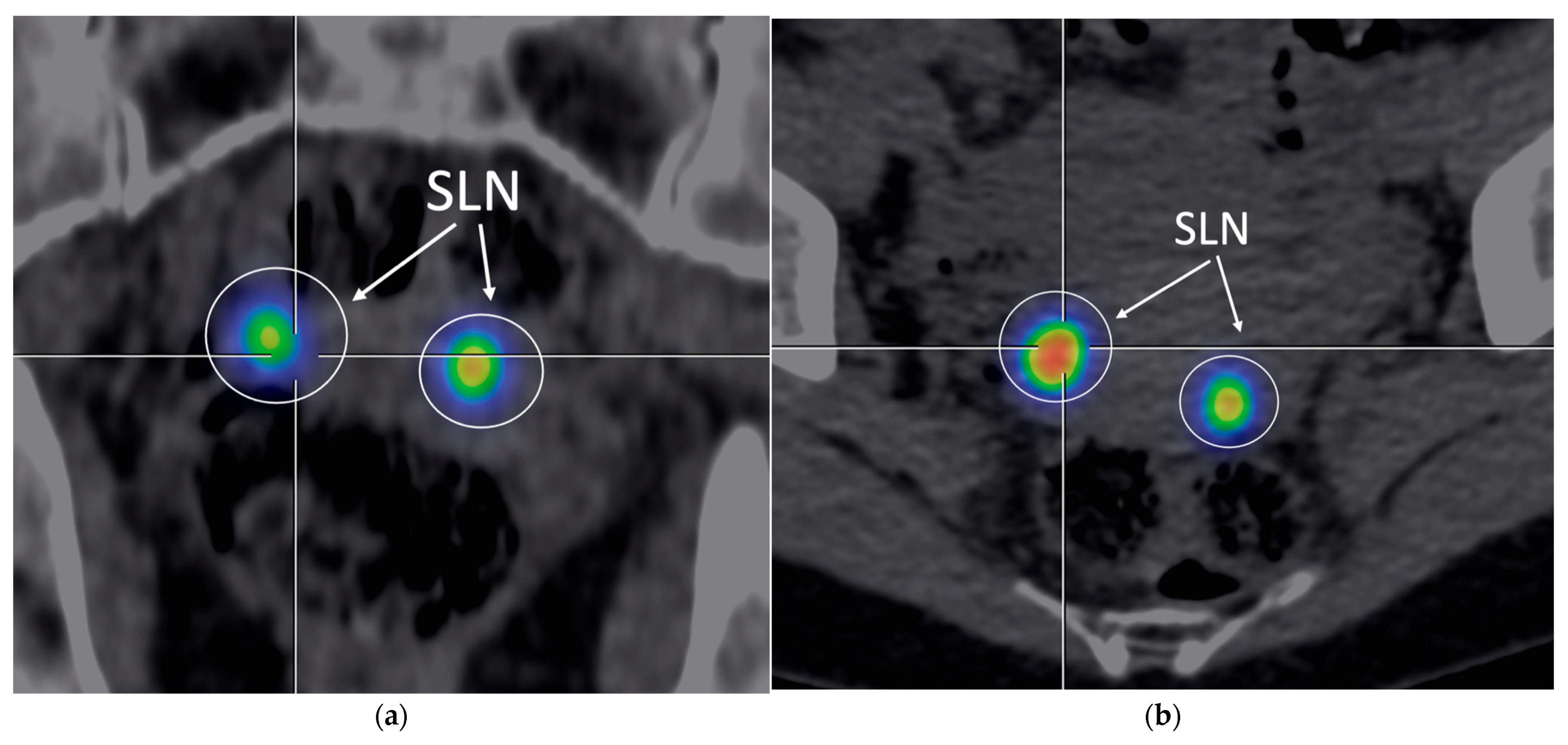

| Feature | Characteristic | n | % |
|---|---|---|---|
| Age (years) | Mean ± SD | 67.5 ± 9.7 | – |
| Histologic Type | Endometrioid | 109 | 87.20% |
| Serous | 10 | 8.00% | |
| Clear-Cell | 6 | 4.80% | |
| Lymphovascular Space Invasion (LVSI) | Present | 17 | 13.60% |
| Absent | 108 | 86.40% | |
| Myometrial Invasion | 0% | 19 | 15.20% |
| <50% | 74 | 59.20% | |
| >50% | 32 | 25.60% | |
| Lymphadenectomy | Performed | 22 | 17.60% |
| Not Performed | 103 | 82.40% | |
| FIGO Stage | IA | 29 | 23.20% |
| IB | 57 | 45.60% | |
| II | 16 | 12.80% | |
| IIIA | 4 | 3.20% | |
| IIIB | 2 | 1.60% | |
| IIIC1 | 13 | 10.40% | |
| IIIC2 | 4 | 3.20% | |
| FIGO Grade | G1 | 60 | 48.00% |
| G2 | 53 | 42.40% | |
| G3 | 12 | 9.60% | |
| BMI Category | <25 | 8 | 6.40% |
| 25–29.9 | 19 | 15.20% | |
| ≥30 | 93 | 74.40% | |
| Total | 125 | 100.00% |
| Parameter | 30-Minute Planar Scintigraphy | SPECT/CT | 18-Hour Planar Scintigraphy |
|---|---|---|---|
| Overall Detection Rate (%) | 72.00% (90/125) * | 87.20% (109/125) * | 94.40% (118/125) |
| Bilateral Detection Rate (%) | 60.00% (75/125) * 95% CI: 51.1–68.4%) | 73.60% (92/125) * 95% CI: 65.1–80.7%) | 80.80% (101/125) 95% CI: 72.9–87.3%) |
| Intraoperative Confirmation (%) | Not verified * | Not verified * | 100.00% |
| Histopathological Confirmation (%) | Not verified * | Not verified * | 100.00% |
| PPV (%) | Not verified * | Not verified * | 100.00% (95% CI: 96.9–100.0) |
| NPV (%) | Not verified * | Not verified * | 100.00% (95% CI: 59.0–100.0) |
| SNR (mean ± SD) | 4.61 ± 1.10 | 4.22 ± 1.10 | 3.51 ± 1.20 |
| C-factor (mean ± SD) | 10.02 ± 2.00 (range: 3.46–21.26) | 10.20 ± 1.30 (range: 3.48–21.5) | 10.30 ± 1.22 (range: 3.61–21.27) |
| Sensitivity, BMI ≥ 30 (%) | 71.00% (66/93) * | 86.00% (80/93) * | 94.00% (87/93) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatkowski, W.; Pniewska, K.; Blecharz, P.; Nowak-Jastrząb, M.; Ryś, J.; Banaś, T.; Pacholczak-Madej, R.; Krzywonos, E.; Rawojć, K.; Kisielewicz, K. 18-Hour Planar Scintigraphy Versus SPECT/CT for Sentinel Lymph Node Detection in Early-Stage Endometrial Cancer. Cancers 2025, 17, 2976. https://doi.org/10.3390/cancers17182976
Szatkowski W, Pniewska K, Blecharz P, Nowak-Jastrząb M, Ryś J, Banaś T, Pacholczak-Madej R, Krzywonos E, Rawojć K, Kisielewicz K. 18-Hour Planar Scintigraphy Versus SPECT/CT for Sentinel Lymph Node Detection in Early-Stage Endometrial Cancer. Cancers. 2025; 17(18):2976. https://doi.org/10.3390/cancers17182976
Chicago/Turabian StyleSzatkowski, Wiktor, Karolina Pniewska, Paweł Blecharz, Małgorzata Nowak-Jastrząb, Janusz Ryś, Tomasz Banaś, Renata Pacholczak-Madej, Emilia Krzywonos, Kamila Rawojć, and Kamil Kisielewicz. 2025. "18-Hour Planar Scintigraphy Versus SPECT/CT for Sentinel Lymph Node Detection in Early-Stage Endometrial Cancer" Cancers 17, no. 18: 2976. https://doi.org/10.3390/cancers17182976
APA StyleSzatkowski, W., Pniewska, K., Blecharz, P., Nowak-Jastrząb, M., Ryś, J., Banaś, T., Pacholczak-Madej, R., Krzywonos, E., Rawojć, K., & Kisielewicz, K. (2025). 18-Hour Planar Scintigraphy Versus SPECT/CT for Sentinel Lymph Node Detection in Early-Stage Endometrial Cancer. Cancers, 17(18), 2976. https://doi.org/10.3390/cancers17182976






