First-in-Human Study of MDG1011, a TCR-T Therapy Directed Against HLA-A*02:01-Restricted PRAME Antigen for High-Risk Myeloid and Lymphoid Neoplasms
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. IMP Manufacture
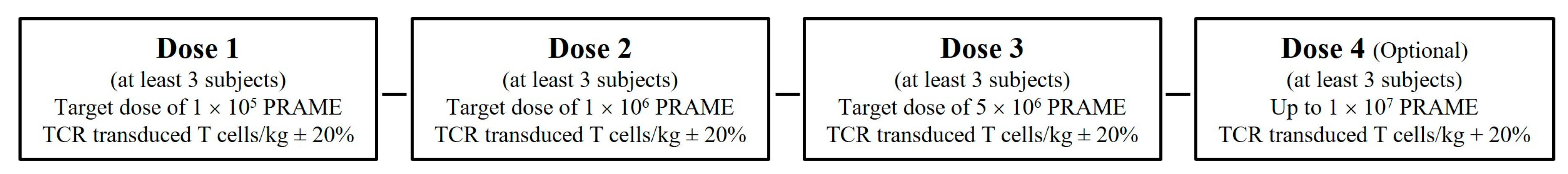
2.2. Clinical Study Design
2.3. Statistical Analysis
2.4. Immune Monitoring
3. Results
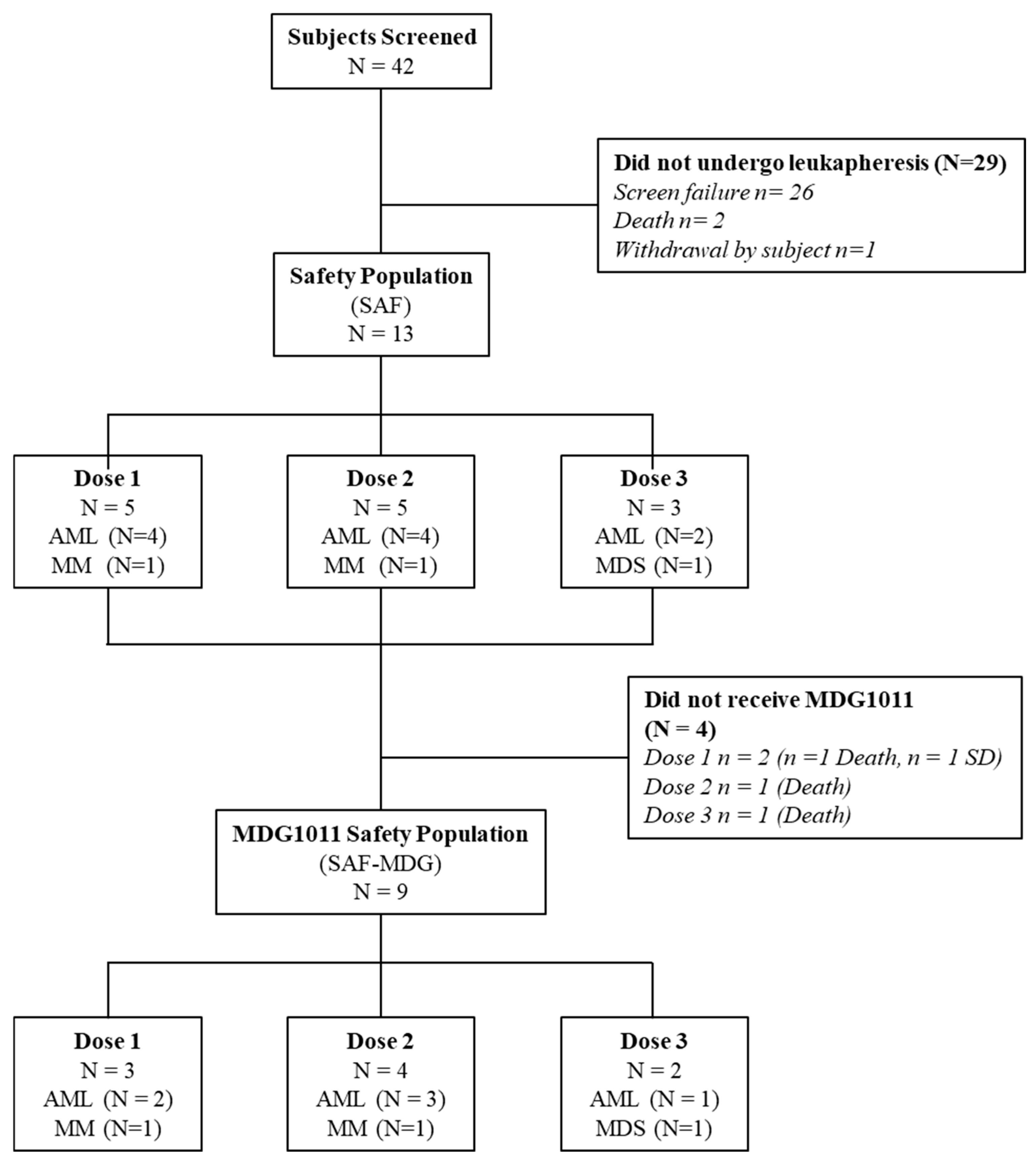
3.1. Patient Disposition
3.2. Baseline Characteristics and Demographics
3.3. IMP Manufacturing Feasibility, Treatment Feasibility, and IMP Exposure
3.4. Biological Activity
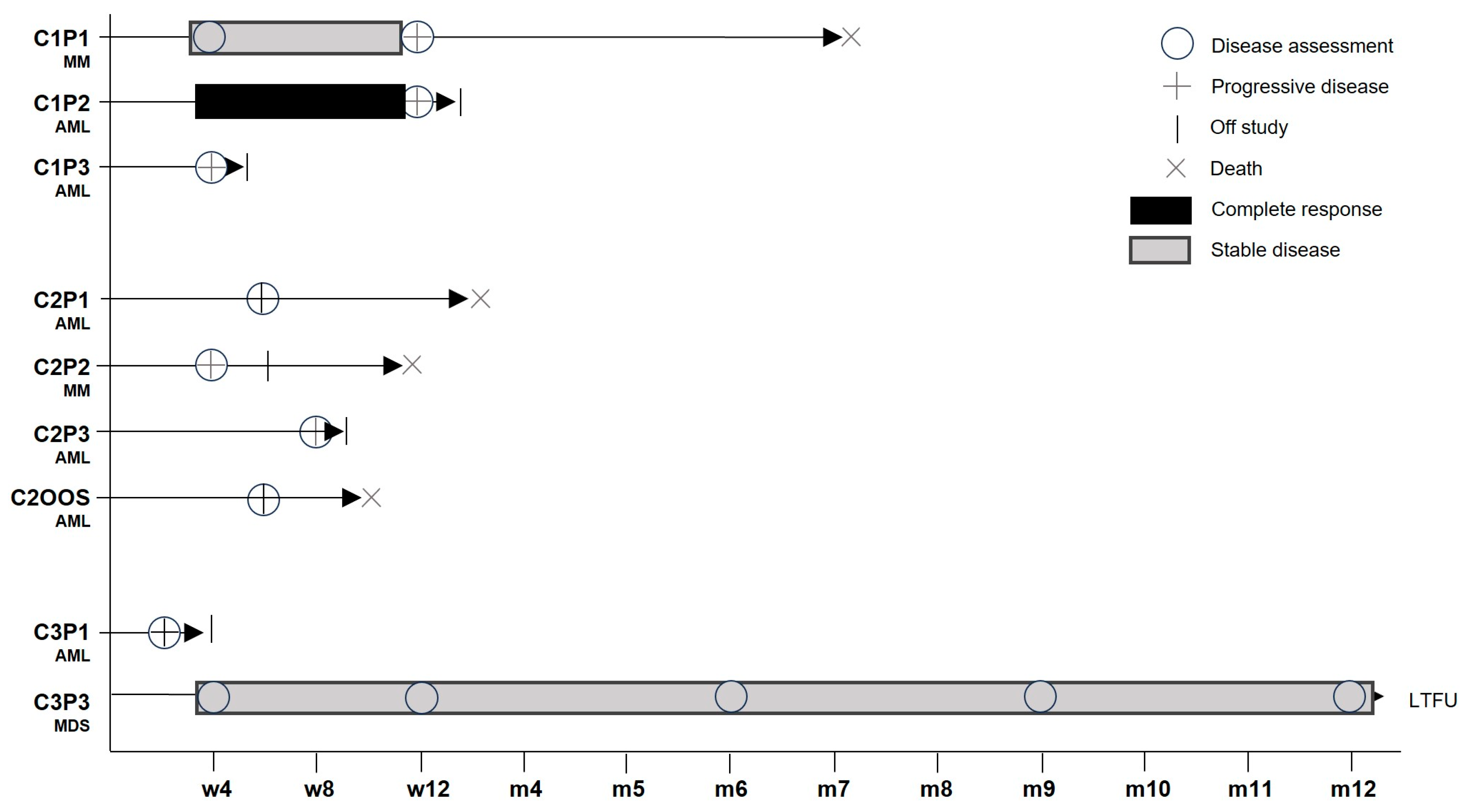
3.5. Clinical Activity
3.6. Safety
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilde, S.; Sommermeyer, D.; Frankenberger, B.; Schiemann, M.; Milosevic, S.; Spranger, S.; Pohla, H.; Uckert, W.; Busch, D.H.; Schendel, D.J. Dendritic cells pulsed with RNA encoding allogeneic MHC and antigen induce T cells with superior anti-tumor activity and higher TCR functional avidity. Blood 2009, 114, 2131–2139. [Google Scholar] [CrossRef]
- Sailer, N.; Fetzer, I.; Salvermoser, M.; Braun, M.; Brechtefeld, D.; Krendl, C.; Geiger, C.; Mutze, K.; Noessner, E.; Schendel, D.J.; et al. T-Cells Expressing a Highly Potent PRAME-Specific T-Cell Receptor in Combination with a Chimeric PD1-41BB Co-Stimulatory Receptor Show a Favorable Preclinical Safety Profile and Strong Anti-Tumor Reactivity. Cancers 2022, 14, 1998. [Google Scholar] [CrossRef]
- Bürdek, M.; Prinz, P.U.; Mutze, K.; Tippmer, S.; Geiger, C.; Longinotti, G.; Schendel, D.J. Characterization of a 3S PRAME VLD-Specific T Cell Receptor and Its Use in Investigational Medicinal Products for TCR-T Therapy of Patients with Myeloid Malignancies. Cancers 2025, 17, 242. [Google Scholar] [CrossRef]
- Guillaudeux, T.; Comez, E.; Onno, M.; Drénou, B.; Segretain, D.; Alberti, S.; Lejeune, H.; Fauchet, R.; Jégou, B.; Le Bouteiller, P. Expression of HLA Class I genes in meiotic and post-meiotic human spermatogenic cells. Biol. Reprod. 1996, 55, 99–110. [Google Scholar] [CrossRef]
- Yang, J.; Chen, M.; Ye, J.; Ma, H. Targeting PRAME for acute myeloid leukemia therapy. Front. Immunol. 2024, 15, 1378277. [Google Scholar] [CrossRef]
- Wermke, M.; Araujo, D.M.; Chatterjee, M.; Tsimberidou, A.M.; Holderried, T.A.W.; Jazaeri, A.A.; Reshef, R.; Bokemeyer, C.; Alsdorf, W.; Wetzko, K.; et al. Autologous T cell therapy for PRAME+ advanced solid tumors in HLA-A*02 + patients: A phase 1 trial. Nat. Med. 2025, 31, 2365–2374. [Google Scholar] [CrossRef]
- Kirkey, D.C.; Loeb, A.M.; Castro, S.; McKay, C.N.; Perkins, L.; Pardo, L.; Leonti, A.R.; Tang, T.T.; Loken, M.R.; Brodersen, L.E.; et al. Therapeutic targeting of PRAME with mTCRCAR T cells in acute myeloid leukemia. Blood Adv. 2023, 7, 1178–1189. [Google Scholar] [CrossRef]
- Greiner, J.; Schmitt, M.; Li, L.; Giannopoulos, K.; Bosch, K.; Schmitt, A.; Dohner, K.; Schlenk, R.F.; Pollack, J.R.; Dohner, H.; et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood 2006, 108, 4109–4117. [Google Scholar] [CrossRef]
- Falkenburg, J.H.; Heslop, H.E.; Barrett, A.J. T Cell Therapy in Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2008, 14, 136–141. [Google Scholar] [CrossRef]
- Amir, A.L.; van der Steen, D.M.; van Loenen, M.M.; Hagedoorn, R.S.; de Boer, R.; Kester, M.D.; de Ru, A.H.; Lugthart, G.J.; van Kooten, C.; Hiemstra, P.S.; et al. PRAME-specific allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin. Cancer Res. 2011, 17, 5615–5625. [Google Scholar] [CrossRef]
- Lulla, P.D.; Mamonkin, M.; Brenner, M.K. Adoptive Cell Therapy for Acute Myeloid Leukemia and T-Cell Acute Lymphoblastic Leukemia. Cancer J. 2019, 25, 199–207. [Google Scholar] [CrossRef]
- Qin, Y.-Z.; Zhu, H.-H.; Liu, Y.-R.; Wang, Y.-Z.; Shi, H.-X.; Lai, Y.-Y.; Xu, L.-P.; Liu, D.-H.; Jiang, Q.; Li, L.-D.; et al. PRAME and WT1 transcripts constitute a good molecular marker combination for monitoring minimal residual disease in myelodysplastic syndromes. Leuk. Lymphoma 2013, 54, 1442–1449. [Google Scholar] [CrossRef]
- Shiseki, M.; Ishii, M.; Ohwashi, M.; Wang, Y.-H.; Tanaka, N.; Osanai, S.; Yoshinaga, K.; Mori, N.; Tanaka, J. High PRAME expression is associated with poor survival and early disease progression in myelodysplastic syndromes with a low bone marrow blast percentage. Leuk. Lymphoma 2021, 62, 2448–2456. [Google Scholar] [CrossRef]
- Pellat-Deceunynck, C.; Mellerin, M.-P.; Labarrière, N.; Jego, G.; Moreau-Aubry, A.; Harousseau, J.-L.; Jotereau, F.; Bataille, R. The cancer germ-line genes MAGE-1, MAGE-3 and PRAME are commonly expressed by human myeloma cells. Eur. J. Immunol. 2000, 30, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Lu, J.; Bao, L.; Zhu, H.; Li, J.; Li, L.; Lai, Y.; Shi, H.; Wang, Y.; Liu, Y.; et al. Bortezomib improves progression-free survival in multiple myeloma patients overexpressing preferentially expressed antigen of melanoma. Chin. Med. J. 2014, 127, 1666–1671. [Google Scholar]
- Yang, L.; Wang, Y.-Z.; Zhu, H.-H.; Chang, Y.; Li, L.-D.; Chen, W.-M.; Long, L.-Y.; Zhang, Y.-H.; Liu, Y.-R.; Lu, J.; et al. PRAME Gene Copy Number Variation Is Related to Its Expression in Multiple Myeloma. DNA Cell Biol. 2017, 36, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Yang, L.; Wang, F.; Liu, Y.; Xu, N.; Shi, Z.-Y.; Chen, W.-M.; Li, K.; Qin, Y.-Z. PRAME promotes proliferation of multiple myeloma cells through CTMP/Akt/p21/CCND3 axis by ubiquitinating CTMP and p21. Heliyon 2024, 10, e34094. [Google Scholar] [CrossRef]
- Nienhuis, A.W.; Dunbar, C.E.; Sorrentino, B.P. Genotoxicity of Retroviral Integration In Hematopoietic Cells. Mol. Ther. 2006, 13, 1031–1049. [Google Scholar] [CrossRef]
- Cesana, D.; Cicalese, M.P.; Calabria, A.; Merli, P.; Caruso, R.; Volpin, M.; Rudilosso, L.; Migliavacca, M.; Barzaghi, F.; Fossati, C.; et al. A case of T-cell acute lymphoblastic leukemia in retroviral gene therapy for ADA-SCID. Nat. Commun. 2024, 15, 3662. [Google Scholar] [CrossRef]
- Du, M.; Hari, P.; Hu, Y.; Mei, H. Biomarkers in individualized management of chimeric antigen receptor T cell therapy. Biomark. Res. 2020, 8, 13. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, H.; Cao, J.; Zhang, C.; Liu, H.; Huang, H.; Cheng, H.; Qiao, J.; Wang, Y.; Wang, Y.; et al. Characteristics and Risk Factors of Cytokine Release Syndrome in Chimeric Antigen Receptor T Cell Treatment. Front. Immunol. 2021, 12, 611366. [Google Scholar] [CrossRef]
- Shah, D.; Soper, B.; Shopland, L. Cytokine release syndrome and cancer immunotherapies—Historical challenges and promising futures. Front. Immunol. 2023, 14, 1190379. [Google Scholar] [CrossRef] [PubMed]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Chinnasamy, N.; Gros, A.; Robbins, P.F.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Phan, G.Q.; Hughes, M.S.; Kammula, U.S.; et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013, 36, 133–151. [Google Scholar] [CrossRef]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef]
- Fischer, L.; Grieb, N.; Born, P.; Weiss, R.; Seiffert, S.; Boldt, A.; Fricke, S.; Franz, P.; Heyn, S.; Kubasch, A.S.; et al. Cellular dynamics following CAR T cell therapy are associated with response and toxicity in relapsed/refractory myeloma. Leukemia 2024, 38, 372–382. [Google Scholar] [CrossRef]
- Gattinoni, L.; Zhong, X.-S.; Palmer, D.C.; Ji, Y.; Hinrichs, C.S.; Yu, Z.; Wrzesinski, C.; Boni, A.; Cassard, L.; Garvin, L.M.; et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009, 15, 808–813. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, C.; Lu, Y.; Wu, Z.; Guo, Y.; Liu, Y.; Wei, J.; Wang, C.; Yang, Q.; Han, W. Characteristics of premanufacture CD8+T cells determine CAR-T efficacy in patients with diffuse large B-cell lymphoma. Signal Transduct. Target. Ther. 2023, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Cao, K.; Wang, M. A Correlation Between Differentiation Phenotypes of Infused T Cells and Anti-Cancer Immunotherapy. Front. Immunol. 2021, 12, 745109. [Google Scholar] [CrossRef]
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy. Cell Metab. 2017, 26, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, J.; Zhang, X.; Li, J.; Wang, Z.; Zhang, Y.; Qiu, L.; Wu, Q.; Sun, Z.; Ye, X.; et al. Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: First-in-human clinical study. Blood Cancer J. 2022, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Fløisand, Y.; Remberger, M.; Bigalke, I.; Josefsen, D.; Vålerhaugen, H.; Inderberg, E.M.; Olaussen, R.W.; Gjertsen, B.T.; Goedkoop, R.; Geiger, C.; et al. WT1 and PRAME RNA-loaded dendritic cell vaccine as maintenance therapy in de novo AML after intensive induction chemotherapy. Leukemia 2023, 37, 1842–1849. [Google Scholar] [CrossRef]
- Damiani, D.; Tiribelli, M. CAR-T Cells in Acute Myeloid Leukemia: Where Do We Stand? Biomedicines 2024, 12, 1194. [Google Scholar] [CrossRef] [PubMed]

| Dose 1 | Dose 2 | Dose 3 | All Patients | ||
|---|---|---|---|---|---|
| SAF | N = 5 | N = 5 | N = 3 | N = 13 | |
| Age (years) | |||||
| Median (min, max) | 64.0 (58, 71) | 60.0 (55, 77) | 69.0 (65, 80) | 65.0 (55, 80) | |
| Age group n (%) | |||||
| <65 years | 3 (60.0%) | 3 (60.0%) | - | 6 (46.2%) | |
| ≥65 years | 2 (40.0%) | 2 (40.0%) | 3 (100%) | 7 (53.8%) | |
| Sex n (%) | |||||
| Male | 3 (60.0%) | 2 (40.0%) | 1 (33.3%) | 6 (46.2%) | |
| Female | 2 (40.0%) | 3 (60.0%) | 2 (66.7%) | 7 (53.8%) | |
| Disease entity n (%) | n’ = 5 | n’ = 5 | n’ = 3 | n’ = 13 | |
| Acute myeloid leukemia (AML) | 4 (80.0%) | 4 (80.0%) | 2 (66.7%) | 10 (76.9%) | |
| Myelodysplastic syndrome (MDS) | - | - | 1 (33.3%) | 1 (7.7%) | |
| Multiple myeloma (MM) | 1 (20.0%) | 1 (20.0%) | - | 2 (15.4%) | |
| SAF-MDG1011 | N = 3 | N = 4 | N = 2 | N = 9 | |
| Prior lines of treatment | |||||
| 1 | - | 1 (25%) | 1 (50%) | 2 (22.2%) | |
| 2 | - | 1 (25%) | 1 (50%) | 2 (22.2%) | |
| ≥3 | 3 (100%) | 2 (50%) | - | 5 (55.5%) | |
| Prior HSCT | |||||
| 3 (100% | 3 (75%) | 1 (50%) | 7 (77.7%) | ||
| Bridging therapy | |||||
| Hydroxycarbamid | 1 (33%) | - | 1 (50%) | 2 (22.2%) | |
| Cytarabine | - | 1 (25%) | - | 1 (11.1%) | |
| Mitoxantrone | - | 1 (25%) | - | 1 (11.1%) | |
| Gilteritinib | - | 1 (25%) | - | 1 (11.1%) | |
| Azacitidine | - | - | 1 (50%) | 1 (11.1%) | |
| Carfilzomib | - | 1 (25%) | - | 1 (11.1%) | |
| Radiotherapy | 1 (33%) | - | - | 1 (11.1%) | |
| Cohort 1 | Cohort 2 | Cohort 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | C1P1 | C1P2 | C1P3 | C2P1 | OOS | C2P2 | C2P3 | C3P1 | C3P3 |
| Indication | MM | AML | AML | AML | AML | MM | AML | AML | MDS |
| IMP number | MAR-004 | MAR-006 | MAR-010 | MAR-012 | MAR-014 | MAR-018 | MAR-022 | MAR-028 | MAR-033 |
| Blasts (%) (leukapheresis) | 0% | 13% | 57% | 1% | 82% | 0% | 26% | 7% | 17% |
| Source | BM | PB | PB | BM | BM | BM | BM | PB | PB |
| PRAME mRNA at SCR | 1134 | 4714 | 327 | 1891 | 975 | <LoQ | 1814 | 24,247 | 1797 b |
| PRAME mRNA at Week 4 | 545 | 6607 | 300 a | 424 | <LoQ | 112 | 1059 | 11,087 a | <LoQ b |
| Change (%) | 52% decrease | 40% increase | 8% decrease | 78% decrease | 93% decrease | 60% increase | 42% decrease | 54% decrease | 96% decrease |
| Onset After Beginning MDG1011 Administration (SAF-MDG1011) | Entire Study (SAF) | |
|---|---|---|
| N = 9 | N = 13 | |
| Patients presenting with any | n (%) nae | n (%) nae |
| TEAE | 9 (100.0%) 68 | 13 (100.0%) 124 |
| TEAE related to MDG1011 | 6 (66.7%) 18 | 6 (46.2%) 21 a |
| TEAE related to cyclophosphamide | 5 (55.6%) 17 | 6 (46.2%) 30 |
| TEAE related to fludarabine | 4 (44.4%) 16 | 6 (46.2%) 30 |
| TEAE related to LDC b | 5 (55.6%) 17 | 6 (46.2%) 31 |
| TEAE related to clinical trial procedure other than MDG1011, cyclophosphamide, or fludarabine | 3 (33.3%) 4 | 4 (30.8%) 7 |
| Serious TEAE | 7 (77.8%) 12 | 12 (92.3%) 28 |
| TEAE of special interest | 3 (33.3%) 6 | 4 (30.8%) 9 |
| TEAE with toxicity grade ≥ 3 | 8 (88.9%) 26 | 13 (100.0%) 54 |
| TEAE due to disease progression | 6 (66.7%) 14 | 9 (69.2%) 23 |
| TEAE leading to death | 4 (44.4%) 5 | 8 (61.5%) 9 |
| Onset After Beginning MDG1011 Administration (SAF-MDG1011) | |||||
|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | All Patients | ||
| System Organ Class | N = 3 | N = 4 | N = 2 | N = 9 | |
| Preferred Term | n (%) nae | n (%) nae | n (%) nae | n (%) nae | |
| Any TEAE related to MDG1011 | 2 (66.7%) 2 | 2 (50.0%) 6 | 2 (100.0%) 10 | 6 (66.7%) 18 | |
| Grade 1—Mild | 1 (33.3%) | 1 (25.0%) | - | 2 (22.2%) | |
| Grade 2—Moderate | 1 (33.3%) | - | 1 (50%) | 2 (22.2%) | |
| Grade 3—Severe | - | - | - | - | |
| Grade 4—Life-threatening | - | 1 (25%) | 1 (50%) | 2 (22.2%) | |
| Grade 5—Fatal | - | - | - | - | |
| General disorders and administration site conditions | - | 1 (25.0%) 1 | 1 (50.0%) 1 | 2 (22.2%) 2 | |
| Fatigue | - | 1 (25.0%) 1 | - | 1 (11.1%) 1 | |
| Pyrexia | - | - | 1 (50.0%) 1 | 1 (11.1%) 1 | |
| Immune system disorders | - | 1 (25.0%) 1 | 1 (50.0%) 1 | 2 (22.2%) 2 | |
| Cytokine release syndrome | - | 1 (25.0%) 1 | 1 (50.0%) 1 | 2 (22.2%) 2 | |
| Investigations | 1 (33.3%) 1 | - | 1 (50.0%) 7 | 2 (22.2%) 8 | |
| Aspartate aminotransferase increased | 1 (33.3%) 1 | - | 1 (50.0%) 1 | 2 (22.2%) 2 | |
| Alanine aminotransferase increased | - | - | 1 (50.0%) 1 | 1 (11.1%) 1 | |
| Gamma-glutamyltransferase increased | - | 1 (50.0%) 1 | 1 (11.1%) 1 | ||
| Platelet count decreased | - | - | 1 (50.0%) 2 | 1 (11.1%) 2 | |
| White blood cell count decreased | - | - | 1 (50.0%) 2 | 1 (11.1%) 2 | |
| Blood and lymphatic system disorders | - | - | 1 (50.0%) 1 | 1 (11.1%) 1 | |
| Febrile neutropenia | - | - | 1 (50.0%) 1 | 1 (11.1%) 1 | |
| Infections and infestations | 1 (33.3%) 1 | - | - | 1 (11.1%) 1 | |
| Oropharyngeal candidiasis | 1 (33.3%) 1 | - | - | 1 (11.1%) 1 | |
| Injury, poisoning and procedural complications | - | 1 (25.0%) 1 | - | 1 (11.1%) 1 | |
| Infusion-related reaction | - | 1 (25.0%) 1 | - | 1 (11.1%) 1 | |
| Nervous system disorders | - | 1 (25.0%) 2 | - | 1 (11.1%) 2 | |
| Dizziness | - | 1 (25.0%) 1 | - | 1 (11.1%) 1 | |
| Headache | - | 1 (25.0%) 1 | - | 1 (11.1%) 1 | |
| Skin and subcutaneous tissue disorders | - | 1 (25.0%) 1 | - | 1 (11.1%) 1 | |
| Rash maculo-papular | - | 1 (25.0%) 1 | - | 1 (11.1%) 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.; Wermke, M.; Vučinić, V.; Wagner-Drouet, E.; Mackensen, A.; Zeiser, R.; Bug, G.; Schmitt, M.; Herr, W.; Prinz, P.U.; et al. First-in-Human Study of MDG1011, a TCR-T Therapy Directed Against HLA-A*02:01-Restricted PRAME Antigen for High-Risk Myeloid and Lymphoid Neoplasms. Cancers 2025, 17, 2968. https://doi.org/10.3390/cancers17182968
Thomas S, Wermke M, Vučinić V, Wagner-Drouet E, Mackensen A, Zeiser R, Bug G, Schmitt M, Herr W, Prinz PU, et al. First-in-Human Study of MDG1011, a TCR-T Therapy Directed Against HLA-A*02:01-Restricted PRAME Antigen for High-Risk Myeloid and Lymphoid Neoplasms. Cancers. 2025; 17(18):2968. https://doi.org/10.3390/cancers17182968
Chicago/Turabian StyleThomas, Simone, Martin Wermke, Vladan Vučinić, Eva Wagner-Drouet, Andreas Mackensen, Robert Zeiser, Gesine Bug, Michael Schmitt, Wolfgang Herr, Petra U. Prinz, and et al. 2025. "First-in-Human Study of MDG1011, a TCR-T Therapy Directed Against HLA-A*02:01-Restricted PRAME Antigen for High-Risk Myeloid and Lymphoid Neoplasms" Cancers 17, no. 18: 2968. https://doi.org/10.3390/cancers17182968
APA StyleThomas, S., Wermke, M., Vučinić, V., Wagner-Drouet, E., Mackensen, A., Zeiser, R., Bug, G., Schmitt, M., Herr, W., Prinz, P. U., Bürdek, M., Raffegerst, S., Tafuri, A., Geiger, C., Crame, K., Goedkoop, R., Pinkernell, K., & Schendel, D. J. (2025). First-in-Human Study of MDG1011, a TCR-T Therapy Directed Against HLA-A*02:01-Restricted PRAME Antigen for High-Risk Myeloid and Lymphoid Neoplasms. Cancers, 17(18), 2968. https://doi.org/10.3390/cancers17182968







