Salvage Surgery: A Concrete Opportunity in Unresectable Non-Small Cell Lung Cancer Following Definitive Chemo-Immunotherapy †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
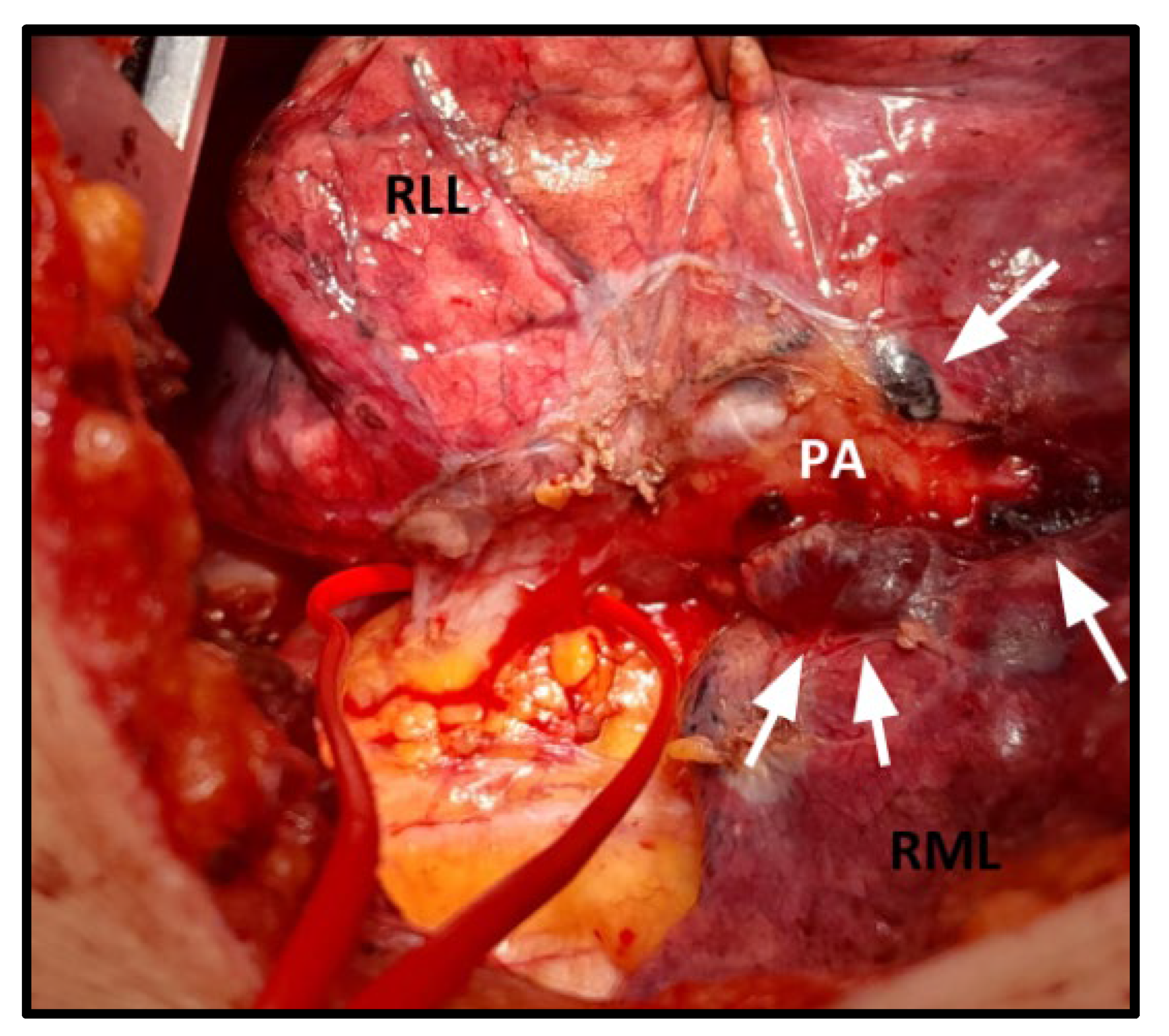
2.2. Pre-Operative Work-Up and Surgical Procedure
2.3. Histopathological Evaluation
2.4. Post-Operative Management and Data Collection
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Surgical and Postoperative Outcomes
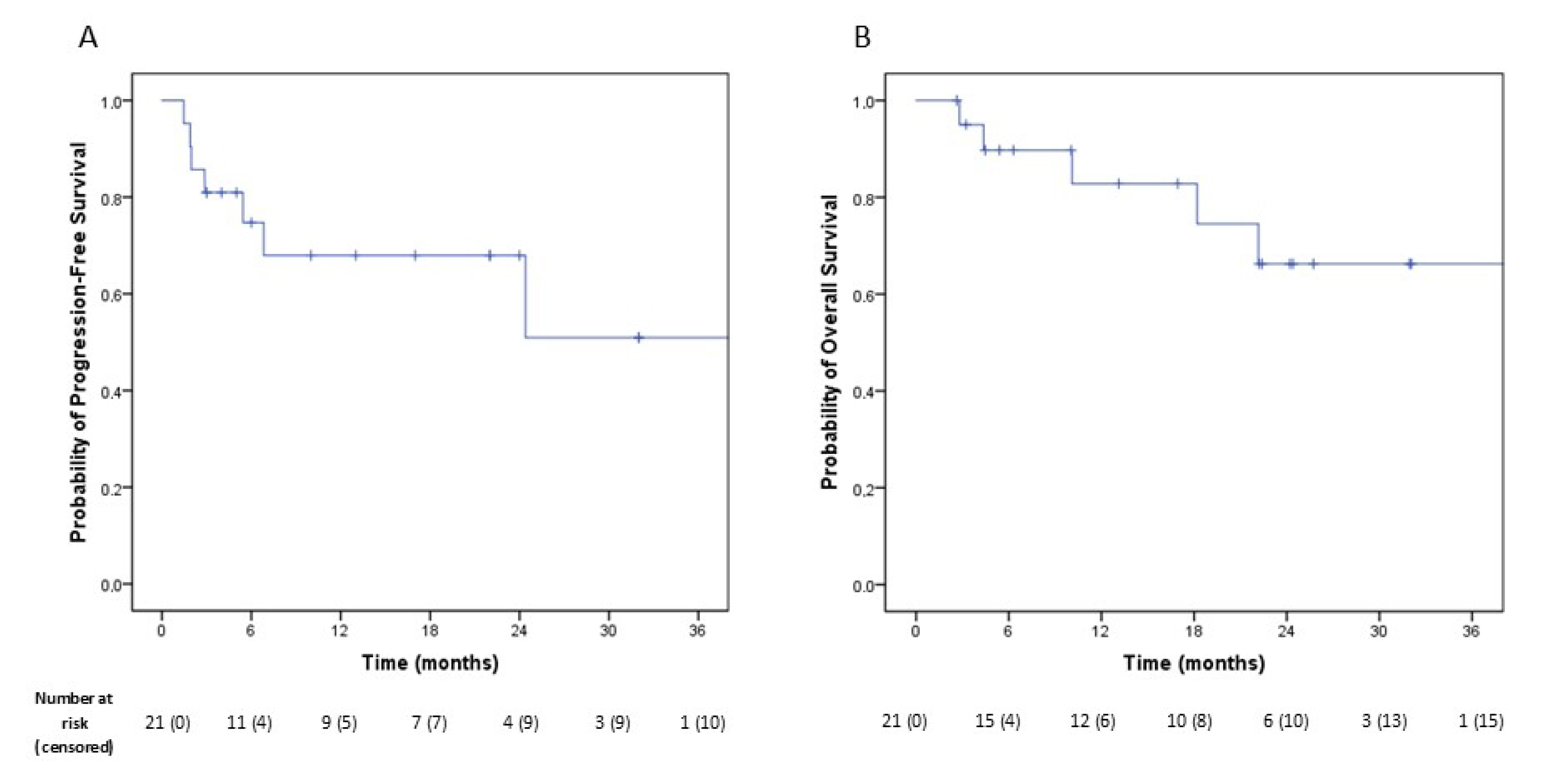
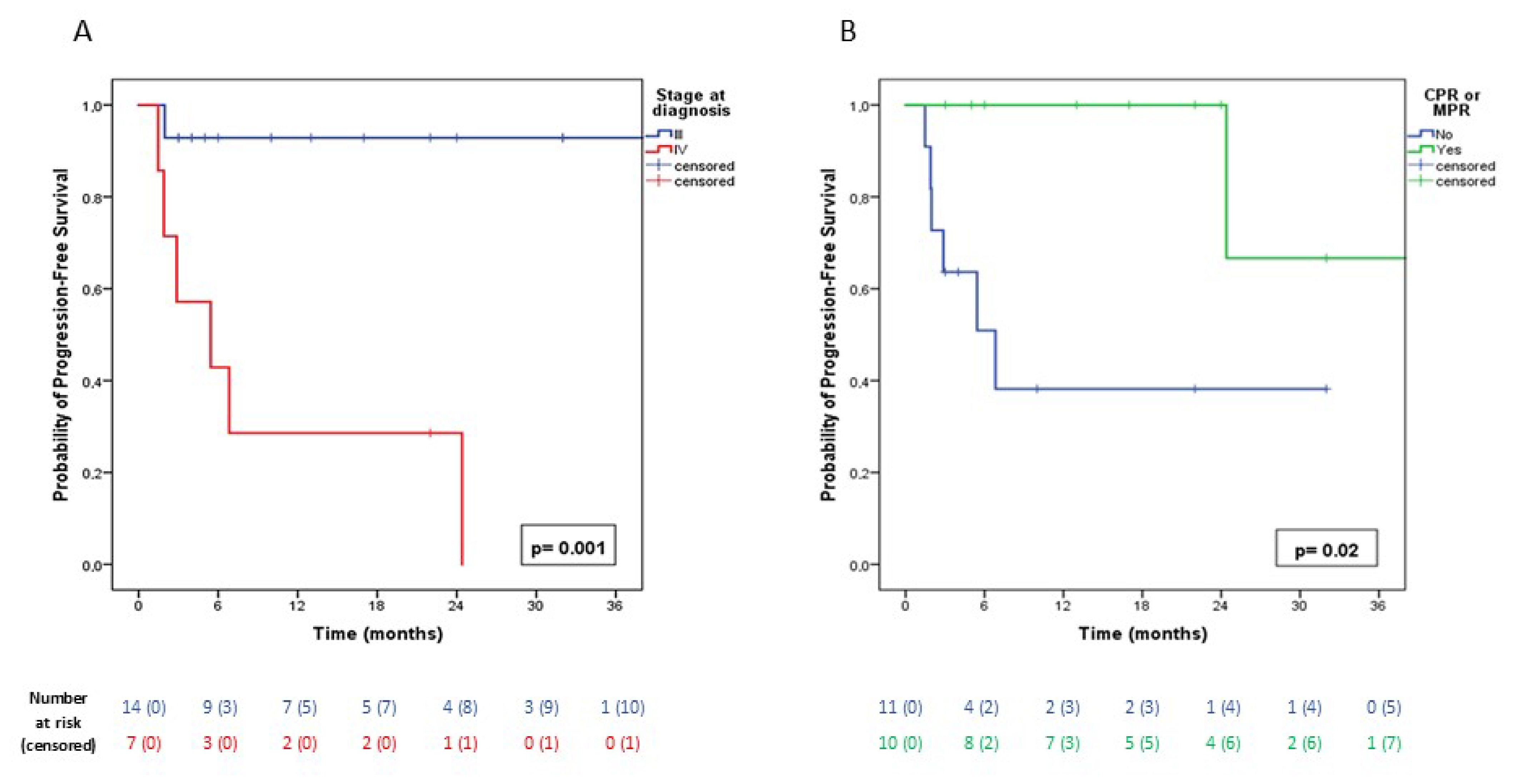
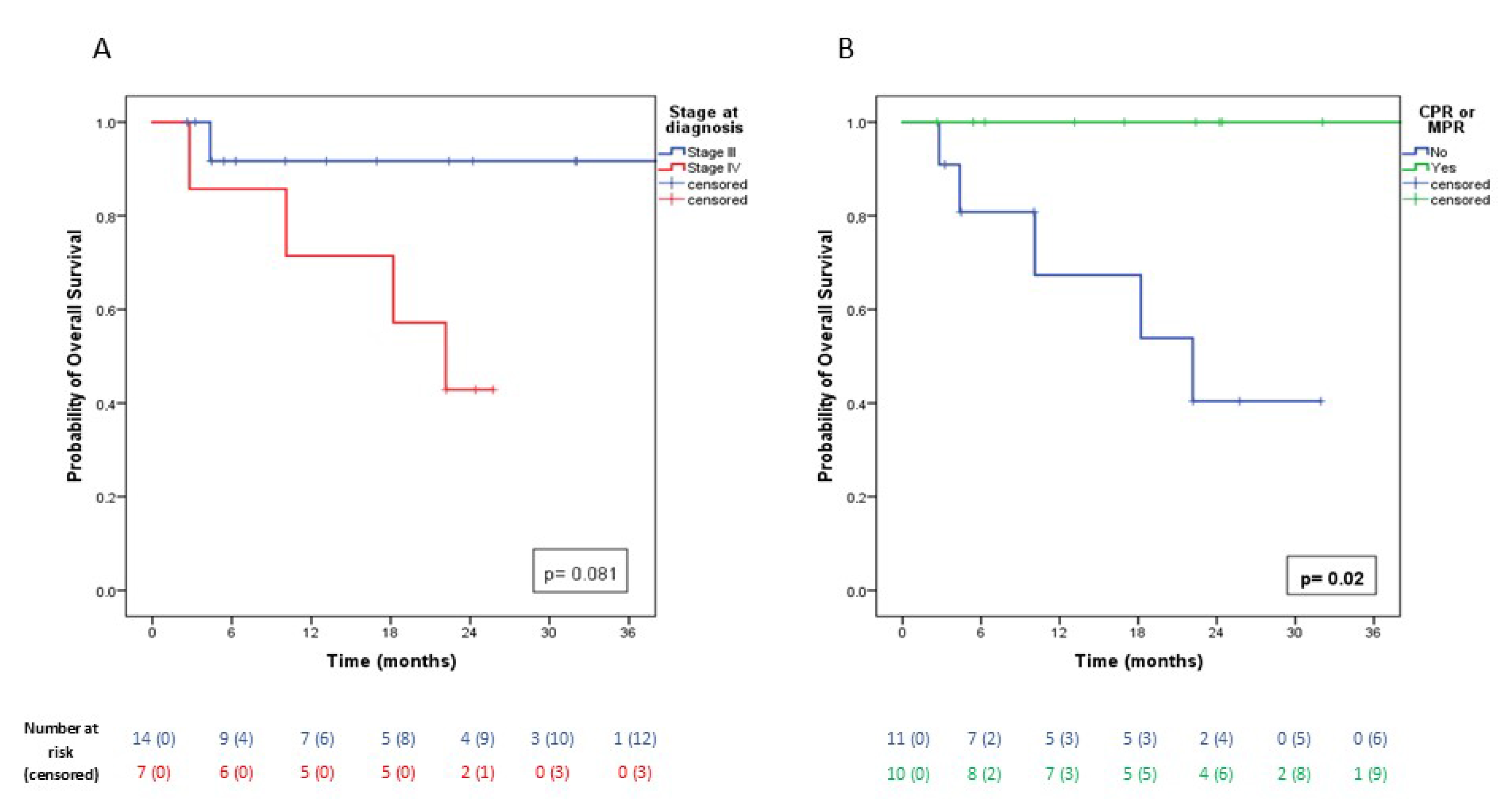
3.3. Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-Small Cell Lung Cancer |
| IQR | Interquartile range |
| ICIs | Immune Checkpoint Inhibitors |
| pCR | Complete Pathological Response |
| MPR | Major Pathological Response |
| PFS | Progression-Free Survival |
| OS | Overall Survival |
| CT | Computed Tomography |
| 18 F FDG PET | 18 fluorodeoxyglucose positron emission tomography |
| MRI | Magnetic Resonance Imaging |
| EBUS | Endobronchial ultrasound |
| ESTS | European Society of Thoracic Surgery |
| PD-L1 | Programmed Cell Death Ligand 1 |
| TNM | Tumor Node Metastasis |
| MTB | Multidisciplinary Tumor Board |
| HER 2 SBRT | Human epidermal growth factor receptor 2 Stereotactic body radiation therapy |
| VATS | Video-assisted thoracoscopic surgery |
| IASCL | International Association for the Study of Lung Cancer |
| BMI | Body Mass Index |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CEAVNO | Comitato Etico Regione Toscana-Area Vasta Nord Ovest |
| SPSS | Statistical Package for the Social Sciences |
| SD | Standard Deviation |
| ECOG-PS COPD OSAS FEV1 FVC DLCO | Eastern Cooperative Oncology Group Performance Status Chronic Obstructive Pulmonary Disease Obstructive Sleep Apnea Syndrome Forced Expiratory Volume in 1 s Forced Vital Capacity Diffusing capacity for carbon monoxide |
| OR | Odd Ratio |
| SE | Standard error |
| CI | Confidence Interval |
| RUL | Right Upper Lobe |
| RLL | Right Lower Lobe |
| RML | Right Middle Lobe |
| LUL | Left Upper Lobe |
| LLL | Left Lower Lobe |
References
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 13, 2342–2350. [Google Scholar] [CrossRef]
- Bauman, J.E.; Mulligan, M.S.; Martins, R.G.; Kurland, B.F.; Eaton, K.D.; Wood, D.E. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: Surgical and oncologic outcomes. Ann. Thorac. Surg. 2008, 86, 1632–1638. [Google Scholar] [CrossRef]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef]
- Van Schil, P.E.; Berzenji, L.; Yogeswaran, S.K.; Hendriks, J.M.; Lauwers, P. Surgical management of stage IIIA non-small cell lung cancer. Front. Oncol. 2017, 26, 249. [Google Scholar] [CrossRef]
- Bott, M.; Cools-Lartigue, J.; See Tan, K.; Dycoco, J.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; Molena, D.; Park, B.J.; et al. Safety and feasibility of lung resection after immunotherapy for metastatic or unresectable tumors. Ann. Thorac. Surg. 2018, 106, 178–183. [Google Scholar] [CrossRef]
- Guerrera, F.; Lococo, F.; Fournel, L.; Viggiano, D.; Mangiameli, G.; Mastromarino, M.G.; Guggino, G.; Seitlinger, J.; Bertoglio, P.; Luzzi, L.; et al. The outcomes of salvage surgery for non-small cell lung cancer after immune checkpoint inhibitor or targeted therapy treatment. A multi-center international real-life study. Eur. J. Surg. Oncol. 2025, 51, 109592. [Google Scholar] [CrossRef]
- Feldman, H.A.; Zhou, N.; Deboever, N.; Hofstetter, W.; Mehran, R.; Rajaram, R.; Rice, D.; Roth, J.A.; Sepesi, B.; Swisher, S.; et al. Intraoperative challenges after induction therapy for non-small cell lung cancer: Effect of nodal disease on technical complexity. JTCVS Open 2022, 25, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kuroda, H.; Tanahashi, M.; Takao, M.; Ohde, Y.; Yokoi, K.; Tarukawa, T. Survival benefits of salvage surgery for primary lung cancer based on routine clinical practice. Thorac. Cancer 2021, 12, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Van Schil, P.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejacket, V.; on behalf of the International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Lerut, T.; Weder, W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Travis, W.D.; Dacic, S.; Wistuba, I.; Sholl, L.; Adusumilli, P.; Bubendorf, L.; Bunn, P.; Cascone, T.; Chaft, J.; Chen, G.; et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J. Thorac. Oncol. 2020, 15, 709–740. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events; Version 5.0; US Department of Health and Human Services: Washington, DC, USA, 2017.
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the eastern cooperative oncology group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; Kar, G.; Spicer, J.D.; García-Campelo, R.; Weder, W.; Daniel, D.B.; Spigel, D.R.; Hussein, M.; Mazieres, J.; Oliveira, J.; et al. Neoadjuvant durvalumab alone or combined with novel immuno-oncology agents in resectable lung cancer: The phase II NeoCOAST platform trial. Cancer Discov. 2023, 13, 2394–2411. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-smallcell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lee, J.S.; Ciuleanu, T.E.; Caro, R.B.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J. Clin. Onc. 2023, 41, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar] [CrossRef]
- Chaft, J.E.; Hellmann, M.D.; Velez, M.J.; Travis, W.D.; Rusch, V.W. Initial experience with lung cancer resection after treatment with T-cell checkpoint inhibitors. Ann. Thorac. Surg. 2017, 104, e217–e218. [Google Scholar] [CrossRef]
- Ueno, T.; Yamashita, M.; Yamashita, N.; Uomoto, M.; Kawamata, O.; Sano, Y.; Inokawa, H.; Hirayama, S.; Okazaki, M.; Toyooka, S. Safety of salvage lung resection after immunotherapy for unresectable non-small cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 812–817. [Google Scholar] [CrossRef]
- Schiavon, M.; Cannone, G.; Bertolaccini, L.; Gallina, F.T.; Pezzuto, F.; Lorenzoni, G.; Facciolo, F.; Spaggiari, L.; Calabrese, F.; Rea, F.; et al. Safety and efficacy of salvage surgery after treatment with immune-checkpoint adjuvant inhibitors for advanced non-small cell lung cancer: A multicentric study. J. Surg. Oncol. 2025, 131, 371–379. [Google Scholar] [CrossRef]
- Higuchi, M.; Inomata, S.; Yamaguchi, H.; Saito, T.; Suzuki, H. Salvage surgery for advanced non-small cell lung cancer following previous immunotherapy: A retrospective study. J. Cardiothorac. Surg. 2023, 18, 235. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Le, A.; Bott, M.; Yang, C.-F.J.; Gossot, D.; Melfi, F.; Tian, D.H.; Guo, A. Meta-analysis of neoadjuvant immunotherapy for patients with resectable non-small cell lung cancer. Curr. Oncol. 2021, 28, 4686–4701. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.K.; Nakagawa, K.; Nakayama, Y.; Ohe, Y.; Yotsukura, M.; Uchida, S.; Asakura, K.; Yoshida, Y.; Watanabe, S.-I. Salvage surgery compared to surgery after induction chemoradiation therapy for advanced lung cancer. Ann. Thorac. Surg. 2022, 114, 2087–2092. [Google Scholar] [CrossRef]
- Song, W.; Di, S.; Liu, J.; Fan, B.; Zhao, J.; Zhou, S.; Chen, S.; Dong, H.; Yue, C.; Gong, T. Salvage surgery or advanced non-small cell lung cancer after targeted therapy: A case series. Thorac. Cancer 2020, 11, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cao, X.; Ai, B.; Xiao, H.; Huang, Q.; Zhang, Z.; Chu, Q.; Zhang, L.; Dai, X.; Liao, Y. Salvage surgery following downstaging of advanced non-small cell lung cancer by targeted therapy. Thorac. Cancer 2021, 12, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Hamaji, M.; Ozasa, H.; Sakamori, Y.; Terada, K.; Yoshizawa, A.; Kikuchi, R.; Sakaguchi, Y.; Sonobe, M.; Muranishi, Y.; Miyahara, R.; et al. Characteristics and outcomes of salvage surgery after immune checkpoint inhibitor therapy for initially unresectable non-small cell lung cancer. J. Thorac. Dis. 2024, 16, 6094–6100. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, E.; Mastromarino, M.G.; Bacchin, D.; La Rosa, A.; Aprile, V.; Korasidis, S.; Ambrogi, M.C.; Lucchi, M. Salvage surgery: A concrete opportunity in unresectable non-small cell lung cancer following definitive chemo-immunotherapy. In Proceedings of the 33rd Meeting of the European Society of Thoracic Surgeons (ESTS), Budapest, Hungary, 25–27 May 2025. [Google Scholar]
| Variable | Description |
|---|---|
| Sex (n, %) | |
| M | 13 (61.9) |
| F | 8 (38.1) |
| Median age * (years, IQR) | 68 (9) |
| Smoking status (n, %) | |
| Current smokers | 10 (47.6) |
| Former smokers | 9 (42.9) |
| Never | 2 (9.5) |
| BMI (n, %) | |
| <25 | 8 (38.1) |
| 25–30 | 9 (42.9) |
| >30 | 4 (19) |
| Comorbidity (n, %) | |
| Cardiovascular | 13 (61.9) |
| Previous malignancy | 6 (28.6) |
| Diabetes Mellitus | 5 (23.8) |
| Respiratory | 4 (19) |
| COPD | 2 (9.5) |
| OSAS | 1 (4.8) |
| Pulmonary fibrosis | 1 (4.8) |
| Gastrointestinal | 3 (14.3) |
| ECOG-PS Score (n, %) | |
| 0 | 18 (85.7) |
| 1 | 3 (14.3) |
| Preoperative pulmonary function test (mean, SD) | |
| FEV1 (%) | 86 (12) |
| FVC (%) | 88 (11) |
| DLCO (%) | 76 (8) |
| PD-L1 (n, %) | |
| <50% | 17 (81) |
| ≥50% | 4 (19) |
| Primary tumor location (n, %) | |
| RUL | 3 (14.3) |
| RML | 1 (4.8) |
| RLL | 5 (23.8) |
| LUL | 9 (42.8) |
| LLL | 3 (14.3) |
| Metastasis location (n, %) Contralateral lung Brain Bone | (N = 7 patients) 3 (43) 3 (43) 1 (14) |
| Immunotherapy drugs associated with traditional chemotherapy (n, %) | |
| Pembrolizumab | 16 (76) |
| Trastuzumab | 1 (4.8) |
| Atezolizumab | 1 (4.8) |
| Durvalumab | 1 (4.8) |
| Monalizumab/Durvalumab | 1 (4.8) |
| Volrustomig | 1 (4.8) |
| Cycles of immunotherapy associated with traditional chemotherapy (n, %) | |
| 2 | 2 (9.5) |
| 3 | 3 (14.3) |
| 4 | 7 (33.3) |
| 6 | 4 (19) |
| 7 | 1 (4.8) |
| 8 | 1 (4.8) |
| 10 | 2 (9.5) |
| 31 | 1 (4.8) |
| Site of radiotherapy (n, %) | |
| Brain | 3 (14.3) |
| Bone (iliac lesion) | 1 (4.8) |
| Primitive lung tumor | 3 (14.3) |
| Variable | OR (95% C.I.) | p-Value |
| Age * | 1.013 (0.904–1.134) | 0.83 |
| Male sex | 43.690 (0.023–84068.084) | 0.33 |
| Former or current smokers | 0.211 (0.019–2.345) | 0.20 |
| BMI * | 0.956 (0.750–1.219) | 0.72 |
| Adenocarcinoma | 1.547 (0.515–4.647) | 0.44 |
| Clinical stage III at diagnosis | 0.418 (0.139–1.258) | 0.12 |
| Anatomical lung resection | 0.826 (0.274–2.490) | 0.73 |
| Major or Complete Pathological Response | 8.283 (0.226–303.978) | 0.25 |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variable | OR (95% C.I.) | p-Value | OR (95% C.I.) | p-Value |
| Age * | 1.023 (0.939–1.114) | 0.61 | ||
| Male sex | 58.638 (0.127–27096.376) | 0.19 | ||
| Former or current smoker | 0.199 (0.020–1.954) | 0.17 | ||
| BMI * | 0.887 (0.716–7.099) | 0.27 | ||
| Adenocarcinoma | 1.971 (0.681–5.707) | 0.21 | ||
| Clinical stage III at diagnosis | 0.257 (0.089–0.743) | 0.012 | 0.292 (0.093–0.912) | 0.034 |
| Anatomical lung resection | 1.265 (0.437–3.658) | 0.66 | ||
| Major or Complete Pathological Response | 0.113 (0.013–0.959) | 0.046 | 0.140 (0.010–2.038) | 0.150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastromarino, M.G.; Guerrini, E.; Caciagli, L.M.; La Rosa, A.; Bacchin, D.; Aprile, V.; Korasidis, S.; Lenzini, A.; Celi, A.; Alì, G.; et al. Salvage Surgery: A Concrete Opportunity in Unresectable Non-Small Cell Lung Cancer Following Definitive Chemo-Immunotherapy. Cancers 2025, 17, 2967. https://doi.org/10.3390/cancers17182967
Mastromarino MG, Guerrini E, Caciagli LM, La Rosa A, Bacchin D, Aprile V, Korasidis S, Lenzini A, Celi A, Alì G, et al. Salvage Surgery: A Concrete Opportunity in Unresectable Non-Small Cell Lung Cancer Following Definitive Chemo-Immunotherapy. Cancers. 2025; 17(18):2967. https://doi.org/10.3390/cancers17182967
Chicago/Turabian StyleMastromarino, Maria Giovanna, Elena Guerrini, Lisa Maria Caciagli, Andrea La Rosa, Diana Bacchin, Vittorio Aprile, Stylianos Korasidis, Alessandra Lenzini, Alessandra Celi, Greta Alì, and et al. 2025. "Salvage Surgery: A Concrete Opportunity in Unresectable Non-Small Cell Lung Cancer Following Definitive Chemo-Immunotherapy" Cancers 17, no. 18: 2967. https://doi.org/10.3390/cancers17182967
APA StyleMastromarino, M. G., Guerrini, E., Caciagli, L. M., La Rosa, A., Bacchin, D., Aprile, V., Korasidis, S., Lenzini, A., Celi, A., Alì, G., Ambrogi, M. C., & Lucchi, M. (2025). Salvage Surgery: A Concrete Opportunity in Unresectable Non-Small Cell Lung Cancer Following Definitive Chemo-Immunotherapy. Cancers, 17(18), 2967. https://doi.org/10.3390/cancers17182967








