Cervical Cancer Screening in Refugee and Migrant Populations: Results of Systematic Review and Meta-Analysis in Cross-Sectional and Cohort Studies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
- Peer-reviewed primary studies in English, designed as cohort or Cross-Sectional;
- Studies reporting outcome measures related to cervical cancer prevention strategies, especially cervical cancer screening;
- Studies that investigated the aforementioned outcomes in populations with migratory backgrounds.
- Articles not in English;
- Articles without abstracts;
- Articles whose study design was one of meta-analysis, trial, review or systematic review, pre-post study, articles appearing as opinions, guidelines, books, commentaries;
- Articles that did not include our reference population;
- Articles that did not provide outcomes related to CCS.
2.2. Report Evaluation and Data Extraction
- -
- Migrant status of the study population, classified as “International Migrants”, “Refugees”, or “Mixed” (when methodological constraints prevented clear distinction).
- -
- The geographical region in which the study was conducted, according to the WHO regional offices grouping [22].
- -
- The economic level of the country of origin, based on the World Bank classification [23]. For studies where the population’s origin was unknown or multiple, the income level was coded as “unspecified”.
2.3. Data Analysis
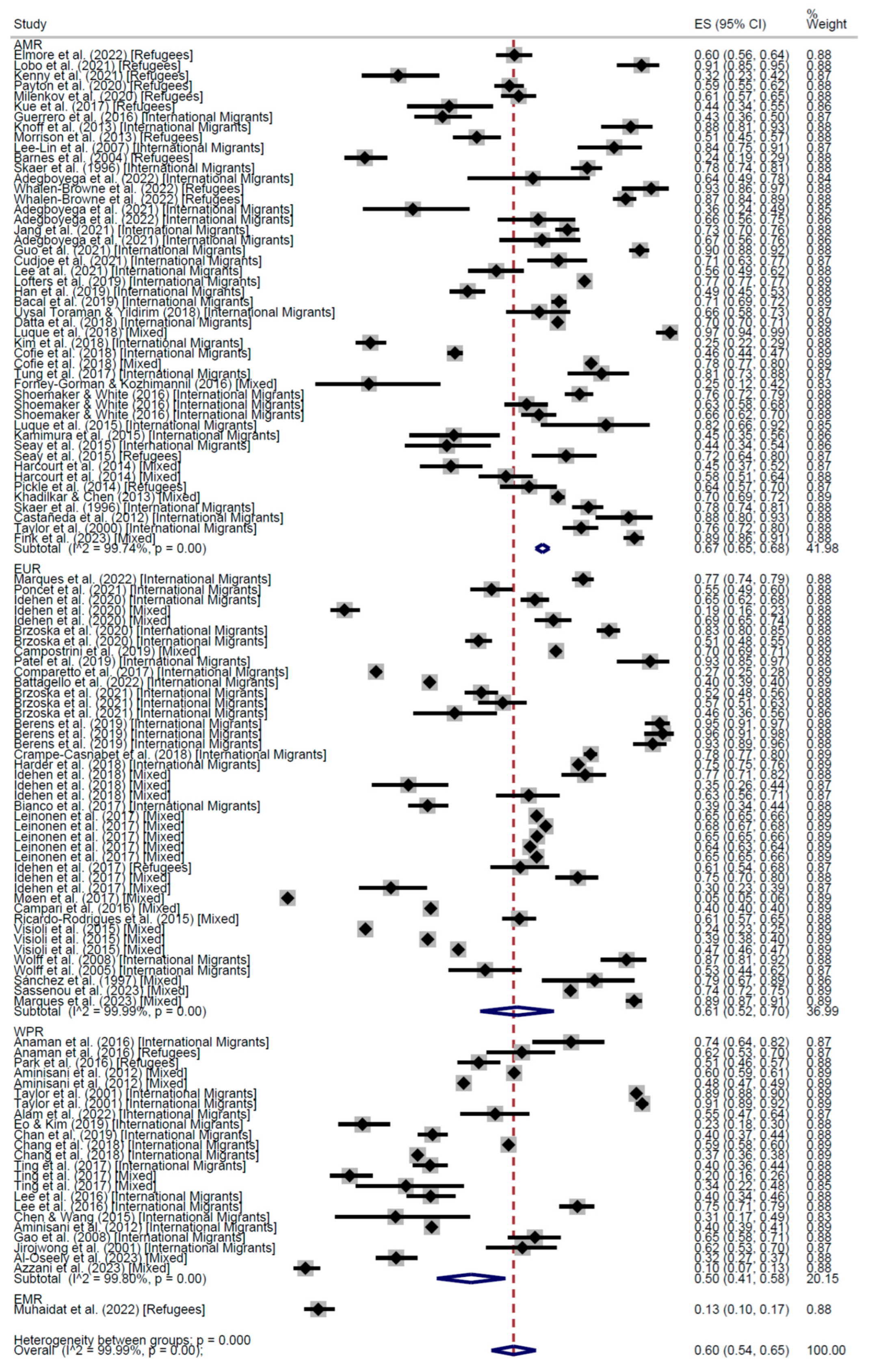
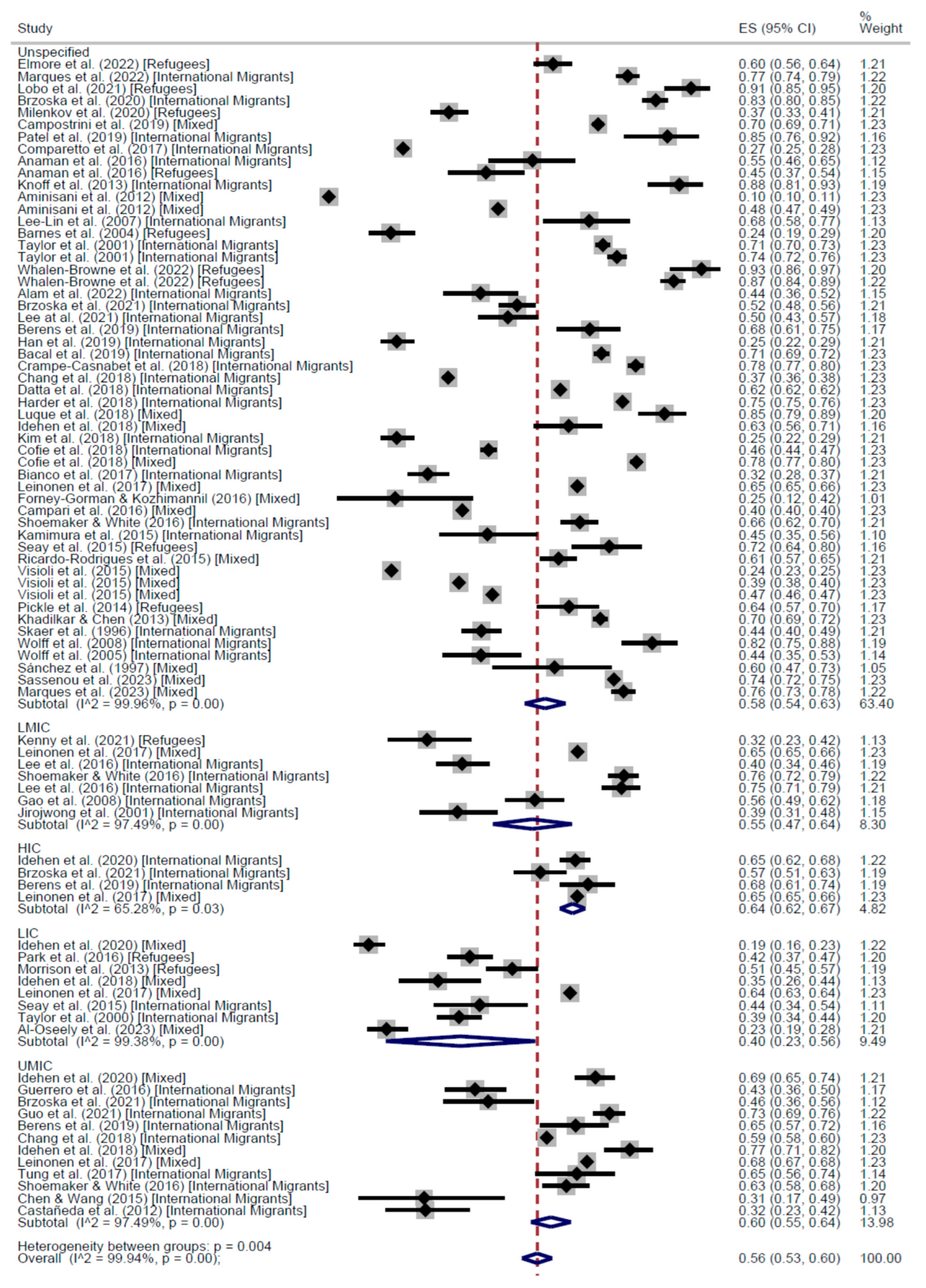
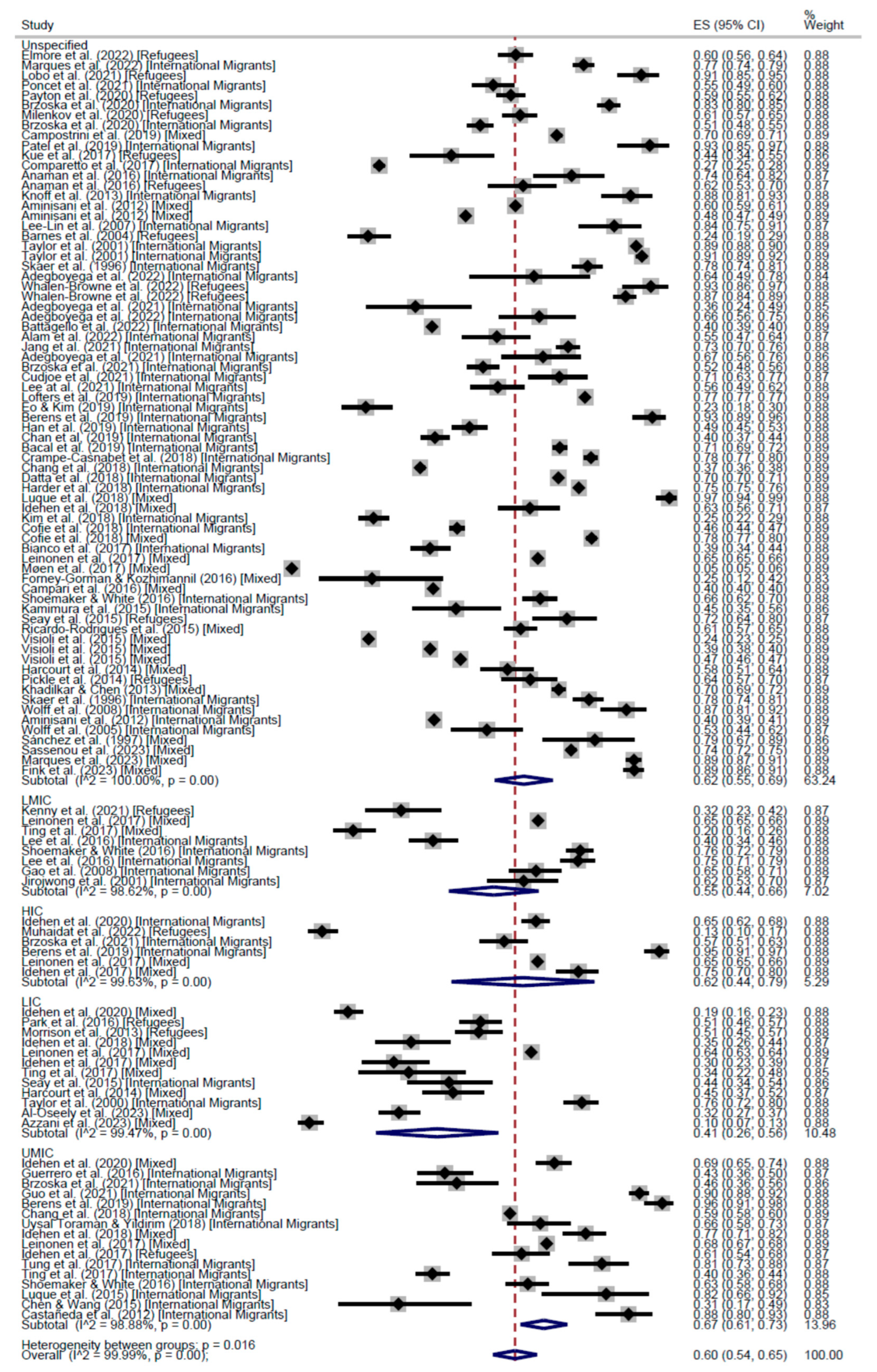
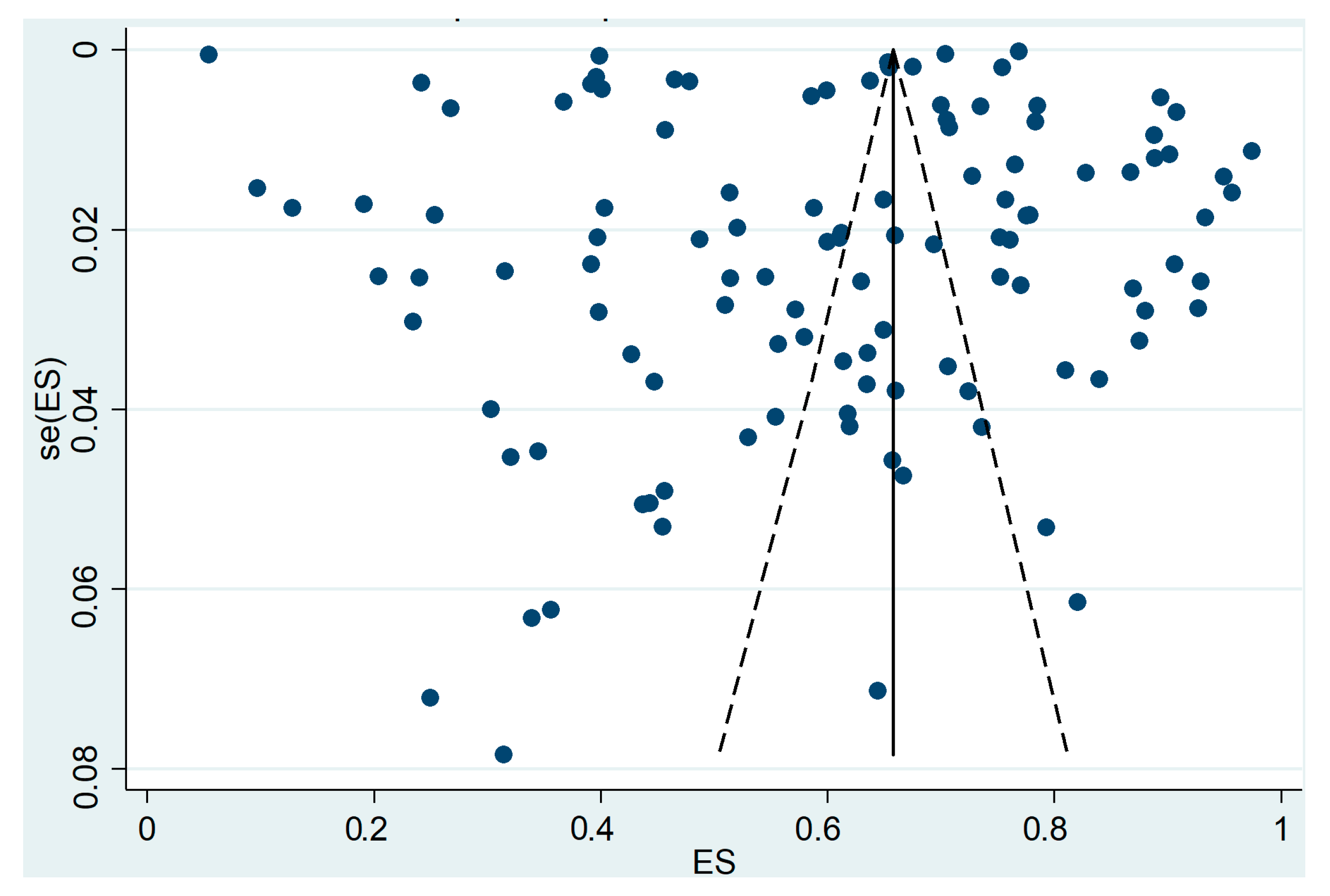
3. Results
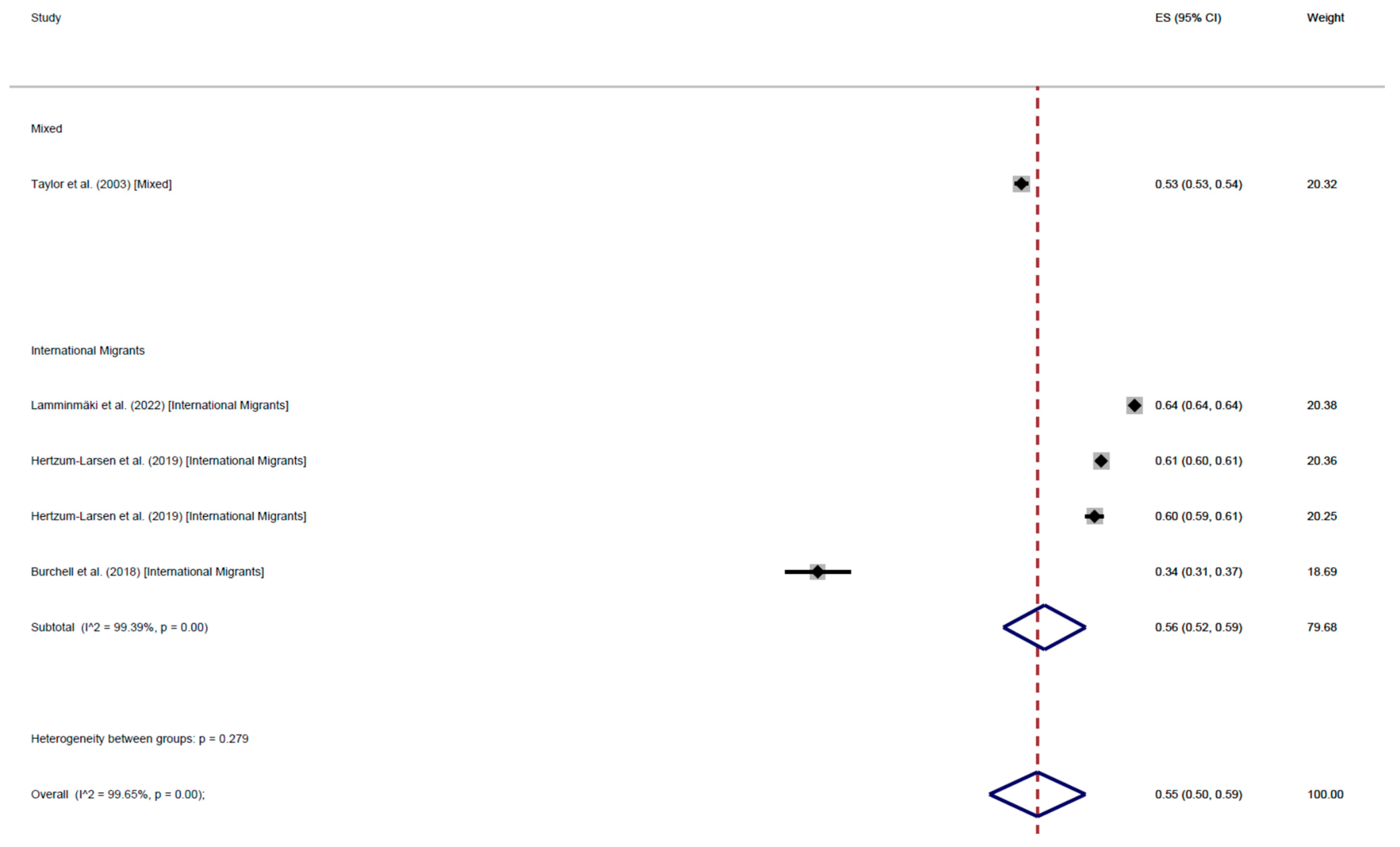
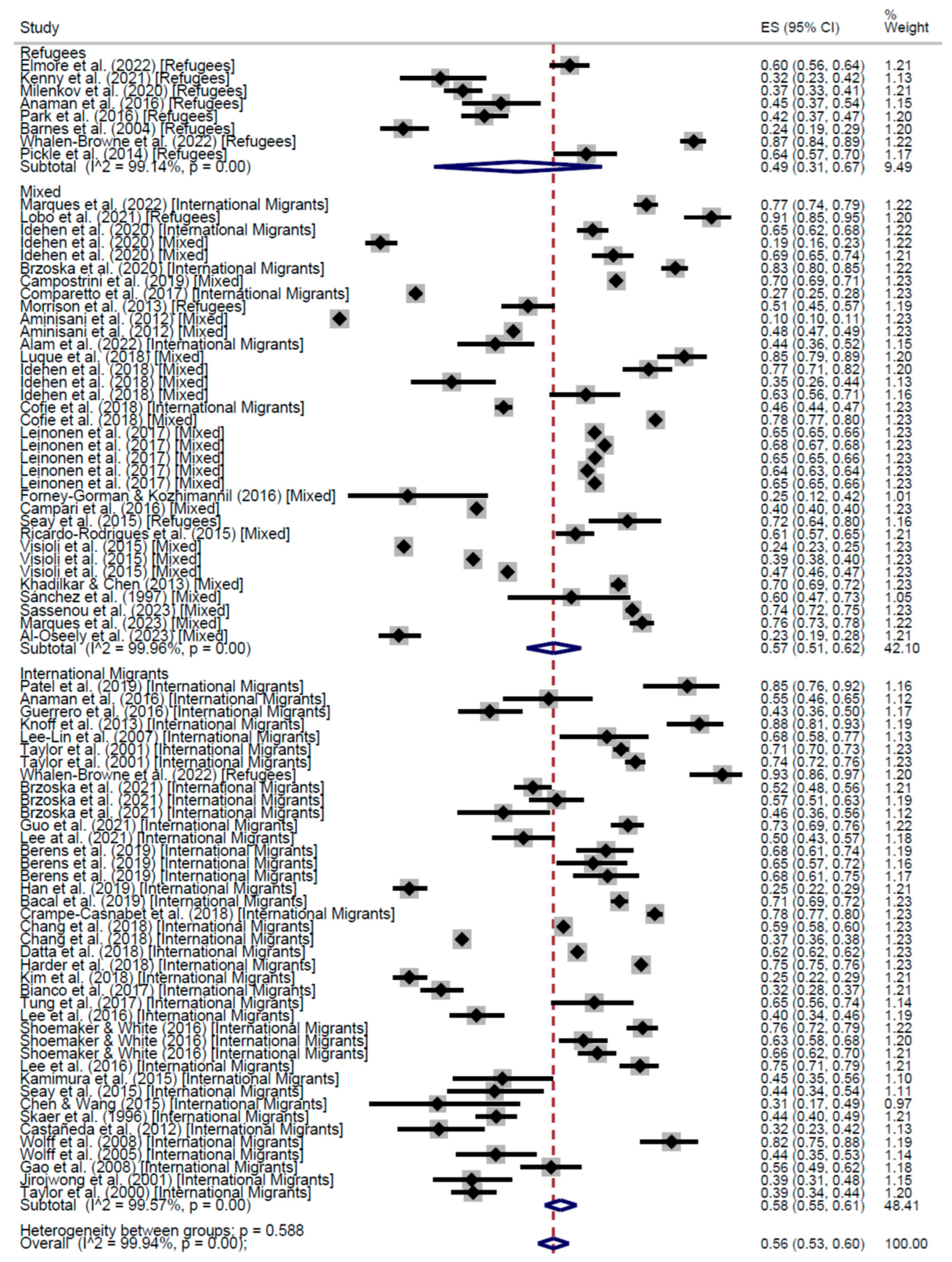
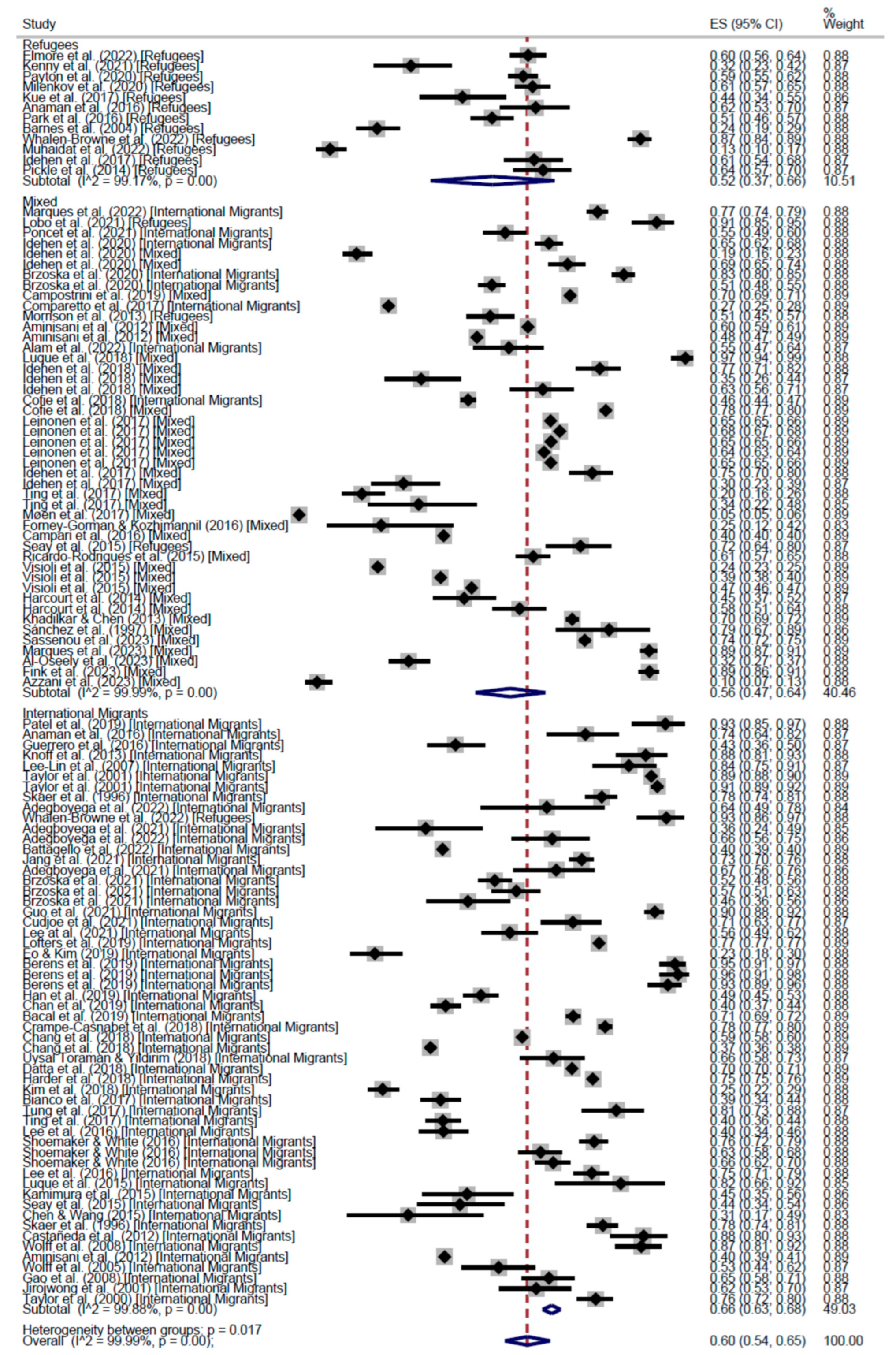
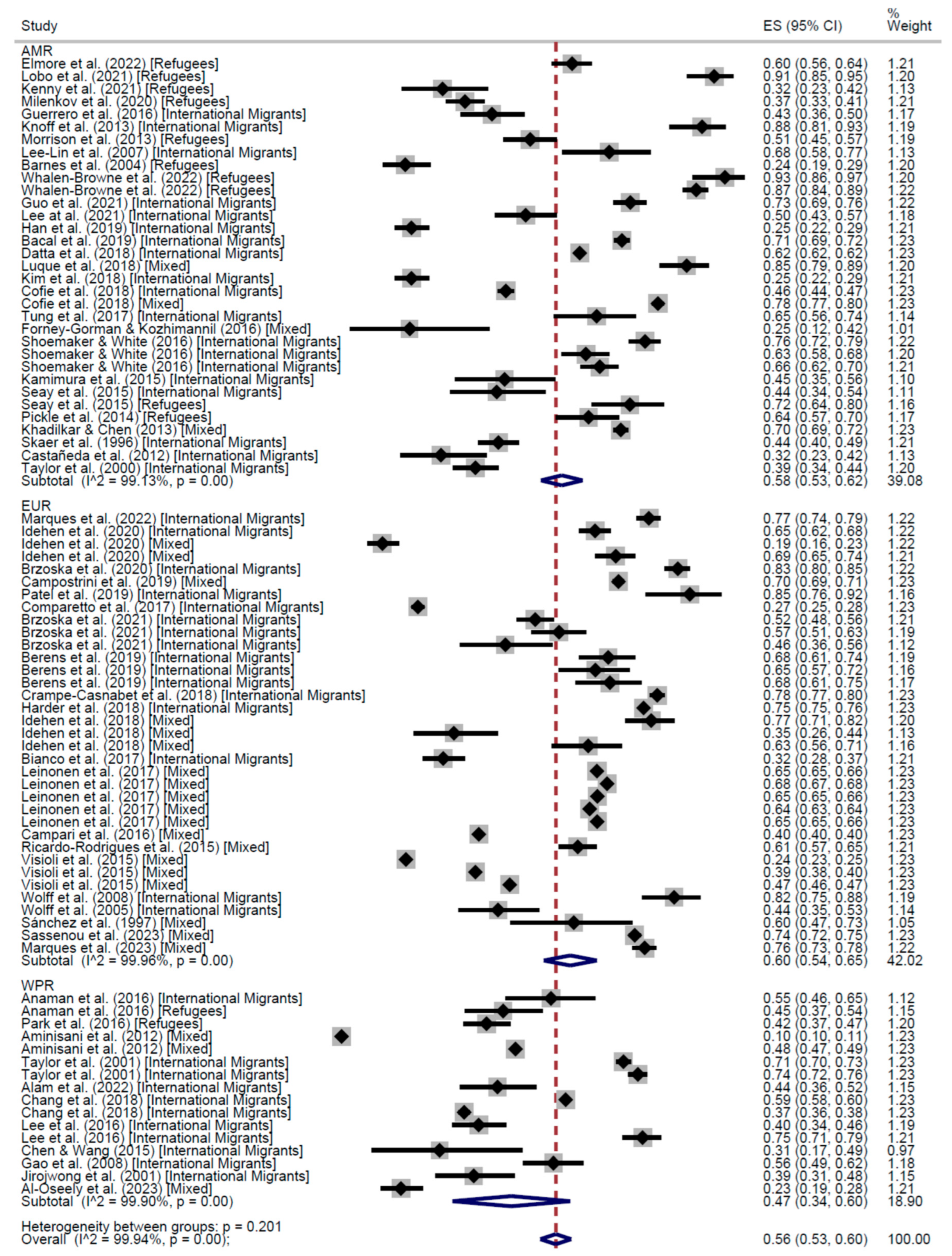
CCS Participation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author (Year) | Population (N) | Region | Economic Level | Study Design | QS |
|---|---|---|---|---|---|
| Payton et al. (2020) [25] | Refugees (782) | AMR | Unspecified | Cross-Sectional | 6 |
| Skaer et al. (1996) [26] | International Migrants (512) | AMR | Unspecified | Cross-Sectional | 4 |
| Skaer et al. (1996) [27] | International Migrants (510) | AMR | Unspecified | Cross-Sectional | 7 |
| Taylor et al. (2000) [28] | International Migrants (406) | AMR | LIC | Cross-Sectional | 6 |
| Taylor et al. (2001) [29] | International Migrants (3392) | WPR | Unspecified | Cross-Sectional | 7 |
| Taylor et al. (2001) [29] | International Migrants (1743) | WPR | Unspecified | Cross-Sectional | 7 |
| Jirojwong et al. (2001) [30] | International Migrants (134) | WPR | LMIC | Cross-Sectional | 4 |
| Wolff et al. (2005) [31] | International Migrants (134) | EUR | Unspecified | Cross-Sectional | 1 |
| Lee-Lin et al. (2007) [32] | International Migrants (100) | AMR | Unspecified | Cross-Sectional | 7 |
| Wolff et al. (2008) [33] | International Migrants (161) | EUR | Unspecified | Cross-Sectional | 4 |
| Gao et al. (2008) [34] | International Migrants (234) | WPR | LMIC | Cross-Sectional | 8 |
| Castañeda et al. (2012) [35] | International Migrants (104) | AMR | UMIC | Cross-Sectional | 2 |
| Aminisani et al. (2012) [36] | International Migrants (12,392) | WPR | Unspecified | Cross-Sectional | 9 |
| Knoff et al. (2013) [37] | International Migrants (125) | AMR | Unspecified | Cross-Sectional | 5 |
| Luque et al. (2015) [38] | International Migrants (39) | AMR | UMIC | Cross-Sectional | 3 |
| Kamimura et al. (2015) [17] | International Migrants (88) | AMR | Unspecified | Cross-Sectional | 2 |
| Seay et al. (2015) [39] | International Migrants (96) | AMR | LIC | Cross-Sectional | 3 |
| Seay et al. (2015) [39] | Unspecified (138) | AMR | Unspecified | Cross-Sectional | 3 |
| Chen & Wang (2015) [40] | International Migrants (35) | WPR | UMIC | Cross-Sectional | 3 |
| Anaman et al. (2016) [41] | International Migrants (110) | WPR | Unspecified | Cross-Sectional | 6 |
| Anaman et al. (2016) [41] | Refugees (144) | WPR | Unspecified | Cross-Sectional | 6 |
| Guerrero et al. (2016) [42] | International Migrants (213) | AMR | UMIC | Cross-Sectional | 4 |
| Lee et al. (2016) [43] | International Migrants (281) | WPR | LMIC | Cross-Sectional | 4 |
| Shoemaker & White (2016) [44] | International Migrants (659) | AMR | LMIC | Cross-Sectional | 6 |
| Shoemaker & White (2016) [44] | International Migrants (351) | AMR | UMIC | Cross-Sectional | 6 |
| Shoemaker & White (2016) [44] | International Migrants (526) | AMR | Unspecified | Cross-Sectional | 6 |
| Lee et al. (2016) [45] | International Migrants (427) | WPR | LMIC | Cross-Sectional | 6 |
| Comparetto et al. (2017) [46] | Unspecified (4609) | EUR | Unspecified | Cross-Sectional | 6 |
| Bianco et al. (2017) [47] | International Migrants (419) | EUR | Unspecified | Cross-Sectional | 5 |
| Tung et al. (2017) [48] | International Migrants (121) | AMR | UMIC | Cross-Sectional | 4 |
| Ting et al. (2017) [49] | International Migrants (549) | WPR | UMIC | Cross-Sectional | 4 |
| Ting et al. (2017) [49] | Unspecified (255) | WPR | LMIC | Cross-Sectional | 4 |
| Ting et al. (2017) [49] | Unspecified (5) | WPR | HIC | Cross-Sectional | 4 |
| Ting et al. (2017) [49] | Unspecified (56) | WPR | LIC | Cross-Sectional | 4 |
| Bacal et al. (2019) [50] | International Migrants (2782) | AMR | Unspecified | Cross-Sectional | 8 |
| Crampe-Casnabet et al. (2018) [51] | International Migrants (2637) | EUR | Unspecified | Cross-Sectional | 5 |
| Chang et al. (2018) [52] | International Migrants (9067) | WPR | UMIC | Cross-Sectional | 9 |
| Chang et al. (2018) [52] | International Migrants (6868) | WPR | Unspecified | Cross-Sectional | 9 |
| Uysal Toraman & Yildirim (2018) [53] | International Migrants (156) | AMR | UMIC | Cross-Sectional | 5 |
| Datta et al. (2018) [54] | International Migrants (826,387) | AMR | Unspecified | Cross-Sectional | 6 |
| Harder et al. (2018) [55] | International Migrants (48,218) | EUR | Unspecified | Cross-Sectional | 8 |
| Hagopian et al. (2018) [56] | International Migrants (271) | AMR | Unspecified | Cohort | 4 |
| Burchell et al. (2018) [57] | Unspecified (949) | AMR | Unspecified | Cohort | 4 |
| Kim et al. (2018) [58] | International Migrants (560) | AMR | Unspecified | Cross-Sectional | 5 |
| Cofie et al. (2018) [59] | Unspecified (3080) | AMR | Unspecified | Cross-Sectional | 5 |
| Patel et al. (2019) [60] | International Migrants (82) | EUR | Unspecified | Cross-Sectional | 5 |
| Lofters et al. (2019) [61] | International Migrants (3,630,981) | AMR | Unspecified | Cross-Sectional | 9 |
| Eo & Kim (2019) [62] | International Migrants (196) | WPR | Unspecified | Cross-Sectional | 5 |
| Berens et al. (2019) [63] | International Migrants (239) | EUR | HIC | Cross-Sectional | 3 |
| Berens et al. (2019) [63] | International Migrants (163) | EUR | UMIC | Cross-Sectional | 3 |
| Berens et al. (2019) [63] | International Migrants (179) | EUR | Unspecified | Cross-Sectional | 3 |
| Han et al. (2019) [64] | International Migrants (560) | AMR | Unspecified | Cross-Sectional | 4 |
| Chan et al., (2019) [65] | International Migrants (776) | WPR | Unspecified | Cross-Sectional | 5 |
| Hertzum-Larsen et al. (2019) [66] | Unspecified (12,500) | EUR | HIC | Cohort | 8 |
| Hertzum-Larsen et al. (2019) [66] | Unspecified (44,829) | EUR | Unspecified | Cohort | 8 |
| Idehen et al. (2020) [67] | Unspecified (816) | EUR | HIC | Cross-Sectional | 9 |
| Idehen et al. (2020) [67] | Unspecified (523) | EUR | LIC | Cross-Sectional | 9 |
| Idehen et al. (2020) [67] | Unspecified (451) | EUR | UMIC | Cross-Sectional | 9 |
| Brzoska et al. (2020) [68] | Unspecified (755) | EUR | Unspecified | Cross-Sectional | 7 |
| Brzoska et al. (2020) [69] | Unspecified (983) | EUR | Unspecified | Cross-Sectional | 8 |
| Poncet et al. (2021) [70] | Unspecified (387) | EUR | Unspecified | Cross-Sectional | 5 |
| Adegboyega et al. (2021) [71] | International Migrants (59) | AMR | Unspecified | Cross-Sectional | 5 |
| Jang et al. (2021) [72] | International Migrants (999) | AMR | Unspecified | Cross-Sectional | 6 |
| Adegboyega et al. (2021) [73] | International Migrants (99) | AMR | Unspecified | Cross-Sectional | 6 |
| Brzoska et al. (2021) [74] | International Migrants (292) | EUR | HIC | Cross-Sectional | 6 |
| Brzoska et al. (2021) [74] | International Migrants (103) | EUR | UMIC | Cross-Sectional | 6 |
| Brzoska et al. (2021) [74] | International Migrants (634) | EUR | Unspecified | Cross-Sectional | 6 |
| Guo et al. (2021) [75] | International Migrants (652) | AMR | UMIC | Cross-Sectional | 4 |
| Cudjoe et al. (2021) [76] | International Migrants (167) | AMR | Unspecified | Cross-Sectional | 5 |
| Lee at al. (2021) [77] | International Migrants (230) | AMR | Unspecified | Cross-Sectional | 4 |
| Marques et al. (2022) [78] | Unspecified (1100) | EUR | Unspecified | Cross-Sectional | 7 |
| Adegboyega et al. (2022) [79] | International Migrants (45) | AMR | Unspecified | Cross-Sectional | 5 |
| Adegboyega et al. (2022) [80] | International Migrants (108) | AMR | Unspecified | Cross-Sectional | 4 |
| Battagello et al. (2022) [81] | International Migrants (26,355) | EUR | Unspecified | Cross-Sectional | 8 |
| Lamminmäki et al. (2022) [82] | International Migrants (85,272) | EUR | Unspecified | Cohort | 8 |
| Alam et al. (2022) [83] | Unspecified (148) | WPR | Unspecified | Cross-Sectional | 5 |
| Barnes et al. (2004) [84] | Refugees (283) | AMR | Unspecified | Cross-Sectional | 5 |
| Morrison et al. (2013) [85] | Unspecified (310) | AMR | LIC | Cross-Sectional | 7 |
| Pickle et al. (2014) [86] | Refugees (203) | AMR | Unspecified | Cross-Sectional | 5 |
| Park et al. (2016) [87] | Refugees (385) | WPR | LIC | Cross-Sectional | 5 |
| Kue et al. (2017) [88] | Refugees (97) | AMR | Unspecified | Cross-Sectional | 5 |
| Idehen et al. (2017) [89] | Refugees (197) | EUR | UMIC | Cross-Sectional | 6 |
| Idehen et al. (2017) [89] | Unspecified (291) | EUR | HIC | Cross-Sectional | 6 |
| Idehen et al. (2017) [89] | Unspecified (132) | EUR | LIC | Cross-Sectional | 6 |
| Milenkov et al. (2020) [90] | Refugees (542) | AMR | Unspecified | Cross-Sectional | 6 |
| Lobo et al. (2021) [91] | Unspecified (149) | AMR | Unspecified | Cross-Sectional | 5 |
| Kenny et al. (2021) [92] | Refugees (106) | AMR | LMIC | Cross-Sectional | 5 |
| Elmore et al. (2022) [93] | Refugees (525) | AMR | Unspecified | Cross-Sectional | 10 |
| Whalen-Browne et al. (2022) [94] | International Migrants (99) | AMR | Unspecified | Cross-Sectional | 8 |
| Muhaidat et al. (2022) [95] | Refugees (359) | EMR | HIC | Cross-Sectional | 4 |
| Sánchez et al. (1997) [96] | Unspecified (58) | EUR | Unspecified | Cross-Sectional | 7 |
| Taylor et al. (2003) [97] | International Migrants (22,787) | WPR | LIC | Cohort | 5 |
| Aminisani et al. (2012) [98] | Unspecified (11,477) | WPR | Unspecified | Cross-Sectional | 8 |
| Aminisani et al. (2012) [98] | Unspecified (19,907) | WPR | Unspecified | Cross-Sectional | 8 |
| Khadilkar & Chen (2013) [99] | Unspecified (3420) | AMR | Unspecified | Cross-Sectional | 7 |
| Harcourt et al. (2014) [100] | Unspecified (181) | AMR | LIC | Cross-Sectional | 4 |
| Harcourt et al. (2014) [100] | Unspecified (238) | AMR | Unspecified | Cross-Sectional | 4 |
| Ricardo-Rodrigues et al. (2015) [101] | Unspecified (570) | EUR | Unspecified | Cross-Sectional | 6 |
| Visioli et al. (2015) [102] | Unspecified (13,535) | EUR | Unspecified | Cross-Sectional | 8 |
| Visioli et al. (2015) [102] | Unspecified (16,427) | EUR | Unspecified | Cross-Sectional | 8 |
| Visioli et al. (2015) [102] | Unspecified (22,319) | EUR | Unspecified | Cross-Sectional | 8 |
| Forney-Gorman & Kozhimannil (2016) [103] | Unspecified (36) | AMR | Unspecified | Cross-Sectional | 7 |
| Campari et al. (2016) [104] | Unspecified (516,291) | EUR | Unspecified | Cross-Sectional | 6 |
| Leinonen et al. (2017) [105] | Unspecified (112,801) | EUR | HIC | Cross-Sectional | 9 |
| Leinonen et al. (2017) [105] | Unspecified (58,644) | EUR | UMIC | Cross-Sectional | 9 |
| Leinonen et al. (2017) [105] | Unspecified (58,888) | EUR | LMIC | Cross-Sectional | 9 |
| Leinonen et al. (2017) [105] | Unspecified (19,260) | EUR | LIC | Cross-Sectional | 9 |
| Leinonen et al. (2017) [105] | Unspecified (67,999) | EUR | Unspecified | Cross-Sectional | 9 |
| Møen et al. (2017) [106] | Unspecified (152,800) | EUR | Unspecified | Cross-Sectional | 8 |
| Cofie et al. (2018) [107] | Unspecified (4278) | AMR | Unspecified | Cross-Sectional | 8 |
| Luque et al. (2018) [108] | Unspecified (196) | AMR | Unspecified | Cross-Sectional | 6 |
| Idehen et al. (2018) [109] | Unspecified (167) | EUR | Unspecified | Cross-Sectional | 7 |
| Idehen et al. (2018) [109] | Unspecified (257) | EUR | UMIC | Cross-Sectional | 7 |
| Idehen et al. (2018) [109] | Unspecified (113) | EUR | LIC | Cross-Sectional | 7 |
| Campostrini et al. (2019) [110] | Unspecified (5576) | EUR | Unspecified | Cross-Sectional | 7 |
| Sassenou et al. (2023) [111] | Unspecified (4891) | EUR | Unspecified | Cross-Sectional | 5 |
| Marques et al. (2023) [112] | Unspecified (1100) | EUR | Unspecified | Cross-Sectional | 7 |
| Al-Oseely et al. (2023) [113] | Unspecified (355) | WPR | LIC | Cross-Sectional | 6 |
| Fink et al. (2023) [114] | Unspecified (675) | AMR | Unspecified | Cross-Sectional | 4 |
| Azzani et al. (2023) [115] | Unspecified (370) | WPR | LIC | Cross-Sectional | 6 |
References
- Khan, I.; Harshithkumar, R.; More, A.; Mukherjee, A. Human Papilloma Virus: An Unraveled Enigma of Universal Burden of Malignancies. Pathogens 2023, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.; Durden, A.; Sundararajan, S.; Al-Booz, H.; Newton, C. Review of invasive cervical cancer. Obstet. Gynaecol. Reprod. Med. 2023, 33, 281–285. [Google Scholar] [CrossRef]
- Seyoum, A.; Assefa, N.; Gure, T.; Seyoum, B.; Mulu, A.; Mihret, A. Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection Among Sub-Saharan African Women: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 890880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 30 October 2024).
- Ardekani, A.; Sepidarkish, M.; Mollalo, A.; Afradiasbagharani, P.; Rouholamin, S.; Rezaeinejad, M.; Farid-Mojtahedi, M.; Mahjour, S.; Almukhtar, M.; Nourollahpour Shiadeh, M.; et al. Worldwide prevalence of human papillomavirus among pregnant women: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2374. [Google Scholar] [CrossRef]
- Zhetpisbayeva, I.; Kassymbekova, F.; Sarmuldayeva, S.; Semenova, Y.; Glushkova, N. Cervical Cancer Prevention in Rural Areas. Ann. Glob. Health 2023, 89, 75. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, E.; Boily, M.-C.; Ali, H.; Baandrup, L.; Bauer, H.; Beddows, S.; Brisson, J.; Brotherton, J.M.L.; Cummings, T.; et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2015, 15, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Kinney, W.K.; Cheung, L.C.; Gage, J.C.; Fetterman, B.; Poitras, N.E.; Lorey, T.S.; Wentzensen, N.; Befano, B.; Schussler, J.; et al. Why does cervical cancer occur in a state-of-the-art screening program? Gynecol. Oncol. 2017, 146, 546–553. [Google Scholar] [CrossRef]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and Cervical Cancer: A Review of Epidemiology and Screening Uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef]
- WHO. Cervical Cancer. World Health Organization Website. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer?gclid=CjwKCAiAk9itBhASEiwA1my_69sUl9sTor9BNLOUnMPxJFd1RbaSs_I4-9Z3mfyvs1BAOfQFMkOwFxoCLtwQAvD_BwE (accessed on 28 January 2024).
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef] [PubMed]
- Elemes, S.; Stachteas, P.; Haidich, A.B.; Mamopoulos, A.; Smyrnakis, E. The Impact of the COVID-19 Pandemic on Breast and Cervical Cancer Screening: A Systematic Review. In Vivo 2023, 37, 1455–1476. [Google Scholar] [CrossRef]
- Swanson, A.A.; Pantanowitz, L. The evolution of cervical cancer screening. J. Am. Soc. Cytopathol. 2024, 13, 10–15. [Google Scholar] [CrossRef]
- Waller, J.; McCaffery, K.; Forrest, S.; Szarewski, A.; Cadman, L.; Austin, J.; Wardle, J. Acceptability of unsupervised HPV self-sampling using written instructions. J. Med. Screen. 2006, 13, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Strelow, B.; O’Laughlin, D. Barriers to cervical cancer screening among immigrants. JAAPA 2022, 35, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, A.; Myers, K.; Ashby, J.; Trinh, H.N.; Nourian, M.M.; Reel, J.J. Women in Free Clinics: An Assessment of Health-Related Quality of Life for Prevention and Health Education. J. Community Health 2015, 40, 793–801. [Google Scholar] [CrossRef]
- Ponce-Chazarri, L.; Ponce-Blandón, J.A.; Immordino, P.; Giordano, A.; Morales, F. Barriers to Breast Cancer-Screening Adherence in Vulnerable Populations. Cancers 2023, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Vahabi, M.; Lofters, A. Acceptability, Feasibility and Uptake of HPV Self-Sampling Among Immigrant Minority Women: A Focused Literature Review. J. Immigr. Minor. Health 2019, 21, 1380–1393. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Moher, D.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Convention Relating to the Status of Refugees. United Nations Conference of Plenipotentiaries on the Status of Refugees and Stateless Persons. 1951. Available online: https://www.ohchr.org/sites/default/files/refugees.pdf (accessed on 26 December 2023).
- WHO. Organizational Structure. Available online: https://www.who.int/about/structure (accessed on 25 July 2024).
- The World Bank. World Development Indicators—Historical Classification by Income. Available online: https://datacatalog.worldbank.org/search/dataset/0037712 (accessed on 25 July 2024).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 July 2024).
- Payton, C.; Zeidan, A.; Bogen, H.; Altshuler, M. Women’s Health Screening and Mapped Community Resources for Refugees Resettled in Philadelphia, Pennsylvania. J. Health Care Poor Underserved 2020, 31, 958–972. [Google Scholar] [CrossRef]
- Skaer, T.L.; Robison, L.M.; Sclar, D.A.; Harding, G.H. Knowledge, attitudes, and patterns of cancer screening: A self-report among foreign born Hispanic women utilizing rural migrant health clinics. J. Rural Health 1996, 12, 169–177. [Google Scholar] [CrossRef]
- Skaer, T.L.; Robison, L.M.; Sclar, D.A.; Harding, G.H. Cancer-screening determinants among Hispanic women using migrant health clinics. J. Health Care Poor Underserved 1996, 7, 338–354. [Google Scholar] [CrossRef]
- Taylor, V.M.; Jackson, J.C.; Yasui, Y.; Schwartz, S.M.; Kuniyuki, A.; Fischer, M.; Tu, S.P. Pap Testing Stages of Adoption among Cambodian Immigrants. Asian Am. Pac. Isl. J. Health 2000, 8, 58–68. [Google Scholar]
- Taylor, R.J.; Mamoon, H.A.; Morrell, S.L.; Wain, G.V. Cervical screening in migrants to Australia. Aust. N. Z. J. Public Health 2001, 25, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Jirojwong, S.; Maclennan, R.; Manderson, L. Health beliefs and Pap smears among Thai women in Brisbane, Australia. Asia-Pac. J. Public Health 2001, 13, 20–23. [Google Scholar] [CrossRef]
- Wolff, H.; Stalder, H.; Epiney, M.; Walder, A.; Irion, O.; Morabia, A. Health care and illegality: A survey of undocumented pregnant immigrants in Geneva. Soc. Sci. Med. (1982) 2005, 60, 2149–2154. [Google Scholar] [CrossRef]
- Lee-Lin, F.; Pett, M.; Menon, U.; Lee, S.; Nail, L.; Mooney, K.; Itano, J. Cervical cancer beliefs and pap test screening practices among Chinese American immigrants. Oncol. Nurs. Forum 2007, 34, 1203–1209. [Google Scholar] [CrossRef]
- Wolff, H.; Epiney, M.; Lourenco, A.P.; Costanza, M.C.; Delieutraz-Marchand, J.; Andreoli, N.; Dubuisson, J.B.; Gaspoz, J.M.; Irion, O. Undocumented migrants lack access to pregnancy care and prevention. BMC Public Health 2008, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Paterson, J.; DeSouza, R.; Lu, T. Demographic predictors of cervical cancer screening in Chinese women in New Zealand. N. Z. Med. J. 2008, 121, 8–17. [Google Scholar]
- Castañeda, S.F.; Rosenbaum, R.P.; Gonzalez, P.; Holscher, J.T. Breast and cervical cancer screening among rural midwestern latina migrant and seasonal farmworkers. J. Prim. Care Community Health 2012, 3, 104–110. [Google Scholar] [CrossRef]
- Aminisani, N.; Armstrong, B.K.; Canfell, K. Participation in cervical screening by older asian and middle eastern migrants in new South wales, australia. Health Promot. Perspect. 2012, 2, 274–286. [Google Scholar] [CrossRef]
- Knoff, J.S.; Harlow, S.D.; Yassine, M.; Soliman, A.S. Cervical cancer screening practice and knowledge among Hispanic migrant and seasonal farmworkers of Michigan. J. Prim. Care Community Health 2013, 4, 209–215. [Google Scholar] [CrossRef]
- Luque, J.S.; Tarasenko, Y.N.; Maupin, J.N.; Alfonso, M.L.; Watson, L.C.; Reyes-Garcia, C.; Ferris, D.G. Cultural beliefs and understandings of cervical cancer among Mexican immigrant women in Southeast Georgia. J. Immigr. Minor. Health 2015, 17, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Seay, J.S.; Carrasquillo, O.; Campos, N.G.; McCann, S.; Amofah, A.; Pierre, L.; Kobetz, E. Cancer Screening Utilization Among Immigrant Women in Miami, Florida. Prog. Community Health Partnersh. 2015, 9, 11–20. [Google Scholar] [CrossRef]
- Chen, W.T. Chinese female immigrants english-speaking ability and breast and cervical cancer early detection practices in the New York metropolitan area. Asian Pac. J. Cancer Prev. 2013, 14, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Anaman, J.A.; Correa-Velez, I.; King, J. A survey of cervical screening among refugee and non-refugee African immigrant women in Brisbane, Australia. Health Promot. J. Aust. 2017, 28, 217–224. [Google Scholar] [CrossRef]
- Guerrero, N.; Zhang, X.; Rangel, G.; Gonzalez-Fagoaga, J.E.; Martinez-Donate, A. Cervical and Breast Cancer Screening Among Mexican Migrant Women, 2013. Prev. Chronic Dis. 2016, 13, E104. [Google Scholar] [CrossRef]
- Lee, F.H.; Wang, H.H.; Yang, Y.M.; Huang, J.J.; Tsai, H.M. Influencing Factors of Intention to Receive Pap Tests in Vietnamese Women who Immigrated to Taiwan for Marriage. Asian Nurs. Res. 2016, 10, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.L.; White, M.C. Breast and cervical cancer screening among Asian subgroups in the USA: Estimates from the National Health Interview Survey, 2008, 2010, and 2013. Cancer Causes Control 2016, 27, 825–829. [Google Scholar] [CrossRef]
- Lee, F.H.; Wang, H.H.; Tsai, H.M.; Lin, M.L. Factors associated with receiving Pap tests among married immigrant women of Vietnamese origin in southern Taiwan. Women Health 2016, 56, 243–256. [Google Scholar] [CrossRef]
- Comparetto, C.; Epifani, C.; Manca, M.C.; Lachheb, A.; Bravi, S.; Cipriani, F.; Bellomo, F.; Olivieri, S.; Fiaschi, C.; Di Marco, L.; et al. Uptake of cervical cancer screening among the migrant population of Prato Province, Italy. Int. J. Gynaecol. Obstet. 2017, 136, 309–314. [Google Scholar] [CrossRef]
- Bianco, A.; Larosa, E.; Pileggi, C.; Nobile, C.G.A.; Pavia, M. Cervical and breast cancer screening participation and utilisation of maternal health services: A cross-sectional study among immigrant women in Southern Italy. BMJ Open 2017, 7, e016306. [Google Scholar] [CrossRef] [PubMed]
- Tung, W.C.; Lu, M.; Granner, M. Perceived Benefits and Barriers of Cervical Cancer Screening Among Chinese American Women. Oncol. Nurs. Forum 2017, 44, 247–254. [Google Scholar] [CrossRef]
- Ting, Y.H.; Tse, H.Y.; Lam, W.C.; Chan, K.S.; Leung, T.Y. The pattern of cervical smear abnormalities in marginalised women in Hong Kong. Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2017, 23, 28–34. [Google Scholar] [CrossRef]
- Bacal, V.; Blinder, H.; Momoli, F.; Wu, K.Y.; McFaul, S. Is Immigrant Status Associated with Cervical Cancer Screening Among Women in Canada? Results From a Cross-Sectional Study. J. Obstet. Gynaecol. Can. 2019, 41, 824–831.e1. [Google Scholar] [CrossRef]
- Crampe-Casnabet, C.; Franck, J.E.; Ringa, V.; Coeuret-Pellicer, M.; Chauvin, P.; Menvielle, G. Role of obesity in differences in cervical cancer screening rates by migration history. The CONSTANCES survey. Cancer Epidemiol. 2018, 58, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Seo, S.S.; Myong, J.P.; Koo, J.W.; Jeong, J. Factors Associated with Cervical Cancer Screening among Married Female Immigrants with Korean Husbands in South Korea. Int. J. Environ. Res. Public Health 2018, 15, 2528. [Google Scholar] [CrossRef]
- Uysal Toraman, A.; Yildirim, N. Knowledge About Cervical Cancer Risk Factors and Practices of Pap Testing Among Turkish Immigrant Women in the United States. J. Immigr. Minor. Health 2018, 20, 1222–1229. [Google Scholar] [CrossRef]
- Datta, G.D.; Blair, A.; Sylvestre, M.P.; Gauvin, L.; Drouin, M.; Mayrand, M.H. Cervical cancer screening in Montreal: Building evidence to support primary care and policy interventions. Prev. Med. 2018, 111, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Harder, E.; Juul, K.E.; Jensen, S.M.; Thomsen, L.T.; Frederiksen, K.; Kjaer, S.K. Factors associated with non-participation in cervical cancer screening—A nationwide study of nearly half a million women in Denmark. Prev. Med. 2018, 111, 94–100. [Google Scholar] [CrossRef]
- Hagopian, G.S.; Lieber, M.; Dottino, P.R.; Margaret Kemeny, M.; Li, X.; Overbey, J.; Clark, L.D.; Beddoe, A.M. The impact of nativity on cervical cancer survival in the public hospital system of Queens, New York. Gynecol. Oncol. 2018, 149, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Burchell, A.N.; Kendall, C.E.; Cheng, S.Y.; Lofters, A.; Cotterchio, M.; Bayoumi, A.M.; Glazier, R.H.; Antoniou, T.; Raboud, J.; Yudin, M.H.; et al. Cervical cancer screening uptake among HIV-positive women in Ontario, Canada: A population-based retrospective cohort study. Prev. Med. 2018, 107, 14–20. [Google Scholar] [CrossRef]
- Kim, K.; Xue, Q.L.; Walton-Moss, B.; Nolan, M.T.; Han, H.R. Decisional balance and self-efficacy mediate the association among provider advice, health literacy and cervical cancer screening. Eur. J. Oncol. Nurs. 2018, 32, 55–62. [Google Scholar] [CrossRef]
- Cofie, L.E.; Hirth, J.M.; Guo, F.; Berenson, A.B.; Markides, K.; Wong, R. HPV Vaccination Among Foreign-Born Women: Examining the National Health Interview Survey 2013–2015. Am. J. Prev. Med. 2018, 54, 20–27. [Google Scholar] [CrossRef]
- Patel, H.; Sherman, S.M.; Tincello, D.; Moss, E.L. Awareness of and attitudes towards cervical cancer prevention among migrant Eastern European women in England. J. Med. Screen. 2020, 27, 40–47. [Google Scholar] [CrossRef]
- Lofters, A.K.; Kopp, A.; Vahabi, M.; Glazier, R.H. Understanding those overdue for cancer screening by five years or more: A retrospective cohort study in Ontario, Canada. Prev. Med. 2019, 129, 105816. [Google Scholar] [CrossRef]
- Eo, Y.S.; Kim, J.S. Associations of health belief and health literacy with Pap smear practice among Asian immigrant women. Eur. J. Oncol. Nurs. 2019, 42, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Berens, E.M.; Mohwinkel, L.M.; van Eckert, S.; Reder, M.; Kolip, P.; Spallek, J. Uptake of Gynecological Cancer Screening and Performance of Breast Self-Examination Among 50-Year-Old Migrant and Non-migrant Women in Germany: Results of a Cross-Sectional Study (InEMa). J. Immigr. Minor. Health 2019, 21, 674–677. [Google Scholar] [CrossRef]
- Han, H.R.; Kim, K.; Cudjoe, J.; Kim, M.T. Familiarity, Navigation, and Comprehension: Key Dimensions of Health Literacy in Pap Test Use among Korean American Women. J. Health Commun. 2019, 24, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.N.S.; So, W.K.W.; Choi, K.C.; Gurung, S. Development of an explanatory model to explore cervical cancer screening behaviour among South Asian women: The influence of multilevel factors. Eur. J. Oncol. Nurs. 2019, 40, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hertzum-Larsen, R.; Kjær, S.K.; Frederiksen, K.; Thomsen, L.T. Participation in cervical cancer screening among immigrants and Danish-born women in Denmark. Prev. Med. 2019, 123, 55–64. [Google Scholar] [CrossRef]
- Idehen, E.E.; Virtanen, A.; Lilja, E.; Tuomainen, T.P.; Korhonen, T.; Koponen, P. Cervical Cancer Screening Participation among Women of Russian, Somali, and Kurdish Origin Compared with the General Finnish Population: A Register-Based Study. Int. J. Environ. Res. Public Health 2020, 17, 7899. [Google Scholar] [CrossRef]
- Brzoska, P.; Aksakal, T.; Yilmaz-Aslan, Y. Disparities in the use of regular pap smears among migrant and non-migrant women in Austria: A population-based survey of 7633 women. J. Med. Screen. 2020, 28, 372–376. [Google Scholar] [CrossRef]
- Brzoska, P.; Aksakal, T.; Yilmaz-Aslan, Y. Utilization of cervical cancer screening among migrants and non-migrants in Germany: Results from a large-scale population survey. BMC Public Health 2020, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Poncet, L.; Panjo, H.; Ringa, V.; Andro, A. Do vulnerable groups access prevention services? Cervical cancer screening and HIV testing among homeless migrant women in the Paris metropolitan area. PLoS ONE 2021, 16, e0255900. [Google Scholar] [CrossRef]
- Adegboyega, A.; Wiggins, A.T.; Williams, L.B.; Dignan, M. HPV Testing Behaviors and Willingness to Use HPV Self-sampling at Home Among African American (AA) and Sub-Saharan African Immigrant (SAI) Women. J. Racial Ethn. Health Disparities 2021, 9, 2485–2494. [Google Scholar] [CrossRef]
- Jang, S.H.; Meischke, H.; Ko, L.K. The impact of medical tourism on cervical cancer screening among immigrant women in the U.S. BMC Women’s Health 2021, 21, 414. [Google Scholar] [CrossRef]
- Adegboyega, A.; Wu, J.R.; Mudd-Martin, G. Acculturation Strategies and Pap Screening Uptake among Sub-Saharan African Immigrants (SAIs). Int. J. Environ. Res. Public Health 2021, 18, 13204. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, P.; Wahidie, D.; Yilmaz-Aslan, Y. An Intersectional Perspective on the Utilization of Cervical Cancer Screening among Migrants. A Cross-Sectional Analysis of Survey Data from Austria. Cancers 2021, 13, 6082. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.M.; Tom, L.; Leung, I.; O’Brian, C.; Zumpf, K.; Simon, M. Associations between Fatalistic Cancer Beliefs and Cancer-Screening Behaviors in Chinese American Immigrant Women. J. Immigr. Minor. Health 2021, 23, 699–706. [Google Scholar] [CrossRef]
- Cudjoe, J.; Budhathoki, C.; Roter, D.; Gallo, J.J.; Sharps, P.; Han, H.R. Exploring Health Literacy and the Correlates of Pap Testing Among African Immigrant Women: Findings from the AfroPap Study. J. Cancer Educ. 2021, 36, 441–451. [Google Scholar] [CrossRef]
- Lee, H.Y.; Choi, Y.J.; Shin, J.; Yoon, Y.J.; An, S. Adherence to Cervical Cancer Screening in Korean American Immigrant Women: Identifying Malleable Variables for Intervention Development. J. Transcult. Nurs. 2021, 32, 230–238. [Google Scholar] [CrossRef]
- Marques, P.; Geraldes, M.; Gama, A.; Heleno, B.; Dias, S. Non-attendance in cervical cancer screening among migrant women in Portugal: A cross-sectional study. Women’s Health 2022, 18, 17455057221093034. [Google Scholar] [CrossRef] [PubMed]
- Adegboyega, A.; Wiggins, A.T.; Obielodan, O.; Dignan, M.; Schoenberg, N. Beliefs associated with cancer screening behaviors among African Americans and Sub-Saharan African immigrant adults: A cross-sectional study. BMC Public Health 2022, 22, 2219. [Google Scholar] [CrossRef]
- Adegboyega, A.; Aroh, A.; Williams, L.B.; Mudd-Martin, G. Social support and cervical cancer screening among sub-Saharan African immigrant (SAI) women. Cancer Causes Control 2022, 33, 823–830. [Google Scholar] [CrossRef]
- Battagello, J.; Monetti, D.; Rizzato, S.; Rosano, A.; Stocco, C.F.; Zamberlan, S.; Rugge, M.; Zorzi, M. Young immigrant women and cervical cancer screening: Participation and lesions detected at the first screening round. Epidemiol. Prev. 2022, 46, 173–180. [Google Scholar] [CrossRef]
- Lamminmäki, M.; Leivonen, A.; Sarkeala, T.; Virtanen, A.; Heinävaara, S. Health inequalities among Russian-born immigrant women in Finland: Longitudinal analysis on cervical cancer incidence and participation in screening. J. Migr. Health 2022, 6, 100117. [Google Scholar] [CrossRef]
- Alam, Z.; Ann Dean, J.; Janda, M. Cervical screening uptake: A cross-sectional study of self-reported screening attitudes, behaviours and barriers to participation among South Asian immigrant women living in Australia. Women’s Health 2022, 18, 17455057221096240. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.M.; Harrison, C.L. Refugee women’s reproductive health in early resettlement. J. Obstet. Gynecol. Neonatal Nurs. 2004, 33, 723–728. [Google Scholar] [CrossRef]
- Morrison, T.B.; Flynn, P.M.; Weaver, A.L.; Wieland, M.L. Cervical cancer screening adherence among Somali immigrants and refugees to the United States. Health Care Women Int. 2013, 34, 980–988. [Google Scholar] [CrossRef]
- Pickle, S.; Altshuler, M.; Scott, K.C. Cervical Cancer Screening Outcomes in a Refugee Population. J. Immigr. Refug. Stud. 2014, 12, 1–8. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Yang, W.; Lee, H.; Park, S.M. Cervical Cancer Screening and Its Associated Factors Among North Korean Defectors Living in South Korea. J. Immigr. Minor. Health 2018, 20, 66–72. [Google Scholar] [CrossRef]
- Kue, J.; Hanegan, H.; Tan, A. Perceptions of Cervical Cancer Screening, Screening Behavior, and Post-Migration Living Difficulties Among Bhutanese-Nepali Refugee Women in the United States. J. Community Health 2017, 42, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Idehen, E.E.; Korhonen, T.; Castaneda, A.; Juntunen, T.; Kangasniemi, M.; Pietilä, A.M.; Koponen, P. Factors associated with cervical cancer screening participation among immigrants of Russian, Somali and Kurdish origin: A population-based study in Finland. BMC Women’s Health 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Milenkov, A.R.; Felini, M.; Baker, E.; Acharya, R.; Longanga Diese, E.; Onsa, S.; Fernando, S.; Chor, H. Uptake of cancer screenings among a multiethnic refugee population in North Texas, 2014–2018. PLoS ONE 2020, 15, e0230675. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.J.; Lin, J.G.; Vais, S.; Wang, D.; Adegoke, T.M.; Wu, W.J.; Steer-Massaro, C. Pap Smear and Mammogram Screening Rates in a Refugee and General OB/GYN Clinic: A Retrospective Review. J. Immigr. Minor. Health 2021, 24, 111–117. [Google Scholar] [CrossRef]
- Kenny, D.X.; Hsueh, K.; Walters, R.W.; Coté, J.J. Human Papillomavirus Vaccination and Pap Smear Rates Among Burmese Refugee Girls in a Healthcare System in Omaha, Nebraska. J. Community Health 2021, 46, 1170–1176. [Google Scholar] [CrossRef]
- Elmore, C.E.; Mitchell, E.M.; Debnam, K.; Keim-Malpass, J.; Laughon, K.; Tanabe, K.O.; Hauck, F.R. Predictors of cervical cancer screening for refugee women attending an international family medicine clinic in the United States. Cancer Causes Control 2022, 33, 1295–1304. [Google Scholar] [CrossRef]
- Whalen-Browne, M.; Talavlikar, R.; Brown, G.; McBrien, K.; Wiedmeyer, M.L.; Norrie, E.; Fabreau, G. Cervical Cancer Screening by Refugee Category in a Refugee Health Primary Care Clinic in Calgary, Canada, 2011–2016. J. Immigr. Minor. Health 2022, 24, 1534–1542. [Google Scholar] [CrossRef]
- Muhaidat, N.; Alshrouf, M.A.; Alshajrawi, R.N.; Miqdadi, Z.R.; Amro, R.; Rabab’ah, A.O.; Qatawneh, S.A.; Albandi, A.M.; Fram, K. Cervical Cancer Screening among Female Refugees in Jordan: A Cross-Sectional Study. Healthcare 2022, 10, 1343. [Google Scholar] [CrossRef]
- Sánchez, V.; Rohlfs, I.; Borràs, J.M.; Borrell, C. Migration within Spain, level of education, and cervical cancer screening. Eur. J. Cancer Prev. 1997, 6, 31–37. [Google Scholar] [CrossRef]
- Taylor, R.J.; Morrell, S.L.; Mamoon, H.A.; Macansh, S.; Ross, J.; Wain, G.V. Cervical cancer screening in a Vietnamese nominal cohort. Ethn. Health 2003, 8, 251–261. [Google Scholar] [CrossRef]
- Aminisani, N.; Armstrong, B.K.; Canfell, K. Cervical cancer screening in Middle Eastern and Asian migrants to Australia: A record linkage study. Cancer Epidemiol. 2012, 36, e394–e400. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, A.; Chen, Y. Rate of cervical cancer screening associated with immigration status and number of years since immigration in Ontario, Canada. J. Immigr. Minor. Health 2013, 15, 244–248. [Google Scholar] [CrossRef]
- Harcourt, N.; Ghebre, R.G.; Whembolua, G.L.; Zhang, Y.; Warfa Osman, S.; Okuyemi, K.S. Factors associated with breast and cervical cancer screening behavior among African immigrant women in Minnesota. J. Immigr. Minor. Health 2014, 16, 450–456. [Google Scholar] [CrossRef]
- Ricardo-Rodrigues, I.; Jiménez-García, R.; Hernández-Barrera, V.; Carrasco-Garrido, P.; Jiménez-Trujillo, I.; López de Andrés, A. Social disparities in access to breast and cervical cancer screening by women living in Spain. Public Health 2015, 129, 881–888. [Google Scholar] [CrossRef]
- Visioli, C.B.; Crocetti, E.; Zappa, M.; Iossa, A.; Andersson, K.L.; Bulgaresi, P.; Alfieri, A.; Amunni, G. Participation and risk of high grade cytological lesions among immigrants and Italian-born women in an organized cervical cancer screening program in Central Italy. J. Immigr. Minor. Health 2015, 17, 670–678. [Google Scholar] [CrossRef]
- Forney-Gorman, A.; Kozhimannil, K.B. Differences in Cervical Cancer Screening Between African-American Versus African-Born Black Women in the United States. J. Immigr. Minor. Health 2016, 18, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Campari, C.; Fedato, C.; Iossa, A.; Petrelli, A.; Zorzi, M.; Anghinoni, E.; Bietta, C.; Brachini, A.; Brezzi, S.; Cogo, C.; et al. Cervical cancer screening in immigrant women in Italy: A survey on participation, cytology and histology results. Eur. J. Cancer Prev. 2016, 25, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, M.K.; Campbell, S.; Ursin, G.; Tropé, A.; Nygård, M. Barriers to cervical cancer screening faced by immigrants: A registry-based study of 1.4 million women in Norway. Eur. J. Public Health 2017, 27, 873–879. [Google Scholar] [CrossRef]
- Møen, K.A.; Kumar, B.; Qureshi, S.; Diaz, E. Differences in cervical cancer screening between immigrants and nonimmigrants in Norway: A primary healthcare register-based study. Eur. J. Cancer Prev. 2017, 26, 521–527. [Google Scholar] [CrossRef]
- Cofie, L.E.; Hirth, J.M.; Wong, R. Chronic comorbidities and cervical cancer screening and adherence among US-born and foreign-born women. Cancer Causes Control 2018, 29, 1105–1113. [Google Scholar] [CrossRef]
- Luque, J.S.; Tarasenko, Y.N.; Li, H.; Davila, C.B.; Knight, R.N.; Alcantar, R.E. Utilization of Cervical Cancer Screening Among Hispanic Immigrant Women in Coastal South Carolina. J. Racial Ethn. Health Disparities 2018, 5, 588–597. [Google Scholar] [CrossRef]
- Idehen, E.E.; Koponen, P.; Härkänen, T.; Kangasniemi, M.; Pietilä, A.M.; Korhonen, T. Disparities in cervical screening participation: A comparison of Russian, Somali and Kurdish immigrants with the general Finnish population. Int. J. Equity Health 2018, 17, 56. [Google Scholar] [CrossRef]
- Campostrini, S.; Carrozzi, G.; Severoni, S.; Masocco, M.; Salmaso, S.; WHO Migration Health Programme; Office of the Regional Director; WHO Regional Office for Europe; PASSI National Coordinating Group. Migrant health in Italy: A better health status difficult to maintain-country of origin and assimilation effects studied from the Italian risk factor surveillance data. Popul. Health Metr. 2019, 17, 14. [Google Scholar] [CrossRef]
- Sassenou, J.; Ringa, V.; Zins, M.; Ozguler, A.; Paquet, S.; Panjo, H.; Franck, J.E.; Menvielle, G.; Rigal, L. Combined influence of immigration status and income on cervical cancer screening uptake. Prev. Med. Rep. 2023, 36, 102363. [Google Scholar] [CrossRef]
- Marques, P.; Geraldes, M.; Gama, A.; Heleno, B.; Dias, S. What is the role of attitudinal barriers on cervical cancer screening non-attendance? Findings from a cross-sectional study with migrant women in Portugal. BMC Women’s Health 2023, 23, 52. [Google Scholar] [CrossRef]
- Al-Oseely, S.; Abdul Manaf, R.; Ismail, S. Factors affecting cervical cancer screening among Yemeni immigrant women in Klang Valley, Malaysia: A Cross-Sectional study. PLoS ONE 2023, 18, e0290152. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.; Abdulcadir, J.; Johnson-Agbakwu, C.E. Rates of Cervical Cancer Screening and Dysplasia Among Refugees in a Health Care Safety Net System. J. Immigr. Minor. Health 2023, 25, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Azzani, M.; Ba-Alawi, E.; Atroosh, W.M.; Yadav, H. Awareness of cervical cancer and its associated socio-demographic factors among Yemeni immigrant women in Malaysia. BMC Women’s Health 2023, 23, 19. [Google Scholar] [CrossRef]
- Bhatta, M.P.; Johnson, D.C.; Lama, M.; Maharjan, B.; Lhaki, P.; Shrestha, S. Cervical Cancer and Human Papillomavirus Vaccine Awareness Among Married Bhutanese Refugee and Nepali Women in Eastern Nepal. J. Community Health 2020, 45, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Elmore, C.E.; Keim-Malpass, J.; Mitchell, E.M. Health Inequity in Cervical Cancer Control Among Refugee Women in the United States by Country of Origin. Health Equity 2021, 5, 119–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miranda, P.Y.; Yao, N.; Snipes, S.A.; BeLue, R.; Lengerich, E.; Hillemeier, M.M. Citizenship, length of stay, and screening for breast, cervical, and colorectal cancer in women, 2000–2010. Cancer Causes Control 2017, 28, 589–598. [Google Scholar] [CrossRef]
- Islam, R.M.; Billah, B.; Hossain, M.N.; Oldroyd, J. Barriers to Cervical Cancer and Breast Cancer Screening Uptake in Low-Income and Middle-Income Countries: A Systematic Review. Asian Pac. J. Cancer Prev. 2017, 18, 1751–1763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marques, P.; Nunes, M.; Antunes, M.D.L.; Heleno, B.; Dias, S. Factors associated with cervical cancer screening participation among migrant women in Europe: A scoping review. Int. J. Equity Health 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, N.Y.; Hossain, S.Z.; Mackey, M.; Adam, S.; Brennan, P. HPV and Cervical Cancer Awareness and Screening Practices among Migrant Women: A Narrative Review. Healthcare 2024, 12, 709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holt, H.K.; Zhang, X.; Hu, S.Y.; Zhao, F.H.; Smith, J.S.; Qiao, Y.L. Inequalities in Cervical Cancer Screening Uptake Between Chinese Migrant Women and Local Women: A Cross-Sectional Study. Cancer Control J. Moffitt Cancer Cent. 2021, 28, 1073274820985792. [Google Scholar] [CrossRef] [PubMed]
- Dossier Statistico Immigrazione 2023. Italia, Europa e le Nuove Politiche Migratorie. Dati e Riflessioni Nel Nuovo Rapporto a Cura Del Centro Studi e Ricerche Idos. Available online: https://integrazionemigranti.gov.it/it-it/Ricerca-news/Dettaglio-news/id/3478/Dossier-statistico-immigrazione-023#:~:text=A%20fine%202022%20si%20stimano,essi%20%C3%A8%20costituito%20da%20minorenn (accessed on 14 August 2024).
- Graci, D.; Piazza, N.; Ardagna, S.; Casuccio, A.; Drobov, A.; Geraci, F.; Immordino, A.; Pirrello, A.; Restivo, V.; Rumbo, R.; et al. Barriers to and Facilitators for Accessing HPV Vaccination in Migrant and Refugee Populations: A Systematic Review. Vaccines 2024, 12, 256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Essa-Hadad, J.; Gorelik, Y.; Vervoort, J.; Jansen, D.; Edelstein, M. Understanding the health system barriers and enablers to childhood MMR and HPV vaccination among disadvantaged, minority or underserved populations in middle- and high-income countries: A systematic review. Eur. J. Public Health 2024, 34, 368–374. [Google Scholar] [CrossRef]
- Rosato, I.; Dalla Zuanna, T.; Tricarico, V.; Barbiellini Amidei, C.; Canova, C. Adherence to Cervical Cancer Screening Programs in Migrant Populations: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Refugee and Migrant Health: Global Competency Standards for Health Workers (The Standards). 2021. Available online: https://www.who.int/publications/i/item/9789240030626 (accessed on 31 October 2024).
- Ghebrendrias, S.; Pfeil, S.; Crouthamel, B.; Chalmiers, M.; Kully, G.; Mody, S. An Examination of Misconceptions and Their Impact on Cervical Cancer Prevention Practices among Sub-Saharan African and Middle Eastern Refugees. Health Equity 2021, 5, 382–389. [Google Scholar] [CrossRef]


| Adherence | Participation at Least Once | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IM | R | Mix | Total | IM | R | Mix | Total | ||
| Cross-Sectional | 57% (95% CI: 53–61) | 55% (95% CI: 40–70) | 55% (95% CI: 48–62) | 56% (95% CI: 53–60) | 66% (95% CI: 63–68) | 52% (95% CI: 37–66) | 56% (95% CI: 47–64) | 60% (95% CI: 54–65) | |
| Cohort | 62% (95% CI: 62–62) | - | 52% (95% CI: 46–59) | 55% (95% CI: 50–59) | 61% (95% CI: 52–70) | - | 52% (95% CI: 46–59) | 56% (95% CI: 52–61) | |
| AMR | EUR | WPR | Total | AMR | EUR | WPR | EMR | Total | |
| Cross-Sectional | 58% (95% CI: 53–62) | 60% (95% CI: 54–65) | 47% (95% CI: 34–60) | 56% (95% CI: 53–60) | 67% (95% CI: 65–68) | 61% (95% CI: 52–70) | 50% (95% CI: 41–58) | 13% (95% CI: 10–17) | 60% (95% CI: 54–65) |
| High income | Low income | Unspecified | Total | High income | Low income | Unspecified | Total | ||
| Cross-Sectional | 63% (95% CI: 60–65) | 47% (95% CI: 42–52) | 58% (95% CI: 54–63) | 56% (95% CI: 53–60) | 66% (95% CI: 62–69) | 47% (95% CI: 41–52) | 62% (95% CI: 55–69) | 60% (95% CI: 54–65) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restivo, V.; Graci, D.; Immordino, A.; Mancuso, D.G.; Morales, F.; Pace, C.; Pirrello, A.; Casuccio, A.; Immordino, P. Cervical Cancer Screening in Refugee and Migrant Populations: Results of Systematic Review and Meta-Analysis in Cross-Sectional and Cohort Studies. Cancers 2025, 17, 2966. https://doi.org/10.3390/cancers17182966
Restivo V, Graci D, Immordino A, Mancuso DG, Morales F, Pace C, Pirrello A, Casuccio A, Immordino P. Cervical Cancer Screening in Refugee and Migrant Populations: Results of Systematic Review and Meta-Analysis in Cross-Sectional and Cohort Studies. Cancers. 2025; 17(18):2966. https://doi.org/10.3390/cancers17182966
Chicago/Turabian StyleRestivo, Vincenzo, Davide Graci, Angelo Immordino, Daniele Giacomo Mancuso, Fátima Morales, Chiara Pace, Alessandra Pirrello, Alessandra Casuccio, and Palmira Immordino. 2025. "Cervical Cancer Screening in Refugee and Migrant Populations: Results of Systematic Review and Meta-Analysis in Cross-Sectional and Cohort Studies" Cancers 17, no. 18: 2966. https://doi.org/10.3390/cancers17182966
APA StyleRestivo, V., Graci, D., Immordino, A., Mancuso, D. G., Morales, F., Pace, C., Pirrello, A., Casuccio, A., & Immordino, P. (2025). Cervical Cancer Screening in Refugee and Migrant Populations: Results of Systematic Review and Meta-Analysis in Cross-Sectional and Cohort Studies. Cancers, 17(18), 2966. https://doi.org/10.3390/cancers17182966