Evaluation of a Novel Pan-RAS Inhibitor in 3D Bioprinted Tumor Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioprinting 3D Tumor Models
2.2. High-Throughput Drug Treatment
2.3. High-Content Imaging
2.4. ATP Cell Viability Assay
2.5. Apoptosis Assay
2.6. Generative AI Disclosure
3. Results
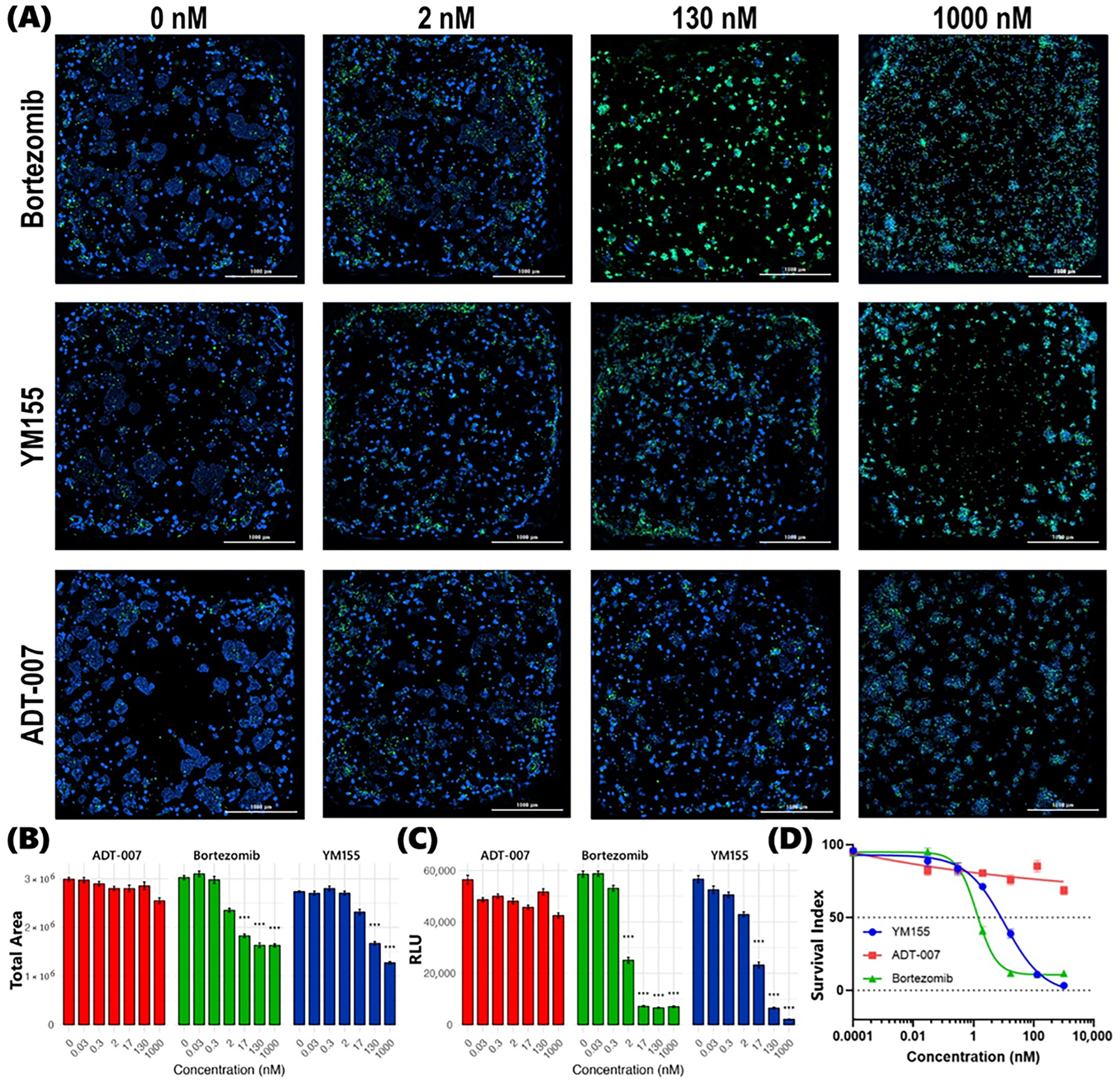
3.1. RAS Selective Inhibition of CRC Cell Growth by ADT-007
3.2. RAS Selective Apoptosis Induction by ADT-007
3.3. Differential Response in KRAS-Mutant CRC BEST
3.4. Differential Response in WT RAS CRC BEST
3.5. Summary of Differential Response in CRC BEST
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BEST | Bioprinted ex vivo Slice Tissue |
| CRC | Colorectal cancer |
| WT | Wild-type |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Deo, S.V.S.; Kumar, S.; Bhoriwal, S.; Shukla, N.K.; Sharma, A.; Thulkar, S.; Das, P.; Bhagat, P.; Dhall, K.; Pathy, S.; et al. Colorectal Cancers in Low- and Middle-Income Countries—Demographic Pattern and Clinical Profile of 970 Patients Treated at a Tertiary Care Cancer Center in India. JCO Glob. Oncol. 2021, 7, 1110–1115. [Google Scholar] [CrossRef]
- Abdelgadir, O.; Kuo, Y.-F.; Khan, M.F.; Okorodudu, A.O.; Cheng, Y.-W.; Dong, J. Mortality Outcome Associated with Specific KRAS, NRAS, and BRAF Hot-Spot Mutations in Metastatic Colorectal Cancer Patients: A Retrospective Cohort Study. Diagnostics 2025, 15, 590. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The frequency of ras mutations in cancer. Cancer Res. 2020, 80, 2669–2974. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.G.; Esposito, D.; Bagni, R.K.; McCormick, F. Dragging ras back in the ring. Cancer Cell 2014, 25, 272–281. [Google Scholar] [CrossRef]
- Charette, N.; Vandeputte, C.; Stärkel, P. Ras in digestive oncology: From molecular biology to clinical implications. Curr. Opin. Oncol. 2014, 26, 454–461. [Google Scholar] [CrossRef]
- Piazza, G.A.; Chandrasekaran, P.; Maxuitenko, Y.Y.; Budhwani, K.I. Assessment of KRASG12C inhibitors for colorectal cancer. Front. Oncol. 2024, 14, 1412435. [Google Scholar] [CrossRef] [PubMed]
- Porru, M.; Pompili, L.; Caruso, C.; Biroccio, A.; Leonetti, C. Targeting kras in metastatic colorectal cancer: Current strategies and emerging opportunities. J. Exp. Clin. Cancer Res. 2018, 37, 57. [Google Scholar] [CrossRef]
- Bteich, F.; Mohammadi, M.; Li, T.; Bhat, M.A.; Sofianidi, A.; Wei, N.; Kuang, C. Targeting KRAS in Colorectal Cancer: A Bench to Bedside Review. Int. J. Mol. Sci. 2023, 24, 12030. [Google Scholar] [CrossRef]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission Possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef]
- Papke, B.; Der, C.J. Drugging RAS: Know the enemy. Science 2017, 355, 1158–1163. [Google Scholar] [CrossRef]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Mackay, H.; Hedley, D.; Major, P.; Townsley, C.; Mackenzie, M.; Vincent, M.; Degendorfer, P.; Tsao, M.-S.; Nicklee, T.; Birle, D.; et al. A Phase II Trial with Pharmacodynamic Endpoints of the Proteasome Inhibitor Bortezomib in Patients with Metastatic Colorectal Cancer. Clin. Cancer Res. 2005, 11, 5526–5533. [Google Scholar] [CrossRef]
- Cusack, J.C.; Liu, R.; Houston, M.; Abendroth, K.; Elliott, P.J.; Adams, J.; Baldwin, A.S. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: Implications for systemic nuclear factor-κB inhibition. Cancer Res. 2001, 61, 3535–3540. [Google Scholar]
- Altieri, D.C. Survivin, cancer networks and pathway-directed drug discovery. Nat. Rev. Cancer 2008, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.; Werner, T.A.; Verde, P.E.; Stoecklein, N.H.; Knoefel, W.T.; Srinivasula, S.M. Prognostic and Clinicopathological Significance of Survivin in Colorectal Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e65338. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Carlstedt, A.; Emmerich, C.; Mustafa, A.-H.M.; Göder, A.; Knauer, S.K.; Linnebacher, M.; Heinzel, T.; Krämer, O.H. Survivin antagonizes chemotherapy-induced cell death of colorectal cancer cells. Oncotarget 2018, 9, 27835–27850. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Ji, S.-Y.; Mia-Jan, K.; Cho, M.-Y. Chemoresistance of CD133+ colon cancer may be related with increased survivin expression. Biochem. Biophys. Res. Commun. 2015, 463, 229–234. [Google Scholar] [CrossRef]
- Kim, S.T.; Sohn, I.; Do, I.-G.; Jang, J.; Kim, S.H.; Jung, I.H.; Park, J.O.; Park, Y.S.; Talasaz, A.; Lee, J.; et al. Transcriptome analysis of CD133-positive stem cells and prognostic value of survivin in colorectal cancer. Cancer Genom. Proteom. 2014, 11, 259–266. [Google Scholar] [CrossRef]
- Rauch, A.; Hennig, D.; Schäfer, C.; Wirth, M.; Marx, C.; Heinzel, T.; Schneider, G.; Krämer, O.H. Survivin and YM155: How faithful is the liaison? Biochim. Biophys. Acta-Rev. Cancer 2014, 1845, 202–220. [Google Scholar] [CrossRef]
- Xiao, M.; Li, W. Recent Advances on Small-Molecule Survivin Inhibitors. Curr. Med. Chem. 2015, 22, 1136–1146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukuda, S.; Pelus, L.M. Survivin, a cancer target with an emerging role in normal adult tissues. Mol. Cancer Ther. 2006, 5, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Takeuchi, M.; Kinoyama, I.; Minematsu, T.; Shirasuna, K.; Matsuhisa, A.; Kita, A.; Tominaga, F.; Yamanaka, K.; Kudoh, M.; et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007, 67, 8014–8021. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRAS G12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Jänne, P.; Rybkin, I.; Spira, A.; Riely, G.; Papadopoulos, K.; Sabari, J.; Johnson, M.; Heist, R.; Bazhenova, L.; Barve, M.; et al. KRYSTAL-1: Activity and Safety of Adagrasib (MRTX849) in Advanced/Metastatic Non–Small-Cell Lung Cancer (NSCLC) Harboring KRAS G12C Mutation. Eur. J. Cancer 2020, 138, S1–S2. [Google Scholar] [CrossRef]
- Vasan, N.; Boyer, J.L.; Herbst, R.S. A RAS Renaissance: Emerging Targeted Therapies for KRAS-Mutated Non–Small Cell Lung Cancer. Clin. Cancer Res. 2014, 20, 3921–3930. [Google Scholar] [CrossRef]
- Ryan, M.B.; de la Cruz, F.F.; Phat, S.; Myers, D.T.; Wong, E.; Shahzade, H.A.; Hong, C.B.; Corcoran, R.B. Vertical pathway inhibition overcomes adaptive feedback resistance to KrasG12C inhibition. Clin. Cancer Res. 2020, 26, 1633–1643. [Google Scholar] [CrossRef]
- McCormick, F. KRAS as a therapeutic target. Clin. Cancer Res. 2015, 21, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Block, C.; Geisen, C.; Haas, K.; Weber, C.; Winde, G.; Möröy, T.; Müller, O. Sulindac sulfide inhibits Ras signaling. Oncogene 1998, 17, 1769–1776. [Google Scholar] [CrossRef]
- Sattler, M.; Mohanty, A.; Kulkarni, P.; Salgia, R. Precision oncology provides opportunities for targeting KRAS-inhibitor resistance. Trends Cancer 2023, 9, 42–54. [Google Scholar] [CrossRef]
- Shima, F.; Yoshikawa, Y.; Ye, M.; Araki, M.; Matsumoto, S.; Liao, J.; Hu, L.; Sugimoto, T.; Ijiri, Y.; Takeda, A.; et al. In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras-effector interaction. Proc. Natl. Acad. Sci. USA 2013, 110, 8182–8187. [Google Scholar] [CrossRef]
- Foote, J.B.; Mattox, T.E.; Keeton, A.B.; Chen, X.; Smith, F.T.; Berry, K.; Holmes, T.W.; Wang, J.; Huang, C.-H.; Ward, A.; et al. A Pan-RAS Inhibitor with a Unique Mechanism of Action Blocks Tumor Growth and Induces Antitumor Immunity in Gastrointestinal Cancer. Cancer Res. 2024, 85, 956–972. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, L.; Maldonato, B.J.; Wang, Y.; Holderfield, M.; Aronchik, I.; Winters, I.P.; Salman, Z.; Blaj, C.; Menard, M.; et al. Translational and Therapeutic Evaluation of RAS-GTP Inhibition by RMC-6236 in RAS-Driven Cancers. Cancer Discov. 2024, 14, 994–1017. [Google Scholar] [CrossRef] [PubMed]
- Bandi, D.S.R.; Nagaraju, G.P.; Sarvesh, S.; Carstens, J.L.; Foote, J.B.; Graff, E.C.; Fang, Y.-H.D.; Keeton, A.B.; Chen, X.; Valiyaveettil, J.; et al. ADT-1004: A first-in-class, oral pan-RAS inhibitor with robust antitumor activity in preclinical models of pancreatic ductal adenocarcinoma. Mol. Cancer 2025, 24, 76. [Google Scholar] [CrossRef]
- Budhwani, K.I.; Patel, Z.H.; Guenter, R.E.; Charania, A.A. A hitchhiker’s guide to cancer models. Trends Biotechnol. 2022, 40, 1361–1373. [Google Scholar] [CrossRef]
- Bollenbecker, S.; Patel, Z.; Punjani, Z.; Charania, A.; Patel, H.; Abbott, A.; Kunkle, K.; Sewell-Loftin, M.K.; Grossman, G.; Budhwani, K. Predictive efficacy biomarker for chemotherapy agents against triple-negative breast cancer bioprinted organoid tumors (BOTs) using solid tumor biopsy-on-a-chip. Cancer Res. 2023, 83, P6-01. [Google Scholar] [CrossRef]
- Patel, Z.H.; Charania, A.A.; Punjani, Z.; Patel, H.K.; Sewell-Loftin, M.K.; Saleh, M.N.; Budhwani, K.I. Evaluating anticancer agents on 3D bioprinted organoid tumors (BOT) to reduce cost and accelerate therapeutic discovery. J. Clin. Oncol. 2022, 40, e13500. [Google Scholar] [CrossRef]
- Ghajar, C.M.; Bissell, M.J. Tumor Engineering: The Other Face of Tissue Engineering. Tissue Eng. Part A 2010, 16, 2153–2156. [Google Scholar] [CrossRef]
- Lee, G.Y.; A Kenny, P.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef]
- Kawai, S.; Shibuya, K.; Yamazaki, M.; Fujii, E.; Nakano, K.; Suzuki, M. Three-dimensional culture models mimic colon cancer heterogeneity induced by different microenvironments. Sci. Rep. 2020, 10, 3156. [Google Scholar] [CrossRef]
- Zuaiter, D.; Ahirwar, P.; Pokal, A.G.; Patel, Z.H.; Charania, A.A.; Crawford, C.L.; Sewell-Loftin, M.K.; Tsung, A.; Kim, A.; Budhwani, K.I. Characterizing differential efficacy and phenotypic response to proteasome and survivin inhibitors in colorectal cancers using a high throughput organoid assay. J. Clin. Oncol. 2024, 42, 153. [Google Scholar] [CrossRef]
- Moyer, M.P.; Manzano, L.A.; Merriman, R.L.; Stauffer, J.S.; Tanzer, L.R. NCM460 a Normal Human Colon Mucosal Epithelial Cell Line. Vitr. Cell. Dev. Biol.-Anim. 1996, 32, 315–317. [Google Scholar] [CrossRef]
- Zimmermann, S.; Gretzinger, S.; Scheeder, C.; Schwab, M.; Oelmeier, S.A.; Osberghaus, A.; Gottwald, E.; Hubbuch, J. High-throughput cell quantification assays for use in cell purification development—Enabling technologies for cell production. Biotechnol. J. 2016, 11, 676–686. [Google Scholar] [CrossRef]
- Pitts, T.M.; Morrow, M.; Kaufman, S.A.; Tentler, J.J.; Eckhardt, S.G. Vorinostat and bortezomib exert synergistic antiproliferative and proapoptotic effects in colon cancer cell models. Mol. Cancer Ther. 2009, 8, 342–349. [Google Scholar] [CrossRef]
- Suzuki, E.; Demo, S.; Deu, E.; Keats, J.; Arastu-Kapur, S.; Bergsagel, P.L.; Bennett, M.K.; Kirk, C.J. Molecular mechanisms of bortezomib resistant adenocarcinoma cells. PLoS ONE 2011, 6, e27996. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- NCI. NCI-60 Growth Inhibition Data. In NCI DTP Data; NCI: Bethesda, MD, USA, 2012. [Google Scholar]
- Santini, M.T.; Rainaldi, G.; Romano, R.; Ferrante, A.; Clemente, S.; Motta, A.; Indovina, P.L. MG-63 human osteosarcoma cells grown in monolayer and as three-dimensional tumor spheroids present a different metabolic profile: A 1H NMR study. FEBS Lett. 2004, 557, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Boykin, L.A.; Jayashankar, S.; Budhwani, K.K.K.; Budhwani, B.M.K.; Samal, D.; Crawford, C.L.; Tsung, A.; Budhwani, K.I. Protocol for spatial characterization of ECM collagen-GAG in colorectal cancer tumor microenvironment. arXiv 2025, arXiv:2025.06.25.661355. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Nobrega, D.D.; Eiler, L.C.; Ahirwar, P.; Nallapu, S.; Rawal, U.P.; Crawford, C.L.; Buchsbaum, D.J.; Keeton, A.B.; Maxuitenko, Y.Y.; Chen, X.; et al. Evaluation of a Novel Pan-RAS Inhibitor in 3D Bioprinted Tumor Models. Cancers 2025, 17, 2958. https://doi.org/10.3390/cancers17182958
De Nobrega DD, Eiler LC, Ahirwar P, Nallapu S, Rawal UP, Crawford CL, Buchsbaum DJ, Keeton AB, Maxuitenko YY, Chen X, et al. Evaluation of a Novel Pan-RAS Inhibitor in 3D Bioprinted Tumor Models. Cancers. 2025; 17(18):2958. https://doi.org/10.3390/cancers17182958
Chicago/Turabian StyleDe Nobrega, Daniela D., Logan C. Eiler, Parmanand Ahirwar, Sonika Nallapu, Urvi P. Rawal, Chelsea L. Crawford, Donald J. Buchsbaum, Adam B. Keeton, Yulia Y. Maxuitenko, Xi Chen, and et al. 2025. "Evaluation of a Novel Pan-RAS Inhibitor in 3D Bioprinted Tumor Models" Cancers 17, no. 18: 2958. https://doi.org/10.3390/cancers17182958
APA StyleDe Nobrega, D. D., Eiler, L. C., Ahirwar, P., Nallapu, S., Rawal, U. P., Crawford, C. L., Buchsbaum, D. J., Keeton, A. B., Maxuitenko, Y. Y., Chen, X., Piazza, G. A., Tsung, A., & Budhwani, K. I. (2025). Evaluation of a Novel Pan-RAS Inhibitor in 3D Bioprinted Tumor Models. Cancers, 17(18), 2958. https://doi.org/10.3390/cancers17182958






