Dendritic Cells of Leukemic Origin (DCleu) Modulate the Expression of Inhibitory Checkpoint Molecules and Their Ligands on T Cells and Blasts in AML Relapse After Allogeneic Stem Cell Transplantation
Simple Summary
Abstract
1. Introduction
1.1. Acute Myeloid Leukemia Relapse After Allogeneic Hematopoietic Stem Cell Transplantation
1.2. Immune Checkpoint Molecules and Immune Escape
1.3. DC-Based Immunotherapy
1.4. Innate and Adaptive Immune System and Antileukemic Activity
1.5. Aim of the Study
2. Materials and Methods
2.1. Sample Collection
2.2. Patients
2.3. Flow Cytometry and Sample Preparation
2.4. Dendritic Cell Culture (DCC)
2.5. T-Cell-Enriched Mixed Lymphocyte Culture (MLC) and Functional Analyses (Intracellular Cytokine Assay (InCyt), Degranulation Assay (DEG), Cytotoxicity Fluorolysis Assay (CTX))
2.6. Statistical Methods
3. Results
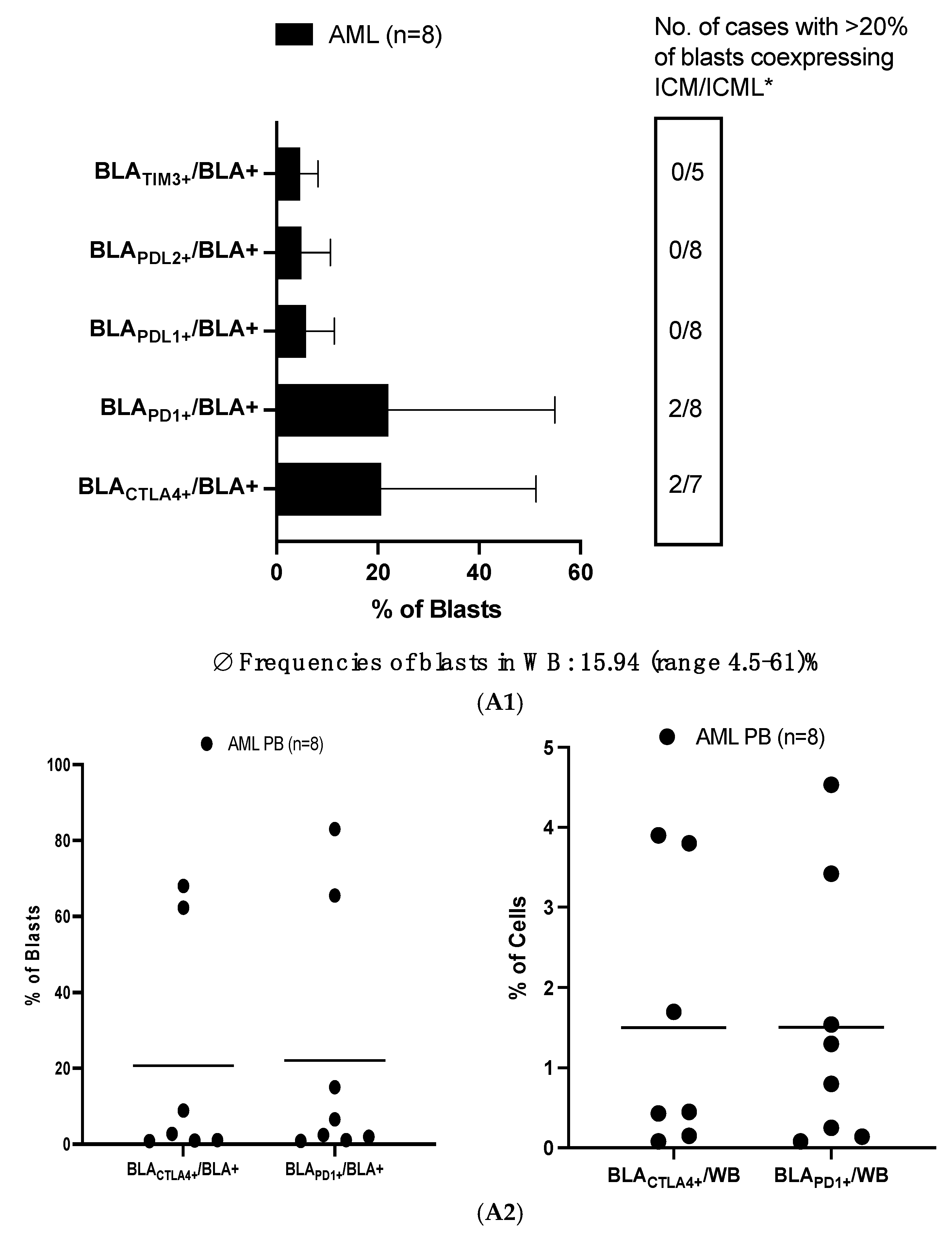
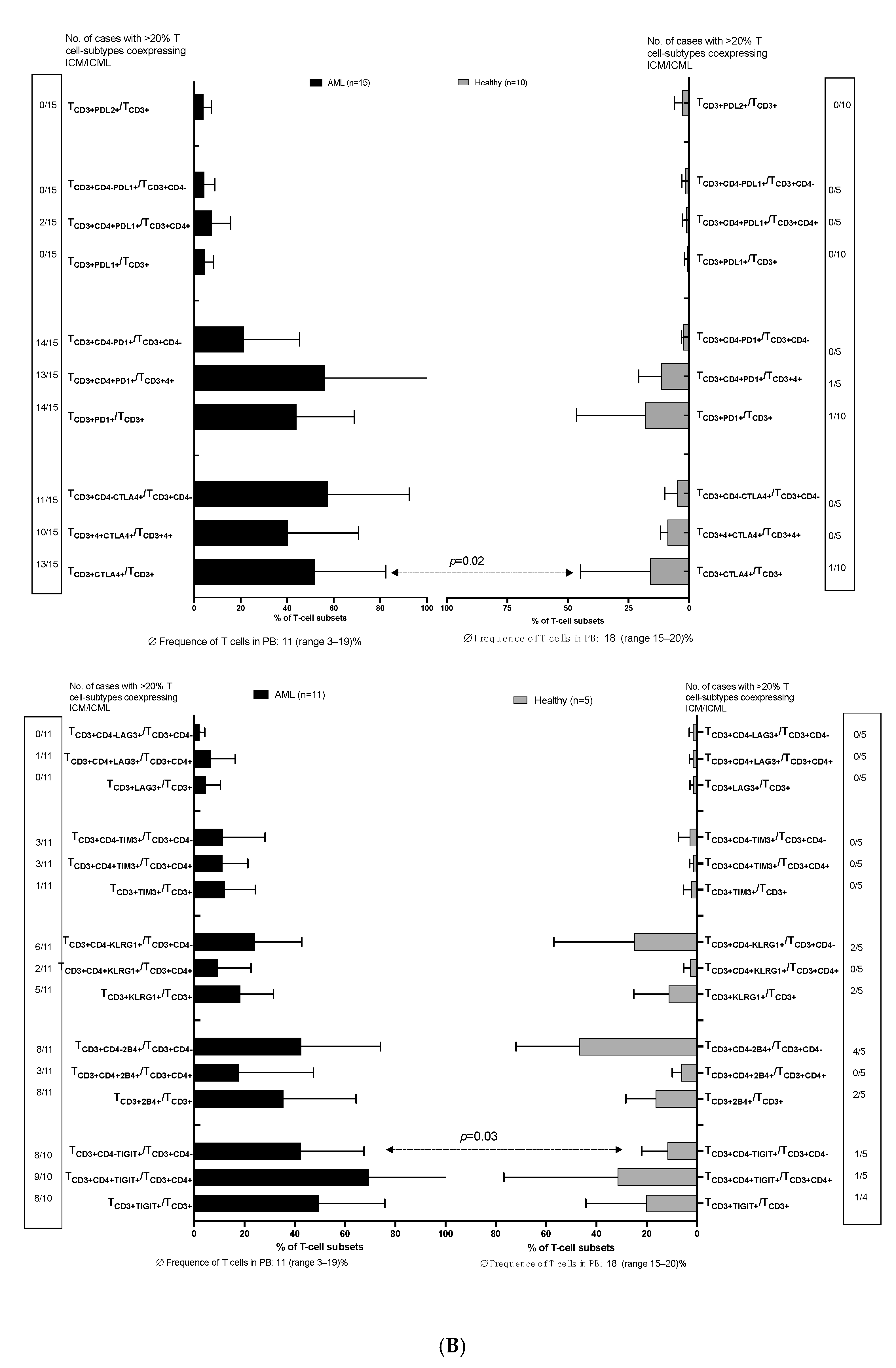
3.1. ICM/ICML Expressing Uncultured Blasts and T-Cells from AML Patients at Relapse After Allo-HCT, and on Immune Cells from Healthy Donors
3.1.1. High Frequencies of Uncultured AML-Blasts Co-Express ICM (CTLA4 and PD1)
3.1.2. Higher Frequencies of Uncultured T-Cell (Subtypes) from AML Patients vs. Healthy Donors Co-Express ICM (CTLA4 and PD1)
3.2. Increased Frequencies of (Antigen-Specific) Intracellular IFNγ-Producing or Degranulating Immune Cells After Stimulation of Uncultured WB (AML and Healthy) with vs. Without LAA/SEB
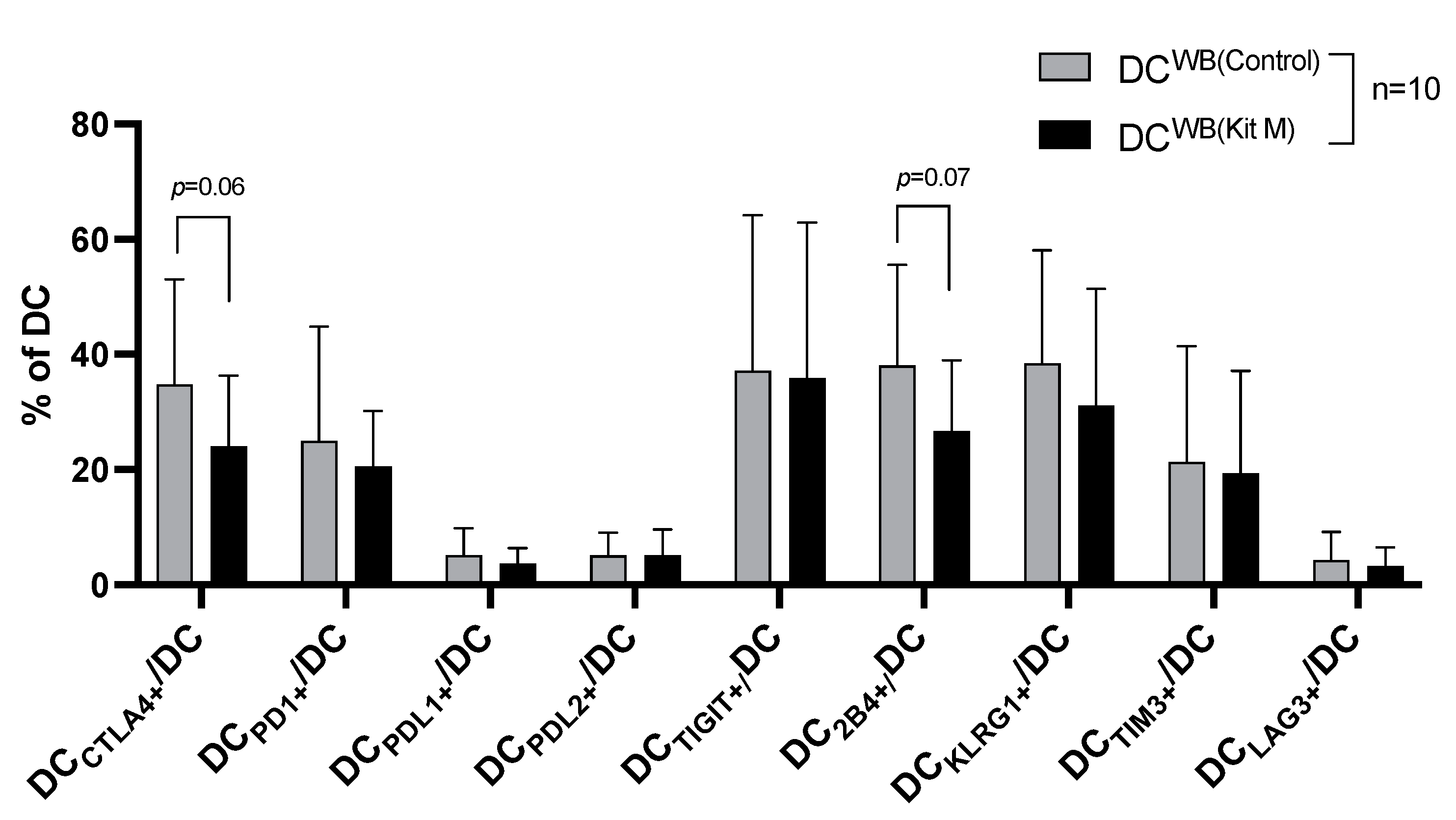
3.3. Characteristics of DC from AML and Healthy WB After Culture with (vs. Without) Kit M
3.3.1. Increased Frequencies of (Leukemia)-Derived DC/DCleu in AML or Healthy WB After Culture with (vs. Without) Kit M
3.3.2. Decreased Frequencies of ICM/ICML Expressing DC from AML WB After Culture with (vs. Without) Kit M
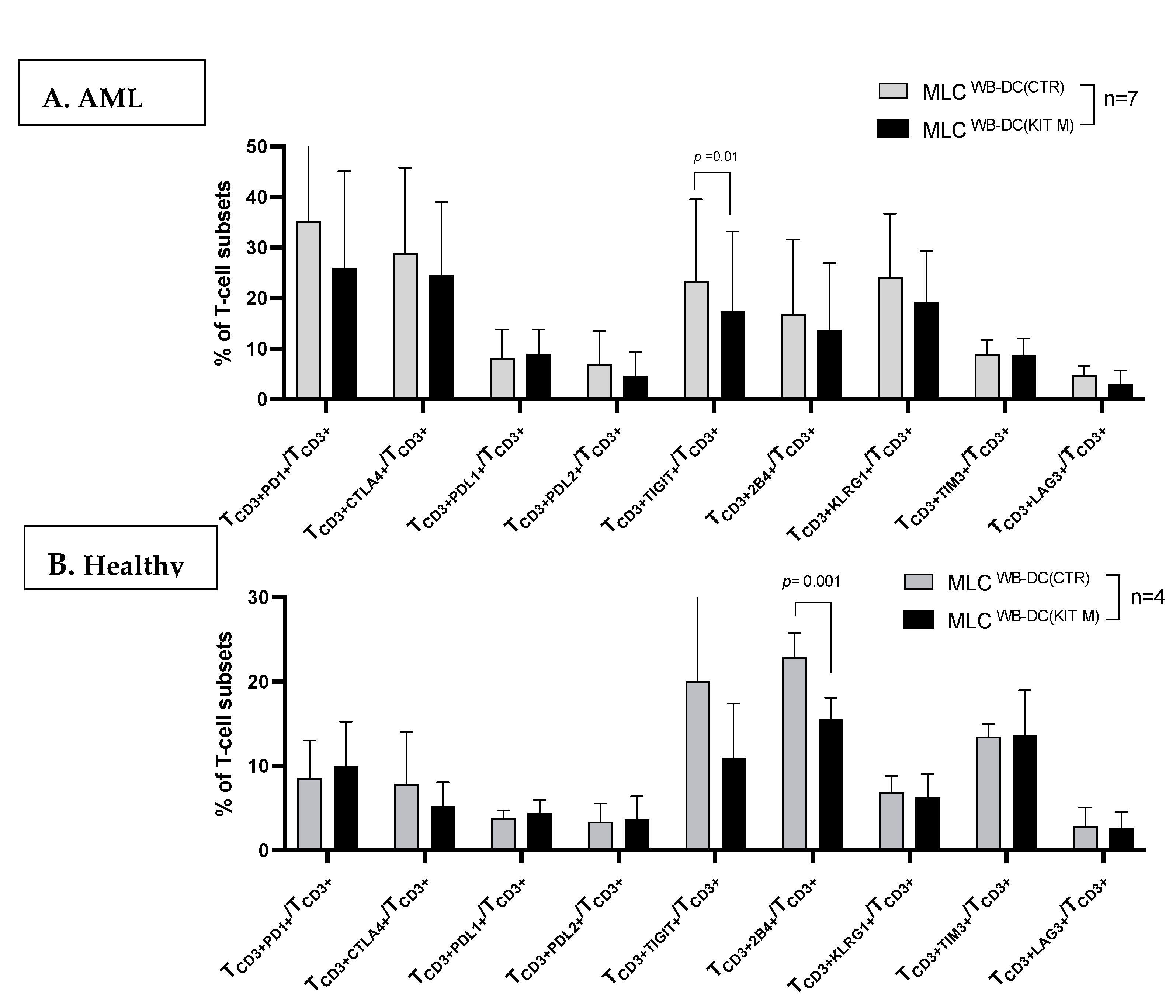
3.4. Characteristics of T-Cells After MLC with Kit M-Pretreated (vs. Not Pretreated) Patients’ and Healthy Donors’ WB
3.4.1. Activated and Memory T-Cells Increased After MLC (With Kit M-Pretreated vs. Not Pretreated Patients’ and Healthy Donors’ WB)
3.4.2. Decreased Frequencies of ICM/ICML-Expressing T-Cells After MLC of Kit M-Pretreated vs. Not Pretreated AML Patients’ and Healthy Donors’ Samples
3.5. Increased Frequencies of (Antigen-Specific) Intracellular IFNγ-Producing or -Degranulating Immune-Activated and Memory T or Innate Cells After MLC with Kit M-Pretreated (vs. Not Pretreated) Patients’ or Healthy Donors’ WB
3.6. Increased Antileukemic Cytotoxicity After MLC in Kit-M-Treated AML Patients’ WB
3.7. Correlations of Frequencies of ICM/ICML-Expressing Cells with Ex Vivo Achieved Blast Lysis or Patients’ Response to Relapse Treatment
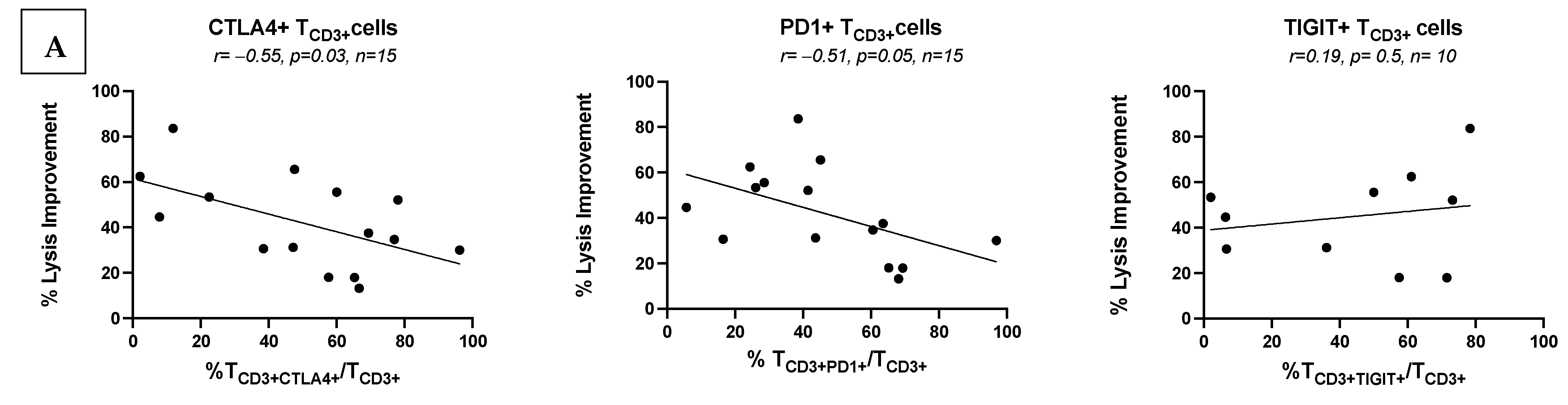
3.7.1. Low Frequencies of ICM/ICML-Expressing Uncultured AML Patients’ T-Cells Correlate with Ex Vivo Achieved Blast Lysis After MLC, and with Clinical Response to Initial Salvage Therapy
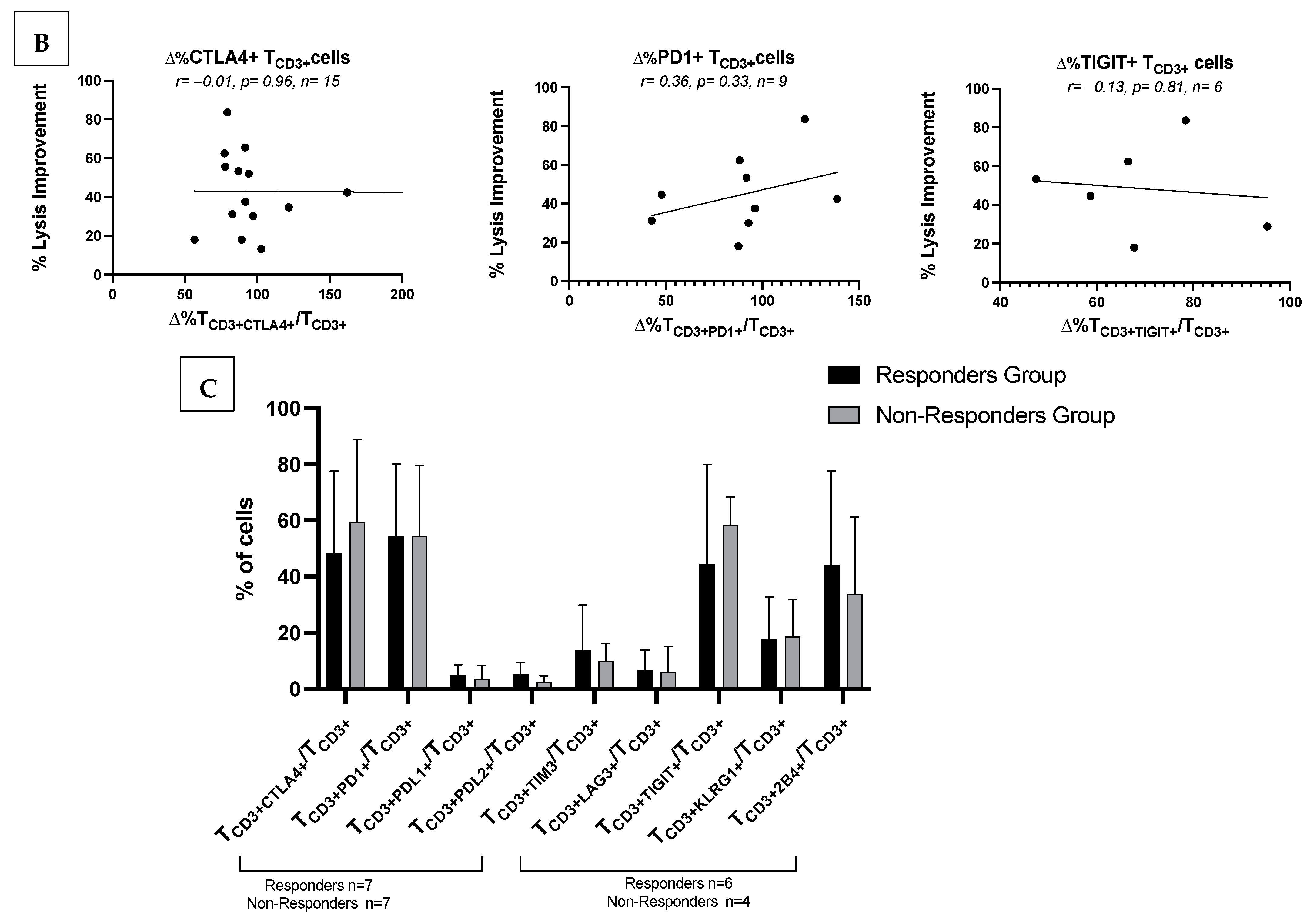
3.7.2. No Correlations of Frequencies of ICM/ICML-Expressing T-Cells After MLC of Kit M-Pretreated vs. Not Pretreated AML Patients with Ex Vivo Achieved Blast Lysis or with Patients’ Response to Relapse Treatment
4. Discussion
4.1. T-Cell Exhaustion Responsible for the Immunosuppressive Leukemic Microenvironment
4.2. Higher Frequencies of ICM-Expressing Uncultured Blasts and T-Cells Found in AML Patients That Relapsed After Allo-HCT Compared to Healthy Donors
4.3. ICM-Expressing B- or NK Cells, DC, or Blasts
4.3.1. Enhanced Intracellular Cytokine-Producing or Degranulating Immune Cells in Uncultured and Cultured (After MLC in Kit-M-Treated (vs. Untreated)) AML Patients and Healthy Donors’ WB
4.3.2. DC-Based Immunotherapy
4.3.3. Provision of Immunoreactive Cells and Increased Blastolytic Activity After MLC in Kit-M-Treated WB
4.4. Potential of ICM/ICML Monitoring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Stratmann, S.; Yones, S.A.; Mayrhofer, M.; Norgren, N.; Skaftason, A.; Sun, J.; Smolinska, K.; Komorowski, J.; Herlin, M.K.; Sundström, C.; et al. Genomic characterization of relapsed acute myeloid leukemia reveals novel putative therapeutic targets. Blood Adv. 2021, 5, 900–912. [Google Scholar] [CrossRef]
- Giacopelli, B.; Wang, M.; Cleary, A.; Wu, Y.-Z.; Schultz, A.R.; Schmutz, M.; Blachly, J.S.; Eisfeld, A.-K.; Mundy-Bosse, B.; Vosberg, S.; et al. DNA methylation epitypes highlight underlying developmental and disease pathways in acute myeloid leukemia. Genome Res. 2021, 31, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Toffalori, C.; Zito, L.; Gambacorta, V.; Riba, M.; Oliveira, G.; Bucci, G.; Barcella, M.; Spinelli, O.; Greco, R.; Crucitti, L.; et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat. Med. 2019, 25, 603–611. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Chan, S.M.; Daver, N.G.; Jonas, B.A.; Pollyea, D.A. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am. J. Hematol. 2019, 94, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Vago, L.; Gojo, I. Immune escape and immunotherapy of acute myeloid leukemia. J. Clin. Investig. 2020, 130, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Sauerer, T.; Velázquez, G.F.; Schmid, C. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: Immune escape mechanisms and current implications for therapy. Mol. Cancer 2023, 22, 180. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Noviello, M.; Manfredi, F.; Ruggiero, E.; Perini, T.; Oliveira, G.; Cortesi, F.; de Simone, P.; Toffalori, C.; Gambacorta, V.; Greco, R.; et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat. Commun. 2019, 10, 1065. [Google Scholar] [CrossRef]
- Kim, M.J.; Ha, S.-J. Differential Role of PD-1 Expressed by Various Immune and Tumor Cells in the Tumor Immune Microenvironment: Expression, Function, Therapeutic Efficacy, and Resistance to Cancer Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 767466. [Google Scholar] [CrossRef]
- Oyewole-Said, D.; Konduri, V.; Vazquez-Perez, J.; Weldon, S.A.; Levitt, J.M.; Decker, W.K. Beyond T-Cells: Functional Characterization of CTLA-4 Expression in Immune and Non-Immune Cell Types. Front. Immunol. 2020, 11, 608024. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Köhler, N.; Ruess, D.A.; Kesselring, R.; Zeiser, R. The Role of Immune Checkpoint Molecules for Relapse After Allogeneic Hematopoietic Cell Transplantation. Front. Immunol. 2021, 12, 634435. [Google Scholar] [CrossRef] [PubMed]
- Amberger, D.C.; Schmetzer, H.M. Dendritic Cells of Leukemic Origin: Specialized Antigen-Presenting Cells as Potential Treatment Tools for Patients with Myeloid Leukemia. Transfus. Med. Hemotherapy 2020, 47, 432–443. [Google Scholar] [CrossRef]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef]
- van Acker, H.H.; Versteven, M.; Lichtenegger, F.S.; Roex, G.; Campillo-Davo, D.; Lion, E.; Subklewe, M.; van Tendeloo, V.F.; Berneman, Z.N.; Anguille, S. Dendritic Cell-Based Immunotherapy of Acute Myeloid Leukemia. J. Clin. Med. 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Klauer, L.K.; Schutti, O.; Ugur, S.; Doraneh-Gard, F.; Amberger, D.C.; Rogers, N.; Krämer, D.; Rank, A.; Schmid, C.; Eiz-Vesper, B.; et al. Interferon Gamma Secretion of Adaptive and Innate Immune Cells as a Parameter to Describe Leukaemia-Derived Dendritic-Cell-Mediated Immune Responses in Acute Myeloid Leukaemia in vitro. Transfus. Med. Hemotherapy 2022, 49, 44–61. [Google Scholar] [CrossRef]
- Schutti, O.; Klauer, L.; Baudrexler, T.; Burkert, F.; Schmohl, J.; Hentrich, M.; Bojko, P.; Kraemer, D.; Rank, A.; Schmid, C.; et al. Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients’ Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays. Int. J. Mol. Sci. 2024, 25, 6983. [Google Scholar] [CrossRef]
- Unterfrauner, M.; Rejeski, H.A.; Hartz, A.; Bohlscheid, S.; Baudrexler, T.; Feng, X.; Rackl, E.; Li, L.; Rank, A.; Filippini Velázquez, G.; et al. Granulocyte-Macrophage-Colony-Stimulating-Factor Combined with Prostaglandin E1 Create Dendritic Cells of Leukemic Origin from AML Patients’ Whole Blood and Whole Bone Marrow That Mediate Antileukemic Processes after Mixed Lymphocyte Culture. Int. J. Mol. Sci. 2023, 24, 17436. [Google Scholar] [CrossRef]
- Atzler, M.; Baudrexler, T.; Amberger, D.C.; Rogers, N.; Rabe, A.; Schmohl, J.; Wang, R.; Rank, A.; Schutti, O.; Hirschbühl, K.; et al. In Vivo Induction of Leukemia-Specific Adaptive and Innate Immune Cells by Treatment of AML-Diseased Rats and Therapy-Refractory AML Patients with Blast Modulating Response Modifiers. Int. J. Mol. Sci. 2024, 25, 13469. [Google Scholar] [CrossRef]
- Velázquez, G.F.; Anand, P.; Abdulmajid, J.; Feng, X.; Weller, J.F.; Hirschbühl, K.; Schmetzer, H.; Schmid, C. Clinical stabilization of a highly refractory acute myeloid leukaemia under individualized treatment with immune response modifying drugs by in vivo generation of dendritic cells of leukaemic origin (DCleu) and modulation of effector cells and immune escape mechanisms. Biomark. Res. 2025, 13, 104. [Google Scholar] [CrossRef]
- Robertson, F.C.; Berzofsky, J.A.; Terabe, M. NKT cell networks in the regulation of tumor immunity. Front. Immunol. 2014, 5, 543. [Google Scholar] [CrossRef]
- Rackl, E.; Li, L.; Klauer, L.K.; Ugur, S.; Pepeldjiyska, E.; Seidel, C.L.; Gunsilius, C.; Weinmann, M.; Doraneh-Gard, F.; Reiter, N.; et al. Dendritic Cell-Triggered Immune Activation Goes along with Provision of (Leukemia-Specific) Integrin Beta 7-Expressing Immune Cells and Improved Antileukemic Processes. Int. J. Mol. Sci. 2022, 24, 463. [Google Scholar] [CrossRef]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef]
- Pepeldjiyska, E.; Li, L.; Gao, J.; Seidel, C.L.; Blasi, C.; Özkaya, E.; Schmohl, J.; Kraemer, D.; Schmid, C.; Rank, A.; et al. Leukemia derived dendritic cell (DCleu) mediated immune response goes along with reduced (leukemia-specific) regulatory T-cells. Immunobiology 2022, 227, 152237. [Google Scholar] [CrossRef] [PubMed]
- El Dosoky, W.; Aref, S.; El Menshawy, N.; Ramez, A.; Abou Zaid, T.; Aref, M.; Atia, D. Prognostic effect of CTLA4/LAG3 Expression by T-Cells Subsets on Acute Myeloid Leukemia Patients. Asian Pac. J. Cancer Prev. 2024, 25, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Hutten, T.J.A.; Norde, W.J.; Woestenenk, R.; Wang, R.C.; Maas, F.; Kester, M.; Falkenburg, J.H.F.; Berglund, S.; Luznik, L.; Jansen, J.H.; et al. Increased Coexpression of PD-1, TIGIT, and KLRG-1 on Tumor-Reactive CD8+ T Cells During Relapse after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 666–677. [Google Scholar] [CrossRef]
- Huang, S.; Liang, C.; Zhao, Y.; Deng, T.; Tan, J.; Zha, X.; Li, Y.; Chen, S. Increased TOX expression concurrent with PD-1, Tim-3, and CD244 expression in T cells from patients with acute myeloid leukemia. Cytom. B Clin. Cytom. 2022, 102, 143–152. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, L.; Schell, T.D.; Zhang, J.; Claxton, D.F.; Ehmann, W.C.; Rybka, W.B.; George, M.R.; Zeng, H.; Zheng, H. T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8+ T-Cell Exhaustion and Poor Clinical Outcome in AML Patients. Clin. Cancer Res. 2016, 22, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, J.; Claxton, D.F.; Ehmann, W.C.; Rybka, W.B.; Zhu, L.; Zeng, H.; Schell, T.D.; Zheng, H. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J. 2015, 5, e330. [Google Scholar] [CrossRef]
- Noviello, M.; Manfredi, F.; Perini, T.; Oliveira, G.; Cortesi, F.; Toffalori, C.; Gambacorta, V.; Bondanza, A.; Greco, R.; Peccatori, J.; et al. Multiple Inhibitory Receptors Are Expressed on Central Memory and Memory Stem T Cells Infiltrating the Bone Marrow of AML Patients Relapsing after Allo-HSCT. Blood 2016, 128, 4564. [Google Scholar] [CrossRef]
- Strauss, L.; Mahmoud, M.A.A.; Weaver, J.D.; Tijaro-Ovalle, N.M.; Christofides, A.; Wang, Q.; Pal, R.; Yuan, M.; Asara, J.; Patsoukis, N.; et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 2020, 5, eaay1863. [Google Scholar] [CrossRef]
- Bolkun, L.; Tynecka, M.; Walewska, A.; Bernatowicz, M.; Piszcz, J.; Cichocka, E.; Wandtke, T.; Czemerska, M.; Wierzbowska, A.; Moniuszko, M.; et al. The Association between Immune Checkpoint Proteins and Therapy Outcomes in Acute Myeloid Leukaemia Patients. Cancers 2023, 15, 4487. [Google Scholar] [CrossRef]
- Suo, S.; Le Nguyen, X.T.; Li, F.; Zhao, D.; Qiao, J.; Liang, C.; Hoang, D.H.; Chen, F.; Rodriguez, I.; Ghoda, L.; et al. Acute Myeloid Leukemia Cell-Intrinsic PD-1 Functions Promoting Leukemia Development By Recruiting SHP2. Blood 2021, 138, 1173. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y. Targeting NK Cell Checkpoint Receptors or Molecules for Cancer Immunotherapy. Front. Immunol. 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wei, R.; Lin, Y.; Kwok, H.F. Clinical and Recent Patents Applications of PD-1/PD-L1 Targeting Immunotherapy in Cancer Treatment-Current Progress, Strategy, and Future Perspective. Front. Immunol. 2020, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Pistillo, M.P.; Tazzari, P.L.; Palmisano, G.L.; Pierri, I.; Bolognesi, A.; Ferlito, F.; Capanni, P.; Polito, L.; Ratta, M.; Pileri, S.; et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood 2003, 101, 202–209. [Google Scholar] [CrossRef]
- Kleffel, S.; Posch, C.; Barthel, S.R.; Mueller, H.; Schlapbach, C.; Guenova, E.; Elco, C.P.; Lee, N.; Juneja, V.R.; Zhan, Q.; et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015, 162, 1242–1256. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liu, S.; Guo, L.; Zhang, B.; Zhang, J.; Ye, Q. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology 2017, 66, 1920–1933. [Google Scholar] [CrossRef]
- Pu, N.; Gao, S.; Yin, H.; Li, J.-A.; Wu, W.; Fang, Y.; Zhang, L.; Rong, Y.; Xu, X.; Wang, D.; et al. Cell-intrinsic PD-1 promotes proliferation in pancreatic cancer by targeting CYR61/CTGF via the hippo pathway. Cancer Lett. 2019, 460, 42–53. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Zhang, C.; Wang, Y.; Cheng, T.; Duan, L.; Tong, Z.; Tan, S.; Zhang, H.; Saw, P.E.; et al. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 6640–6650. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Fontana, V.; Boccardo, S.; Merlo, D.F.; Margallo, E.; Laurent, S.; Morabito, A.; Rijavec, E.; Dal Bello, M.G.; Mora, M.; et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2012, 61, 1463–1472. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koh, J.; Kim, K.; Chie, E.K.; Kim, S.; Lee, K.B.; Jang, J.-Y.; Kim, S.W.; Oh, D.-Y.; Bang, Y.-J. Clinical Implications of Cytotoxic T Lymphocyte Antigen-4 Expression on Tumor Cells and Tumor-Infiltrating Lymphocytes in Extrahepatic Bile Duct Cancer Patients Undergoing Surgery Plus Adjuvant Chemoradiotherapy. Target. Oncol. 2017, 12, 211–218. [Google Scholar] [CrossRef]
- Paulsen, E.-E.; Kilvaer, T.K.; Rakaee, M.; Richardsen, E.; Hald, S.M.; Andersen, S.; Busund, L.-T.; Bremnes, R.M.; Donnem, T. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: Diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol. Immunother. 2017, 66, 1449–1461. [Google Scholar] [CrossRef]
- Cheng, P.; Eksioglu, E.A.; Chen, X.; Kandell, W.; Le Trinh, T.; Cen, L.; Qi, J.; Sallman, D.A.; Zhang, Y.; Tu, N.; et al. S100A9-induced overexpression of PD-1/PD-L1 contributes to ineffective hematopoiesis in myelodysplastic syndromes. Leukemia 2019, 33, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Carenza, C.; Calcaterra, F.; Oriolo, F.; Di Vito, C.; Ubezio, M.; Della Porta, M.G.; Mavilio, D.; Della Bella, S. Costimulatory Molecules and Immune Checkpoints Are Differentially Expressed on Different Subsets of Dendritic Cells. Front. Immunol. 2019, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Amberger, D.C.; Doraneh-Gard, F.; Gunsilius, C.; Weinmann, M.; Möbius, S.; Kugler, C.; Rogers, N.; Böck, C.; Ködel, U.; Werner, J.-O.; et al. PGE1-Containing Protocols Generate Mature (Leukemia-Derived) Dendritic Cells Directly from Leukemic Whole Blood. Int. J. Mol. Sci. 2019, 20, 4590. [Google Scholar] [CrossRef]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef]
- Logan, C.; Koura, D.; Taplitz, R. Updates in infection risk and management in acute leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 135–139. [Google Scholar] [CrossRef]
- Teague, R.M.; Kline, J. Immune evasion in acute myeloid leukemia: Current concepts and future directions. J. Immunother. Cancer 2013, 1, 13. [Google Scholar] [CrossRef]
- Plett, C.; Klauer, L.K.; Amberger, D.C.; Ugur, S.; Rabe, A.; Fischer, Z.; Deen, D.; Hirn-Lopez, A.; Gunsilius, C.; Werner, J.-O.; et al. Immunomodulatory kits generating leukaemia derived dendritic cells do not induce blast proliferation ex vivo: IPO-38 as a novel marker to quantify proliferating blasts in acute myeloid leukaemia. Clin. Immunol. 2022, 242, 109083. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.-B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Penter, L.; Liu, Y.; Wolff, J.O.; Yang, L.; Taing, L.; Jhaveri, A.; Southard, J.; Patel, M.; Cullen, N.M.; Pfaff, K.L.; et al. Mechanisms of response and resistance to combined decitabine and ipilimumab for advanced myeloid disease. Blood 2023, 141, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.S.; Flamand, Y.; Penter, L.; Keng, M.; Tomlinson, B.K.; Mendez, L.M.; Koller, P.; Cullen, N.; Arihara, Y.; Pfaff, K.; et al. Ipilimumab plus decitabine for patients with MDS or AML in posttransplant or transplant-naïve settings. Blood 2023, 141, 1884–1888. [Google Scholar] [CrossRef]
- Wong, K.K.; Hassan, R.; Yaacob, N.S. Hypomethylating Agents and Immunotherapy: Therapeutic Synergism in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Front. Oncol. 2021, 11, 624742. [Google Scholar] [CrossRef]
- Geng, S.; Xu, R.; Huang, X.; Li, M.; Deng, C.; Lai, P.; Wang, Y.; Wu, P.; Chen, X.; Weng, J.; et al. Dynamics of PD-1 expression are associated with treatment efficacy and prognosis in patients with intermediate/high-risk myelodysplastic syndromes under hypomethylating treatment. Front. Immunol. 2022, 13, 950134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Filippini Velázquez, G.; Bohlscheid, S.; Unterfrauner, M.; Anand, P.; Rejeski, H.A.; Hartz, A.; Baudrexler, T.; Schmid, C.; Schmetzer, H.M. Dendritic Cells of Leukemic Origin (DCleu) Modulate the Expression of Inhibitory Checkpoint Molecules and Their Ligands on T Cells and Blasts in AML Relapse After Allogeneic Stem Cell Transplantation. Cancers 2025, 17, 2948. https://doi.org/10.3390/cancers17182948
Feng X, Filippini Velázquez G, Bohlscheid S, Unterfrauner M, Anand P, Rejeski HA, Hartz A, Baudrexler T, Schmid C, Schmetzer HM. Dendritic Cells of Leukemic Origin (DCleu) Modulate the Expression of Inhibitory Checkpoint Molecules and Their Ligands on T Cells and Blasts in AML Relapse After Allogeneic Stem Cell Transplantation. Cancers. 2025; 17(18):2948. https://doi.org/10.3390/cancers17182948
Chicago/Turabian StyleFeng, Xiaojia, Giuliano Filippini Velázquez, Sophia Bohlscheid, Marianne Unterfrauner, Philipp Anand, Hazal Aslan Rejeski, Anne Hartz, Tobias Baudrexler, Christoph Schmid, and Helga Maria Schmetzer. 2025. "Dendritic Cells of Leukemic Origin (DCleu) Modulate the Expression of Inhibitory Checkpoint Molecules and Their Ligands on T Cells and Blasts in AML Relapse After Allogeneic Stem Cell Transplantation" Cancers 17, no. 18: 2948. https://doi.org/10.3390/cancers17182948
APA StyleFeng, X., Filippini Velázquez, G., Bohlscheid, S., Unterfrauner, M., Anand, P., Rejeski, H. A., Hartz, A., Baudrexler, T., Schmid, C., & Schmetzer, H. M. (2025). Dendritic Cells of Leukemic Origin (DCleu) Modulate the Expression of Inhibitory Checkpoint Molecules and Their Ligands on T Cells and Blasts in AML Relapse After Allogeneic Stem Cell Transplantation. Cancers, 17(18), 2948. https://doi.org/10.3390/cancers17182948






