Cell Line-Dependent Internalization, Persistence, and Immunomodulatory Effects of Staphylococcus aureus in Triple-Negative Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Breast Cell Lines
2.3. Labeling of S. aureus with eFluor450
2.4. Gentamicin Protection Assay
2.5. Measurement of S. aureus Internalization by Flow Cytometry
2.6. Cytotoxicity and Inhibition of Proliferation
2.7. Expression of Cell Surface Markers Determined by Flow Cytometry
2.8. Western Blot
2.9. Clearance of Viable Intracellular S. aureus
2.10. Transmission Electron Microscopy
2.11. Statistical Analysis
3. Results
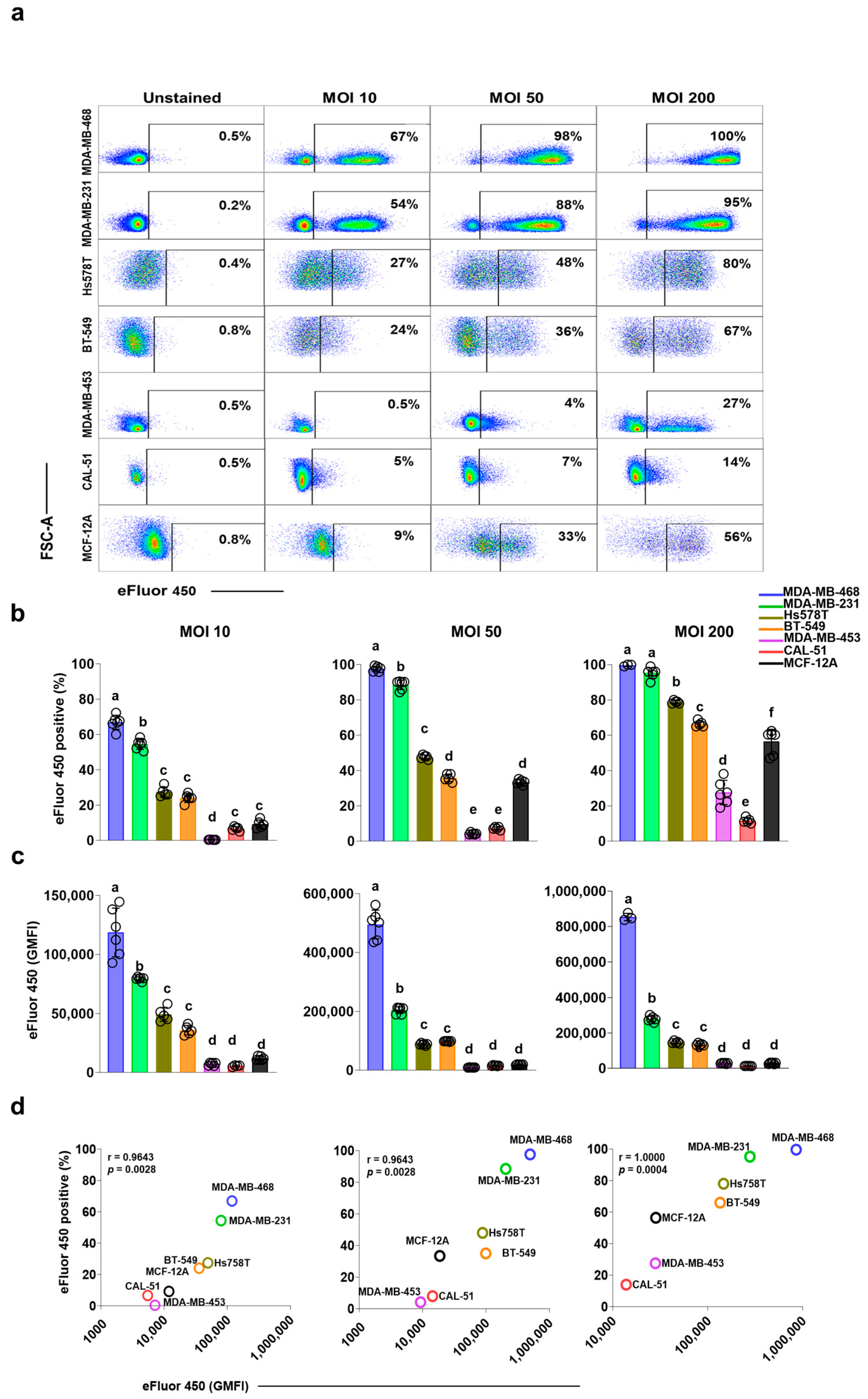
3.1. Breast Cell Lines Exhibit Differential Internalization of Staphylococcus aureus
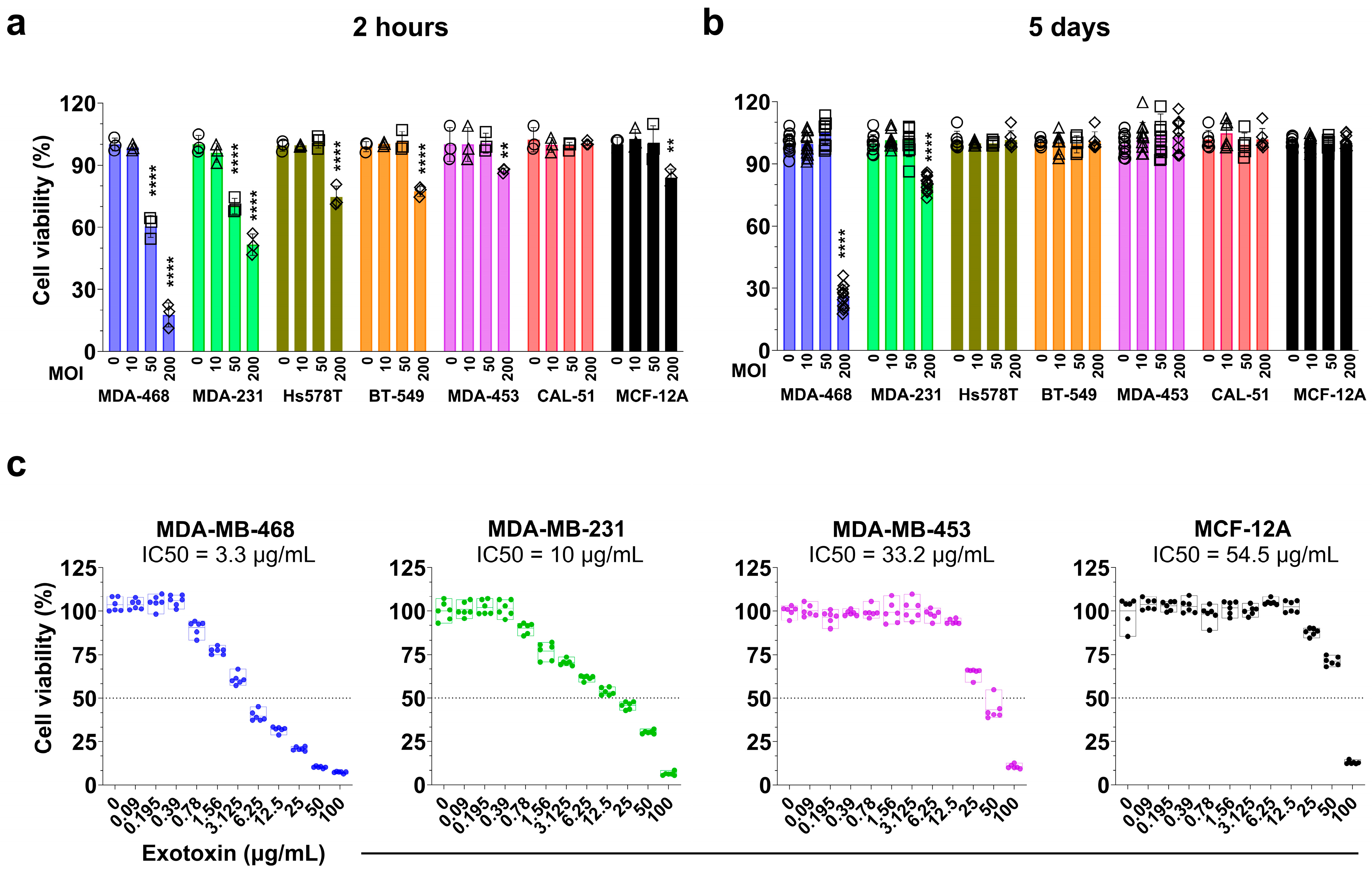
3.2. S. aureus Induces Dose-Dependent Cytotoxicity and Inhibits Proliferation in a Cell Line–Specific Manner
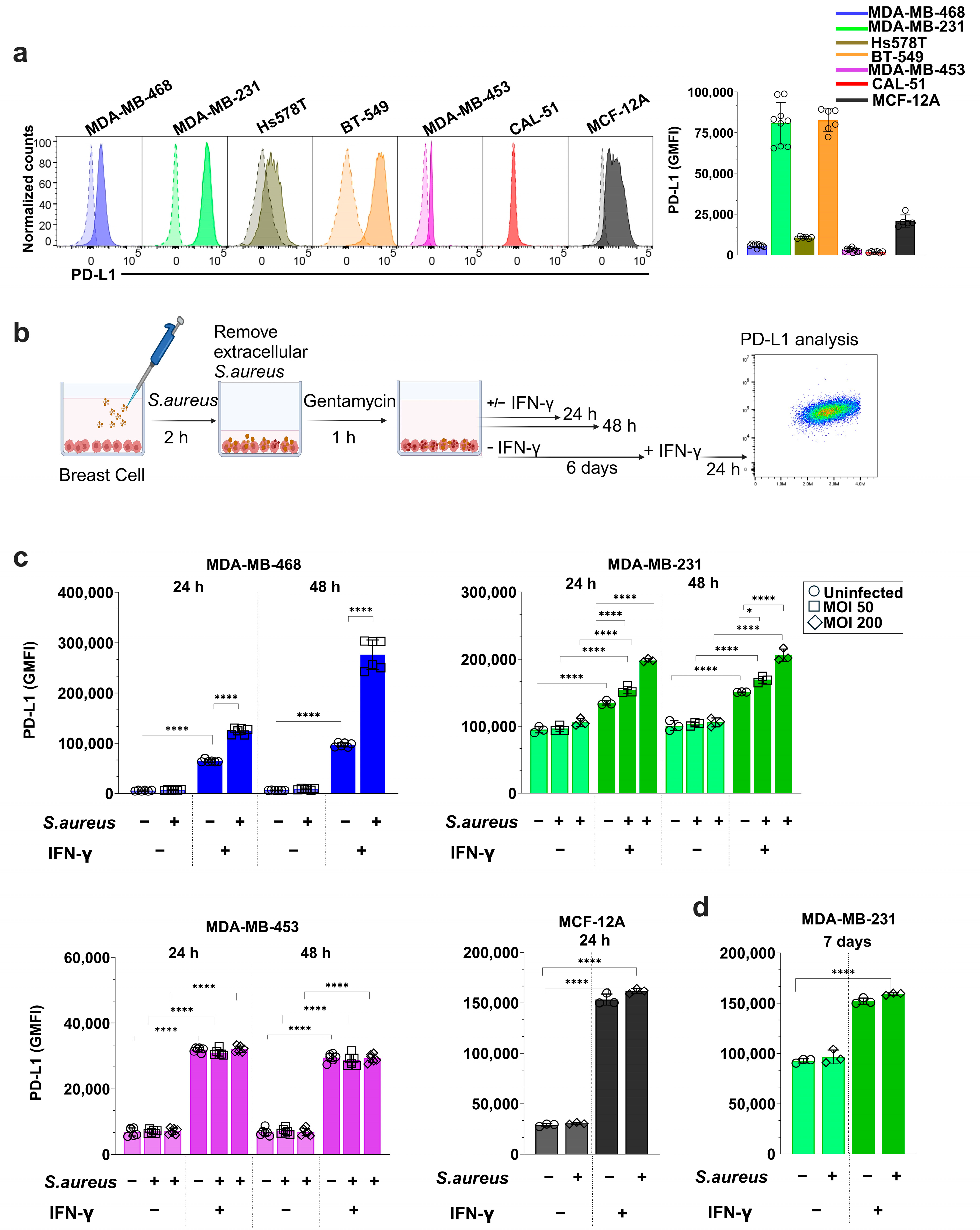
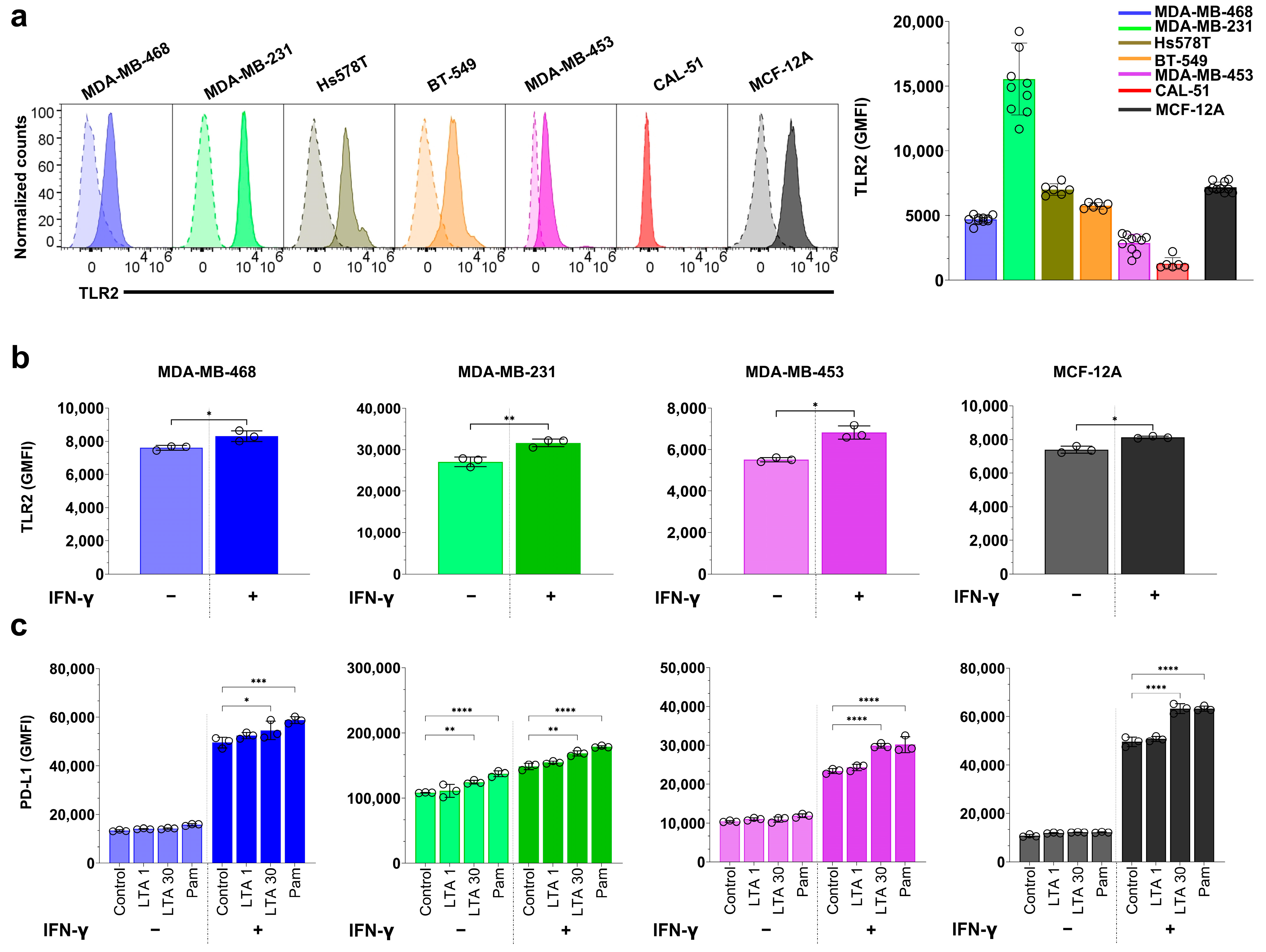
3.3. S. aureus Infection Enhances IFN-γ-Induced PD-L1 Expression in a Cell Line-Dependent Manner
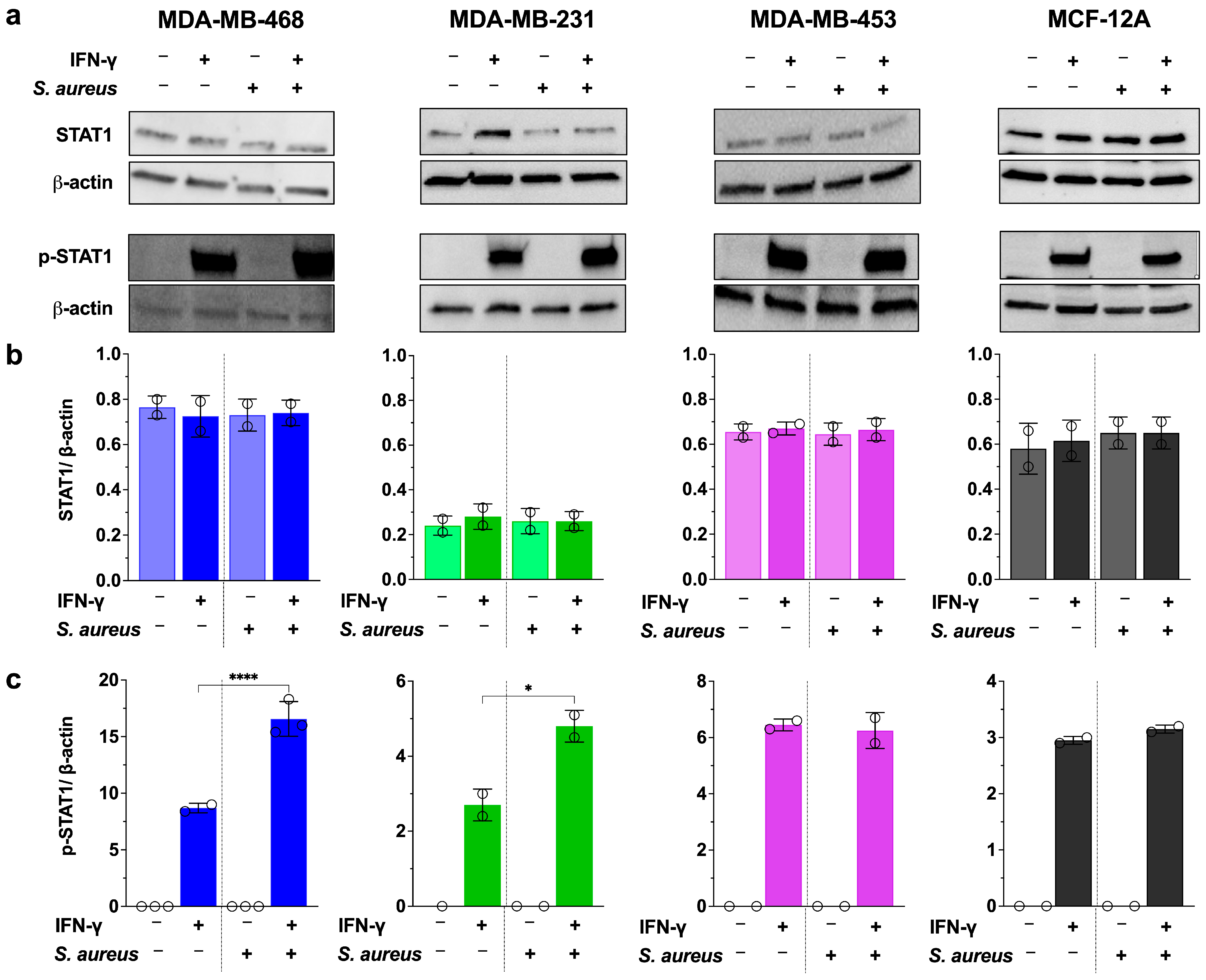
3.4. S. aureus Enhances IFN-γ–Induced PD-L1 Expression Via STAT1 Activation
3.5. TLR2 Agonists Upregulate PD-L1 Expression in Breast Cell Lines
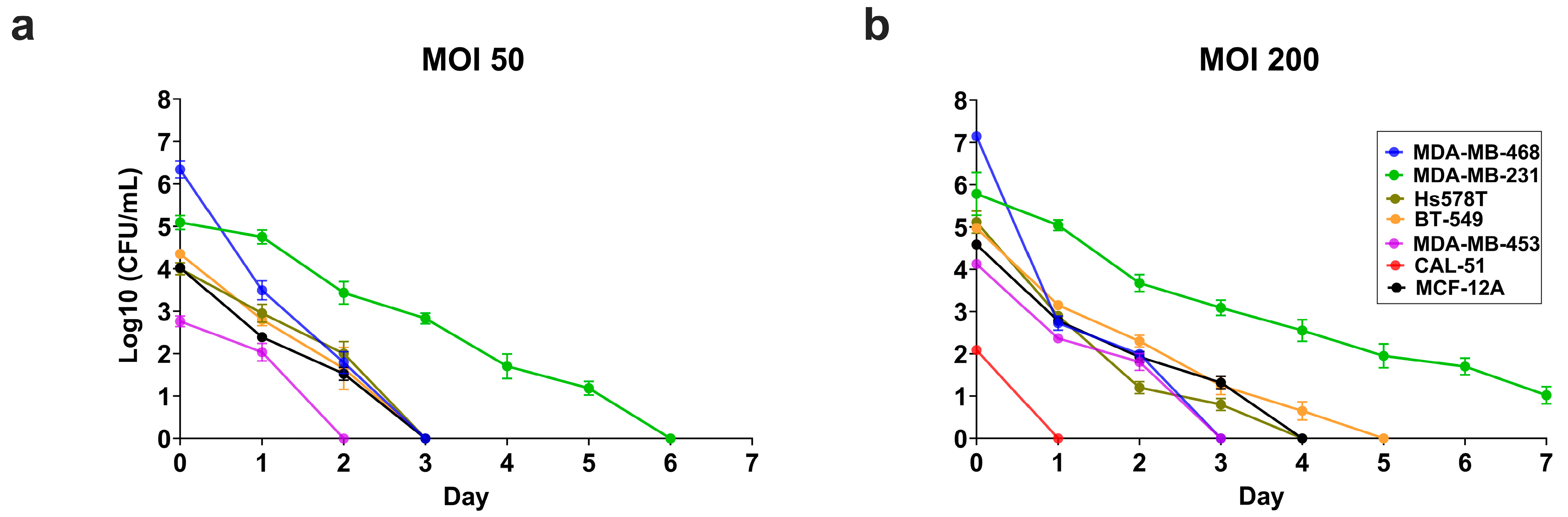
3.6. Intracellular S. aureus Clearance Varies in a Cell Line-Dependant Manner
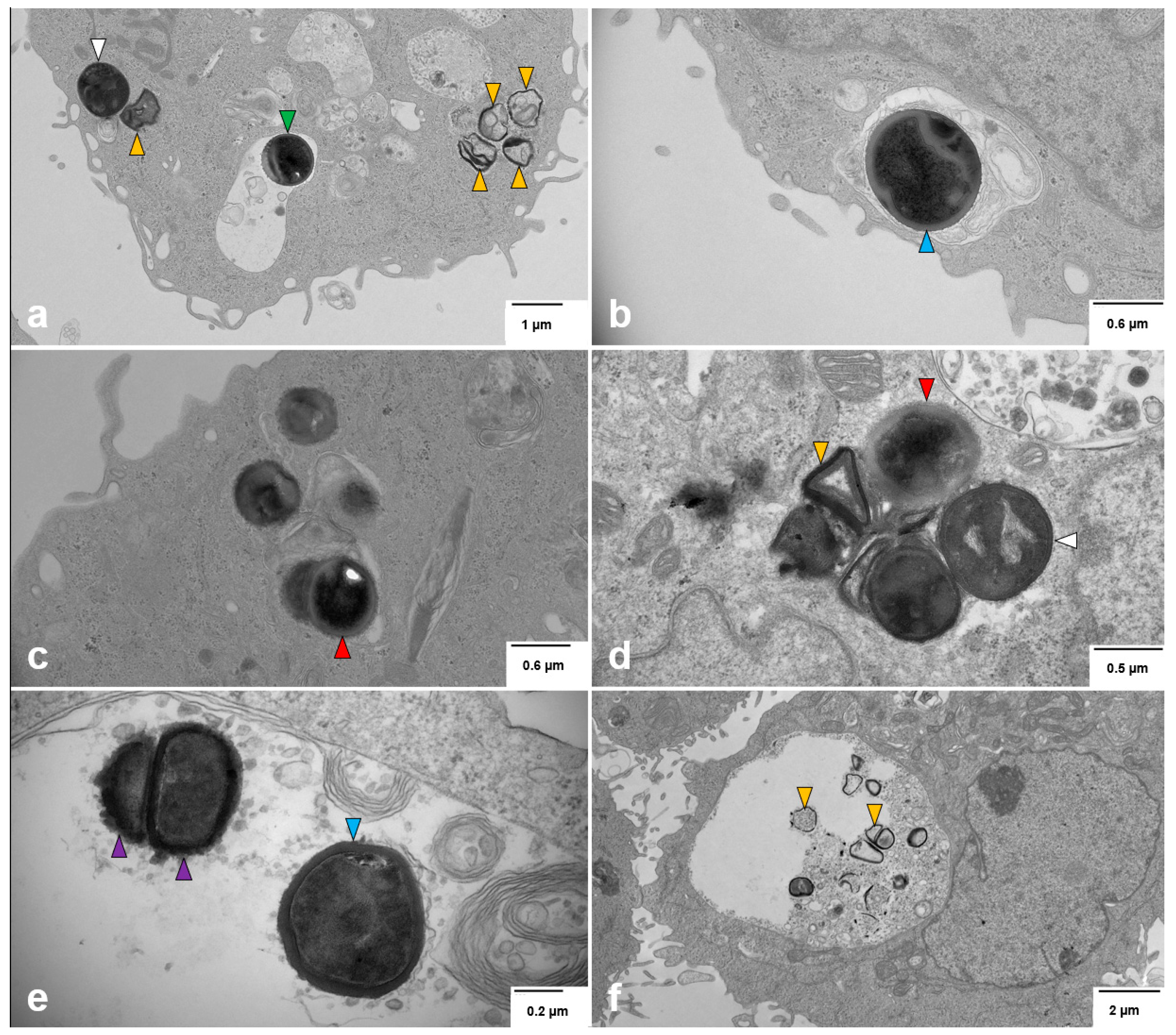
3.7. Intracellular S. aureus Exists in Multiple Forms Within Breast Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EGFR | Epidermal Growth Factor Receptor |
| GMFI | Geometric Mean Fluorescence Intensity |
| ICI | Immune Checkpoint Inhibitor |
| JAK | Janus Kinase |
| LTA | Lipoteichoic Acid |
| MIC | Minimum Inhibitory Concentration |
| MOI | Multiplicity of Infection |
| p-STAT1 | Phosphorylated Signal Transducer and Activator of Transcription 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| TLR2 | Toll-Like Receptor 2 |
References
- Cortés, J.; Guo, Z.; Karantza, V.; Aktan, G. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab (pembro)+ chemotherapy (chemo) vs placebo (PBO)+ chemo for previously untreated, locally recurrent, inoperable or metastatic triple-negative breast cancer (mTNBC). Am. Soc. Clin. Oncol. 2018, 36, 5. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Al-Khadairi, G.; Decock, J. Immune checkpoint inhibitors in triple negative breast cancer treatment: Promising future prospects. Front. Oncol. 2021, 10, 600573. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.-C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef]
- Kumar, S.; Chatterjee, M.; Ghosh, P.; Ganguly, K.K.; Basu, M.; Ghosh, M.K. Targeting PD-1/PD-L1 in cancer immunotherapy: An effective strategy for treatment of triple-negative breast cancer (TNBC) patients. Genes Dis. 2023, 10, 1318–1350. [Google Scholar] [CrossRef]
- Bastaki, S.; Irandoust, M.; Ahmadi, A.; Hojjat-Farsangi, M.; Ambrose, P.; Hallaj, S.; Edalati, M.; Ghalamfarsa, G.; Azizi, G.; Yousefi, M. PD-L1/PD-1 axis as a potent therapeutic target in breast cancer. Life Sci. 2020, 247, 117437. [Google Scholar] [CrossRef]
- Sabatier, R.; Finetti, P.; Mamessier, E.; Adelaide, J.; Chaffanet, M.; Ali, H.R.; Viens, P.; Caldas, C.; Birnbaum, D.; Bertucci, F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015, 6, 5449. [Google Scholar] [CrossRef]
- Nakayama, Y.; Mimura, K.; Tamaki, T.; Shiraishi, K.; Kua, L.F.; Koh, V.; Ohmori, M.; Kimura, A.; Inoue, S.; Okayama, H. Phospho-STAT1 expression as a potential biomarker for anti-PD-1/anti-PD-L1 immunotherapy for breast cancer. Int. J. Oncol. 2019, 54, 2030–2038. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Mao, F.; Cai, L.; Dan, H.; Jiang, L.; Zeng, X.; Li, T.; Zhou, Y.; Chen, Q. PD-1 blockade prevents the progression of oral carcinogenesis. Carcinogenesis 2021, 42, 891–902. [Google Scholar] [CrossRef]
- German, R.; Marino, N.; Hemmerich, C.; Podicheti, R.; Rusch, D.B.; Stiemsma, L.T.; Gao, H.; Xuei, X.; Rockey, P.; Storniolo, A.M. Exploring breast tissue microbial composition and the association with breast cancer risk factors. Breast Cancer Res. 2023, 25, 82. [Google Scholar] [CrossRef]
- Hoskinson, C.; Jiang, R.Y.; Stiemsma, L.T. Elucidating the roles of the mammary and gut microbiomes in breast cancer development. Front. Oncol. 2023, 13, 1198259. [Google Scholar] [CrossRef] [PubMed]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef] [PubMed]
- Kartti, S.; Bendani, H.; Boumajdi, N.; Bouricha, E.M.; Zarrik, O.; El Agouri, H.; Fokar, M.; Aghlallou, Y.; El Jaoudi, R.; Belyamani, L. Metagenomics analysis of breast microbiome highlights the abundance of Rothia genus in tumor tissues. J. Pers. Med. 2023, 13, 450. [Google Scholar] [CrossRef]
- Thyagarajan, S.; Zhang, Y.; Thapa, S.; Allen, M.S.; Phillips, N.; Chaudhary, P.; Kashyap, M.V.; Vishwanatha, J.K. Comparative analysis of racial differences in breast tumor microbiome. Sci. Rep. 2020, 10, 14116. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Wei, Z.; Tian, T.; Bose, D.; Shih, N.N.; Feldman, M.D.; Khoury, T.; De Michele, A.; Robertson, E.S. Prognostic correlations with the microbiome of breast cancer subtypes. Cell Death Dis. 2021, 12, 831. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Al-Saafeen, B.H.; Al-Sbiei, A.; Bashir, G.; Mohamed, Y.A.; Masad, R.J.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Attenuated Salmonella potentiate PD-L1 blockade immunotherapy in a preclinical model of colorectal cancer. Front. Immunol. 2022, 13, 1017780. [Google Scholar] [CrossRef]
- Esposito, M.V.; Fosso, B.; Nunziato, M.; Casaburi, G.; D’Argenio, V.; Calabrese, A.; D’Aiuto, M.; Botti, G.; Pesole, G.; Salvatore, F. Microbiome composition indicate dysbiosis and lower richness in tumor breast tissues compared to healthy adjacent paired tissue, within the same women. BMC Cancer 2022, 22, 30. [Google Scholar] [CrossRef]
- Hieken, T.J.; Chen, J.; Chen, B.; Johnson, S.; Hoskin, T.L.; Degnim, A.C.; Walther-Antonio, M.R.; Chia, N. The breast tissue microbiome, stroma, immune cells and breast cancer. Neoplasia 2022, 27, 100786. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef]
- Rad, S.K.; Yeo, K.K.; Li, R.; Wu, F.; Liu, S.; Nourmohammadi, S.; Murphy, W.M.; Tomita, Y.; Price, T.J.; Ingman, W.V. Enhancement of Doxorubicin Efficacy by Bacopaside II in Triple-Negative Breast Cancer Cells. Biomolecules 2025, 15, 55. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Shukla, D.; Mahor, H.; Srivastava, S.K.; Bodhale, N.; Banerjee, R.; Saha, B. Leishmania surface molecule lipophosphoglycan-TLR2 interaction moderates TPL2-mediated TLR2 signalling for parasite survival. Immunology 2024, 171, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, P.; Mei, W.; Zeng, C. Intratumoral microbiota: Implications for cancer onset, progression, and therapy. Front. Immunol. 2024, 14, 1301506. [Google Scholar] [CrossRef]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022, 185, 1356–1372.e26. [Google Scholar] [CrossRef]
- Bawaneh, A.K. Harnessing the Microbiome to Impact Chemotherapy Responsiveness in Triple-Negative Breast Cancer. Ph.D. Thesis, Wake Forest University, Winston-Salem, NC, USA, 2022. [Google Scholar]
- Hoffman, R.M. Tumor-targeting Salmonella typhimurium A1-R: An overview. In Bacterial Therapy of Cancer: Methods and Protocols; Humana Press: New York, NY, USA, 2016; pp. 1–8. [Google Scholar]
- Murakami, T.; Hiroshima, Y.; Miyake, K.; Kiyuna, T.; Endo, I.; Zhao, M.; Hoffman, R.M. Efficacy of tumor-targeting Salmonella typhimurium A1-R against malignancies in patient-derived orthotopic xenograft (PDOX) murine models. Cells 2019, 8, 599. [Google Scholar] [PubMed]
- Binder, D.C.; Engels, B.; Arina, A.; Yu, P.; Slauch, J.M.; Fu, Y.-X.; Karrison, T.; Burnette, B.; Idel, C.; Zhao, M. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol. Res. 2013, 1, 123–133. [Google Scholar]
- Murakami, T.; Hiroshima, Y.; Zhang, Y.; Zhao, M.; Kiyuna, T.; Hwang, H.K.; Miyake, K.; Homma, Y.; Mori, R.; Matsuyama, R. Tumor-targeting Salmonella typhimurium A1-R promotes tumoricidal CD8+ T cell tumor infiltration and arrests growth and metastasis in a syngeneic pancreatic-cancer orthotopic mouse model. J. Cell. Biochem. 2018, 119, 634–639. [Google Scholar]
- Tuchscherr, L.; Medina, E.; Hussain, M.; Völker, W.; Heitmann, V.; Niemann, S.; Holzinger, D.; Roth, J.; Proctor, R.A.; Becker, K. Staphylococcus aureus phenotype switching: An effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 2011, 3, 129–141. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus aureus: Live-in and let die. Front. Cell. Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef]
- Kubica, M.; Guzik, K.; Koziel, J.; Zarebski, M.; Richter, W.; Gajkowska, B.; Golda, A.; Maciag-Gudowska, A.; Brix, K.; Shaw, L. A potential new pathway for Staphylococcus aureus dissemination: The silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE 2008, 3, e1409. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, J.Y.; Suh, H.B.; Hwangbo, L.; Lee, N.K.; Kim, S.; Lee, J.W.; Choo, K.S.; Nam, K.J.; Kang, T. Characterization of breast cancer subtypes based on quantitative assessment of intratumoral heterogeneity using dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging. Eur. Radiol. 2022, 32, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Roderiquez, G.; Norcross, M.A. Control of Adaptive Immune Responses by Staphylococcus aureus through IL-10, PD-L1 and TLR2. Sci. Rep. 2012, 2, 606. [Google Scholar] [CrossRef]
- Mann, J.E.; Ludwig, M.L.; Kulkarni, A.; Scheftz, E.B.; Murray, I.R.; Zhai, J.; Gensterblum-Miller, E.; Jiang, H.; Brenner, J.C. Microbe-mediated activation of toll-like receptor 2 drives PDL1 expression in HNSCC. Cancers 2021, 13, 4782. [Google Scholar]
- Berg, T.; Jensen, M.-B.; Celik, A.; Talman, M.-L.; Misiakou, M.A.; Knoop, A.S.; Nielsen, F.C.; Ejlertsen, B.; Rossing, M. Molecular subtyping improves breast cancer diagnosis in the Copenhagen Breast Cancer Genomics Study. JCI Insight 2024, 9, e178114. [Google Scholar] [CrossRef]
- Smith, E.; Palethorpe, H.M.; Tomita, Y.; Pei, J.V.; Townsend, A.R.; Price, T.J.; Young, J.P.; Yool, A.J.; Hardingham, J.E. The purified extract from the medicinal plant Bacopa monnieri, bacopaside II, inhibits growth of colon cancer cells in vitro by inducing cell cycle arrest and apoptosis. Cells 2018, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Thomas, N.; Zhang, G.; Prestidge, C.A.; Coenye, T.; Wormald, P.-J.; Vreugde, S. Deferiprone and gallium-protoporphyrin have the capacity to potentiate the activity of antibiotics in Staphylococcus aureus small colony variants. Front. Cell. Infect. Microbiol. 2017, 7, 280. [Google Scholar] [CrossRef]
- Palethorpe, H.M.; Smith, E.; Tomita, Y.; Nakhjavani, M.; Yool, A.J.; Price, T.J.; Young, J.P.; Townsend, A.R.; Hardingham, J.E. Bacopasides I and II act in synergy to inhibit the growth, migration and invasion of breast cancer cell lines. Molecules 2019, 24, 3539. [Google Scholar] [CrossRef]
- Shaghayegh, G.; Cooksley, C.; Bouras, G.S.; Panchatcharam, B.S.; Idrizi, R.; Jana, M.; Ellis, S.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Chronic rhinosinusitis patients display an aberrant immune cell localization with enhanced S aureus biofilm metabolic activity and biomass. J. Allergy Clin. Immunol. 2023, 151, 723–736.e16. [Google Scholar] [CrossRef]
- Jing, X.; Shao, S.; Zhang, Y.; Luo, A.; Zhao, L.; Zhang, L.; Gu, S.; Zhao, X. BRD4 inhibition suppresses PD-L1 expression in triple-negative breast cancer. Exp. Cell Res. 2020, 392, 112034. [Google Scholar] [CrossRef]
- Lee, S.-J.; Jang, B.-C.; Lee, S.-W.; Yang, Y.-I.; Suh, S.-I.; Park, Y.-M.; Oh, S.; Shin, J.-G.; Yao, S.; Chen, L. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-γ-induced upregulation of B7-H1 (CD274). FEBS Lett. 2006, 580, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, G.P.; Shafran, J.S.; Smith, C.L.; Belkina, A.C.; Casey, A.N.; Jafari, N.; Denis, G.V. BET protein targeting suppresses the PD-1/PD-L1 pathway in triple-negative breast cancer and elicits anti-tumor immune response. Cancer Lett. 2019, 465, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Karasar, P.; Esendagli, G. T helper responses are maintained by basal-like breast cancer cells and confer to immune modulation via upregulation of PD-1 ligands. Breast Cancer Res. Treat. 2014, 145, 605–614. [Google Scholar] [CrossRef]
- Xu, P.; Xiong, W.; Lin, Y.; Fan, L.; Pan, H.; Li, Y. Histone deacetylase 2 knockout suppresses immune escape of triple-negative breast cancer cells via downregulating PD-L1 expression. Cell Death Dis. 2021, 12, 779. [Google Scholar] [CrossRef]
- Watkins, R.L.; Pallister, K.B.; Voyich, J.M. The SaeR/S gene regulatory system induces a pro-inflammatory cytokine response during Staphylococcus aureus infection. PLoS ONE 2011, 6, e19939. [Google Scholar] [CrossRef]
- Watkins, R.L.; Zurek, O.W.; Pallister, K.B.; Voyich, J.M. The SaeR/S two-component system induces interferon-gamma production in neutrophils during invasive Staphylococcus aureus infection. Microbes Infect. 2013, 15, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Nishikawa, S.; Miura, T.; Mizuki, M.; Yamada, K.; Madarame, H.; Tagawa, Y.I.; Iwakura, Y.; Nakane, A. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect Immun 2000, 68, 2424–2430. [Google Scholar] [CrossRef]
- McLoughlin, R.M.; Lee, J.C.; Kasper, D.L.; Tzianabos, A.O. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 2008, 181, 1323–1332. [Google Scholar] [CrossRef]
- Yin, H.; Pu, N.; Chen, Q.; Zhang, J.; Zhao, G.; Xu, X.; Wang, D.; Kuang, T.; Jin, D.; Lou, W.; et al. Gut-derived lipopolysaccharide remodels tumoral microenvironment and synergizes with PD-L1 checkpoint blockade via TLR4/MyD88/AKT/NF-kappaB pathway in pancreatic cancer. Cell Death Dis 2021, 12, 1033. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, L.; Du, D.; Mai, L.; Liu, Y.; Morigen, M.; Fan, L. The RIG-I-like receptor signaling pathway triggered by Staphylococcus aureus promotes breast cancer metastasis. Int. Immunopharmacol. 2024, 142, 113195. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B. The function of TLR2 during staphylococcal diseases. Front. Cell. Infect. Microbiol. 2013, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Alva-Murillo, N.; López-Meza, J.E.; Ochoa-Zarzosa, A. Nonprofessional phagocytic cell receptors involved in Staphylococcus aureus internalization. BioMed Res. Int. 2014, 2014, 538546. [Google Scholar] [CrossRef]
- Ji, Z.; Su, J.; Hou, Y.; Yao, Z.; Yu, B.; Zhang, X. EGFR/FAK and c-Src signalling pathways mediate the internalisation of Staphylococcus aureus by osteoblasts. Cell. Microbiol. 2020, 22, e13240. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Dieras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S. LBA16 IMpassion130: Final OS analysis from the pivotal phase III study of atezolizumab+ nab-paclitaxel vs placebo+ nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann. Oncol. 2020, 31, S1148. [Google Scholar] [CrossRef]
- Heshiki, Y.; Vazquez-Uribe, R.; Li, J.; Ni, Y.; Quainoo, S.; Imamovic, L.; Li, J.; Sørensen, M.; Chow, B.K.; Weiss, G.J. Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome 2020, 8, 28. [Google Scholar] [CrossRef]
- Josse, J.; Laurent, F.; Diot, A. Staphylococcal adhesion and host cell invasion: Fibronectin-binding and other mechanisms. Front. Microbiol. 2017, 8, 2433. [Google Scholar] [CrossRef]
- Renard, H.-F.; Boucrot, E. Unconventional endocytic mechanisms. Curr. Opin. Cell Biol. 2021, 71, 120–129. [Google Scholar] [CrossRef]
- Underhill, D.M.; Ozinsky, A.; Hajjar, A.M.; Stevens, A.; Wilson, C.B.; Bassetti, M.; Aderem, A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 1999, 401, 811–815. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.; Khan, N.; Vyas, J.; Hooper, D. Tet38 efflux pump affects Staphylococcus aureus internalization by epithelial cells through interaction with CD36 and contributes to bacterial escape from acidic and nonacidic phagolysosomes. Infect. Immun. 2017, 85, e00862-16. [Google Scholar] [CrossRef] [PubMed]
- Haggar, A. Interaction Between Extracellular Adherence Protein (Eap) from Staphylococcus Aureus and the Human Host; Karolinska Institutet: Stockholm, Sweden, 2005. [Google Scholar]
- Porayath, C.; Suresh, M.K.; Biswas, R.; Nair, B.G.; Mishra, N.; Pal, S. Autolysin mediated adherence of Staphylococcus aureus with fibronectin, gelatin and heparin. Int. J. Biol. Macromol. 2018, 110, 179–184. [Google Scholar] [CrossRef]
- Banville, R.R. Factors affecting growth of Staphylococcus aureus L forms on semidefined medium. J. Bacteriol. 1964, 87, 1192–1197. [Google Scholar] [CrossRef]
- Kawai, Y.; Mercier, R.; Mickiewicz, K.; Serafini, A.; Sório de Carvalho, L.P.; Errington, J. Crucial role for central carbon metabolism in the bacterial L-form switch and killing by β-lactam antibiotics. Nat. Microbiol. 2019, 4, 1716–1726. [Google Scholar] [PubMed]
- Han, J.; Shi, W.; Xu, X.; Wang, S.; Zhang, S.; He, L.; Sun, X.; Zhang, Y. Conditions and mutations affecting Staphylococcus aureus L-form formation. Microbiology 2015, 161, 57–66. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Martinez-Martin, D.; Venturini, C.; Mickiewicz, K.; Flores-Rodriguez, N.; Errington, J.; Iredell, J. L-form switching in Escherichia coli as a common β-lactam resistance mechanism. Microbiol. Spectr. 2022, 10, e02419–e02422. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Mickiewicz, K.; Errington, J. Lysozyme counteracts β-lactam antibiotics by promoting the emergence of L-form bacteria. Cell 2018, 172, 1038–1049.e10. [Google Scholar] [CrossRef]
- Kong, L.-X.; Wang, Z.; Shou, Y.-K.; Zhou, X.-D.; Zong, Y.-W.; Tong, T.; Liao, M.; Han, Q.; Li, Y.; Cheng, L. The FnBPA from methicillin-resistant Staphylococcus aureus promoted development of oral squamous cell carcinoma. J. Oral Microbiol. 2022, 14, 2098644. [Google Scholar] [CrossRef]
- Hattar, K.; Reinert, C.P.; Sibelius, U.; Gökyildirim, M.Y.; Subtil, F.S.; Wilhelm, J.; Eul, B.; Dahlem, G.; Grimminger, F.; Seeger, W. Lipoteichoic acids from Staphylococcus aureus stimulate proliferation of human non-small-cell lung cancer cells in vitro. Cancer Immunol. Immunother. 2017, 66, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Jean, A.T.S.; Swofford, C.A.; Panteli, J.T.; Brentzel, Z.J.; Forbes, N.S. Bacterial delivery of Staphylococcus aureus α-hemolysin causes regression and necrosis in murine tumors. Mol. Ther. 2014, 22, 1266–1274. [Google Scholar] [CrossRef]
- Mahmoodzadeh Hosseini, H.; Imani Fooladi, A.A.; Soleimanirad, J.; Nourani, M.R.; Davaran, S.; Mahdavi, M. Staphylococcal entorotoxin B anchored exosome induces apoptosis in negative esterogen receptor breast cancer cells. Tumor Biol. 2014, 35, 3699–3707. [Google Scholar] [CrossRef]
- Stelzner, K.; Winkler, A.-C.; Liang, C.; Boyny, A.; Ade, C.P.; Dandekar, T.; Fraunholz, M.J.; Rudel, T. Intracellular Staphylococcus aureus perturbs the host cell Ca2+ homeostasis to promote cell death. mBio 2020, 11, e02250-20. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, J.; Zhao, Y.; Wang, S.; Xu, J.; Yin, K. Targeting STING in cancer: Challenges and emerging opportunities. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188983. [Google Scholar] [CrossRef] [PubMed]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.; Pauken, K.E.; Huang, A.C. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.-D.; Mauceri, H.; Beckett, M.; Darga, T. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef]
- Fishbein, S.R.; Mahmud, B.; Dantas, G. Antibiotic perturbations to the gut microbiome. Nat. Rev. Microbiol. 2023, 21, 772–788. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Pinato, D.J.; Gramenitskaya, D.; Altmann, D.M.; Boyton, R.J.; Mullish, B.H.; Marchesi, J.R.; Bower, M. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 287. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Badaoui, S.; Kichenadasse, G.; Karapetis, C.S.; McKinnon, R.A.; Rowland, A.; Sorich, M.J. Efficacy of atezolizumab in patients with advanced NSCLC receiving concomitant antibiotic or proton pump inhibitor treatment: Pooled analysis of five randomized control trials. J. Thorac. Oncol. 2022, 17, 758–767. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rad, S.K.; Li, R.; Yeo, K.K.L.; Cooksley, C.; Shaghayegh, G.; Vreugde, S.; Wu, F.; Tomita, Y.; Price, T.J.; Ingman, W.V.; et al. Cell Line-Dependent Internalization, Persistence, and Immunomodulatory Effects of Staphylococcus aureus in Triple-Negative Breast Cancer. Cancers 2025, 17, 2947. https://doi.org/10.3390/cancers17182947
Rad SK, Li R, Yeo KKL, Cooksley C, Shaghayegh G, Vreugde S, Wu F, Tomita Y, Price TJ, Ingman WV, et al. Cell Line-Dependent Internalization, Persistence, and Immunomodulatory Effects of Staphylococcus aureus in Triple-Negative Breast Cancer. Cancers. 2025; 17(18):2947. https://doi.org/10.3390/cancers17182947
Chicago/Turabian StyleRad, Sima Kianpour, Runhao Li, Kenny K. L. Yeo, Clare Cooksley, Gohar Shaghayegh, Sarah Vreugde, Fangmeinuo Wu, Yoko Tomita, Timothy J. Price, Wendy V. Ingman, and et al. 2025. "Cell Line-Dependent Internalization, Persistence, and Immunomodulatory Effects of Staphylococcus aureus in Triple-Negative Breast Cancer" Cancers 17, no. 18: 2947. https://doi.org/10.3390/cancers17182947
APA StyleRad, S. K., Li, R., Yeo, K. K. L., Cooksley, C., Shaghayegh, G., Vreugde, S., Wu, F., Tomita, Y., Price, T. J., Ingman, W. V., Townsend, A. R., & Smith, E. (2025). Cell Line-Dependent Internalization, Persistence, and Immunomodulatory Effects of Staphylococcus aureus in Triple-Negative Breast Cancer. Cancers, 17(18), 2947. https://doi.org/10.3390/cancers17182947









