Influence of Genetic, Dietary, and Environmental Factors on Natural Killer (NK) Cell Biology and Function: Interplay Between NK Cell Activity and Cancer Onset or Progression
Simple Summary
Abstract
1. Introduction: Natural Killer Cells
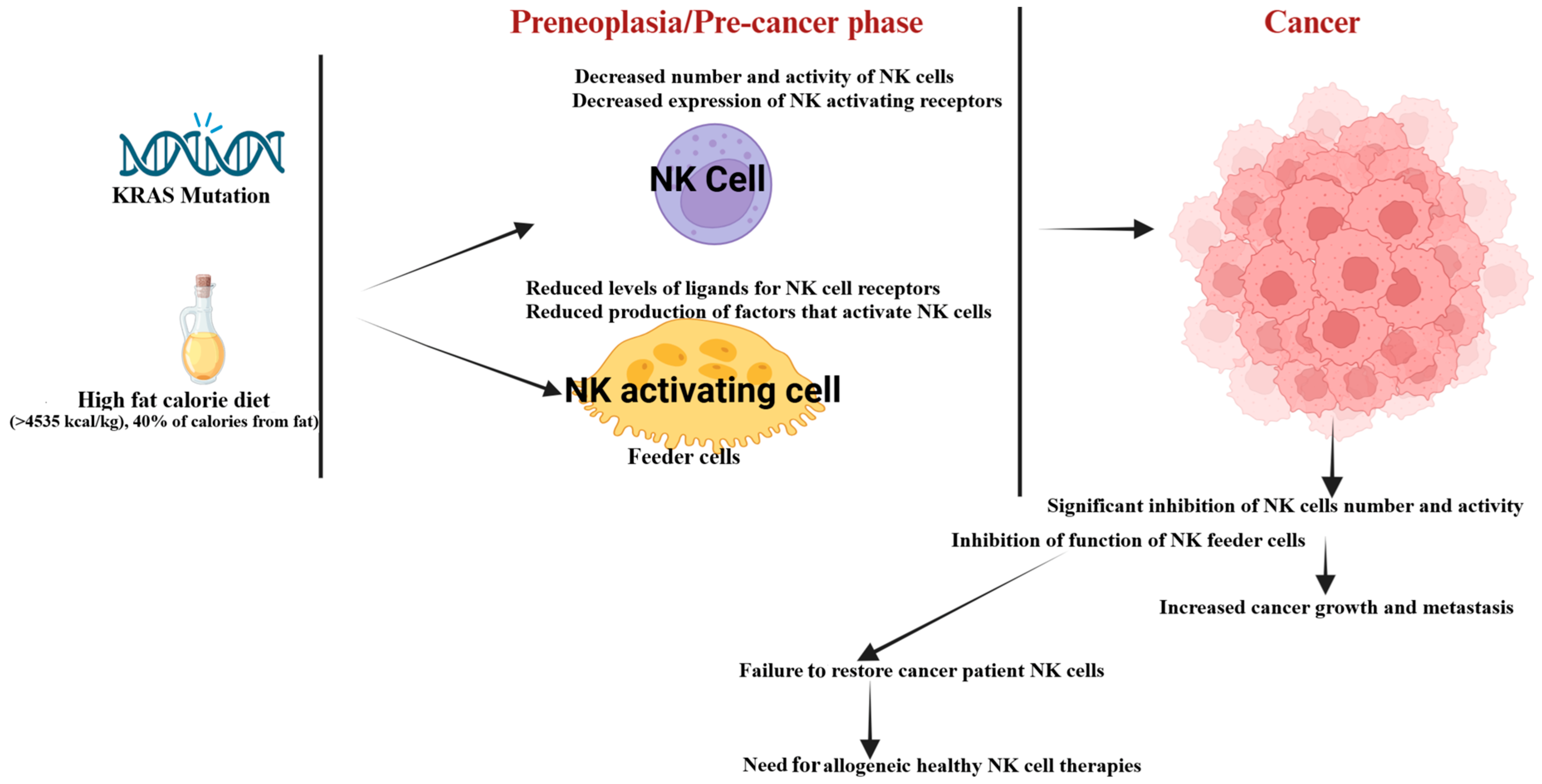
2. Changes in the Number and Function of NK Cells at the Preneoplastic Stage or Due to Genetic and Environmental Factors
3. Reduced NK Cell Activity in the Cancer-Bearing Mice Model
4. Impaired NK Cell Function in Human Cancer Patients
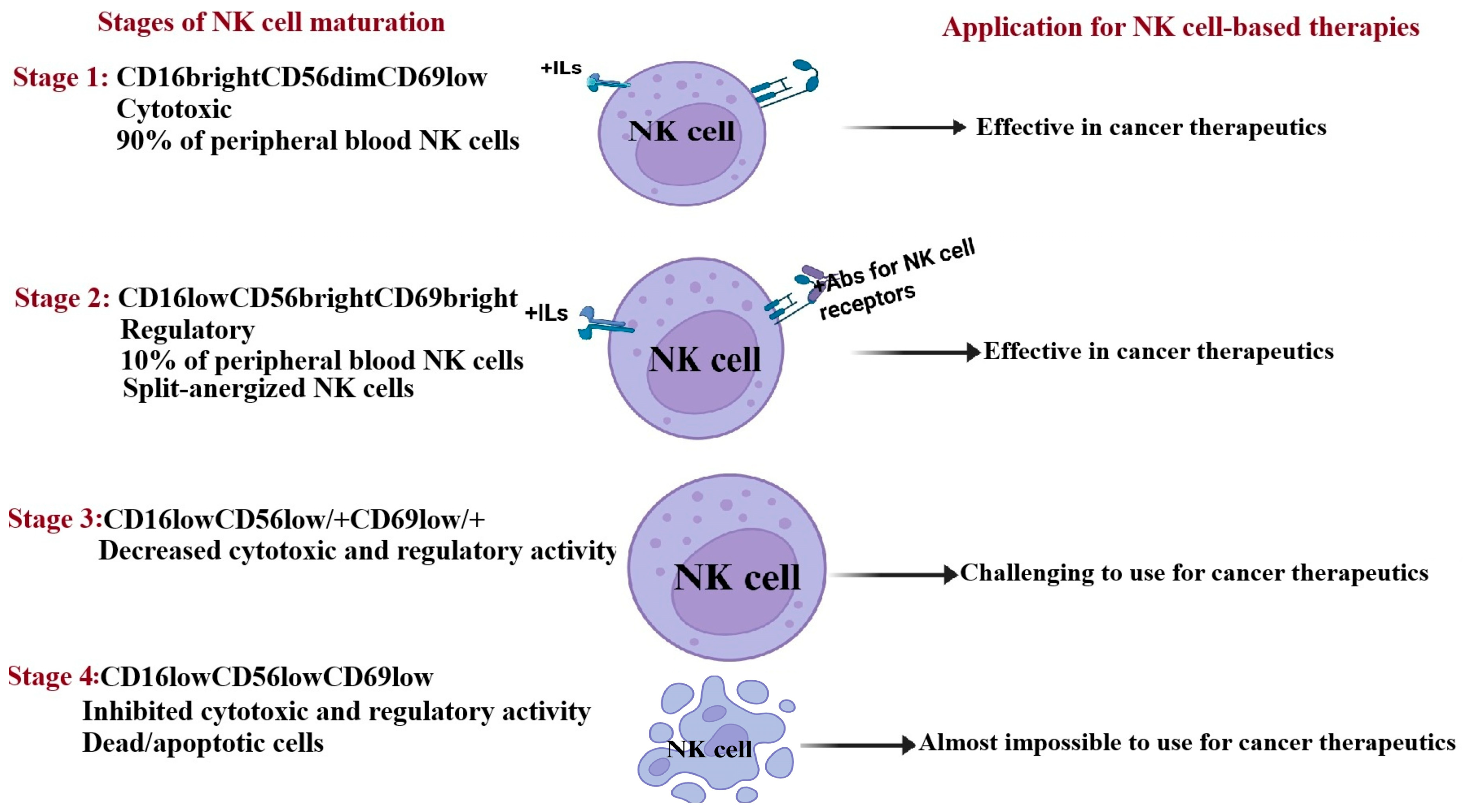
5. The Maturation Stages of Natural Killer Cells
6. Memory-like NK Cells
7. Progress and Challenges in Developing NK Cell-Based Cancer Immunotherapies
7.1. Modifying the Genes of NK Cells to Enhance Their Activity
7.2. Feeder Cells Triggered the Activation and Expansion of NK Cells
7.3. Genetically Engineered Feeder Cells to Stimulate NK Cell Activation and Expansion
7.4. Cytokine-Driven NK Cell Expansion
7.5. Challenges in Developing and Ensuring the Efficacy of NK Cell-Based Therapies
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Moretta, A.; Marcenaro, E.; Parolini, S.; Ferlazzo, G.; Moretta, L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2007, 15, 226–233. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Colucci, F.; Caligiuri, M.A.; Di Santo, J.P. What does it take to make a natural killer? Nat. Rev. Immunol. 2003, 3, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.M.; Rajasekaran, K.; Thakar, M.S.; Malarkannan, S. Clinical Relevance of Natural Killer Cells Following Hematopoietic Stem Cell Transplantation. J. Cancer 2013, 4, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Fildes, J.E.; Yonan, N.; Leonard, C.T. Natural killer cells and lung transplantation, roles in rejection, infection, and tolerance. Transpl. Immunol. 2008, 19, 1–11. [Google Scholar] [CrossRef]
- Farag, S.S.; Caligiuri, M.A. Human natural killer cell development and biology. Blood Rev. 2006, 20, 123–137. [Google Scholar] [CrossRef]
- Lanier, L.L. NK Cell Recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Magister, Š.; Tseng, H.-C.; Bui, V.T.; Kos, J.; Jewett, A. Regulation of split anergy in natural killer cells by inhibition of cathepsins C and H and cystatin F. Oncotarget 2015, 6, 22310–22327. [Google Scholar] [CrossRef]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.-Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef]
- Perišić Nanut, M.; Sabotič, J.; Jewett, A.; Kos, J. Cysteine cathepsins as regulators of the cytotoxicity of NK and T cells. Front. Immunol. 2014, 5, 616. [Google Scholar] [CrossRef]
- Jakoš, T.; Pišlar, A.; Jewett, A.; Kos, J. Cysteine Cathepsins in Tumor-Associated Immune Cells. Front. Immunol. 2019, 10, 2037. [Google Scholar] [CrossRef] [PubMed]
- D’ANgelo, M.E.; Bird, P.I.; Peters, C.; Reinheckel, T.; Trapani, J.A.; Sutton, V.R. Cathepsin H Is an Additional Convertase of Pro-granzyme B. J. Biol. Chem. 2010, 285, 20514–20519. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Nanut, M.P.; Prunk, M.; Sabotič, J.; Dautović, E.; Jewett, A. Cystatin F as a regulator of immune cell cytotoxicity. Cancer Immunol. Immunother. 2018, 67, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.J.; Zanivan, S. The tumor microenvironment is an ecosystem sustained by metabolic interactions. Cell Rep. 2025, 44, 115432. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fu, L.; Wu, R.; Che, L.; Liu, G.; Ran, Q.; Xia, Z.; Liang, X.; Zhao, G. Immunocytes in the tumor microenvironment: Recent updates and interconnections. Front. Immunol. 2025, 16, 1517959. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, J. Cytotoxic and chemotactic dynamics of NK cells quantified by live-cell imaging. Methods Cell Biol. 2022, 173, 49–64. [Google Scholar] [CrossRef]
- Quatrini, L.; Della Chiesa, M.; Sivori, S.; Mingari, M.C.; Pende, D.; Moretta, L. Author response for “Human NK cells, their receptors and function”. Eur. J. Immunol. 2021. [Google Scholar] [CrossRef]
- Strasser, A.; Jost, P.J.; Nagata, S. The Many Roles of FAS Receptor Signaling in the Immune System. Immunity 2009, 30, 180–192. [Google Scholar] [CrossRef]
- Ramaswamy, M.; Clel, S.Y.; Cruz, A.C.; Siegel, R.M. Many Checkpoints on the Road to Cell Death:Regulation of Fas–FasL Interactions and Fas Signaling in Peripheral Immune Responses. In Death Receptors and Cognate Ligands in Cancer; Springer: Berlin/Heidelberg, Germany, 2009; Volume 49, pp. 17–47. [Google Scholar] [CrossRef]
- Chua, H.L.; Serov, Y.; Brahmi, Z. Regulation of FasL expression in natural killer cells. Hum. Immunol. 2004, 65, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Furuke, K.; Shiraishi, M.; Mostowski, H.S.; Bloom, E.T. Fas Ligand Induction in Human NK Cells Is Regulated by Redox Through a Calcineurin-Nuclear Factors of Activated T Cell-Dependent Pathway. J. Immunol. 1999, 162, 1988–1993. [Google Scholar] [CrossRef]
- Daher, M.; Rezvani, K. Next generation natural killer cells for cancer immunotherapy: The promise of genetic engineering. Curr. Opin. Immunol. 2018, 51, 146–153. [Google Scholar] [CrossRef]
- Page, A.; Chuvin, N.; Valladeau-Guilemond, J.; Depil, S. Development of NK cell-based cancer immunotherapies through receptor engineering. Cell. Mol. Immunol. 2024, 21, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Sordo-Bahamonde, C.; Vitale, M.; Lorenzo-Herrero, S.; López-Soto, A.; Gonzalez, S. Mechanisms of Resistance to NK Cell Immunotherapy. Cancers 2020, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Jacobs, M.T.; Fehniger, T.A. Allogeneic natural killer cell therapy. Blood 2023, 141, 856–868. [Google Scholar] [CrossRef]
- Gao, F.; Ye, Y.; Gao, Y.; Huang, H.; Zhao, Y. Influence of KIR and NK Cell Reconstitution in the Outcomes of Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 2022. [Google Scholar] [CrossRef]
- Mehta, R.S.; Rezvani, K. Can we make a better match or mismatch with KIR genotyping? Hematol.-Am. Soc. Hematol. Educ. Program 2016, 2016, 106–118. [Google Scholar] [CrossRef]
- Mohseni, R.; Sharif, P.M.; Behfar, M.; Shojaei, S.; Shoae-Hassani, A.; Jafari, L.; Khosravi, A.; Nikfetrat, Z.; Hamidieh, A.A. Phase I study of safety and efficacy of allogeneic natural killer cell therapy in relapsed/refractory neuroblastomas post autologous hematopoietic stem cell transplantation. Sci. Rep. 2024, 14, 20971. [Google Scholar] [CrossRef]
- Bednarski, J.J.; Zimmerman, C.; Berrien-Elliott, M.M.; Foltz, J.A.; Becker-Hapak, M.; Neal, C.C.; Foster, M.; Schappe, T.; McClain, E.; Pence, P.P.; et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood 2022, 139, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, G.P.; Dimitroulis, J.; Antoniou, D.; Katis, C.; Tsavdaridis, D.; Armenaki, O.; Marosis, C.; Michalopoulou, P.; Grigoratou, T.; Stathopoulos, J. Front-line paclitaxel and irinotecan combination chemotherapy in advanced non-small-cell lung cancer: A phase I–II trial. Br. J. Cancer 2005, 93, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Sochacka-Ćwikła, A.; Mączyński, M.; Regiec, A. FDA-Approved Drugs for Hematological Malignancies—The Last Decade Review. Cancers 2021, 14, 87. [Google Scholar] [CrossRef]
- Heipertz, E.L.; Zynda, E.R.; Stav-Noraas, T.E.; Hungler, A.D.; Boucher, S.E.; Kaur, N.; Vemuri, M.C. Current Perspectives on “Off-The-Shelf” Allogeneic NK and CAR-NK Cell Therapies. Front. Immunol. 2021, 12, 732135. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Lamb, M.G.; Rangarajan, H.G.; Tullius, B.P.; Lee, D.A. Natural killer cell therapy for hematologic malignancies: Successes, challenges, and the future. Stem Cell Res. Ther. 2021, 12, 211. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Kerbauy, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.S.A.; Suck, G.; Nowakowska, P.; Ullrich, E.; Seifried, E.; Bader, P.; Tonn, T.; Seidl, C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016, 65, 477–484. [Google Scholar] [CrossRef]
- Eser, S.; Schnieke, A.; Schneider, G.; Saur, D. Oncogenic KRAS signalling in pancreatic cancer. Br. J. Cancer 2014, 111, 817–822. [Google Scholar] [CrossRef]
- Kaur, K.; Chang, H.-H.; Cook, J.; Eibl, G.; Jewett, A. Suppression of Gingival NK Cells in Precancerous and Cancerous Stages of Pancreatic Cancer in KC and BLT-Humanized Mice. Front. Immunol. 2017, 8, 1606. [Google Scholar] [CrossRef]
- Kaur, K.; Chang, H.-H.; Topchyan, P.; Cook, J.M.; Barkhordarian, A.; Eibl, G.; Jewett, A. Deficiencies in Natural Killer Cell Numbers, Expansion, and Function at the Pre-Neoplastic Stage of Pancreatic Cancer by KRAS Mutation in the Pancreas of Obese Mice. Front. Immunol. 2018, 9, 1229. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, J.-C.; Paik, K.-H.; Kang, J.; Kim, J.; Hwang, J.-H. Serum interleukin-6 is associated with pancreatic ductal adenocarcinoma progression pattern. Medicine 2017, 96, e5926. [Google Scholar] [CrossRef]
- Long, K.B.; Tooker, G.; Tooker, E.; Luque, S.L.; Lee, J.W.; Pan, X.; Beatty, G.L. IL6 Receptor Blockade Enhances Chemotherapy Efficacy in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2017, 16, 1898–1908. [Google Scholar] [CrossRef]
- Nagathihalli, N.S.; Castellanos, J.A.; VanSaun, M.N.; Dai, X.; Ambrose, M.; Guo, Q.; Xiong, Y.; Merchant, N.B. Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells. Oncotarget 2016, 7, 65982–65992. [Google Scholar] [CrossRef]
- Goumas, F.A.; Holmer, R.; Egberts, J.; Gontarewicz, A.; Heneweer, C.; Geisen, U.; Hauser, C.; Mende, M.; Legler, K.; Röcken, C.; et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int. J. Cancer 2015, 137, 1035–1046. [Google Scholar] [CrossRef]
- Lesina, M.; Kurkowski, M.U.; Ludes, K.; Rose-John, S.; Treiber, M.; Klöppel, G.; Yoshimura, A.; Reindl, W.; Sipos, B.; Akira, S.; et al. Stat3/Socs3 Activation by IL-6 Transsignaling Promotes Progression of Pancreatic Intraepithelial Neoplasia and Development of Pancreatic Cancer. Cancer Cell 2011, 19, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, J.; Sheard, M.A.; Tran, H.C.; Wan, Z.; Liu, W.Y.; Asgharzadeh, S.; Sposto, R.; Wu, H.W.; Seeger, R.C.; et al. Lenalidomide overcomes suppression of human natural killer cell anti-tumor functions by neuroblastoma microenvironment-associated IL-6 and TGFβ1. Cancer Immunol. Immunother. 2013, 62, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Arasteh, A.; Paranjpe, A.; Teruel, A.; Yang, W.; Behel, A.; Alva, J.A.; Walter, G.; Head, C.; Ishikawa, T.-O.; et al. Increased Lysis of Stem Cells but Not Their Differentiated Cells by Natural Killer Cells; De-Differentiation or Reprogramming Activates NK Cells. PLoS ONE 2010, 5, e11590. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Cacalano, N.A.; Teruel, A.; Romero, M.; Rashedi, M.; Wang, M.; Nakamura, H. Inhibition of nuclear factor kappa B (NFκB) activity in oral tumor cells prevents depletion of NK cells and increases their functional activation. Cancer Immunol. Immunother. 2005, 55, 1052–1063. [Google Scholar] [CrossRef]
- Kozlowska, A.K.; Tseng, H.-C.; Kaur, K.; Topchyan, P.; Inagaki, A.; Bui, V.T.; Kasahara, N.; Cacalano, N.; Jewett, A. Resistance to cytotoxicity and sustained release of interleukin-6 and interleukin-8 in the presence of decreased interferon-γ after differentiation of glioblastoma by human natural killer cells. Cancer Immunol. Immunother. 2016, 65, 1085–1097. [Google Scholar] [CrossRef]
- Kozlowska, A.; Topchyan, P.; Kaur, K.; Tseng, H.-C.; Teruel, A.; Hiraga, T.; Jewett, A. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J. Cancer 2017, 8, 537–554. [Google Scholar] [CrossRef]
- Young, M.R.; Wheeler, E.; Newby, M. Macrophage-Mediated Suppression of Natural Killer Cell Activity in Mice Bearing Lewis Lung Carcinoma2. JNCI J. Natl. Cancer Inst. 1986, 76, 745–750. [Google Scholar] [CrossRef]
- Kaur, K.; Cook, J.; Park, S.-H.; Topchyan, P.; Kozlowska, A.; Ohanian, N.; Fang, C.; Nishimura, I.; Jewett, A. Novel Strategy to Expand Super-Charged NK Cells with Significant Potential to Lyse and Differentiate Cancer Stem Cells: Differences in NK Expansion and Function between Healthy and Cancer Patients. Front. Immunol. 2017, 8, 297. [Google Scholar] [CrossRef]
- Kaur, K.; Topchyan, P.; Kozlowska, A.K.; Ohanian, N.; Chiang, J.; Maung, P.O.; Park, S.-H.; Ko, M.-W.; Fang, C.; Nishimura, I.; et al. Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. OncoImmunology 2018, 7, e1426518. [Google Scholar] [CrossRef]
- Jewett, A.; Kozlowska, A.K.; Kaur, K.; Topchyan, P. Novel strategies to target cancer stem cells by NK cells studies in humanized mice. Front. Biosci.-Landmark 2017, 22, 370–384. [Google Scholar] [CrossRef]
- Kaur, K.; Kozlowska, A.K.; Topchyan, P.; Ko, M.-W.; Ohanian, N.; Chiang, J.; Cook, J.; Maung, P.O.; Park, S.-H.; Cacalano, N.; et al. Probiotic-Treated Super-Charged NK Cells Efficiently Clear Poorly Differentiated Pancreatic Tumors in Hu-BLT Mice. Cancers 2019, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Topchyan, P.; Jewett, A. Supercharged Natural Killer (sNK) Cells Inhibit Melanoma Tumor Progression and Restore Endogenous NK Cell Function in Humanized BLT Mice. Cancers 2025, 17, 2430. [Google Scholar] [CrossRef] [PubMed]
- Türkseven, M.R.; Oygür, T. Evaluation of natural killer cell defense in oral squamous cell carcinoma. Oral Oncol. 2010, 46, e34–e37. [Google Scholar] [CrossRef] [PubMed]
- Accomando, W.P.; Wiencke, J.K.; Houseman, E.A.; Butler, R.A.; Zheng, S.; Nelson, H.H.; Kelsey, K.T. Decreased NK cells in patients with head and neck cancer determined in archival DNA. Clin. Cancer Res. 2012, 18, 6147–6154. [Google Scholar] [CrossRef]
- Mickel, R.A.; Kessler, D.J.; Taylor, J.M.G.; Lichtenstein, A. Natural-Killer Cell Cyto-Toxicity in the Peripheral-Blood, Cervical Lymph-Nodes, and Tumor of Head and Neck-Cancer Patients. Cancer Res. 1988, 48, 5017–5022. [Google Scholar]
- Kaur, K.; Ko, M.-W.; Chen, F.; Jewett, A. Defective NK cell expansion, cytotoxicity, and lack of ability to differentiate tumors from a pancreatic cancer patient in a long term follow-up: Implication in the progression of cancer. Cancer Immunol. Immunother. 2021, 71, 1033–1047. [Google Scholar] [CrossRef]
- Aparicio-Pagés, M.N.; Verspaget, H.W.; Peña, A.S.; Lamers, C.B. Natural killer cell activity in patients with adenocarcinoma in the upper gastrointestinal tract. J. Clin. Lab. Immunol. 1991, 35, 27–32. [Google Scholar]
- Duan, X.; Deng, L.; Chen, X.; Lu, Y.; Zhang, Q.; Zhang, K.; Hu, Y.; Zeng, J.; Sun, W. Clinical significance of the immunostimulatory MHC class I chain-related molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med. Oncol. 2010, 28, 466–474. [Google Scholar] [CrossRef]
- Peng, Y.-P.; Zhu, Y.; Zhang, J.-J.; Xu, Z.-K.; Qian, Z.-Y.; Dai, C.-C.; Jiang, K.-R.; Wu, J.-L.; Gao, W.-T.; Li, Q.; et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J. Transl. Med. 2013, 11, 262. [Google Scholar] [CrossRef]
- Jewett, A.; Man, Y.-G.; Tseng, H.-C. Dual Functions of Natural Killer Cells in Selection and Differentiation of Stem Cells; Role in Regulation of Inflammation and Regeneration of Tissues. J. Cancer 2013, 4, 12–24. [Google Scholar] [CrossRef]
- Gogali, F.; Paterakis, G.; Rassidakis, G.Z.; Liakou, C.I.; Liapi, C. CD3(-)CD16(-)CD56(bright) immunoregulatory NK cells are increased in the tumor microenvironment and inversely correlate with advanced stages in patients with papillary thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2013, 23, 1561–1568. [Google Scholar] [CrossRef]
- López-Cobo, S.; Pieper, N.; Campos-Silva, C.; García-Cuesta, E.M.; Reyburn, H.T.; Paschen, A.; Valés-Gómez, M. Impaired NK cell recognition of vemurafenib-treated melanoma cells is overcome by simultaneous application of histone deacetylase inhibitors. OncoImmunology 2017, 7, e1392426. [Google Scholar] [CrossRef] [PubMed]
- Ciszak, L.; Kosmaczewska, A.; Werynska, B.; Szteblich, A.; Jankowska, R.; Frydecka, I. Impaired zeta chain expression and IFN-gamma production in peripheral blood T and NK cells of patients with advanced lung cancer. Oncol. Rep. 2009, 21, 173–184. [Google Scholar] [PubMed]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.Y.; Quek, J.K.S.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C.; et al. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef] [PubMed]
- Coënon, L.; Villalba, M. From CD16a Biology to Antibody-Dependent Cell-Mediated Cytotoxicity Improvement. Front. Immunol. 2022, 13, 913215. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.B.; Rocco, A.; Lamb, L.S.; Friedman, G.K.; Hjelmeland, A.B. Regulation of NKG2D Stress Ligands and Its Relevance in Cancer Progression. Cancers 2022, 14, 2339. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Xin, J.; Wang, J.; Yao, C.; Zhang, Z. Role of NKG2D and its ligands in cancer immunotherapy. Am. J. Cancer Res. 2019, 9, 2064–2078. [Google Scholar]
- Boneva, E.; Shivarov, V.; Ivanova, M. A Concise Review of the Role of the NKG2D Receptor and Its Ligands in Cancer. Immuno 2025, 5, 9. [Google Scholar] [CrossRef]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.; Cooley, S.; Verneris, M.; Walcheck, B.; et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013, 121, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. The paradoxical role of IFN-γ in cancer: Balancing immune activation and immune evasion. Pathol.-Res. Pract. 2025, 272, 156046. [Google Scholar] [CrossRef]
- Fanijavadi, S.; Thomassen, M.; Jensen, L.H. Targeting Triple NK Cell Suppression Mechanisms: A Comprehensive Review of Biomarkers in Pancreatic Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 515. [Google Scholar] [CrossRef]
- Sykulev, Y.; Tsygankova, O.; Anikeeva, N.; Maskalenko, N.; Campbell, K. CD16 and NKG2D co-clustering facilitates quality of primary NK cell responses. J. Immunol. 2022, 208, 116.06. [Google Scholar] [CrossRef]
- Srpan, K.; Ambrose, A.; Karampatzakis, A.; Saeed, M.; Cartwright, A.N.; Guldevall, K.; Matos, G.D.S.C.D.; Önfelt, B.; Davis, D.M. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J. Cell Biol. 2018, 217, 3267–3283. [Google Scholar] [CrossRef]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. Sage Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Knudsen, N.H.; Manguso, R.T. Tumor-Derived PGE2 Gives NK Cells a Headache. Immunity 2020, 53, 1131–1132. [Google Scholar] [CrossRef]
- Pietra, G.; Manzini, C.; Rivara, S.; Vitale, M.; Cantoni, C.; Petretto, A.; Balsamo, M.; Conte, R.; Benelli, R.; Minghelli, S.; et al. Melanoma Cells Inhibit Natural Killer Cell Function by Modulating the Expression of Activating Receptors and Cytolytic Activity. Cancer Res. 2012, 72, 1407–1415. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C. NK Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 69–90. [Google Scholar]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Jewett, A.; Tseng, H.-C. Tumor Induced Inactivation of Natural Killer Cell Cytotoxic Function; Implication in Growth, Expansion and Differentiation of Cancer Stem Cells. J. Cancer 2011, 2, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Cacalano, N.; Jewett, A. Split anergized natural killer cells halt inflammation by inducing stem cell differentiation, resistance to NK cell cytotoxicity and prevention of cytokine and chemokine secretion. Oncotarget 2015, 6, 8947–8959. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Kaur, K.; Ko, M.-W.; Ohanian, N.; Cook, J.; Jewett, A. Osteoclast-expanded super-charged NK-cells preferentially select and expand CD8+ T cells. Sci. Rep. 2020, 10, 20363. [Google Scholar] [CrossRef]
- Tartter, P.I.; Steinberg, B.; Barron, D.M.; Martinelli, G. The Prognostic Significance of Natural Killer Cytotoxicity in Patients with Colorectal Cancer. Arch. Surg. 1987, 122, 1264–1268. [Google Scholar] [CrossRef]
- Igarashi, T.; Wynberg, J.; Srinivasan, R.; Becknell, B.; McCoy, J.P.; Takahashi, Y.; Suffredini, D.A.; Linehan, W.M.; Caligiuri, M.A.; Childs, R.W.; et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 2004, 104, 170–177. [Google Scholar] [CrossRef]
- Alici, E.; Sutlu, T.; Björkstrand, B.; Gilljam, M.; Stellan, B.; Nahi, H.; Quezada, H.C.; Gahrton, G.; Ljunggren, H.-G.; Dilber, M.S.; et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood 2008, 111, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Lundqvist, A.; McCoy, P.; Samsel, L.; Fan, Y.; Tawab, A.; Childs, R. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009, 11, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Martín-Antonio, B.; Yang, H.; Ku, S.; Lee, D.A.; Cooper, L.J.N.; Decker, W.K.; Li, S.; Robinson, S.N.; Sekine, T.; et al. Antigen Presenting Cell-Mediated Expansion of Human Umbilical Cord Blood Yields Log-Scale Expansion of Natural Killer Cells with Anti-Myeloma Activity. PLoS ONE 2013, 8, e76781. [Google Scholar] [CrossRef]
- Märklin, M.; Hagelstein, I.; Koerner, S.P.; Rothfelder, K.; Pfluegler, M.S.; Schumacher, A.; Grosse-Hovest, L.; Jung, G.; Salih, H.R. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins for induction of NK and T cell reactivity against acute myeloid leukemia. J. Immunother. Cancer 2019, 7, 143. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Chan, G.C.-F.; Chan, W.K. Designs of NKG2D-based immunotherapeutics for cancer. Front. Immunol. 2025, 16, 1557644. [Google Scholar] [CrossRef]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2010, 89, 216–224. [Google Scholar] [CrossRef]
- Imširović, V.; Wensveen, F.M.; Polić, B.; Jelenčić, V. Maintaining the Balance: Regulation of NK Cell Activity. Cells 2024, 13, 1464. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, D.; Churov, A.; Fu, R. Research Progress on NK Cell Receptors and Their Signaling Pathways. Mediat. Inflamm. 2020, 2020, 6437057. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Cacalano, N.A.; Head, C.; Teruel, A. Coengagement of CD16 and CD94 Receptors Mediates Secretion of Chemokines and Induces Apoptotic Death of Naive Natural Killer Cells. Clin. Cancer Res. 2006, 12, 1994–2003. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Johnson, K.M.; Lee, D.A. Natural killer cells in skin: A unique opportunity to better characterize the many facets of an overlooked secondary lymphoid organ. Front. Immunol. 2025, 16, 1646719. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Kennedy, P.R.; Stacey, K.B.; Worboys, J.D.; Yarwood, A.; Seo, S.; Solloa, E.H.; Mistretta, B.; Chatterjee, S.S.; Gunaratne, P.; et al. Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Adv. 2020, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Rebuli, M.E.; Pawlak, E.A.; Walsh, D.; Martin, E.M.; Jaspers, I. Distinguishing Human Peripheral Blood NK Cells from CD56dimCD16dimCD69+CD103+ Resident Nasal Mucosal Lavage Fluid Cells. Sci. Rep. 2018, 8, 3394. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C.; Tian, Z.; Xiao, W. NK cells in immunotolerant organs. Cell. Mol. Immunol. 2013, 10, 202–212. [Google Scholar] [CrossRef]
- Bui, V.T.; Tseng, H.-C.; Kozlowska, A.; Maung, P.O.; Kaur, K.; Topchyan, P.; Jewett, A. Augmented IFN-γ and TNF-α Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-Like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Front. Immunol. 2015, 6, 576. [Google Scholar] [CrossRef]
- Hashemi, E.; Malarkannan, S. Tissue-Resident NK Cells: Development, Maturation, and Clinical Relevance. Cancers 2020, 12, 1553. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Xin, Z.; Lin, M.; Hao, Z.; Chen, D.; He, T.; Zhao, L.; Wu, D.; Wu, P.; et al. Tissue-resident CD69(+) CXCR6(+) Natural Killer cells with exhausted phenotype accumulate in human non-small cell lung cancer. Eur. J. Immunol. 2022, 52, 1993–2005. [Google Scholar] [CrossRef]
- Marquardt, N.; Kekäläinen, E.; Chen, P.; Lourda, M.; Wilson, J.N.; Scharenberg, M.; Bergman, P.; Al-Ameri, M.; Hård, J.; Mold, J.E.; et al. Unique transcriptional and protein-expression signature in human lung tissue-resident NK cells. Nat. Commun. 2019, 10, 3841. [Google Scholar] [CrossRef]
- De Maria, A.; Bozzano, F.; Cantoni, C.; Moretta, L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc. Natl. Acad. Sci. USA 2011, 108, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Man, Y.-G.; Cacalano, N.; Kos, J.; Tseng, H.-C. Natural killer cells as effectors of selection and differentiation of stem cells: Role in resolution of inflammation. J. Immunotoxicol. 2014, 11, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Teruel, A.; Romero, M.; Head, C.; Cacalano, N. Rapid and potent induction of cell death and loss of NK cell cytotoxicity against oral tumors by F(ab′)2 fragment of anti-CD16 antibody. Cancer Immunol. Immunother. 2008, 57, 1053–1066. [Google Scholar] [CrossRef]
- Jewett, A.; Cavalcanti, M.; Bonavida, B. Pivotal role of endogenous TNF-alpha in the induction of functional inactivation and apoptosis in NK cells. J. Immunol. 1997, 159, 4815–4822. [Google Scholar] [CrossRef]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef]
- Schwartz, R.H. T cell anergy. Annu. Rev. Immunol. 2003, 21, 305–334. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Bonavida, B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J. Immunol. 1996, 156, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Bonavida, B. Target-induced anergy of natural killer cytotoxic function is restricted to the NK—Target conjugate subset. Cell. Immunol. 1995, 160, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bonavida, B.; Lebow, L.T.; Jewett, A. Natural killer cell subsets: Maturation, differentiation and regulation. Nat. Immun. 1993, 12, 194–208. [Google Scholar]
- Vujanovic, L.; Chuckran, C.; Lin, Y.; Ding, F.; Sander, C.A.; Santos, P.M.; Lohr, J.; Mashadi-Hossein, A.; Warren, S.; White, A.; et al. CD56dim CD16− Natural Killer Cell Profiling in Melanoma Patients Receiving a Cancer Vaccine and Interferon-α. Front. Immunol. 2019, 10, 14. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Wagner, J.A.; Cashen, A.F.; Fehniger, T.A. Memory-Like Natural Killer Cells. Blood 2018, 132, SCI-8. [Google Scholar] [CrossRef]
- Peng, H.; Tian, Z. Natural Killer Cell Memory: Progress and Implications. Front. Immunol. 2017, 8, 1143. [Google Scholar] [CrossRef]
- Judge, S.J.; Purl, M.C.; Murphy, W.J.; Canter, R.J. What’s in a name? Memory NK cells for cancer immunotherapy. J. Immunother. Cancer 2025, 13, e010850. [Google Scholar] [CrossRef]
- Neely, H.R.; Mazo, I.B.; Gerlach, C.; von Andrian, U.H. Is There Natural Killer Cell Memory and Can It Be Harnessed by Vaccination? Natural Killer Cells in Vaccination. Cold Spring Harb. Perspect. Biol. 2018, 10, a029488. [Google Scholar] [CrossRef]
- Gang, M.; Wong, P.; Berrien-Elliott, M.M.; Fehniger, T.A. Memory-like natural killer cells for cancer immunotherapy. Semin. Hematol. 2020, 57, 185–193. [Google Scholar] [CrossRef]
- Sun, J.C.; Lopez-Verges, S.; Kim, C.C.; DeRisi, J.L.; Lanier, L.L. NK cells and immune “memory”. J. Immunol. 2011, 186, 1891–1897. [Google Scholar] [CrossRef]
- O’Sullivan Timothy, E.; Sun Joseph, C.; Lanier Lewis, L. Natural Killer Cell Memory. Immunity 2015, 43, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123. [Google Scholar] [CrossRef]
- Beaulieu, A.M. Transcriptional and epigenetic regulation of memory NK cell responses. Immunol. Rev. 2021, 300, 125–133. [Google Scholar] [CrossRef]
- Rückert, T.; Lareau, C.A.; Mashreghi, M.-F.; Ludwig, L.S.; Romagnani, C. Clonal expansion and epigenetic inheritance of long-lasting NK cell memory. Nat. Immunol. 2022, 23, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Rožmanić, C.; Lisnić, B.; Matešić, M.P.; Mihalić, A.; Hiršl, L.; Park, E.; Brizić, A.L.; Indenbirken, D.; Viduka, I.; Šantić, M.; et al. Perinatal murine cytomegalovirus infection reshapes the transcriptional profile and functionality of NK cells. Nat. Commun. 2023, 14, 6412. [Google Scholar] [CrossRef]
- Hamdan, T.A. The Multifaceted Roles of NK Cells in the Context of Murine Cytomegalovirus and Lymphocytic Choriomeningitis Virus Infections. Immune Netw. 2024, 24, e29. [Google Scholar] [CrossRef] [PubMed]
- Jensen, I.J.; Martin, M.D.; Tripathy, S.K.; Badovinac, V.P. Novel Mouse Model of Murine Cytomegalovirus–Induced Adaptive NK Cells. ImmunoHorizons 2022, 6, 8–15. [Google Scholar] [CrossRef]
- van der Ploeg, K.; Sottile, R.; Kontopoulos, T.; Shaffer, B.C.; Papanicolaou, G.A.; Maloy, M.A.; Cho, C.; Robinson, K.S.; Perales, M.-A.; Le Luduec, J.-B.; et al. Emergence of human CMV-induced NKG2C+ NK cells is associated with CD8+ T-cell recovery after allogeneic HCT. Blood Adv. 2023, 7, 5784–5798. [Google Scholar] [CrossRef]
- Malmberg, K.-J.; Beziat, V.; Ljunggren, H.-G. Spotlight on NKG2C and the human NK-cell response to CMV infection. Eur. J. Immunol. 2012, 42, 3141–3145. [Google Scholar] [CrossRef]
- Foley, B.; Cooley, S.; Verneris, M.R.; Curtsinger, J.; Luo, X.; Waller, E.K.; Anasetti, C.; Weisdorf, D.; Miller, J.S. Human Cytomegalovirus (CMV)-Induced Memory-like NKG2C+ NK Cells Are Transplantable and Expand In Vivo in Response to Recipient CMV Antigen. J. Immunol. 2012, 189, 5082–5088. [Google Scholar] [CrossRef]
- Lozada, J.R.; Zhang, B.; Miller, J.S.; Cichocki, F. NK Cells from Human Cytomegalovirus-Seropositive Individuals Have a Distinct Metabolic Profile That Correlates with Elevated mTOR Signaling. J. Immunol. 2023, 211, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Terrén, I.; Orrantia, A.; Astarloa-Pando, G.; Amarilla-Irusta, A.; Zenarruzabeitia, O.; Borrego, F. Cytokine-Induced Memory-Like NK Cells: From the Basics to Clinical Applications. Front. Immunol. 2022, 13, 884648. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, M.; Romee, R. Cytokine-induced memory-like natural killer cells for cancer immunotherapy. Stem Cell Res. Ther. 2021, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.W.; Chase, J.M.; Romee, R.; Schneider, S.E.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Preactivation with IL-12, IL-15, and IL-18 Induces CD25 and a Functional High-Affinity IL-2 Receptor on Human Cytokine-Induced Memory-like Natural Killer Cells. Transplant. Cell. Ther. 2014, 20, 463–473. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Wagner, J.A.; Fehniger, T.A. Human Cytokine-Induced Memory-Like Natural Killer Cells. J. Innate Immun. 2015, 7, 563–571. [Google Scholar] [CrossRef]
- Brillantes, M.; Beaulieu, A.M. Memory and Memory-Like NK Cell Responses to Microbial Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 102. [Google Scholar] [CrossRef]
- Sinha, O.; Abhipsha, S.; Santara, S.S. Power of Memory: A Natural Killer Cell Perspective. Cells 2025, 14, 846. [Google Scholar] [CrossRef]
- Bakhtiyaridovvombaygi, M.; Yazdanparast, S.; Mikanik, F.; Izadpanah, A.; Parkhideh, S.; Shahbaz ghasabeh, A.; Roshandel, E.; Hajifathali, A.; Gharehbaghian, A. Cytokine-Induced Memory-Like NK Cells: Emerging strategy for AML immunotherapy. Biomed. Pharmacother. 2023, 168, 115718. [Google Scholar] [CrossRef]
- Shapiro, R.M.; Sheffer, M.; Booker, M.A.; Tolstorukov, M.Y.; Birch, G.C.; Sade-Feldman, M.; Fang, J.; Li, S.; Lu, W.; Ansuinelli, M.; et al. First-in-human evaluation of memory-like NK cells with an IL-15 super-agonist and CTLA-4 blockade in advanced head and neck cancer. J. Hematol. Oncol. 2025, 18, 17. [Google Scholar] [CrossRef]
- Lopez, M.L.G.; Gebo, A.; Parodi, M.; Persano, S.; Maus-Conn, J.; Mingari, M.C.; Loiacono, F.; Orecchia, P.; Sivori, S.; Cantoni, C.; et al. CD56brightcytokine-induced memory-like NK cells and NK-cell engagers synergize against non-small cell lung cancer cancer-stem cells. J. Immunother. Cancer 2025, 13, e010205. [Google Scholar] [CrossRef]
- Terrazzano, G.; Carbone, E. NK cells blur the frontier between innate and acquired immunity. Front. Immunol. 2013, 3, 40723. [Google Scholar] [CrossRef] [PubMed]
- Terranova-Barberio, M.; Pawlowska, N.; Dhawan, M.; Moasser, M.; Chien, A.J.; Melisko, M.E.; Rugo, H.; Rahimi, R.; Deal, T.; Daud, A.; et al. Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat. Commun. 2020, 11, 3584. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.X.; Jonker, D.J.; Loree, J.M.; Kennecke, H.F.; Berry, S.R.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; Harb, M.; et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 2020, 6, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Lian, G.; Mak, T.S.-K.; Yu, X.; Lan, H.-Y. Challenges and Recent Advances in NK Cell-Targeted Immunotherapies in Solid Tumors. Int. J. Mol. Sci. 2021, 23, 164. [Google Scholar] [CrossRef]
- Merino, A.; Maakaron, J.; Bachanova, V. Advances in NK cell therapy for hematologic malignancies: NK source, persistence and tumor targeting. Blood Rev. 2023, 60, 101073. [Google Scholar] [CrossRef]
- Tong, L.; Jiménez-Cortegana, C.; Tay, A.H.; Wickström, S.; Galluzzi, L.; Lundqvist, A. NK cells and solid tumors: Therapeutic potential and persisting obstacles. Mol. Cancer 2022, 21, 206. [Google Scholar] [CrossRef]
- Belcaid, L.; Klingenberg, K.; Spanggaard, I.; Harsløf, L.; Hoejgaard, M.; Riley, C.; Hutchings, M.; Rohrberg, K.S.; Lassen, U.N. Clinical outcomes in phase I trials across solid tumors and hematological malignancies. J. Clin. Oncol. 2025, 43, e19014. [Google Scholar] [CrossRef]
- Fang, F.; Wang, W.; Chen, M.; Tian, Z.; Xiao, W. Technical advances in NK cell-based cellular immunotherapy. Cancer Biol. Med. 2019, 16, 647–654. [Google Scholar] [CrossRef]
- Maia, A.; Tarannum, M.; Lérias, J.R.; Piccinelli, S.; Borrego, L.M.; Maeurer, M.; Romee, R.; Castillo-Martin, M. Building a Better Defense: Expanding and Improving Natural Killer Cells for Adoptive Cell Therapy. Cells 2024, 13, 451. [Google Scholar] [CrossRef]
- Rea, A.; Santana-Hernández, S.; Villanueva, J.; Sanvicente-García, M.; Cabo, M.; Suarez-Olmos, J.; Quimis, F.; Qin, M.; Llorens, E.; Blasco-Benito, S.; et al. Enhancing human NK cell antitumor function by knocking out SMAD4 to counteract TGFβ and activin A suppression. Nat. Immunol. 2025, 26, 582–594. [Google Scholar] [CrossRef]
- Hoerster, K.; Uhrberg, M.; Wiek, C.; Horn, P.A.; Hanenberg, H.; Heinrichs, S. HLA Class I Knockout Converts Allogeneic Primary NK Cells Into Suitable Effectors for “Off-the-Shelf” Immunotherapy. Front. Immunol. 2021, 11, 586168. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Ba, Y.; Xu, H.; Zuo, A.; Liu, S.; Zhang, Y.; Weng, S.; Ren, Y.; Luo, P.; et al. Revolutionising Cancer Immunotherapy: Advancements and Prospects in Non-Viral CAR-NK Cell Engineering. Cell Prolif. 2024, 58, e13791. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Arasteh, A.; Kaur, K.; Kozlowska, A.; Topchyan, P.; Jewett, A. Differential Cytotoxicity but Augmented IFN-Î3 Secretion by NK Cells after Interaction with Monocytes from Humans, and Those from Wild Type and Myeloid-Specific COX-2 Knockout Mice. Front. Immunol. 2015, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, A.; Guipouy, D.; Lemieux, W.; Harvey, M.; Bordeleau, L.-J.; Guay, D.; Roméro, H.; Li, Y.; Dion, R.; Béland, K.; et al. KLRC1 knockout overcomes HLA-E-mediated inhibition and improves NK cell antitumor activity against solid tumors. Front. Immunol. 2023, 14, 1231916. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Seow, S.V.; Wong, D.; Robinson, M.; Campana, D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Investig. 2019, 129, 2094–2106. [Google Scholar] [CrossRef]
- Mittal, D.; Lepletier, A.; Madore, J.; Aguilera, A.R.; Stannard, K.; Blake, S.J.; Whitehall, V.L.J.; Liu, C.; Bettington, M.L.; Takeda, K.; et al. CD96 Is an Immune Checkpoint That Regulates CD8(+) T-cell Antitumor Function. Cancer Immunol. Res. 2019, 7, 559–571. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; He, F.; Ma, X.; Fan, F.; Meng, M.; Zhuo, Y.; Zhang, L. CD96, a new immune checkpoint, correlates with immune profile and clinical outcome of glioma. Sci. Rep. 2020, 10, 10768. [Google Scholar] [CrossRef]
- Gol, T.M.; Kim, M.; Sinn, R.; Ureña-Bailén, G.; Stegmeyer, S.; Gratz, P.G.; Zahedipour, F.; Roig-Merino, A.; Antony, J.S.; Mezger, M. CRISPR-Cas9-Based Gene Knockout of Immune Checkpoints in Expanded NK Cells. Int. J. Mol. Sci. 2023, 24, 16065. [Google Scholar]
- Overcoming TGFβ and activin A suppression boosts NK cell antitumor function. Nat. Immunol. 2025, 26, 540–541. [CrossRef] [PubMed]
- Nagai, Y.; Kararoudi, M.N.; Elmas, E.; Pereira, M.; Ali, S.A.; Imus, P.H.; Lee, D.A.; Ghiaur, G. CD38 Knockout Primary NK Cells to Prevent “Fratricide” and Boost Daratumumab Activity. Blood 2019, 134, 870. [Google Scholar] [CrossRef]
- Gurney, M.; Stikvoort, A.; Nolan, E.; Kirkham-McCarthy, L.; Khoruzhenko, S.; Shivakumar, R.; Zweegman, S.; Van de Donk, N.W.C.J.; Mutis, T.; Szegezdi, E.; et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica 2020, 107, 437–445. [Google Scholar] [CrossRef]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell. Mol. Immunol. 2024, 21, 1089–1108. [Google Scholar] [CrossRef]
- Boissel, L.; Betancur, M.; Lu, W.; Wels, W.S.; Marino, T.; Van Etten, R.A.; Klingemann, H. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk. Lymphoma 2011, 53, 958–965. [Google Scholar] [CrossRef]
- El-Mayta, R.; Zhang, Z.; Hamilton, A.G.; Mitchell, M.J. Delivery technologies to engineer natural killer cells for cancer immunotherapy. Cancer Gene Ther. 2021, 28, 947–959. [Google Scholar] [CrossRef]
- Guo, X.; Sui, R.; Piao, H. Exosomes-mediated crosstalk between glioma and immune cells in the tumor microenvironment. CNS Neurosci. Ther. 2023, 29, 2074–2085. [Google Scholar] [CrossRef]
- Peng, J.; Liang, Q.; Xu, Z.; Cai, Y.; Peng, B.; Li, J.; Zhang, W.; Kang, F.; Hong, Q.; Yan, Y.; et al. Current Understanding of Exosomal MicroRNAs in Glioma Immune Regulation and Therapeutic Responses. Front. Immunol. 2022, 12, 813747. [Google Scholar] [CrossRef]
- Tang, Z.; Xue, Z.; Liu, X.; Zhang, Y.; Zhao, J.; Liu, J.; Zhang, L.; Guo, Q.; Feng, B.; Wang, J.; et al. Inhibition of hypoxic exosomal miR-423-3p decreases glioma progression by restricting autophagy in astrocytes. Cell Death Dis. 2025, 16, 265. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Comba, A.; Faisal, S.M.; Kadiyala, P.; Baker, G.J.; Alghamri, M.S.; Doherty, R.; Zamler, D.; Nuñez, G.; Castro, M.G.; et al. A novel miR1983-TLR7-IFNβ circuit licenses NK cells to kill glioma cells, and is under the control of galectin-1. OncoImmunology 2021, 10, 1939601. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Aigner, P.; Stoiber, D. Type I Interferons and Natural Killer Cell Regulation in Cancer. Front. Immunol. 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Sin, W.-X.; Yeong, J.P.-S.; Lim, T.J.F.; Su, I.-H.; Connolly, J.E.; Chin, K.-C. IRF-7 Mediates Type I IFN Responses in Endotoxin-Challenged Mice. Front. Immunol. 2020, 11, 640. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.-H.; Li, N. Immune evasion in cancer: Mechanisms and cutting-edge therapeutic approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Bigley, A.B.; Spade, S.; Agha, N.H.; Biswas, S.; Tang, S.; Malik, M.H.; Dai, L.; Masoumi, S.; Patiño-Escobar, B.; Hale, M.; et al. FcεRIγ-negative NK cells persist in vivo and enhance efficacy of therapeutic monoclonal antibodies in multiple myeloma. Blood Adv. 2021, 5, 3021–3031. [Google Scholar] [CrossRef]
- Perussia, B.; Ramoni, C.; Anegon, I.; Cuturi, M.; Faust, J.; Trinchieri, G. Preferential Proliferation of Natural-Killer-Cells Among Peripheral-Blood Mononuclear-Cells Cocultured with B-Lymphoblastoid Cell-Lines. Nat. Immun. Cell Growth Regul. 1987, 6, 171–188. [Google Scholar]
- Rabinowich, H.; Sedlmayr, P.; Herberman, R.B.; Whiteside, T.L. Increased proliferation, lytic activity, and purity of human natural killer cells cocultured with mitogen-activated feeder cells. Cell. Immunol. 1991, 135, 454–470. [Google Scholar] [CrossRef]
- Srivastava, S.; Lundqvist, A.; Childs, R.W. Natural killer cell immunotherapy for cancer: A new hope. Cytotherapy 2008, 10, 775–783. [Google Scholar] [CrossRef]
- Gras Navarro, A.; Björklund, A.T.; Chekenya, M. Therapeutic Potential and Challenges of Natural Killer Cells in Treatment of Solid Tumors. Front. Immunol. 2015, 6, 202. [Google Scholar] [CrossRef]
- Garg, T.K.; Szmania, S.M.; Khan, J.A.; Hoering, A.; Malbrough, P.A.; Moreno-Bost, A.; Greenway, A.D.; Lingo, J.D.; Li, X.; Yaccoby, S.; et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012, 97, 1348–1356. [Google Scholar] [CrossRef]
- Gurney, M.; Kundu, S.; Pandey, S.; O’dWyer, M. Feeder Cells at the Interface of Natural Killer Cell Activation, Expansion and Gene Editing. Front. Immunol. 2022, 13, 802906. [Google Scholar] [CrossRef]
- Michen, S.; Frosch, J.; Füssel, M.; Schackert, G.; Momburg, F.; Temme, A. Artificial feeder cells expressing ligands for killer cell immunoglobulin-like receptors and CD94/NKG2A for expansion of functional primary natural killer cells with tolerance to self. Cytotherapy 2020, 22, 354–368. [Google Scholar] [CrossRef]
- Bui, T.V.A.; Nguyen, N.M.; Nguyen, H.T.P.; Tran, T.P.D.; Nguyen, D.M.Q.; Ngo, T.M.Q.; Nguyen, T.B.; Verhoeyen, E.; Tran, N.T.; Tran, L.S.; et al. In Vitro Expansion and Transduction of Primary NK Cells Using Feeder Cells Expressing Costimulatory Molecules and IL-21. Cancer Sci. 2025, 116, 1847–1860. [Google Scholar] [CrossRef]
- Min, B.; Yang, B.; Kim, Y.-S.; Park, G.M.; Kim, H.; Kim, H.; Kim, E.-J.; Hwang, Y.K.; Shin, E.-C.; Cho, S.; et al. Harnessing novel engineered feeder cells expressing activating molecules for optimal expansion of NK cells with potent antitumor activity. Cell. Mol. Immunol. 2021, 19, 296–298. [Google Scholar] [CrossRef]
- Tseng, H.C.; Kanayama, K.; Kaur, K.; Park, S.H.; Park, S.; Kozlowska, A.; Sun, S.; McKenna, C.E.; Nishimura, I.; Jewett, A. Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: Role in osteoclast-mediated NK cell activation. Oncotarget 2015, 6, 20002–20025. [Google Scholar] [CrossRef]
- Leivas, A.; Perez-Martínez, A.; Blanchard, M.J.; Martín-Clavero, E.; Fernández, L.; Lahuerta, J.J.; Martinez-Lopez, J. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti-myeloma drugs for multiple myeloma. OncoImmunology 2016, 5, e1250051. [Google Scholar] [CrossRef]
- Cichocki, F.; Bjordahl, R.; Gaidarova, S.; Mahmood, S.; Abujarour, R.; Wang, H.; Tuininga, K.; Felices, M.; Davis, Z.B.; Bendzick, L.; et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti–PD-1 therapy. Sci. Transl. Med. 2020, 12, eaaz5618. [Google Scholar] [CrossRef]
- Benson, D.M.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti–PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef]
- Kaur, K.; Chen, P.-C.; Ko, M.-W.; Mei, A.; Senjor, E.; Malarkannan, S.; Kos, J.; Jewett, A. Sequential therapy with supercharged NK cells with either chemotherapy drug cisplatin or anti-PD-1 antibody decreases the tumor size and significantly enhances the NK function in Hu-BLT mice. Front. Immunol. 2023, 14, 1132807. [Google Scholar] [CrossRef]
- Sadeghi, S.; Chen, P.-C.; Jewett, A.; Kaur, K. Combination of NK cell immunotherapy with chemotherapy and radiation enhances NK cell therapy and provides improved prognosis in cancer patients and in humanized BLT mouse model system. In NK Cells in Cancer Immunotherapy: Successes and Challenges; Academic Press: Cambridge, MA, USA, 2023; pp. 301–320. [Google Scholar] [CrossRef]
- Senjor, E.; Ko, M.-W.; Kaur, K.; Chen, P.-C.; Breznik, B.; Chovatiya, N.; Kos, J.; Jewett, A. Multifaceted nature of natural killer cells: Potential mode of interaction and shaping of stem cells. In NK Cells in Cancer Immunotherapy: Successes and Challenges; Academic Press: Cambridge, MA, USA, 2023; pp. 3–25. [Google Scholar] [CrossRef]
- Ko, M.-W.; Kaur, K.; Chen, P.-C.; Breznik, B.; Senjor, E.; Chovatiya, N.; Wong, P.; Turnsek, T.L.; Kos, J.; Jewett, A.; et al. Diagnostic methods to assess the numbers, phenotype, and function of primary and engineered NK cells: Methods to predict prognosis and treatment outcome. In NK Cells in Cancer Immunotherapy: Successes and Challenges; Academic Press: Cambridge, MA, USA, 2023; pp. 281–297. [Google Scholar] [CrossRef]
- Kaur, K.; Safaie, T.; Ko, M.-W.; Wang, Y.; Jewett, A. ADCC against MICA/B Is Mediated against Differentiated Oral and Pancreatic and Not Stem-Like/Poorly Differentiated Tumors by the NK Cells; Loss in Cancer Patients due to Down-Modulation of CD16 Receptor. Cancers 2021, 13, 239. [Google Scholar] [CrossRef]
- Jewett, A.; Kos, J.; Turnsek, T.L.; Chen, P.-C.; Breznik, B.; Senjor, E.; Chovatiya, N.; Kaur, K.; Ko, M.-W. Novel strategies to expand supercharged NK cells with augmented capacity to withstand inactivation by tumors. In Successes and Challenges of NK Immunotherapy; Academic Press: Cambridge, MA, USA, 2021; pp. 101–119. [Google Scholar] [CrossRef]
- Kaur, K.; Ko, M.-W.; Chen, P.-C.; Breznik, B.; Senjor, E.; Wong, P.; Wang, Y.; Chovatiya, N.; Jewett, A. Probiotics in Health and Disease: Distinct Roles of Different Strains in Natural Killer Cell Activation and Regulation. Crit. Rev. Immunol. 2021, 41, 1–19. [Google Scholar] [CrossRef]
- Jewett, A.; Kos, J.; Kaur, K.; Safaei, T.; Sutanto, C.; Chen, W.; Wong, P.; Namagerdi, A.K.; Fang, C.; Fong, Y.; et al. Natural Killer Cells: Diverse Functions in Tumor Immunity and Defects in Pre-neoplastic and Neoplastic Stages of Tumorigenesis. Mol. Ther.-Oncolytics 2020, 16, 41–52. [Google Scholar] [CrossRef]
- Jewett, A.; Kos, J.; Kaur, K.; Turnsek, T.L.; Breznik, B.; Senjor, E.; Wong, P.; Nguyen, K.Y.; Ko, M.-W. Multiple Defects of Natural Killer Cells in Cancer Patients: Anarchy, Dysregulated Systemic Immunity, and Immunosuppression in Metastatic Cancer. Crit. Rev. Immunol. 2020, 40, 93–133. [Google Scholar] [CrossRef]
- Jewett, A.; Kos, J.; Fong, Y.; Ko, M.-W.; Safaei, T.; Nanut, M.P.; Kaur, K. NK cells shape pancreatic and oral tumor microenvironments; role in inhibition of tumor growth and metastasis. Semin. Cancer Biol. 2018, 53, 178–188. [Google Scholar] [CrossRef]
- Kaur, K.; Nanut, M.P.; Ko, M.-W.; Safaie, T.; Kos, J.; Jewett, A. Natural killer cells target and differentiate cancer stem-like cells/undifferentiated tumors: Strategies to optimize their growth and expansion for effective cancer immunotherapy. Curr. Opin. Immunol. 2018, 51, 170–180. [Google Scholar] [CrossRef]
- Kozlowska, A.K.; Kaur, K.; Topchyan, P.; Jewett, A. Adoptive transfer of osteoclast-expanded natural killer cells for immunotherapy targeting cancer stem-like cells in humanized mice. Cancer Immunol. Immunother. 2016, 65, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Breznik, B.; Novak, M.; Majc, B.; Habič, A.; Jewett, A. Natural killer cells in the treatment of glioblastoma: Diverse antitumor functions and potential clinical applications. In NK Cells in Cancer Immunotherapy: Successes and Challenges; Academic Press: Cambridge, MA, USA, 2023; pp. 335–367. [Google Scholar] [CrossRef]
- Breznik, B.; Ko, M.; Chen, P.; Senjor, E.; Majc, B.; Novak, M.; Habič, A.; Jewett, A. P06.07.A Natural killer cells lyse glioblastoma stem cells and increase their sensitivity to chemotherapy. Neuro-Oncology 2022, 24, ii39. [Google Scholar] [CrossRef]
- Breznik, B.; Ko, M.-W.; Tse, C.; Chen, P.-C.; Senjor, E.; Majc, B.; Habič, A.; Angelillis, N.; Novak, M.; Župunski, V.; et al. Infiltrating natural killer cells bind, lyse and increase chemotherapy efficacy in glioblastoma stem-like tumorospheres. Commun. Biol. 2022, 5, 436. [Google Scholar] [CrossRef]
- Roy, A.; Krzykwa, E.; Lemieux, R.; Néron, S. Increased Efficiency of γ-Irradiated versus Mitomycin C-Treated Feeder Cells for the Expansion of Normal Human Cells in Long-Term Cultures. J. Hematother. Stem Cell Res. 2001, 10, 873–880. [Google Scholar] [CrossRef]
- Marr, B.; Jo, D.; Jang, M.; Lee, S.-H. Cytokines in Focus: IL-2 and IL-15 in NK Adoptive Cell Cancer Immunotherapy. Immune Netw. 2025, 25, e17. [Google Scholar] [CrossRef]
- Denman, C.J.; Senyukov, V.V.; Somanchi, S.S.; Phatarpekar, P.V.; Kopp, L.M.; Johnson, J.L.; Singh, H.; Hurton, L.; Maiti, S.N.; Huls, M.H.; et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 2012, 7, e30264. [Google Scholar] [CrossRef] [PubMed]
- Voskens, C.J.; Watanabe, R.; Rollins, S.; Campana, D.; Hasumi, K.; Mann, D.L. Ex-vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J. Exp. Clin. Cancer Res. 2010, 29, 134. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tang, R.; Li, J.; Liu, Y.; Ye, L.; Shao, D.; Jin, M.; Huang, Q.; Shi, J. A New Ex Vivo Method for Effective Expansion and Activation of Human Natural Killer Cells for Anti-Tumor Immunotherapy. Cell Biochem. Biophys. 2015, 73, 723–729. [Google Scholar] [CrossRef]
- Fujisaki, H.; Kakuda, H.; Imai, C.; Mullighan, C.G.; Campana, D. Replicative potential of human natural killer cells. Br. J. Haematol. 2009, 145, 606–613. [Google Scholar] [CrossRef]
- Chang, Y.H.; Connolly, J.; Shimasaki, N.; Mimura, K.; Kono, K.; Campana, D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013, 73, 1777–1786. [Google Scholar] [CrossRef]
- Lapteva, N.; Durett, A.G.; Sun, J.; Rollins, L.A.; Huye, L.L.; Fang, J.; Dandekar, V.; Mei, Z.; Jackson, K.; Vera, J.; et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012, 14, 1131–1143. [Google Scholar] [CrossRef]
- Moseman, J.E.; Foltz, J.A.; Sorathia, K.; Heipertz, E.L.; Lee, D.A. Evaluation of serum-free media formulations in feeder cell–stimulated expansion of natural killer cells. Cytotherapy 2020, 22, 322–328. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Jiang, Q.; Jiang, H.; Hu, L.J.; Zhao, T.; Yu, X.X.; Huang, X.J. Expanded clinical-grade membrane-bound IL-21/4-1BBL NK cell products exhibit activity against acute myeloid leukemia in vivo. Eur. J. Immunol. 2020, 50, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kadu, S.; Xiao, Y.; Johnson, O.; Kelly, A.; O’Connor, R.S.; Lai, M.; Kong, H.; Srivatsa, S.; Tai, V.; et al. Sequential Exposure to IL21 and IL15 During Human Natural Killer Cell Expansion Optimizes Yield and Function. Cancer Immunol. Res. 2023, 11, 1524–1537. [Google Scholar] [CrossRef]
- Shi, J.; Tricot, G.; Szmania, S.; Camet, C.; Rosen, N.; Cottler-Fox, M.; Barlogie, B.; Campana, D.; van Rhee, F. Stimulation with K562 Cells Transfected with 4-1BBL and IL-15 Expands and Activates Natural Killer (NK) Cells with Specific Cytotoxicity for Multiple Myeloma (MM). Blood 2005, 106, 3392. [Google Scholar] [CrossRef]
- Akhkand, S.S.; Soleimani, M.; Zomorrod, M.S.; Kiani, J. Genetically engineered K562 cells augment NK cell cytotoxicity against acute myeloid leukemia and reduce dependency on IL-15. Med. Oncol. 2025, 42, 211. [Google Scholar] [CrossRef]
- Wang, J.; Erickson, C.; Renelt, M.; Gavino, V.; Sun, J.; Li, J.; Bi, M.; Person, A. IL-12p35 and IL-12p40 functionally synergize to active NK cells. J. Immunol. 2024, 212, 0507_4550. [Google Scholar] [CrossRef]
- Jewett, A.; Wang, M.-Y.; Teruel, A.; Poupak, Z.; Bostanian, Z.; Park, N.-H. Cytokine dependent inverse regulation of CD54 (ICAM1) and major histocompatibility complex class I antigens by nuclear factor κB in HEp2 tumor cell line: Effect on the function of natural killer cells. Hum. Immunol. 2003, 64, 505–520. [Google Scholar] [CrossRef]
- Wu, J.; Cherwinski, H.; Spies, T.; Phillips, J.H.; Lanier, L.L. Dap10 and Dap12 Form Distinct, but Functionally Cooperative, Receptor Complexes in Natural Killer Cells. J. Exp. Med. 2000, 192, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Hui-Yuen, J.; McAllister, S.; Koganti, S.; Hill, E.; Bhaduri-McIntosh, S. Establishment of Epstein-Barr virus growth-transformed lymphoblastoid cell lines. J. Vis. Exp. 2011, 57, 3321. [Google Scholar]
- Yap, H.-Y.; Siow, T.-S.; Chow, S.-K.; Teow, S.-Y. Epstein-Barr Virus- (EBV-) Immortalized Lymphoblastoid Cell Lines (LCLs) Express High Level of CD23 but Low CD27 to Support Their Growth. Adv. Virol. 2019, 2019, 6464521. [Google Scholar] [CrossRef] [PubMed]
- Kis, L.L.; Salamon, D.; Persson, E.K.; Nagy, N.; Scheeren, F.A.; Spits, H.; Klein, G.; Klein, E. IL-21 imposes a type II EBV gene expression on type III and type I B cells by the repression of C- and activation of LMP-1-promoter. Proc. Natl. Acad. Sci. USA 2009, 107, 872–877. [Google Scholar] [CrossRef]
- Konjević, G.M.; Vuletić, A.M.; Mirjačić Martinović, K.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Romee, R.; Leong, J.W.; Fehniger, T.A. Utilizing Cytokines to Function-Enable Human NK Cells for the Immunotherapy of Cancer. Scientifica 2014, 2014, 205796. [Google Scholar] [CrossRef]
- Ojo, E.O.; Sharma, A.A.; Liu, R.; Moreton, S.; Checkley-Luttge, M.-A.; Gupta, K.; Lee, G.; Lee, D.A.; Otegbeye, F.; Sekaly, R.-P.; et al. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci. Rep. 2019, 9, 14916. [Google Scholar] [CrossRef]
- Foltz, J.A.; Tran, J.; Wong, P.; Fan, C.; Schmidt, E.; Fisk, B.; Becker-Hapak, M.; Russler-Germain, D.A.; Johnson, J.; Marin, N.D.; et al. Cytokines drive the formation of memory-like NK cell subsets via epigenetic rewiring and transcriptional regulation. Sci. Immunol. 2024, 9, eadk4893. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.S.; Lee, J.K. Development of NK cell expansion methods using feeder cells from human myelogenous leukemia cell line. Blood Res. 2014, 49, 154–161. [Google Scholar] [CrossRef]
- Lapteva, N.; Szmania, S.M.; van Rhee, F.; Rooney, C.M. Clinical Grade Purification and Expansion of Natural Killer Cells. Crit. Rev.™ Oncog. 2014, 19, 121–132. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, Z.; Wei, H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front. Immunol. 2017, 8, 930. [Google Scholar] [CrossRef]
- Nakazawa, T.; Morimoto, T.; Maeoka, R.; Matsuda, R.; Nakamura, M.; Nishimura, F.; Yamada, S.; Nakagawa, I.; Park, Y.-S.; Nakase, H.; et al. Establishment of an efficient ex vivo expansion strategy for human natural killer cells stimulated by defined cytokine cocktail and antibodies against natural killer cell activating receptors. Regen. Ther. 2022, 21, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yu, J.; Caligiuri, M.A. Natural killer cell–based immunotherapy for cancer. J. Immunol. 2025, 214, 1444–1456. [Google Scholar] [CrossRef]
- Fang, F.; Xie, S.; Chen, M.; Li, Y.; Yue, J.; Ma, J.; Shu, X.; He, Y.; Xiao, W.; Tian, Z.; et al. Advances in NK cell production. Cell. Mol. Immunol. 2022, 19, 460–481. [Google Scholar] [CrossRef] [PubMed]
- Cekic, C.; Day, Y.J.; Sag, D.; Linden, J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014, 74, 7250–7259. [Google Scholar] [CrossRef]
- Xing, S.; Ferrari de Andrade, L. NKG2D and MICA/B shedding: A ‘tag game’ between NK cells and malignant cells. Clin. Transl. Immunol. 2020, 9, e1230. [Google Scholar] [CrossRef]
- Buckle, I.; Guillerey, C. Inhibitory Receptors and Immune Checkpoints Regulating Natural Killer Cell Responses to Cancer. Cancers 2021, 13, 4263. [Google Scholar] [CrossRef]
- Morcillo-Martín-Romo, P.; Valverde-Pozo, J.; Ortiz-Bueno, M.; Arnone, M.; Espinar-Barranco, L.; Espinar-Barranco, C.; García-Rubiño, M.E. The Role of NK Cells in Cancer Immunotherapy: Mechanisms, Evasion Strategies, and Therapeutic Advances. Biomedicines 2025, 13, 857. [Google Scholar] [CrossRef]
- Wu, X.; Matosevic, S. Gene-edited and CAR-NK cells: Opportunities and challenges with engineering of NK cells for immunotherapy. Mol. Ther. -Oncolytics 2022, 27, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Gao, X.; Zhang, L.; Yang, E.; Li, Y.; Yu, L. The Advances and Challenges of NK Cell-Based Cancer Immunotherapy. Curr. Oncol. 2021, 28, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lundqvist, A. Immunomodulatory Effects of IL-2 and IL-15; Implications for Cancer Immunotherapy. Cancers 2020, 12, 3586. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A.; Leveson-Gower, D.B.; Gill, S.; Baker, J.; Beilhack, A.; Negrin, R.S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010, 115, 4293–4301. [Google Scholar] [CrossRef]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human Circulating and Tissue-Resident CD56bright Natural Killer Cell Populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef]
- Kuznetsova, A.V.; Glukhova, X.A.; Beletsky, I.P.; Ivanov, A.A. NK cell activity in the tumor microenvironment. Front. Cell Dev. Biol. 2025, 13, 1609479. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, L.; Liu, Y.; Li, Y.; Liu, Y.; Zhang, Z. Targeted modulation of myeloid-derived suppressor cells in the tumor microenvironment: Implications for cancer therapy. Biomed. Pharmacother. 2024, 180, 117590. [Google Scholar] [CrossRef]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J.; Van Waes, C.; Allen, C.T. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016, 58, 59–70. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Najjar, Y.G. Immunotherapy combination approaches: Mechanisms, biomarkers and clinical observations. Nat. Rev. Immunol. 2023, 24, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Duygu, B.; Olieslagers, T.I.; Groeneweg, M.; Voorter, C.E.M.; Wieten, L. HLA Class I Molecules as Immune Checkpoints for NK Cell Alloreactivity and Anti-Viral Immunity in Kidney Transplantation. Front. Immunol. 2021, 12, 680480. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Vago, L.; Eikema, D.-J.; de Wreede, L.C.; Ciceri, F.; Diaz, M.A.; Locatelli, F.; Jindra, P.; Milone, G.; Diez-Martin, J.L.; et al. Natural killer cell alloreactivity in HLA-haploidentical hematopoietic transplantation: A study on behalf of the CTIWP of the EBMT. Bone Marrow Transplant. 2021, 56, 1900–1907. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Guo, C. Crosstalk between macrophages and natural killer cells in the tumor microenvironment. Int. Immunopharmacol. 2021, 101, 108374. [Google Scholar] [CrossRef]
- Du, N.; Guo, F.; Wang, Y.; Cui, J. NK Cell Therapy: A Rising Star in Cancer Treatment. Cancers 2021, 13, 4129. [Google Scholar] [CrossRef]
- Lickefett, B.; Chu, L.; Ortiz-Maldonado, V.; Warmuth, L.; Barba, P.; Doglio, M.; Henderson, D.; Hudecek, M.; Kremer, A.; Markman, J.; et al. Lymphodepletion—An essential but undervalued part of the chimeric antigen receptor T-cell therapy cycle. Front. Immunol. 2023, 14, 1303935. [Google Scholar] [CrossRef]
- Gornalusse, G.G.; Hirata, R.K.; Funk, S.E.; Riolobos, L.; Lopes, V.S.; Manske, G.; Prunkard, D.; Colunga, A.G.; Hanafi, L.-A.; Clegg, D.O.; et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017, 35, 765–772. [Google Scholar] [CrossRef]
- Hotta, A.; Schrepfer, S.; Nagy, A. Genetically engineered hypoimmunogenic cell therapy. Nat. Rev. Bioeng. 2024, 2, 960–979. [Google Scholar] [CrossRef]
- Shaffer, B.C.; Hsu, K.C. Selection of allogeneic hematopoietic cell transplant donors to optimize natural killer cell alloreactivity. Semin. Hematol. 2020, 57, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, B.; Bangolo, A.; Mansour, C.; Elias, D.; Mohamed, A.; Thor, D.C.; Ehsanullah, S.U.; Tran, H.H.-V.; Aguilar, I.K.; Weissman, S.; et al. Engineering Innate Immunity: Recent Advances and Future Directions for CAR-NK and CAR–Macrophage Therapies in Solid Tumors. Cancers 2025, 17, 2397. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, K. Influence of Genetic, Dietary, and Environmental Factors on Natural Killer (NK) Cell Biology and Function: Interplay Between NK Cell Activity and Cancer Onset or Progression. Cancers 2025, 17, 2946. https://doi.org/10.3390/cancers17182946
Kaur K. Influence of Genetic, Dietary, and Environmental Factors on Natural Killer (NK) Cell Biology and Function: Interplay Between NK Cell Activity and Cancer Onset or Progression. Cancers. 2025; 17(18):2946. https://doi.org/10.3390/cancers17182946
Chicago/Turabian StyleKaur, Kawaljit. 2025. "Influence of Genetic, Dietary, and Environmental Factors on Natural Killer (NK) Cell Biology and Function: Interplay Between NK Cell Activity and Cancer Onset or Progression" Cancers 17, no. 18: 2946. https://doi.org/10.3390/cancers17182946
APA StyleKaur, K. (2025). Influence of Genetic, Dietary, and Environmental Factors on Natural Killer (NK) Cell Biology and Function: Interplay Between NK Cell Activity and Cancer Onset or Progression. Cancers, 17(18), 2946. https://doi.org/10.3390/cancers17182946





