Triangulating Timing, Tropism and Burden of Sarcoma Metastases: Toward Precision Surveillance and Therapy in a Real-World-Time Cohort
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Eligibility Criteria
2.3. Data Collection and Quality Assurance
2.4. Surveillance Imaging Protocol
2.5. Definitions and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Subsection
3.2. Baseline Characteristics
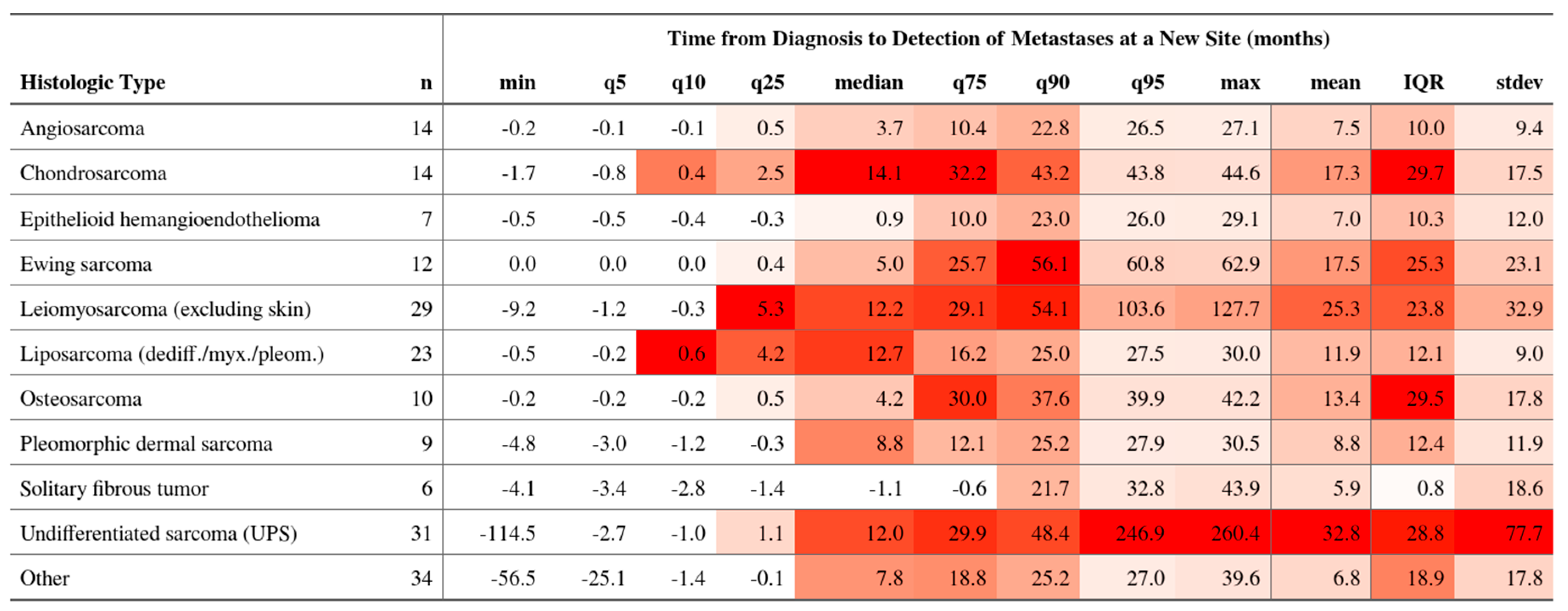
3.3. Incidence and Timing of Metastasis
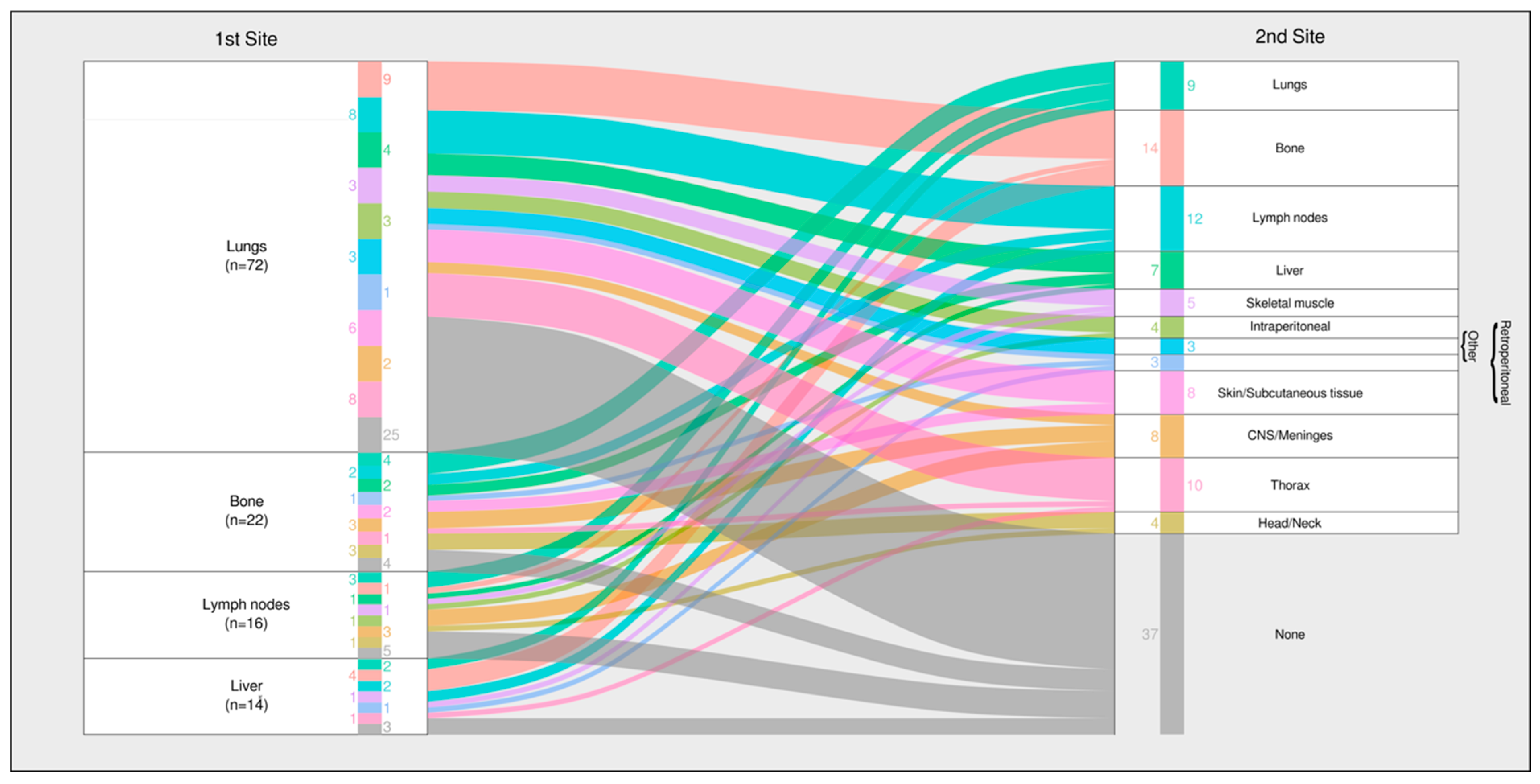
3.4. Anatomic Distribution of First Metastatic Sites
- Leiomyosarcoma and undifferentiated pleomorphic sarcoma (UPS) showed strong pulmonary predilection (78.6% and 72.2%, respectively).
- Ewing sarcoma and epithelioid hemangioendothelioma (EHE) favored bone (83.3% and 66.7%), in line with large Ewing-specific series [20].
- Angiosarcoma and EHE exhibited increased hepatic involvement (14.3% and 66.7%).
3.5. Cumulative Metastatic Burden
3.6. Metastatic Burden at First Presentation
3.7. Temporal Dynamics Across Histotypes
3.8. Metastatic Progression Pathways
- 1.
- Lung-dominant pathway—About half of the patients with an initial pulmonary metastasis remained confined to the lungs, particularly those with leiomyosarcoma, UPS, and osteosarcoma.
- 2.
- Multi-organ pathway—The remainder progressed to extrapulmonary sites, most commonly the bone and lymph nodes, a pattern typical of angiosarcoma and Ewing sarcoma.
3.9. Key Clinical Signals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonescu, C.; Blay, J. WHO Classification of Tumours: Soft Tissue and Bone Tumours, 5th ed.; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef]
- Blay, J.Y.; Hindi, N.; Bollard, J.; Aguiar, S.; Angel, M.; Araya, B.; Badilla, R.; Bernabeu, D.; Campos, F.; Caro-Sánchez, C.H.S.; et al. SELNET clinical practice guidelines for soft tissue sarcoma and GIST. Cancer Treat. Rev. 2022, 102, 102312. [Google Scholar] [CrossRef]
- Outani, H.; Hamada, K.; Yasuda, N.; Ueda, T.; Myoui, A.; Yoshikawa, H.; Okada, S. Impact of surgical resection on survival in patients with metastatic soft tissue sarcoma and comparison between synchronous and metachronous metastases. J. Orthop. Sci. 2022, 27, 892–898. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, X.; Shen, Y.; Wang, F.; Yang, J.; Wang, B.; Chen, Z.; Li, P.; Zhang, X.; Li, S.; et al. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine 2017, 96, e6799. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Gonzalez, M.; Inchaustegui, M.L.; Ruiz-Arellanos, K.; De Souza, F.; Subhawong, T.; Pretell-Mazzini, J. Management of oligometastatic disease in soft tissue sarcomas. J. Cancer Metastasis Treat. 2023, 9, 12. [Google Scholar] [CrossRef]
- Grilley-Olson, J.; Webber, N.; Demos, D.; Christensen, J.; Kirsch, D.G. Multidisciplinary Management of Oligometastatic Soft Tissue Sarcoma. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 939–948. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Ghidini, M.; Bukovec, R.; Trevisan, F.; Turati, L.; Indini, A.; Seghezzi, S.; Lonati, V.; Moleri, G.; et al. Better survival of patients with oligo compared with polymetastatic cancers: A systematic review and meta-analysis of 173 studies. F1000Research 2021, 10, 423. [Google Scholar] [CrossRef]
- Pacifico, P.; Colciago, R.R.; De Felice, F.; Boldrini, L.; Salvestrini, V.; Nardone, V.; Desideri, I.; Greco, C.; Arcangeli, S. A critical review on oligometastatic disease: A radiation oncologist’s perspective. Med. Oncol. 2022, 39, 181. [Google Scholar] [CrossRef]
- Reyes, D.K.; Pienta, K.J. The biology and treatment of oligometastatic cancer. Oncotarget 2015, 6, 8491–8524. [Google Scholar] [CrossRef]
- Pretell-Mazzini, J.; Seldon, C.S.; D’Amato, G.; Subhawong, T.K. Musculoskeletal Metastasis From Soft-tissue Sarcomas: A Review of the Literature. J. Am. Acad. Orthop. Surg. 2022, 30, 493–503. [Google Scholar] [CrossRef]
- Quesada, J.; Amato, R. The molecular biology of soft-tissue sarcomas and current trends in therapy. Sarcoma 2012, 2012, 849456. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Ho, V.K.Y.; Oosten, A.W.; Verhoef, C.; Sleijfer, S. Minimal Increase in Survival Throughout the Years in Patients with Soft Tissue Sarcoma with Synchronous Metastases: Results of a Population-Based Study. Oncologist 2019, 24, e526–e535. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Finley, J.W.; Driscoll, D.; Kraybill, W.G.; Gibbs, J.F. The treatment and outcome of patients with soft tissue sarcomas and synchronous metastases. Sarcoma 2002, 6, 69–73. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Gofrit, B.; Roditi, Y.; Popovtzer, A.; Frank, S.; Sosna, J.; Orevi, M.; Goldberg, S.N. The different clonal origins of metachronous and synchronous metastases. J. Cancer Res. Clin. Oncol. 2023, 149, 11085–11092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, L.; Wu, L.-M.; Shen, W.-H.; Zhou, P.; Lian, C.-L.; Zhang, W.-T.; Wu, S.-G. The patterns of distant metastasis and prognostic factors in patients with primary metastatic Ewing sarcoma of the bone. J. Bone Oncol. 2021, 30, 100385. [Google Scholar] [CrossRef]
- Basile, G.; Mattei, J.C.; Alshaygy, I.; Griffin, A.M.; Catton, C.N.; Chung, P.W.; Shultz, D.B.; Razak, A.R.A.; Demicco, E.G.; Ferguson, P.C.; et al. Curability of patients with lymph node metastases from extremity soft-tissue sarcoma. Cancer 2020, 126, 5098–5108. [Google Scholar] [CrossRef]
- Acem, I.; Martin, E.; van Houdt, W.J.; van de Sande, M.A.J.; Grunhagen, D.J.; Verhoef, C.; Monaco, C. The Association of Metastasis Pattern and Management of Metastatic Disease with Oncological Outcomes in Patients with Malignant Peripheral Nerve Sheath Tumors: A Multicenter Cohort Study. Cancers 2021, 13, 5115. [Google Scholar] [CrossRef]
- Acem, I.; Smit, M.M.; Verhoef, C.; van Houdt, W.J.; Haas, R.L.; van der Hage, J.A.; Grunhagen, D.J.; van de Sande, M.A.J. Management of Soft Tissue Sarcomas in Extremities: Variation in Treatment Recommendations and Surveillance According to Specialty and Continent. Ann. Surg. Oncol. 2021, 28, 7923–7936. [Google Scholar] [CrossRef]
- Walls, G.M.; Zaidi, S.H.; Fotiadis, N.; Jordan, S.; Maruzzo, M.; Hamid, I.; Al-Muderis, O.; Khabra, K.; Benson, C.; Jones, R.L.; et al. Treatments and Outcomes in Oligometastatic Soft Tissue Soft Sarcoma—A Single Centre Retrospective Analysis. Anticancer Res. 2021, 41, 5089–5096. [Google Scholar] [CrossRef]
- Braik, D.; Lemieux, C.; Wilson, B.E.; Salawu, A.; Abdul Razak, A.R. Clinical benefit and fragility evaluation of systemic therapy trials for advanced soft tissue sarcoma. Cancer 2025, 131, e35564. [Google Scholar] [CrossRef] [PubMed]
- LoRusso, P.; Yamamoto, N.; Patel, M.R.; Laurie, S.A.; Bauer, T.M.; Geng, J.; Davenport, T.; Teufel, M.; Li, J.; Lahmar, M.; et al. The MDM2-p53 Antagonist Brigimadlin (BI 907828) in Patients with Advanced or Metastatic Solid Tumors: Results of a Phase Ia, First-in-Human, Dose-Escalation Study. Cancer Discov. 2023, 13, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Guinn, D.; Wilhelm, E.E.; Lieberman, G.; Khozin, S. Assessing function of electronic health records for real-world data generation. BMJ Evid. Based Med. 2019, 24, 95–98. [Google Scholar] [CrossRef]
- Cowie, M.R.; Blomster, J.I.; Curtis, L.H.; Duclaux, S.; Ford, I.; Fritz, F.; Goldman, S.; Janmohamed, S.; Kreuzer, J.; Leenay, M.; et al. Electronic health records to facilitate clinical research. Clin. Res. Cardiol. 2017, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hersh, W.R.; Weiner, M.G.; Embi, P.J.; Logan, J.R.; Payne, P.R.; Bernstam, E.V.; Lehmann, H.P.; Hripcsak, G.; Hartzog, T.H.; Cimino, J.J.; et al. Caveats for the use of operational electronic health record data in comparative effectiveness research. Med. Care 2013, 51, S30–S37. [Google Scholar] [CrossRef]
- Italiano, A.; Mathoulin-Pelissier, S.; Cesne, A.L.; Terrier, P.; Bonvalot, S.; Collin, F.; Michels, J.J.; Blay, J.Y.; Coindre, J.M.; Bui, B. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer 2011, 117, 1049–1054. [Google Scholar] [CrossRef]
- von Konow, A.; Ghanei, I.; Styring, E.; Vult von Steyern, F. Late Local Recurrence and Metastasis in Soft Tissue Sarcoma of the Extremities and Trunk Wall: Better Outcome After Treatment of Late Events Compared with Early. Ann. Surg. Oncol. 2021, 28, 7891–7902. [Google Scholar] [CrossRef]
- Bonvalot, S.; Tetreau, R.; Llacer-Moscardo, C.; Roland, C. The Landmark Series: Multimodal Management of Oligometastatic Sarcoma. Ann. Surg. Oncol. 2024, 31, 7930–7942. [Google Scholar] [CrossRef] [PubMed]
- Szturz, P.; Nevens, D.; Vermorken, J.B. Oligometastatic Disease Management: Finding the Sweet Spot. Front. Oncol. 2020, 10, 617793. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.T.; Moureau-Zabotto, L.; Ouali, M.; Penel, N.; Italiano, A.; Bay, J.O.; Olivier, T.; Sunyach, M.P.; Boudou-Roquette, P.; Salas, S.; et al. Effect on survival of local ablative treatment of metastases from sarcomas: A study of the French sarcoma group. Clin. Oncol. 2015, 27, 48–55. [Google Scholar] [CrossRef]
- Shah, N.K.; Yegya-Raman, N.; Jones, J.A.; Shabason, J.E. Radiation Therapy in Metastatic Soft Tissue Sarcoma: From Palliation to Ablation. Cancers 2021, 13, 4775. [Google Scholar] [CrossRef] [PubMed]



| Factor | Level | Overall | Non-Metastatic | Metastatic | p |
|---|---|---|---|---|---|
| n | 295 | 202 | 93 | ||
| Sex (%) | Male | 151 (51.2) | 101 (50) | 50 (53.8) | 0.634 |
| female | 144 (48.8) | 101 (50) | 43 (46.2) | ||
| Age (median years (IQR) | 58.1 (46.7; 71.2) | 59.1 (47.7; 73.0) | 56.4 (41.8; 67.8) | 0.067 | |
| Follow-up (median months (IQR) | 20.9 (9.9; 36.2) | 18.9 (8.5; 33.4) | 23.9 (14.2; 41.5) | 0.025 | |
| Histologic Type (%) | Angiosarcoma of soft tissues | 9 (3.1) | 3 (1.5) | 6 (6.5) | 0.002 |
| Chondrosarcoma grade II, grade III | 9 (3.1) | 8 (4.0) | 1 (1.1) | ||
| Dedifferentiated liposarcoma | 28 (9.5) | 24 (11.9) | 4 (4.3) | ||
| Ewing sarcoma | 9 (3.1) | 3 (1.5) | 6 (6.5) | ||
| Leiomyosarcoma (excluding skin) | 31 (10.5) | 17 (8.4) | 14 (15.1) | ||
| Myxofibrosarcoma | 22 (7.5) | 20 (9.9) | 2 (2.2) | ||
| Myxoid liposarcoma | 19 (6.4) | 15 (7.4) | 4 (4.3) | ||
| Solitary fibrous tumor | 13 (4.4) | 10 (5.0) | 3 (3.2) | ||
| Undifferentiated sarcoma (UPS) | 53 (18.0) | 35 (17.3) | 18 (19.4) | ||
| Other | 102 (34.6) | 67 (33.29 | 35 (37.6) | ||
| Grading (%) | Suspicious of malignancy | 10 (3.4) | 10 (5.0) | 0 (0.0) | <0.001 |
| G1 | 62 (21.0) | 55 (27.2) | 7 (7.5) | ||
| G2 | 61 (20.7) | 48 (23.8) | 13 (14.0) | ||
| G2/3 | 6 (2.0) | 3 (1.5) | 3 (3.2) | ||
| G3 | 136 (46.1) | 75 (37.1) | 61 (65.6) | ||
| N/A | 20 (6.8) | 11 (5.4) | 9 (9.7) | ||
| Primary Tumor Size (median mm (IQR) | 85.0 (51.8; 135.0) | 80.0 (50.0; 130.0) | 90.0 (63.0; 140.0) | 0.175 | |
| Primary Tumor Site (%) | Extraperitoneal | 37 (11.5) | 25 (11.8) | 12 (10.8) | 0.090 |
| Head/Neck | 15 (4.7) | 10 (4.7) | 5 (4.5) | ||
| Intraperitoneal | 18 (5.6) | 8 (3.8) | 10 (9.0) | ||
| Intrathoracic | 19 (5.9) | 10 (4.7) | 9 (8.1) | ||
| Lower limb | 103 (32.0) | 8 (3.8) | 31 (27.9) | ||
| Lower limb girdle | 46 (14.3) | 29 (13.7) | 17 (15.3) | ||
| Trunk wall | 42 (13.0) | 33 (15.6) | 9 (8.1) | ||
| Upper limb | 22 (6.8) | 14 (6.6) | 8 (7.2) | ||
| Upper limb girdle | 8 (2.5) | 6 (2.8) | 2 (1.8) | ||
| Viscera | 12 (3.7) | 4 (1.9) | 8 (7.2) |
| (a) | ||||||||||||||
| Histologic Type | Bone | CNS/Meninges | Head/Neck | Intraperitoneal | Liver | Lungs | Lymph Nodes | Retroperitoneal | Skeletal Muscle | Skin/Subcutaneous Tissue | Thorax | Other | Total Initial Metastases Sites | Total Patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiosarcoma | 3 (42.9) | 0 (0.0) | 0 (0.0) | 2 (28.6) | 1 (14.3) | 2 (28.6) | 4 (57.1) | 0 (0.0) | 2 (28.6) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 16 (2.3×) | 7 |
| Chondrosarcoma | 2 (28.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (71.4) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (1.1×) | 7 |
| Epithelioid hemangioendothelioma | 2 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 8 (2.7×) | 3 |
| Ewing sarcoma | 5 (83.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (50.0) | 2 (33.3) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 12 (2.0×) | 6 |
| Leiomyosarcoma (excluding skin) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 1 (7.1) | 11 (78.6) | 1 (7.1) | 0 (0.0) | 3 (21.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 18 (1.3×) | 14 |
| Liposarcoma (dediff./myx./pleom.) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (50.0) | 1 (10.0) | 1 (10.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 2 (20.0) | 12 (1.2×) | 10 |
| Osteosarcoma | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.0×) | 4 |
| Pleomorphic dermal sarcoma | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 4 (1.0×) | 4 |
| Solitary fibrous tumor | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.3×) | 3 |
| Undifferentiated sarcoma (UPS) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 1 (5.6) | 13 (72.2) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 18 (1.0×) | 18 |
| Other | 1 (5.9) | 1 (5.9) | 1 (5.9) | 3 (17.6) | 5 (29.4) | 11 (64.7) | 2 (11.8) | 3 (17.6) | 1 (5.9) | 1 (5.9) | 2 (11.8) | 1 (5.9) | 32 (1.9×) | 17 |
| Total | 17 (18.3) | 2 (2.2) | 1 (1.1) | 7 (7.5) | 11 (11.8) | 58 (62.4) | 14 (15.1) | 4 (4.3) | 9 (9.7) | 5 (5.4) | 3 (3.2) | 5 (5.4) | 136 (1.5×) | 93 |
| (b) | ||||||||||||||
| Histologic Type | Bone | CNS/Meninges | Head/Neck | Intraperitoneal | Liver | Lungs | Lymph Nodes | Retroperitoneal | Skeletal Muscle | Skin/Subcutaneous Tissue | Thorax | Other | Total Metastasis Sites | |
| Angiosarcoma | 4 (57.1) | 1 (14.3) | 1 (14.3) | 2 (28.6) | 2 (28.6) | 4 (57.1) | 5 (71.4) | 0 (0.0) | 3 (42.9) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 24 (3.4×) | |
| Chondrosarcoma | 4 (57.1) | 1 (14.3) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 7 (100.0) | 3 (42.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 1 (14.3) | 19 (2.7×) | |
| Epithelioid hemangioendothelioma | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 12 (4.0×) | |
| Ewing sarcoma | 6 (100.0) | 1 (16.7) | 1 (16.7) | 0 (0.0) | 1 (16.7) | 6 (100.0) | 2 (33.3) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 0 (0.0) | 1 (16.7) | 21 (3.5×) | |
| Leiomyosarcoma (excluding skin) | 6 (42.9) | 1 (7.1) | 0 (0.0) | 2 (14.3) | 3 (21.4) | 14 (100.0) | 4 (28.6) | 2 (14.3) | 5 (35.7) | 2 (14.3) | 0 (0.0) | 2 (14.3) | 41 (2.9×) | |
| Liposarcoma (dediff./myx./pleom.) | 3 (30.0) | 2 (20.0) | 0 (0.0) | 1 (10.0) | 1 (10.0) | 8 (80.0) | 2 (20.0) | 3 (30.0) | 4 (40.0) | 3 (30.0) | 3 (30.0) | 2 (20.0) | 32 (3.2×) | |
| Osteosarcoma | 2 (50.0) | 0 (0.0) | 1 (25.0) | 1 (25.0) | 0 (0.0) | 3 (75.0) | 1 (25.0) | 1 (25.0) | 1 (25.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 11 (2.8×) | |
| Pleomorphic dermal sarcoma | 2 (50.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (50.0) | 2 (50.0) | 1 (25.0) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (2.8×) | |
| Solitary fibrous tumor | 2 (66.7) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 9 (3.0×) | |
| Undifferentiated sarcoma (UPS) | 1 (5.6) | 1 (5.6) | 0 (0.0) | 2 (11.1) | 1 (5.6) | 14 (77.8) | 4 (22.2) | 2 (11.1) | 3 (16.7) | 2 (11.1) | 2 (11.1) | 1 (5.6) | 33 (1.8×) | |
| Other | 4 (23.5) | 1 (5.9) | 1 (5.9) | 5 (29.4) | 6 (35.3) | 15 (88.2) | 6 (35.3) | 5 (29.4) | 2 (11.8) | 1 (5.9) | 6 (35.3) | 1 (5.9) | 53 (3.1×) | |
| Total | 37 (39.8) | 9 (9.7) | 5 (5.4) | 15 (16.1) | 18 (19.4) | 78 (83.9) | 30 (32.3) | 15 (16.1) | 21 (22.6) | 15 (16.1) | 15 (16.1) | 8 (8.6) | 266 (2.9×) | |
| Metastasis Site | Angiosarcoma | Chondrosarcoma | Epithelioid Hemangioendothelioma | Ewing Sarcoma | Leiomyosarcoma (Excluding Skin) | Liposarcoma (dediff./myx./pleom./NOS) | Osteosarcoma | Pleomorphic Dermal Sarcoma | Solitary Fibrous Tumor | Undifferentiated Sarcoma (UPS) | Other | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone | 4 (57.1) | 4 (57.1) | 3 (100.0) | 6 (100.0) | 6 (42.9) | 3 (30.0) | 2 (50.0) | 2 (66.7) | 1 (5.6) | 4 (23.5) | 37 (39.8) | |
| CNS/Meninges | 1 (14.3) | 1 (14.3) | 0 (0.0) | 1 (16.7) | 1 (7.1) | 2 (20.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (5.6) | 1 (5.9) | 9 (9.7) |
| Head/Neck | 1 (14.3) | 1 (14.3) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 5 (5.4) |
| Intraperitoneal | 2 (28.6) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 2 (14.3) | 1 (10.0) | 1 (25.0) | 0 (0.0) | 1 (33.3) | 2 (11.1) | 5 (29.4) | 15 (16.1) |
| Liver | 2 (28.6) | 0 (0.0) | 2 (66.7) | 1 (16.7) | 3 (21.4) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 1 (5.6) | 6 (35.3) | 18 (19.4) |
| Lungs | 4 (57.1) | 7 (100.0) | 3 (100.0) | 5 (100.0) | 14 (100.0) | 8 (80.0) | 3 (75.0) | 2 (50.0) | 2 (66.7) | 14 (77.8) | 15 (88.2) | 78 (83.9) |
| Lymph nodes | 5 (71.4) | 3 (42.9) | 0 (0.0) | 2 (33.3) | 4 (28.6) | 2 (20.0) | 1 (25.0) | 2 (50.0) | 1 (33.3) | 4 (22.2) | 6 (35.3) | 30 (32.3) |
| Skeletal muscle | 3 (42.9) | 0 (0.0) | 1 (33.3) | 1 (16.7) | 5 (35.7) | 4 (40.0) | 1 (25.0) | 1 (25.0) | 0 (0.0) | 3 (16.7) | 2 (11.8) | 21 (22.6) |
| Skin/Subcutaneous tissue | 1 (14.3) | 0 (0.0) | 3 (100.0) | 1 (16.7) | 2 (14.3) | 3 (30.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (11.1) | 1 (5.9) | 15 (16.1) |
| Thorax | 1 (14.3) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 3 (30.0) | 1 (25.0) | 0 (0.0) | 1 (33.3) | 2 (11.1) | 0 (0.0) | 6 (35.3) | 15 (16.1) |
| Retroperitoneal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 2 (14.3) | 3 (30.0) | 1 (25.0) | 1 (25.0) | 0 (0.0) | 2 (11.1) | 5 (29.4) | 15 (16.1) |
| Other | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (16.7) | 2 (14.3) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 1 (5.9) | 8 (8.6) |
| Total Metastasis Sites | 24 (3.4×) | 19 (2.7×) | 12 (4.0×) | 21 (3.5×) | 41 (2.9×) | 32 (3.2×) | 11 (2.8×) | 11 (2.8×) | 9 (3.0×) | 33 (1.8×) | 53 (3.1×) | 266 (2.9×) |
| Total Patients | 10 | 18 | 5 | 9 | 31 | 52 | 7 | 6 | 13 | 53 | 91 | 295 |
| …without Metastasis | 3 | 11 | 2 | 3 | 17 | 42 | 3 | 2 | 10 | 35 | 74 | 202 |
| …with Metastasis | 7 | 7 | 3 | 6 | 14 | 10 | 4 | 4 | 3 | 18 | 17 | 93 |
| Prevalence of Metastasis | 70.0% | 38.9% | 60.0% | 66.7% | 45.2% | 19.2% | 57.1% | 66.7% | 23.1% | 34.0% | 18.7% | 31.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heesen, P.; Feusi, D.; Vogel, B.; Studer, G.; Fuchs, B.; on behalf of the Swiss Sarcoma Network. Triangulating Timing, Tropism and Burden of Sarcoma Metastases: Toward Precision Surveillance and Therapy in a Real-World-Time Cohort. Cancers 2025, 17, 2944. https://doi.org/10.3390/cancers17182944
Heesen P, Feusi D, Vogel B, Studer G, Fuchs B, on behalf of the Swiss Sarcoma Network. Triangulating Timing, Tropism and Burden of Sarcoma Metastases: Toward Precision Surveillance and Therapy in a Real-World-Time Cohort. Cancers. 2025; 17(18):2944. https://doi.org/10.3390/cancers17182944
Chicago/Turabian StyleHeesen, Philip, Dario Feusi, Bettina Vogel, Gabriela Studer, Bruno Fuchs, and on behalf of the Swiss Sarcoma Network. 2025. "Triangulating Timing, Tropism and Burden of Sarcoma Metastases: Toward Precision Surveillance and Therapy in a Real-World-Time Cohort" Cancers 17, no. 18: 2944. https://doi.org/10.3390/cancers17182944
APA StyleHeesen, P., Feusi, D., Vogel, B., Studer, G., Fuchs, B., & on behalf of the Swiss Sarcoma Network. (2025). Triangulating Timing, Tropism and Burden of Sarcoma Metastases: Toward Precision Surveillance and Therapy in a Real-World-Time Cohort. Cancers, 17(18), 2944. https://doi.org/10.3390/cancers17182944






