Cancer Growth and Invasion Are Increased in the Tight Skin (TSK) Mouse
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Mice
2.3. Isolation of Dorsal Skin and Bulk RNA Sequencing
2.4. Isolation of Dorsal Skin and Single-Cell RNA Sequencing
2.5. Syngeneic Tumor Growth in Mice and Single-Cell RNA Sequencing Analysis
2.6. Immunohistochemistry and Antibodies
2.7. Pathology Image Analyses
2.8. Statistical Analysis
3. Results
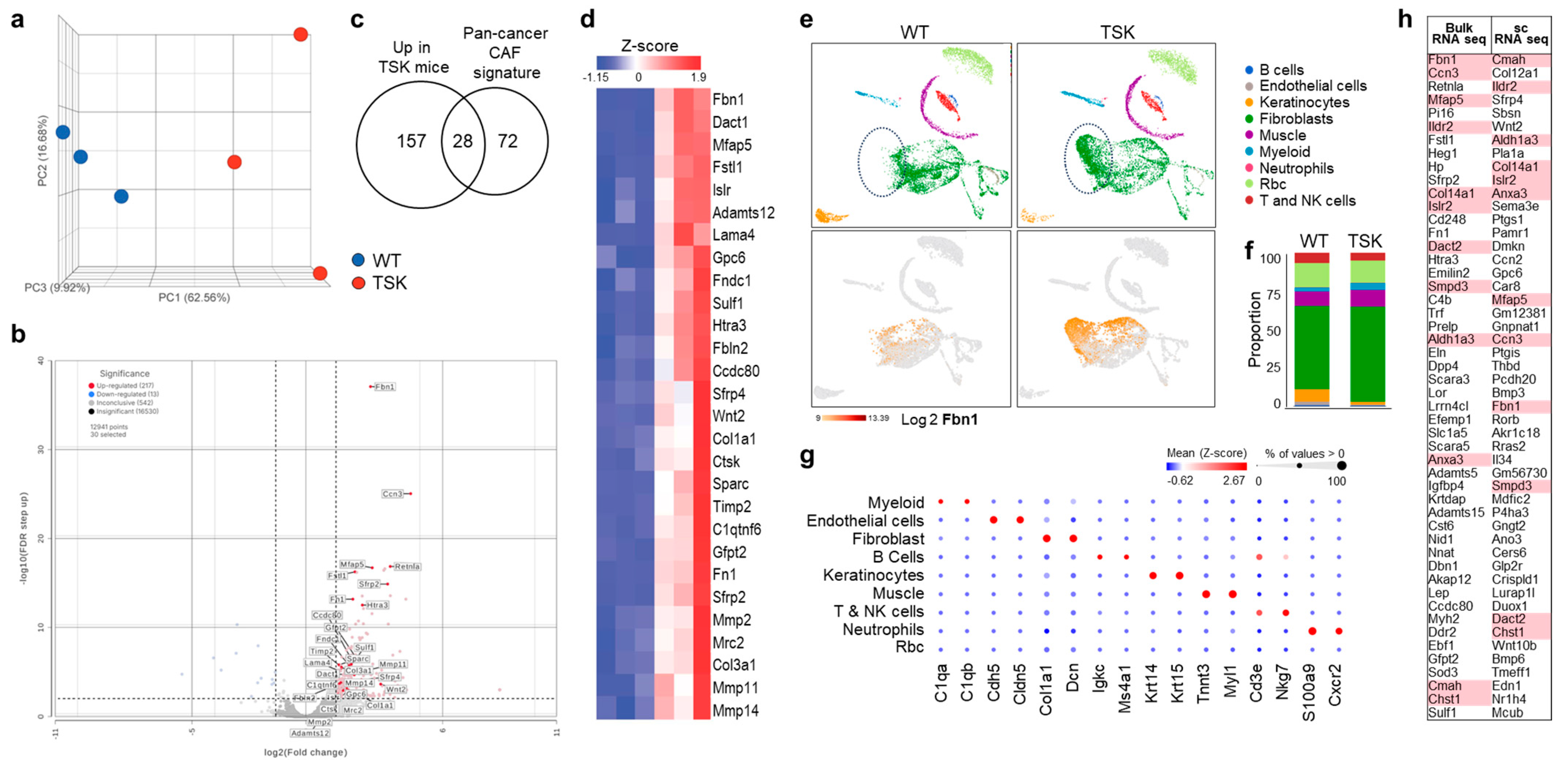
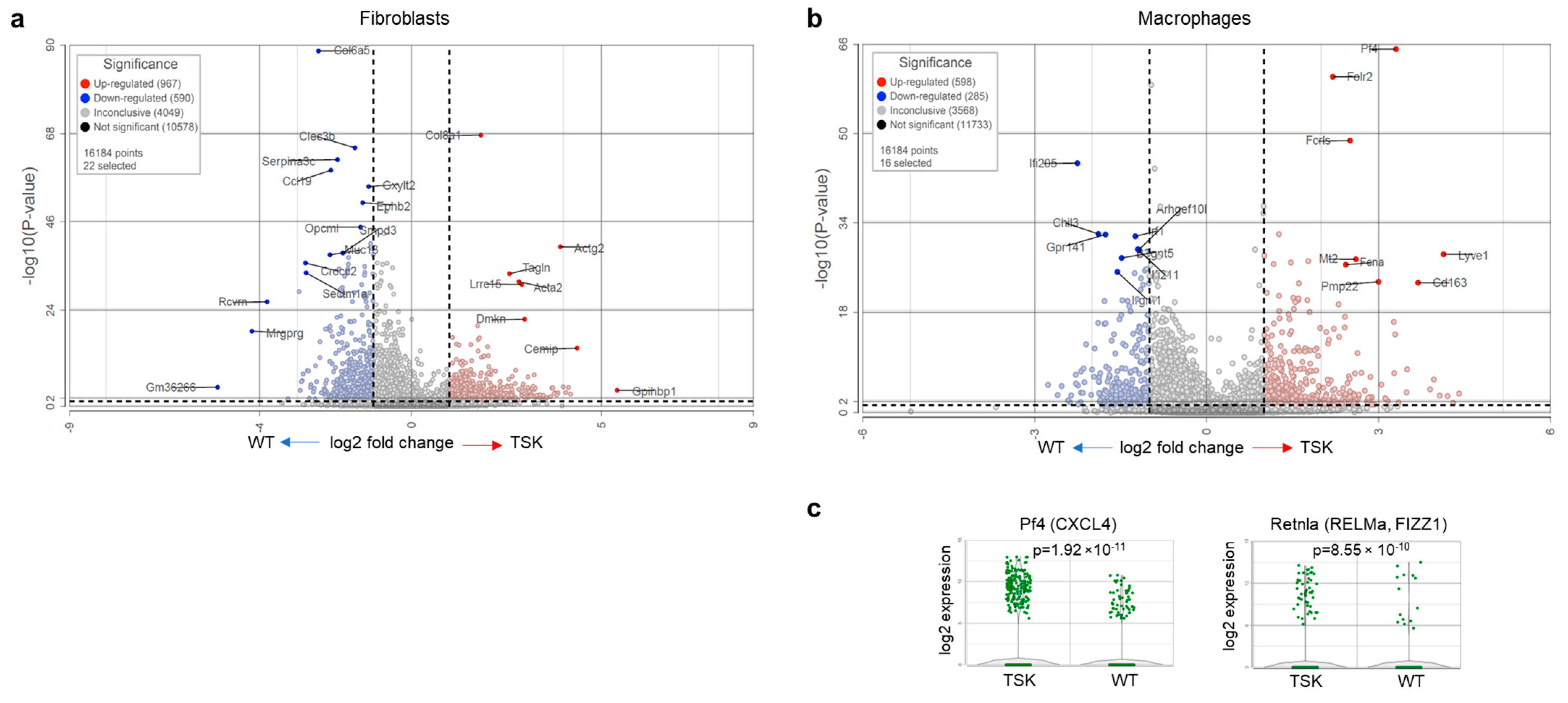
3.1. Dorsal Skin Fibroblasts from TSK Mice Exhibit Features of CAFs
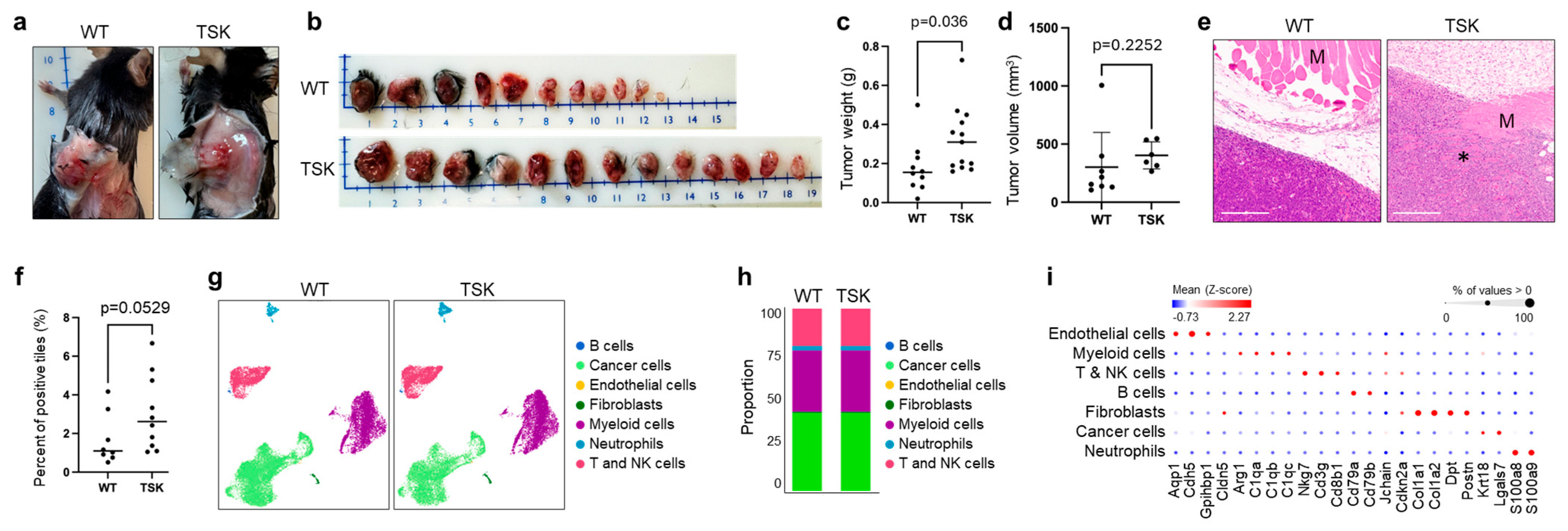
3.2. Melanoma
3.3. Breast Cancer
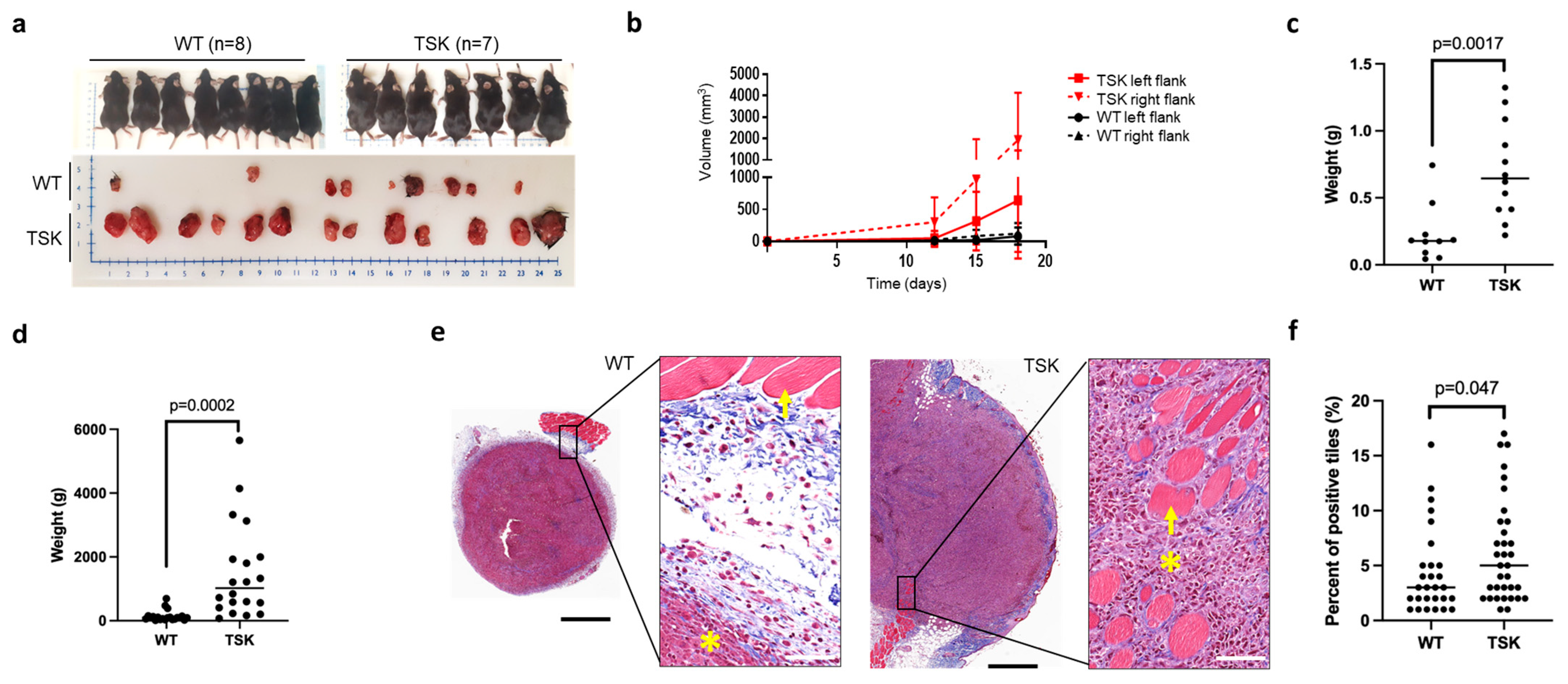
3.4. Ovarian Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSK | Tight skin |
| WT | Wild type |
| CAF | Cancer-associated fibroblasts |
| TAM | Tumor-associated macrophages |
| ECM | Extracellular matrix |
| TCGB | Technology Center for Genomics & Bioinformatics |
| UCLA | University of California, Los Angeles |
| UMI | Unique molecular identifier |
| FDR | False Discovery Rate |
| UMAP | Uniform manifold approximation and projection |
| TCGA | The Cancer Genome Atlas |
| AOCS | Australian Ovarian Cancer Study |
References
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kobayashi, A.; Watabe, K. The role of tumor-associated macrophage in tumor progression. Front. Biosci. 2012, 4, 787–798. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Canellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Grauel, A.L.; Nguyen, B.; Ruddy, D.; Laszewski, T.; Schwartz, S.; Chang, J.; Chen, J.; Piquet, M.; Pelletier, M.; Yan, Z.; et al. TGFβ-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat. Commun. 2020, 11, 6315. [Google Scholar] [CrossRef]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- De Jaeghere, E.A.; Denys, H.G.; De Wever, O. Fibroblasts Fuel Immune Escape in the Tumor Microenvironment. Trends Cancer 2019, 5, 704–723. [Google Scholar] [CrossRef]
- Decitre, M.; Gleyzal, C.; Raccurt, M.; Peyrol, S.; Aubert-Foucher, E.; Csiszar, K.; Sommer, P. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab. Investig. 1998, 78, 143–151. [Google Scholar] [PubMed]
- Peyrol, S.; Raccurt, M.; Gerard, F.; Gleyzal, C.; Grimaud, J.A.; Sommer, P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am. J. Pathol. 1997, 150, 497–507. [Google Scholar] [PubMed]
- Jeay, S.; Pianetti, S.; Kagan, H.M.; Sonenshein, G.E. Lysyl oxidase inhibits ras-mediated transformation by preventing activation of NF-kappa B. Mol. Cell. Biol. 2003, 23, 2251–2263. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef]
- Parekh, A.; Weaver, A.M. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell Adhes. Migr. 2009, 3, 288–292. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.H.; Kim, H.N.; Wang, C.J.; Kwak, M.K.; Hur, E.; Suh, K.Y.; An, S.S.; Levchenko, A. Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nat. Mater. 2016, 15, 792–801. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.H.; Levchenko, A. Topotaxis: A New Mechanism of Directed Cell Migration in Topographic ECM Gradients. Biophys. J. 2018, 114, 1257–1263. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Engvall, E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986, 103, 2499–2509. [Google Scholar] [CrossRef]
- Keene, D.R.; Maddox, B.K.; Kuo, H.J.; Sakai, L.Y.; Glanville, R.W. Extraction of extendable beaded structures and their identification as fibrillin-containing extracellular matrix microfibrils. J. Histochem. Cytochem. 1991, 39, 441–449. [Google Scholar] [CrossRef]
- Reinhardt, D.P.; Keene, D.R.; Corson, G.M.; Pöschl, E.; Bächinger, H.P.; Gambee, J.E.; Sakai, L.Y. Fibrillin-1: Organization in microfibrils and structural properties. J. Mol. Biol. 1996, 258, 104–116. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.S.; Cain, S.A.; Morgan, A.; Dallas, S.L.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-1 regulates the bioavailability of TGFβ1. J. Cell Biol. 2007, 176, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.M.; Raza, S.; van Nimwegen, E.; Freeman, T.C.; Hume, D.A. Co-expression of FBN1 with mesenchyme-specific genes in mouse cell lines: Implications for phenotypic variability in Marfan syndrome. Eur. J. Hum. Genet. 2010, 18, 1209–1215. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, W.; Ramirez, F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J. Cell Biol. 1995, 129, 1165–1176. [Google Scholar] [CrossRef]
- Davis, M.R.; Arner, E.; Duffy, C.R.; De Sousa, P.A.; Dahlman, I.; Arner, P.; Summers, K.M. Expression of FBN1 during adipogenesis: Relevance to the lipodystrophy phenotype in Marfan syndrome and related conditions. Mol. Genet. Metab. 2016, 119, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Millstein, J.; Budden, T.; Goode, E.L.; Anglesio, M.S.; Talhouk, A.; Intermaggio, M.P.; Leong, H.S.; Chen, S.; Elatre, W.; Gilks, B.; et al. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann. Oncol. 2020, 31, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, X.; Lu, H.; Zhang, C.; Li, X.; Zhang, T.; Shen, J.; Wen, J. Succinylation Inhibits the Enzymatic Hydrolysis of the Extracellular Matrix Protein Fibrillin 1 and Promotes Gastric Cancer Progression. Adv. Sci. 2022, 9, e2200546. [Google Scholar] [CrossRef] [PubMed]
- Ovali, M.A.; Bozgeyik, I. Asprosin, a C-Terminal Cleavage Product of Fibrillin 1 Encoded by the FBN1 Gene, in Health and Disease. Mol. Syndromol. 2022, 13, 175–183. [Google Scholar] [CrossRef]
- Bona, C.; Saito, S. Fibrillin-1 protein in tight skin mice and scleroderma. Clin. Rev. Allergy Immunol. 2000, 18, 119–126. [Google Scholar] [CrossRef]
- Saito, S.; Nishimura, H.; Brumeanu, T.D.; Casares, S.; Stan, A.C.; Honjo, T.; Bona, C.A. Characterization of mutated protein encoded by partially duplicated fibrillin-1 gene in tight skin (TSK) mice. Mol. Immunol. 1999, 36, 169–176. [Google Scholar] [CrossRef]
- Siracusa, L.D.; McGrath, R.; Ma, Q.; Moskow, J.J.; Manne, J.; Christner, P.J.; Buchberg, A.M.; Jimenez, S.A. A tandem duplication within the fibrillin 1 gene is associated with the mouse tight skin mutation. Genome Res. 1996, 6, 300–313. [Google Scholar] [CrossRef][Green Version]
- Pablos, J.L.; Everett, E.T.; Norris, J.S. The tight skin mouse: An animal model of systemic sclerosis. Clin. Exp. Rheumatol. 2004, 22, S81–S85. [Google Scholar][Green Version]
- Kasturi, K.N.; Shibata, S.; Muryoi, T.; Bona, C.A. Tight-skin mouse an experimental model for scleroderma. Int. Rev. Immunol. 1994, 11, 253–271. [Google Scholar] [CrossRef]
- Lemaire, R.; Korn, J.H.; Schiemann, W.P.; Lafyatis, R. Fibulin-2 and fibulin-5 alterations in tsk mice associated with disorganized hypodermal elastic fibers and skin tethering. J. Investig. Dermatol. 2004, 123, 1063–1069. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mark, M.E.; Wooley, P.H.; Lawrence, W.D.; Mayes, M.D. Increased dermal elastic fibers in the tight skin mouse. Clin. Exp. Rheumatol. 2004, 22, 617–620. [Google Scholar]
- Kielty, C.M.; Raghunath, M.; Siracusa, L.D.; Sherratt, M.J.; Peters, R.; Shuttleworth, C.A.; Jimenez, S.A. The Tight skin mouse: Demonstration of mutant fibrillin-1 production and assembly into abnormal microfibrils. J. Cell Biol. 1998, 140, 1159–1166. [Google Scholar] [CrossRef]
- Manne, J.; Markova, M.; Siracusa, L.D.; Jimenez, S.A. Collagen content in skin and internal organs of the tight skin mouse: An animal model of scleroderma. Biochem. Res. Int. 2013, 2013, 436053. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Casciola-Rosen, L. Cancer and scleroderma: A paraneoplastic disease with implications for malignancy screening. Curr. Opin. Rheumatol. 2015, 27, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Rosen, A. Cancer and systemic sclerosis: Novel insights into pathogenesis and clinical implications. Curr. Opin. Rheumatol. 2011, 23, 530–535. [Google Scholar] [CrossRef]
- Lepri, G.; Catalano, M.; Bellando-Randone, S.; Pillozzi, S.; Giommoni, E.; Giorgione, R.; Botteri, C.; Matucci-Cerinic, M.; Antonuzzo, L.; Guiducci, S. Systemic Sclerosis Association with Malignancy. Clin. Rev. Allergy Immunol. 2022, 63, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Sugiyama, D.; Kumagai, S.; Morinobu, A. Cancer incidence in systemic sclerosis: Meta-analysis of population-based cohort studies. Arthritis Rheum. 2013, 65, 1913–1921. [Google Scholar] [CrossRef]
- Morrisroe, K.; Hansen, D.; Huq, M.; Stevens, W.; Sahhar, J.; Ngian, G.S.; Ferdowsi, N.; Hill, C.; Roddy, J.; Walker, J.; et al. Incidence, Risk Factors, and Outcomes of Cancer in Systemic Sclerosis. Arthritis Care Res. 2020, 72, 1625–1635. [Google Scholar] [CrossRef]
- Kaşifoğlu, T.; Yaşar Bilge, Ş.; Yıldız, F.; Özen, G.; Pehlivan, Y.; Yılmaz, N.; Tarhan, F.; Yılmaz, S.; Küçük, A.; Emmungil, H.; et al. Risk factors for malignancy in systemic sclerosis patients. Clin. Rheumatol. 2016, 35, 1529–1533. [Google Scholar] [CrossRef]
- Martínez-Urbistondo, M.; González-Guzmán, A.; Fernández-Guitián, R.; Blanco-Valencia, X.P.; Esteban-Sampedro, J.; Martín-Portugués, M.; Durán-Del Campo, P.; Tutor, P.; Mellor-Pita, S.; Ortega-de la Puente, A.; et al. Neoplasm related mortality risk in Systemic Sclerosis: A nationwide study. BMC Rheumatol. 2025, 9, 27. [Google Scholar] [CrossRef]
- Danciu, C.; Oprean, C.; Coricovac, D.E.; Andreea, C.; Cimpean, A.; Radeke, H.; Soica, C.; Dehelean, C. Behaviour of four different B16 murine melanoma cell sublines: C57BL/6J skin. Int. J. Exp. Pathol. 2015, 96, 73–80. [Google Scholar] [CrossRef]
- Le Naour, A.; Rossary, A.; Vasson, M.P. EO771, is it a well-characterized cell line for mouse mammary cancer model? Limit and uncertainty. Cancer Med. 2020, 9, 8074–8085. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Recouvreux, M.S.; Haro, M.; Taylan, E.; Taylor-Harding, B.; Walts, A.E.; Karlan, B.Y.; Orsulic, S. INHBA(+) cancer-associated fibroblasts generate an immunosuppressive tumor microenvironment in ovarian cancer. npj Precis. Oncol. 2024, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Jia, D.; Liu, Z.; Deng, N.; Tan, T.Z.; Huang, R.Y.; Taylor-Harding, B.; Cheon, D.J.; Lawrenson, K.; Wiedemeyer, W.R.; Walts, A.E.; et al. A COL11A1-correlated pan-cancer gene signature of activated fibroblasts for the prioritization of therapeutic targets. Cancer Lett. 2016, 382, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Shay, T.; Kang, J. Immunological Genome Project and systems immunology. Trends Immunol. 2013, 34, 602–609. [Google Scholar] [CrossRef]
- Lemaire, R.; Farina, G.; Bayle, J.; Dimarzio, M.; Pendergrass, S.A.; Milano, A.; Perbal, B.; Whitfield, M.L.; Lafyatis, R. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-β- and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. J. Investig. Dermatol. 2010, 130, 1514–1523. [Google Scholar] [CrossRef]
- Peng, L.; Wei, Y.; Shao, Y.; Li, Y.; Liu, N.; Duan, L. The Emerging Roles of CCN3 Protein in Immune-Related Diseases. Mediat. Inflamm. 2021, 2021, 5576059. [Google Scholar] [CrossRef]
- Li, J.; Ye, L.; Owen, S.; Weeks, H.P.; Zhang, Z.; Jiang, W.G. Emerging role of CCN family proteins in tumorigenesis and cancer metastasis (Review). Int. J. Mol. Med. 2015, 36, 1451–1463. [Google Scholar] [CrossRef]
- Yeger, H.; Perbal, B. CCN family of proteins: Critical modulators of the tumor cell microenvironment. J. Cell Commun. Signal. 2016, 10, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, R.; Farina, G.; Kissin, E.; Shipley, J.M.; Bona, C.; Korn, J.H.; Lafyatis, R. Mutant fibrillin 1 from tight skin mice increases extracellular matrix incorporation of microfibril-associated glycoprotein 2 and type I collagen. Arthritis Rheum. 2004, 50, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ye, L.; Bennett, S.; Xu, H.; He, D.; Xu, J. Molecular structure and function of microfibrillar-associated proteins in skeletal and metabolic disorders and cancers. J. Cell. Physiol. 2021, 236, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hecht, I.; Toporik, A.; Podojil, J.R.; Vaknin, I.; Cojocaru, G.; Oren, A.; Aizman, E.; Liang, S.C.; Leung, L.; Dicken, Y.; et al. ILDR2 Is a Novel B7-like Protein That Negatively Regulates T Cell Responses. J. Immunol. 2018, 200, 2025–2037. [Google Scholar] [CrossRef]
- Podojil, J.R.; Hecht, I.; Chiang, M.Y.; Vaknin, I.; Barbiro, I.; Novik, A.; Neria, E.; Rotman, G.; Miller, S.D. ILDR2-Fc Is a Novel Regulator of Immune Homeostasis and Inducer of Antigen-Specific Immune Tolerance. J. Immunol. 2018, 200, 2013–2024. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Tashkin, D.P.; Roth, M.D.; Clements, P.J.; Khanna, D.; Furst, D.E.; Mayes, M.; Charles, J.; Tseng, C.H.; Elashoff, R.M.; et al. Changes in plasma CXCL4 levels are associated with improvements in lung function in patients receiving immunosuppressive therapy for systemic sclerosis-related interstitial lung disease. Arthritis Res. Ther. 2016, 18, 305. [Google Scholar] [CrossRef]
- Lande, R.; Lee, E.Y.; Palazzo, R.; Marinari, B.; Pietraforte, I.; Santos, G.S.; Mattenberger, Y.; Spadaro, F.; Stefanantoni, K.; Iannace, N.; et al. CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9-mediated interferon-α production in systemic sclerosis. Nat. Commun. 2019, 10, 1731. [Google Scholar] [CrossRef]
- van Bon, L.; Affandi, A.J.; Broen, J.; Christmann, R.B.; Marijnissen, R.J.; Stawski, L.; Farina, G.A.; Stifano, G.; Mathes, A.L.; Cossu, M.; et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 2014, 370, 433–443. [Google Scholar] [CrossRef]
- van der Kroef, M.; Carvalheiro, T.; Rossato, M.; de Wit, F.; Cossu, M.; Chouri, E.; Wichers, C.G.K.; Bekker, C.P.J.; Beretta, L.; Vazirpanah, N.; et al. CXCL4 triggers monocytes and macrophages to produce PDGF-BB, culminating in fibroblast activation: Implications for systemic sclerosis. J. Autoimmun. 2020, 111, 102444. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, K.; Schaefer, G.J.L.; Kim, H.; Schumacher, D.; Bleckwehl, T.; Long, Q.; Klinkhammer, B.M.; Peisker, F.; Koch, L.; Nagai, J.; et al. Platelet-instructed SPP1+ macrophages drive myofibroblast activation in fibrosis in a CXCL4-dependent manner. Cell Rep. 2023, 42, 112131. [Google Scholar] [CrossRef]
- Affandi, A.J.; Carvalheiro, T.; Ottria, A.; de Haan, J.J.; Brans, M.A.D.; Brandt, M.M.; Tieland, R.G.; Lopes, A.P.; Fernández, B.M.; Bekker, C.P.J.; et al. CXCL4 drives fibrosis by promoting several key cellular and molecular processes. Cell Rep. 2022, 38, 110189. [Google Scholar] [CrossRef]
- Sanin, D.E.; Ge, Y.; Marinkovic, E.; Kabat, A.M.; Castoldi, A.; Caputa, G.; Grzes, K.M.; Curtis, J.D.; Thompson, E.A.; Willenborg, S.; et al. A common framework of monocyte-derived macrophage activation. Sci. Immunol. 2022, 7, eabl7482. [Google Scholar] [CrossRef]
- Liu, T.; Yu, H.; Ullenbruch, M.; Jin, H.; Ito, T.; Wu, Z.; Liu, J.; Phan, S.H. The in vivo fibrotic role of FIZZ1 in pulmonary fibrosis. PLoS ONE 2014, 9, e88362. [Google Scholar] [CrossRef]
- Fang, D.; Chen, B.; Lescoat, A.; Khanna, D.; Mu, R. Immune cell dysregulation as a mediator of fibrosis in systemic sclerosis. Nat. Rev. Rheumatol. 2022, 18, 683–693. [Google Scholar] [CrossRef]
- Saito, S.; Kasturi, K.; Bona, C. Genetic and immunologic features associated with scleroderma-like syndrome of TSK mice. Curr. Rheumatol. Rep. 1999, 1, 34–37. [Google Scholar] [CrossRef]
- Saito, E.; Fujimoto, M.; Hasegawa, M.; Komura, K.; Hamaguchi, Y.; Kaburagi, Y.; Nagaoka, T.; Takehara, K.; Tedder, T.F.; Sato, S. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J. Clin. Investig. 2002, 109, 1453–1462. [Google Scholar] [CrossRef][Green Version]
- Mok, S.C.; Bonome, T.; Vathipadiekal, V.; Bell, A.; Johnson, M.E.; Wong, K.K.; Park, D.C.; Hao, K.; Yip, D.K.; Donninger, H.; et al. A gene signature predictive for outcome in advanced ovarian cancer identifies a survival factor: Microfibril-associated glycoprotein 2. Cancer Cell 2009, 16, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.S.; Yeung, T.L.; Yip, K.P.; Pradeep, S.; Balasubramanian, L.; Liu, J.; Wong, K.K.; Mangala, L.S.; Armaiz-Pena, G.N.; Lopez-Berestein, G.; et al. Calcium-dependent FAK/CREB/TNNC1 signalling mediates the effect of stromal MFAP5 on ovarian cancer metastatic potential. Nat. Commun. 2014, 5, 5092. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, X.; Ying, H.; Xu, J.; Yang, H.; Sun, K.; He, L.; Li, M.; Ji, Y.; Liang, T.; et al. Targeting MFAP5 in cancer-associated fibroblasts sensitizes pancreatic cancer to PD-L1-based immunochemotherapy via remodeling the matrix. Oncogene 2023, 42, 2061–2073. [Google Scholar] [CrossRef]
- Peng, Z.; Ren, Z.; Tong, Z.; Zhu, Y.; Zhu, Y.; Hu, K. Interactions between MFAP5 + fibroblasts and tumor-infiltrating myeloid cells shape the malignant microenvironment of colorectal cancer. J. Transl. Med. 2023, 21, 405. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, T.; Fang, M.; Huang, W.; Sun, Z.; Xiao, J.; Yan, W. MFAP5 promotes tumor progression and bone metastasis by regulating ERK/MMP signaling pathways in breast cancer. Biochem. Biophys. Res. Commun. 2018, 498, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.L.; Leung, C.S.; Yip, K.P.; Sheng, J.; Vien, L.; Bover, L.C.; Birrer, M.J.; Wong, S.T.C.; Mok, S.C. Anticancer Immunotherapy by MFAP5 Blockade Inhibits Fibrosis and Enhances Chemosensitivity in Ovarian and Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 6417–6428. [Google Scholar] [CrossRef]
- Martins, V.; Gonzalez De Los Santos, F.G.; Wu, Z.; Capelozzi, V.; Phan, S.H.; Liu, T. FIZZ1-induced myofibroblast transdifferentiation from adipocytes and its potential role in dermal fibrosis and lipoatrophy. Am. J. Pathol. 2015, 185, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Nejatifar, F.; Mirbolouk, N.; Masooleh, I.S.; Kazemnejad, E.; Ghavidel-Parsa, B.; Ghanbari, A.M.; Zayeni, H. Association between neutrophil/lymphocyte ratio and disease severity in scleroderma patients. Heliyon 2023, 9, e20576. [Google Scholar] [CrossRef]
- Wareing, N.; Mohan, V.; Taherian, R.; Volkmann, E.R.; Lyons, M.A.; Wilhalme, H.; Roth, M.D.; Estrada-Y-Martin, R.M.; Skaug, B.; Mayes, M.D.; et al. Blood Neutrophil Count and Neutrophil-to-Lymphocyte Ratio for Prediction of Disease Progression and Mortality in Two Independent Systemic Sclerosis Cohorts. Arthritis Care Res. 2023, 75, 648–656. [Google Scholar] [CrossRef]
- Dominguez, C.X.; Müller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A.; et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15+ Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020, 10, 232–253. [Google Scholar] [CrossRef]
- Krishnamurty, A.T.; Shyer, J.A.; Thai, M.; Gandham, V.; Buechler, M.B.; Yang, Y.A.; Pradhan, R.N.; Wang, A.W.; Sanchez, P.L.; Qu, Y.; et al. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 2022, 611, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Wang, C.; Oberg, A.L.; Riska, S.M.; Torres, M.; Kumka, J.; Multinu, F.; Sagar, G.; Roy, D.; Jung, D.B.; et al. Genes associated with bowel metastases in ovarian cancer. Gynecol. Oncol. 2019, 154, 495–504. [Google Scholar] [CrossRef]
- Purcell, J.W.; Tanlimco, S.G.; Hickson, J.; Fox, M.; Sho, M.; Durkin, L.; Uziel, T.; Powers, R.; Foster, K.; McGonigal, T.; et al. LRRC15 Is a Novel Mesenchymal Protein and Stromal Target for Antibody-Drug Conjugates. Cancer Res. 2018, 78, 4059–4072. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Luke, J.J.; Hollebecque, A.; Powderly, J.D., 2nd; Spira, A.I.; Subbiah, V.; Naumovski, L.; Chen, C.; Fang, H.; Lai, D.W.; et al. First-in-Human Phase I Study of ABBV-085, an Antibody-Drug Conjugate Targeting LRRC15, in Sarcomas and Other Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3556–3566. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recouvreux, M.S.; Taylor-Harding, B.; Rowat, A.C.; Karlan, B.Y.; Orsulic, S. Cancer Growth and Invasion Are Increased in the Tight Skin (TSK) Mouse. Cancers 2025, 17, 2943. https://doi.org/10.3390/cancers17182943
Recouvreux MS, Taylor-Harding B, Rowat AC, Karlan BY, Orsulic S. Cancer Growth and Invasion Are Increased in the Tight Skin (TSK) Mouse. Cancers. 2025; 17(18):2943. https://doi.org/10.3390/cancers17182943
Chicago/Turabian StyleRecouvreux, Maria Sol, Barbie Taylor-Harding, Amy C. Rowat, Beth Y. Karlan, and Sandra Orsulic. 2025. "Cancer Growth and Invasion Are Increased in the Tight Skin (TSK) Mouse" Cancers 17, no. 18: 2943. https://doi.org/10.3390/cancers17182943
APA StyleRecouvreux, M. S., Taylor-Harding, B., Rowat, A. C., Karlan, B. Y., & Orsulic, S. (2025). Cancer Growth and Invasion Are Increased in the Tight Skin (TSK) Mouse. Cancers, 17(18), 2943. https://doi.org/10.3390/cancers17182943





