Physical Activity Telecoaching in Post-Surgical NSCLC Patients: A Mixed-Methods Pilot Study Exploring Feasibility, Acceptability and Actual Usage
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
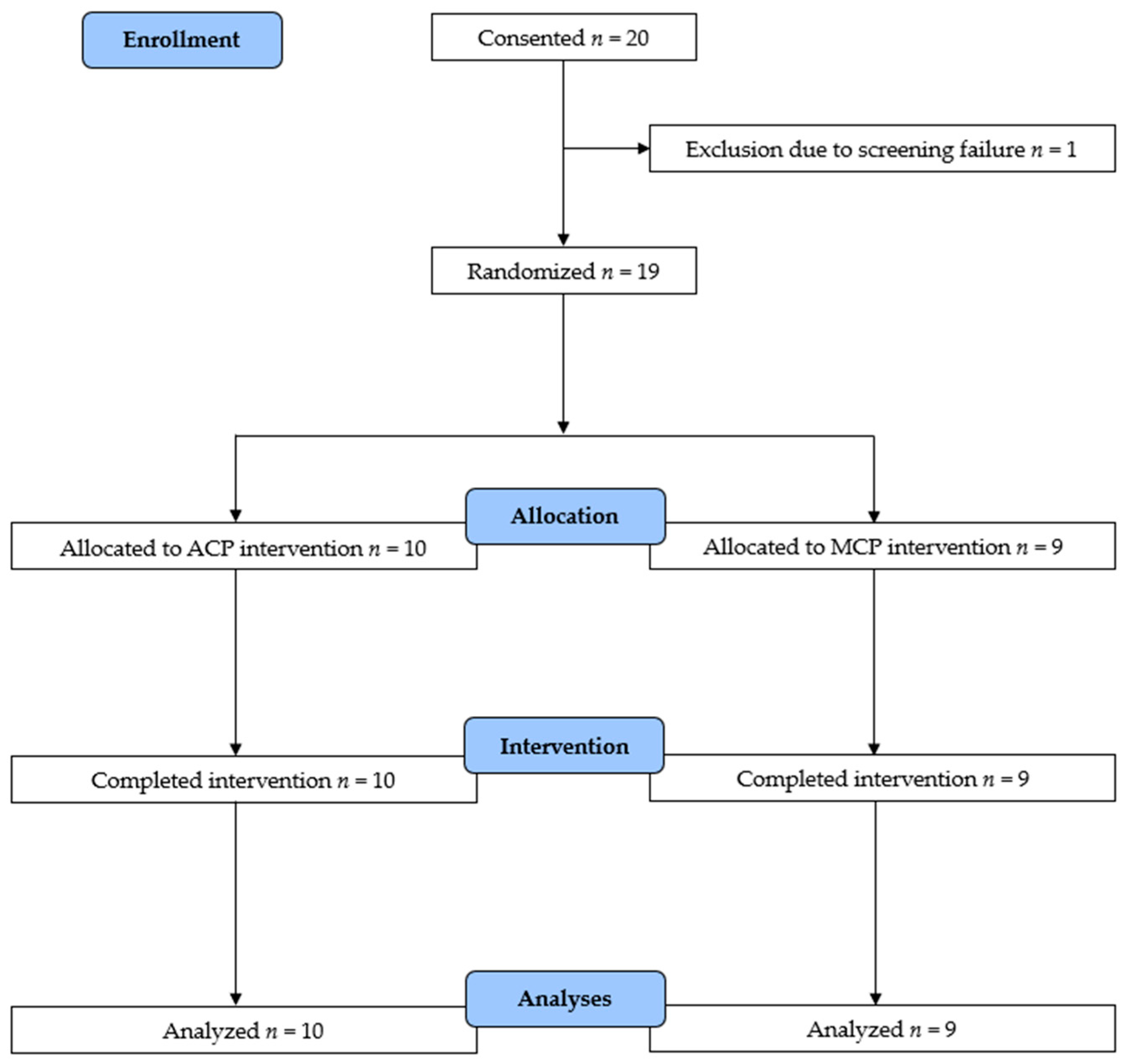
2.2. Participants
2.3. Telecoaching Intervention
2.4. Data Collection
2.4.1. Baseline Characteristics
2.4.2. Primary Outcome Measures
2.4.3. Acceptability
2.4.4. Feasibility
2.4.5. Actual Usage
2.4.6. Safety
2.5. Secondary Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Acceptability
3.1.1. Experiences/Expectations Before the Intervention
3.1.2. Experiences/Feedback at the End of the Intervention
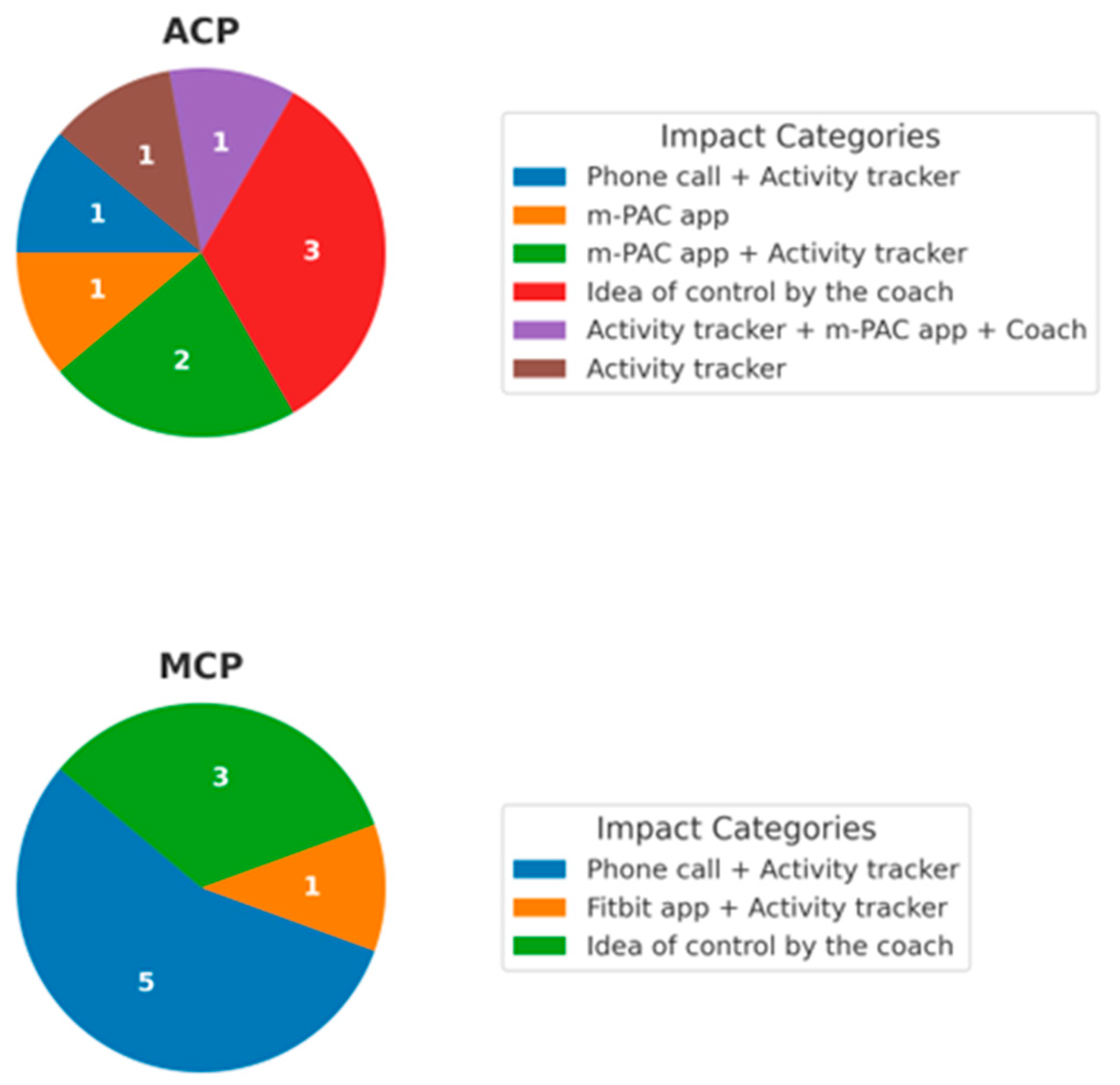
3.1.3. Impact of Different Telecoaching Components
3.2. Actual Usage
3.3. Feasibility
3.4. Safety
3.5. Secondary Outcomes
4. Discussion
4.1. Comparison of Findings to Previous Research
4.2. Strengths and Limitations
4.3. Clinical Importance and Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Xu, Y.; Liu, J.; Feng, L.; Yu, J.; Chen, D. Global burden of lung cancer in 2022 and projections to 2050: Incidence and mortality estimates from GLOBOCAN. Cancer Epidemiol. 2024, 93, 102693. [Google Scholar] [CrossRef]
- Sher, T.; Dy, G.K.; Adjei, A.A. Small cell lung cancer. Mayo Clin. Proc. 2008, 83, 355–367. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Hopstaken, J.S.; de Ruiter, J.C.; Damhuis, R.A.; de Langen, A.J.; van Diessen, J.N.; Klomp, H.M.; Klompenhouwer, E.G.; Hartemink, K.J. Stage I non-small cell lung cancer: Treatment modalities, Dutch daily practice and future perspectives. Cancer Treat. Res. Commun. 2021, 28, 100404. [Google Scholar] [CrossRef]
- Nesbitt, J.C.; Putnam, J.B., Jr.; Walsh, G.L.; Roth, J.A.; Mountain, C.F. Survival in early-stage non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 466–472. [Google Scholar] [CrossRef]
- Haesevoets, S.; Arents, E.; Cops, D.; Quadflieg, K.; Criel, M.; Ruttens, D.; Daenen, M.; Stevens, D.; Surmont, V.; Demeyer, H.; et al. The impact of lung surgery, with or without (neo-)adjuvant therapy, on physical functioning in patients with nonsmall cell lung cancer: A scoping review. Eur. Respir. Rev. 2025, 34, 240156. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Krebs, P.; Coups, E.J.; Feinstein, M.B.; Burkhalter, J.E.; Steingart, R.M.; Logue, A.; Park, B.J.; Ostroff, J.S. Health behaviors of early-stage non-small cell lung cancer survivors. J. Cancer Surviv. 2012, 6, 37–44. [Google Scholar] [CrossRef]
- Kenny, P.M.; King, M.T.; Viney, R.C.; Boyer, M.J.; Pollicino, C.A.; McLean, J.M.; Fulham, M.J.; McCaughan, B.C. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J. Clin. Oncol. 2008, 26, 233–241. [Google Scholar] [CrossRef]
- D’Silva, A.; Gardiner, P.A.; Boyle, T.; Bebb, D.G.; Johnson, S.T.; Vallance, J.K. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among lung cancer survivors: A quantile regression approach. Lung Cancer 2018, 119, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Avancini, A.; Sartori, G.; Gkountakos, A.; Casali, M.; Trestini, I.; Tregnago, D.; Bria, E.; Jones, L.W.; Milella, M.; Lanza, M.; et al. Physical Activity and Exercise in Lung Cancer Care: Will Promises Be Fulfilled? Oncologist 2020, 25, e555–e569. [Google Scholar] [CrossRef]
- Messina, G.; Tartaglia, N.; Ambrosi, A.; Porro, C.; Campanozzi, A.; Valenzano, A.; Corso, G.; Fiorelli, A.; Polito, R.; Santini, M.; et al. The Beneficial Effects of Physical Activity in Lung Cancer Prevention and/or Treatment. Life 2022, 12, 782. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. Jama 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Physical Activity Guidelines for Americans. Available online: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf (accessed on 9 July 2025).
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Cmaj 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Bade, B.C.; Thomas, D.D.; Scott, J.B.; Silvestri, G.A. Increasing physical activity and exercise in lung cancer: Reviewing safety, benefits, and application. J. Thorac. Oncol. 2015, 10, 861–871. [Google Scholar] [CrossRef]
- Hung, R.; Krebs, P.; Coups, E.J.; Feinstein, M.B.; Park, B.J.; Burkhalter, J.; Ostroff, J.S. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. J. Pain. Symptom Manag. 2011, 41, 426–435. [Google Scholar] [CrossRef]
- Coups, E.J.; Park, B.J.; Feinstein, M.B.; Steingart, R.M.; Egleston, B.L.; Wilson, D.J.; Ostroff, J.S. Physical activity among lung cancer survivors: Changes across the cancer trajectory and associations with quality of life. Cancer Epidemiol. Biomark. Prev. 2009, 18, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.A.; Cheville, A.L.; Liu, H.; Novotny, P.J.; Wampfler, J.A.; Garces, Y.I.; Clark, M.M.; Yang, P. Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health Qual. Life Outcomes 2016, 14, 66. [Google Scholar] [CrossRef]
- Rabbani, S.A.; Patni, M.A.; El-Tanani, M.; Rangraze, I.R.; Wali, A.F.; Babiker, R.; Satyam, S.M.; El-Tanani, Y.; Almetwally, A.A.M.S. Impact of Lifestyle Modifications on Cancer Mortality: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 307. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Vardy, J.L.; O’Callaghan, C.J.; Gill, S.; Friedenreich, C.M.; Wong, R.K.S.; Dhillon, H.M.; Coyle, V.; Chua, N.S.; Jonker, D.J.; et al. Structured Exercise after Adjuvant Chemotherapy for Colon Cancer. N. Engl. J. Med. 2025, 393, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Cavalheri, V.; Tahirah, F.; Nonoyama, M.; Jenkins, S.; Hill, K. Exercise training for people following lung resection for non-small cell lung cancer-a Cochrane systematic review. Cancer Treat. Rev. 2014, 40, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Troosters, T.; Blondeel, A.; Rodrigues, F.M.; Janssens, W.; Demeyer, H. Strategies to Increase Physical Activity in Chronic Respiratory Diseases. Clin. Chest Med. 2019, 40, 397–404. [Google Scholar] [CrossRef]
- Sallis, J.F.; Cervero, R.B.; Ascher, W.; Henderson, K.A.; Kraft, M.K.; Kerr, J. An ecological approach to creating active living communities. Annu. Rev. Public. Health 2006, 27, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Wood, C.E.; Johnston, M.; Abraham, C.; Francis, J.J.; Hardeman, W. Behaviour change techniques: The development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technol. Assess. 2015, 19, 1–188. [Google Scholar] [CrossRef]
- Pimenta, S.; Hansen, H.; Demeyer, H.; Slevin, P.; Cruz, J. Role of digital health in pulmonary rehabilitation and beyond: Shaping the future. ERJ Open Res. 2023, 9, 00212-2022. [Google Scholar] [CrossRef] [PubMed]
- Diciolla, N.S.; Yuste-Sánchez, M.J.; Torres-Lacomba, M.; Paixão, C.; Marques, A. Physical activity coaching in people with chronic obstructive pulmonary disease: A systematic literature review with meta-analysis. Ann. Behav. Med. 2025, 59, kaaf044. [Google Scholar] [CrossRef]
- Hume, E.; Muse, H.; Wallace, K.; Wilkinson, M.; Marshall, K.H.; Nair, A.; Clark, S.; Vogiatzis, I. Feasibility and acceptability of a physical activity behavioural modification tele-coaching intervention in lung transplant recipients. Chronic. Respir. Dis. 2022, 19, 14799731221116588. [Google Scholar] [CrossRef]
- Breuls, S.; Zlamalova, T.; Raisova, K.; Blondeel, A.; Wuyts, M.; Dvoracek, M.; Zurkova, M.; Yserbyt, J.; Janssens, W.; Wuyts, W.; et al. Physical activity coaching in patients with interstitial lung diseases: A randomized controlled trial. Chronic Respir. Dis. 2024, 21, 14799731241235231. [Google Scholar] [CrossRef]
- Chen, H.M.; Tsai, C.M.; Wu, Y.C.; Lin, K.C.; Lin, C.C. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br. J. Cancer 2015, 112, 438–445. [Google Scholar] [CrossRef]
- Chang, N.W.; Lin, K.C.; Lee, S.C.; Chan, J.Y.-H.; Lee, Y.H.; Wang, K.Y. Effects of an early postoperative walking exercise programme on health status in lung cancer patients recovering from lung lobectomy. J. Clin. Nurs. 2014, 23, 3391–3402. [Google Scholar] [CrossRef]
- Ha, D.; Ries, A.L.; Mazzone, P.J.; Lippman, S.M.; Fuster, M.M. Exercise capacity and cancer-specific quality of life following curative intent treatment of stage I-IIIA lung cancer. Support. Care Cancer 2018, 26, 2459–2469. [Google Scholar] [CrossRef]
- Gresham, G.; Hendifar, A.E.; Spiegel, B.; Neeman, E.; Tuli, R.; Rimel, B.J.; Figlin, R.A.; Meinert, C.L.; Piantadosi, S.; Shinde, A.M. Wearable activity monitors to assess performance status and predict clinical outcomes in advanced cancer patients. NPJ Digit. Med. 2018, 1, 27. [Google Scholar] [CrossRef]
- Loeckx, M.; Rabinovich, R.A.; Demeyer, H.; Louvaris, Z.; Tanner, R.; Rubio, N.; Frei, A.; De Jong, C.; Gimeno-Santos, E.; Rodrigues, F.M.; et al. Smartphone-Based Physical Activity Telecoaching in Chronic Obstructive Pulmonary Disease: Mixed-Methods Study on Patient Experiences and Lessons for Implementation. JMIR Mhealth Uhealth 2018, 6, e200. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, H.; Mohan, D.; Burtin, C.; Vaes, A.W.; Heasley, M.; Bowler, R.P.; Casaburi, R.; Cooper, C.B.; Corriol-Rohou, S.; Frei, A.; et al. Objectively Measured Physical Activity in Patients with COPD: Recommendations from an International Task Force on Physical Activity. Chronic Obstr. Pulm. Dis. 2021, 8, 528–550. [Google Scholar] [CrossRef]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Troosters, T.; Gosselink, R.; Decramer, M. Six minute walking distance in healthy elderly subjects. Eur. Respir. J. 1999, 14, 270–274. [Google Scholar] [CrossRef]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.M.A.; Garssen, B.; Bonke, B.; De Haes, J.C.J.M. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.; Aaronson, N.K.; Ahmedzai, S.; Kaasa, S.; Sullivan, M. The EORTC QLQ-LC13: A modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur. J. Cancer 1994, 30A, 635–642. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Vorrink, S.N.; Kort, H.S.; Troosters, T.; Zanen, P.; Lammers, J.-W.J. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur. Respir. J. 2016, 48, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Donnachie, C.; Wyke, S.; Mutrie, N.; Hunt, K. ‘It’s like a personal motivator that you carried around wi’ you’: Utilising self-determination theory to understand men’s experiences of using pedometers to increase physical activity in a weight management programme. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; van der Pols, J.C.; Dobson, A.J. Regression to the mean: What it is and how to deal with it. Int. J. Epidemiol. 2005, 34, 215–220. [Google Scholar] [CrossRef]
- Demeyer, H.; Louvaris, Z.; Frei, A.; Rabinovich, R.A.; de Jong, C.; Gimeno-Santos, E.; Loeckx, M.; Buttery, S.C.; Rubio, N.; Van der Molen, T.; et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: A multicentre randomised controlled trial. Thorax 2017, 72, 415–423. [Google Scholar] [CrossRef]
- Number of Spartphone Mobile Network Subscriptions Worldwide from 2016 to 2022, with Forecasts from 2023 to 2028. Available online: https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/ (accessed on 9 July 2025).
| Variable | All Participants | MCP Group n = 9 | ACP Group n = 10 | p-Value |
|---|---|---|---|---|
| Sex (male), n (%) | 12 (63) | 5 (55) | 7 (70) | 0.43 |
| Age (years) | 68 ± 6 | 69 ± 7 | 66 ± 5 | 0.18 |
| BMI (kg/m2) | 25 ± 5 | 24 ± 3 | 26 ± 6 | 0.45 |
| Timing since surgery (days) | 145 ± 50 | 165 ± 58 | 127 ± 35 | 0.21 |
| 6MWD (m) | 548 ± 72 | 513 ± 51 | 579 ± 76 | 0.05 |
| 6MWD (% pred) | 95 ± 11 | 91 ± 8 | 98 ± 13 | 0.16 |
| MFI-20 (20–100) | 50 (33;57) | 39 (31;52) | 56 (48;62) | 0.04 |
| HADS Anxiety score (0–21) | 5.0 (3.0;8.25) | 5.0 (0.75;6.75) | 6.0 (3.75;9.25) | 0.32 |
| HADS Depression score (0–21) | 3.5 (1;7) | 3.5 (1.5;8.0) | 4.0 (1.0;7.0) | 0.97 |
| mMRC score (0–4) | 1 (1;2) | 1 (1;2) | 1 (0;1.25) | 0.24 |
| EORTC-QLQ-LC13 score (0–100) | 13.9 (8.3;25) | 11.1 (6.9;25.0) | 19.4 (11.8;23.6) | 0.45 |
| Step count (steps/day) | 7820 ± 2799 | 9070 ± 2520 | 6696 ± 2656 | 0.04 |
| Moving time (min/day) | 95.2 ± 29.7 | 108.4 ± 27.4 | 83.2 ± 27.6 | 0.04 |
| Importance PA (0–10) | 8.0 (8.0;10.0) | 9.0 (8.0;10.0) | 8.0 (7.0;9.3) | 0.24 |
| Motivation PA (0–10) | 8.0 (8.0;9.0) | 8.0 (8.0;8.5) | 8.0 (7.8;9.0) | 0.78 |
| Confidence PA (0–10) | 7.0 (7.0;8.0) | 7.0 (6.0;8.0) | 7.0 (7.0;8.3) | 0.66 |
| Season of intervention start | ||||
| Spring, n (%) | 3 (16) | 1 (11) | 2 (20) | 0.80 |
| Summer, n (%) | 7 (37) | 4 (44) | 3 (30) | |
| Autumn, n (%) | 5 (26) | 3 (33) | 2 (20) | |
| Winter, n (%) | 4 (21) | 1 (11) | 3 (30) |
| Component | MCP Group | ACP Group | p-Value |
|---|---|---|---|
| Wearing activity tracker | 10 (9;10) | 10 (8;10) | >0.99 |
| Feedback activity tracker | 9 (8;10) | 10 (9;10) | 0.40 |
| Phone calls with coach | 9 (9;10) | 10 (9;10) | 0.39 |
| Daily activity goal | 9 (7;10) | ||
| Daily feedback | 9 (6;10) | ||
| Performance graphs | 10 (8;10) | ||
| Tip of the week | 7.5 (6;9) |
| Overall | MCP Group | ACP Group | |||||
|---|---|---|---|---|---|---|---|
| Variable | Within-Group Difference | p-Value | Δ | p-Value | Δ | p-Value | p-Value (Between-Group Δ) |
| Step count (steps/day) | −253 ± 2426 | 0.33 | −1266 ± 3054 | 0.44 | 759 ± 933 | 0.04 | 0.08 |
| Moving time (min/day) | −1.7 ± 26.6 | 0.33 | −12.9 ± 33.3 | 0.52 | 9.4 ± 11.0 | 0.05 | 0.06 |
| 6MWD (m) | 15 ± 42 | 0.03 | 29 ± 37 | 0.05 | 3 ± 44 | 0.31 | 0.18 |
| MFI-20 | −6 (−14;0) | 0.10 | 0 (−6;18) | 0.40 | −14 (−17;−8) | 0.005 | < 0.001 |
| HADS Anxiety score | 1 (−1;2) | 0.39 | 1 (−2;3) | 0.57 | 1 (−1;3) | 0.57 | 0.90 |
| HADS Depression score | −1 (−2;1) | 0.36 | −1 (−3;0) | 0.18 | −1 (−2;2) | 0.91 | 0.52 |
| mMRC score | 0 (−1;0) | 0.19 | 0 (−1;0) | 0.18 | 0 (−1;0) | 0.48 | 0.84 |
| EORTC-QLQ-LC13 score | −3 (−6;0) | 0.05 | −3 (−3;1) | 0.33 | −4 (−9;1) | 0.09 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arents, E.; Haesevoets, S.; Hermans, F.; Quadflieg, K.; Cops, D.; Criel, M.; Ruttens, D.; Surmont, V.; Salhi, B.; Derom, E.; et al. Physical Activity Telecoaching in Post-Surgical NSCLC Patients: A Mixed-Methods Pilot Study Exploring Feasibility, Acceptability and Actual Usage. Cancers 2025, 17, 2886. https://doi.org/10.3390/cancers17172886
Arents E, Haesevoets S, Hermans F, Quadflieg K, Cops D, Criel M, Ruttens D, Surmont V, Salhi B, Derom E, et al. Physical Activity Telecoaching in Post-Surgical NSCLC Patients: A Mixed-Methods Pilot Study Exploring Feasibility, Acceptability and Actual Usage. Cancers. 2025; 17(17):2886. https://doi.org/10.3390/cancers17172886
Chicago/Turabian StyleArents, Eva, Sarah Haesevoets, Fien Hermans, Kirsten Quadflieg, Dries Cops, Maarten Criel, David Ruttens, Veerle Surmont, Bihiyga Salhi, Eric Derom, and et al. 2025. "Physical Activity Telecoaching in Post-Surgical NSCLC Patients: A Mixed-Methods Pilot Study Exploring Feasibility, Acceptability and Actual Usage" Cancers 17, no. 17: 2886. https://doi.org/10.3390/cancers17172886
APA StyleArents, E., Haesevoets, S., Hermans, F., Quadflieg, K., Cops, D., Criel, M., Ruttens, D., Surmont, V., Salhi, B., Derom, E., Troosters, T., Stevens, D., Burtin, C., & Demeyer, H. (2025). Physical Activity Telecoaching in Post-Surgical NSCLC Patients: A Mixed-Methods Pilot Study Exploring Feasibility, Acceptability and Actual Usage. Cancers, 17(17), 2886. https://doi.org/10.3390/cancers17172886





