Long-Term Particulate Matter (PM) Exposure Promotes Non-Small-Cell Lung Cancer (NSCLC) Angiogenesis Through Up-Regulation of VEGFA
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
2.4. Western Blot Analysis
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) Assay
2.6. Tube Formation Assay
2.7. EPCs Migration Assay
2.8. Immunohistochemistry (IHC) Staining
2.9. Bioinformatics and the GEO Dataset Analysis
2.10. Statistical Analysis
3. Results
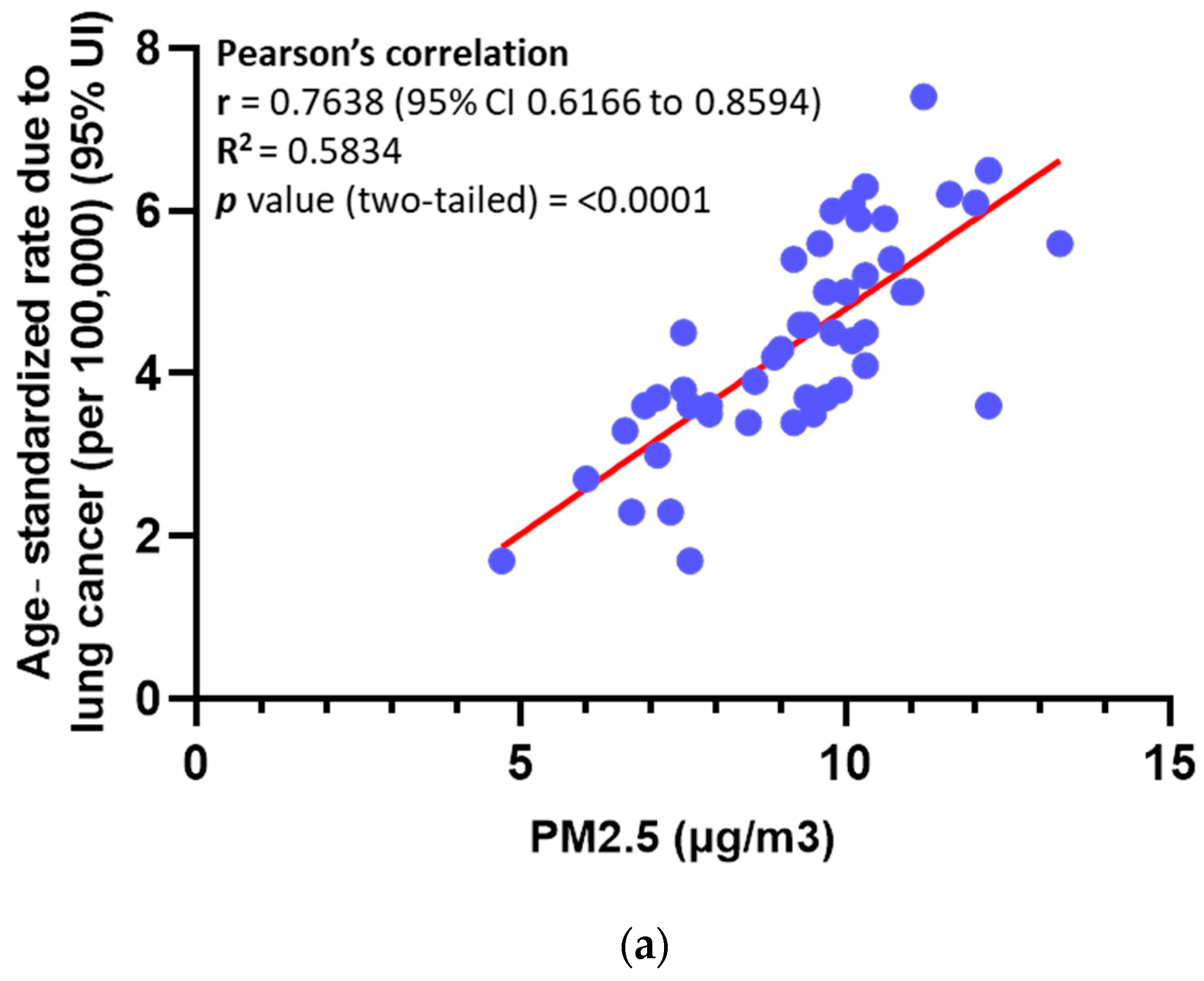
3.1. Elevated Levels of PM in Ambient Air Are Significantly Associated with an Increased Mortality Rate Among Lung Cancer Patients
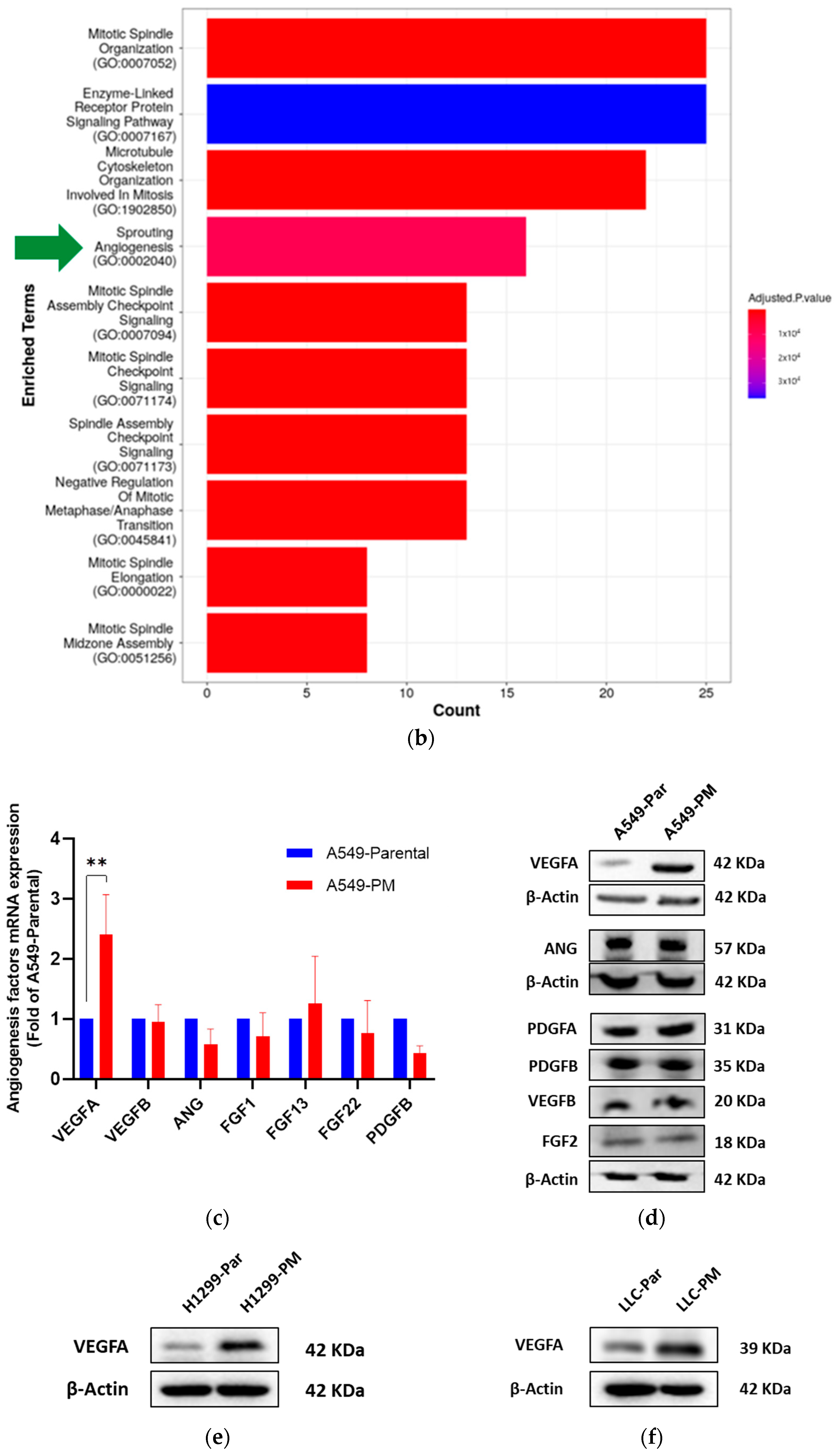
3.2. PM Promotes Angiogenesis in NSCLC
3.3. PM Long-Term Exposure Increases Vascular Endothelial Growth Factor A (VEGFA) Expression
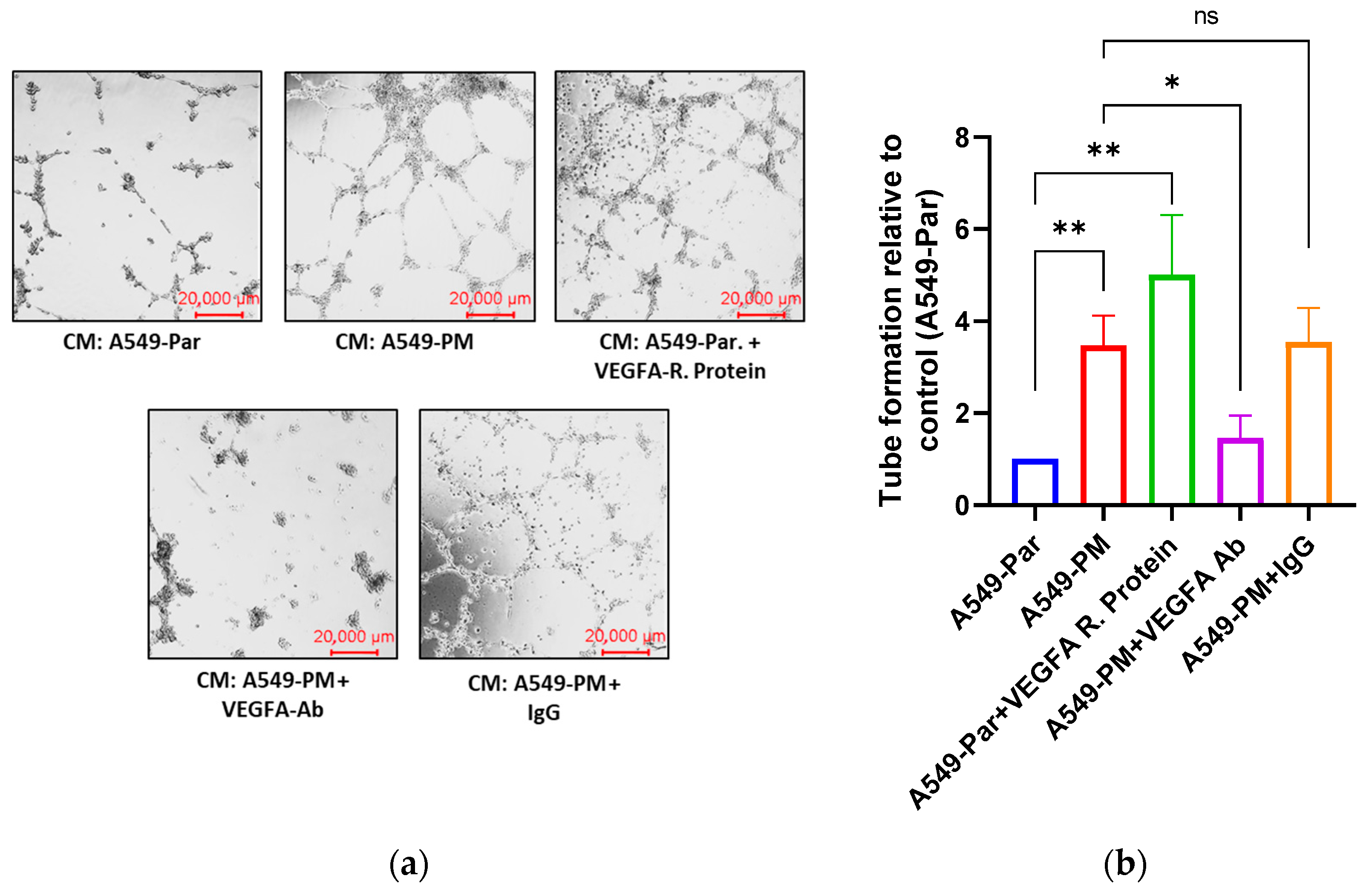
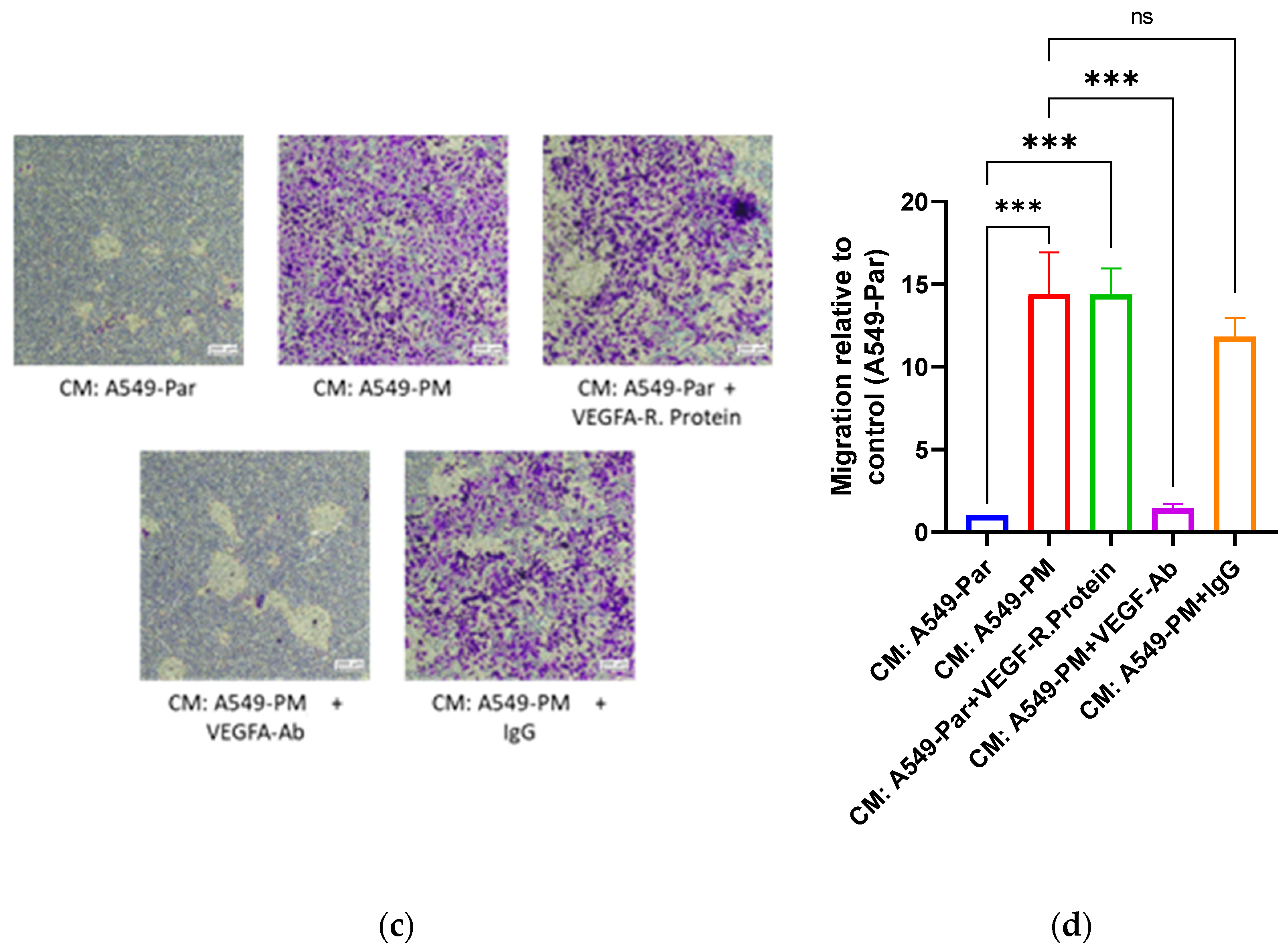
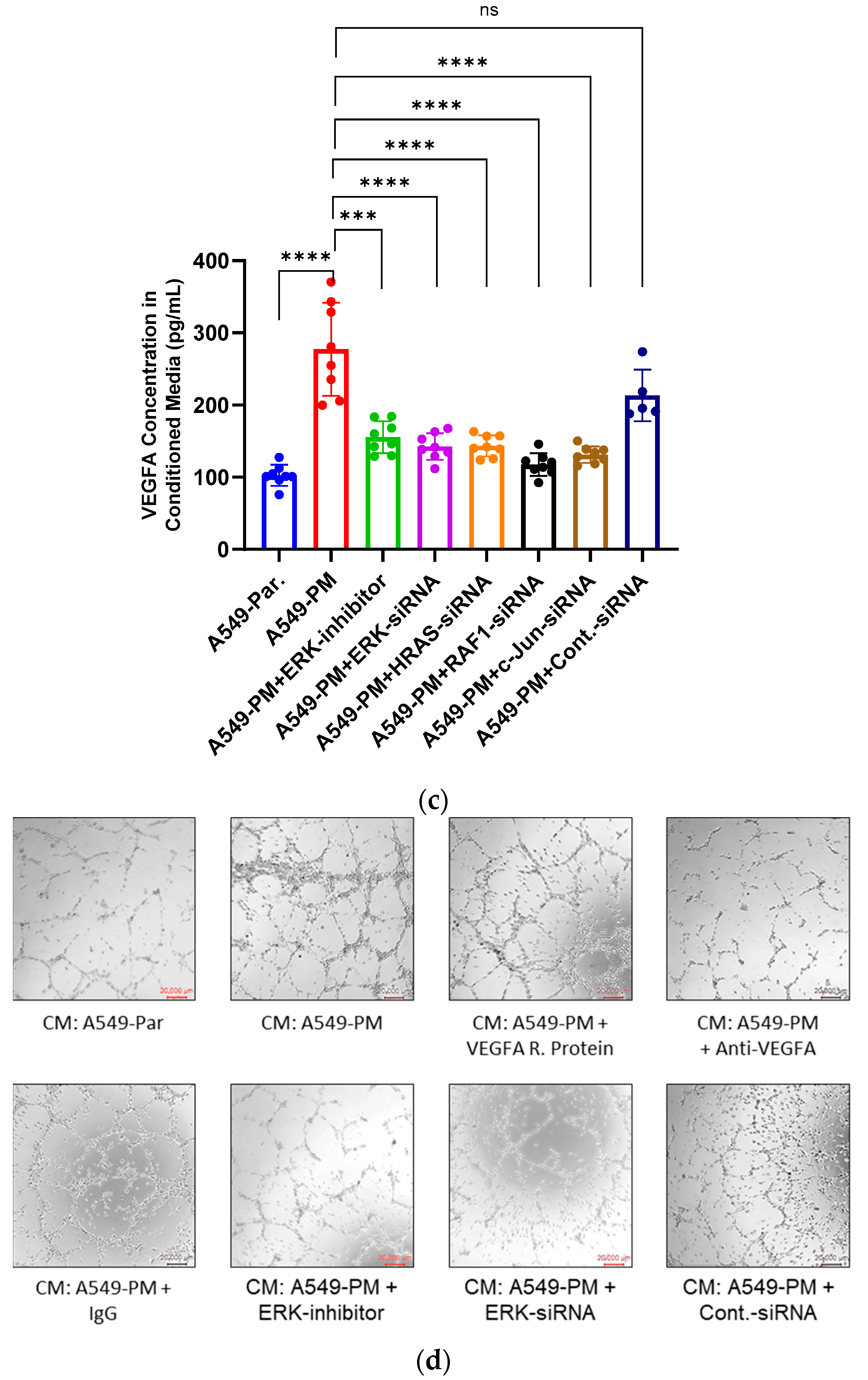
3.4. PM Exposure Promotes Angiogenesis Through Up-Regulation of VEGFA In Vitro
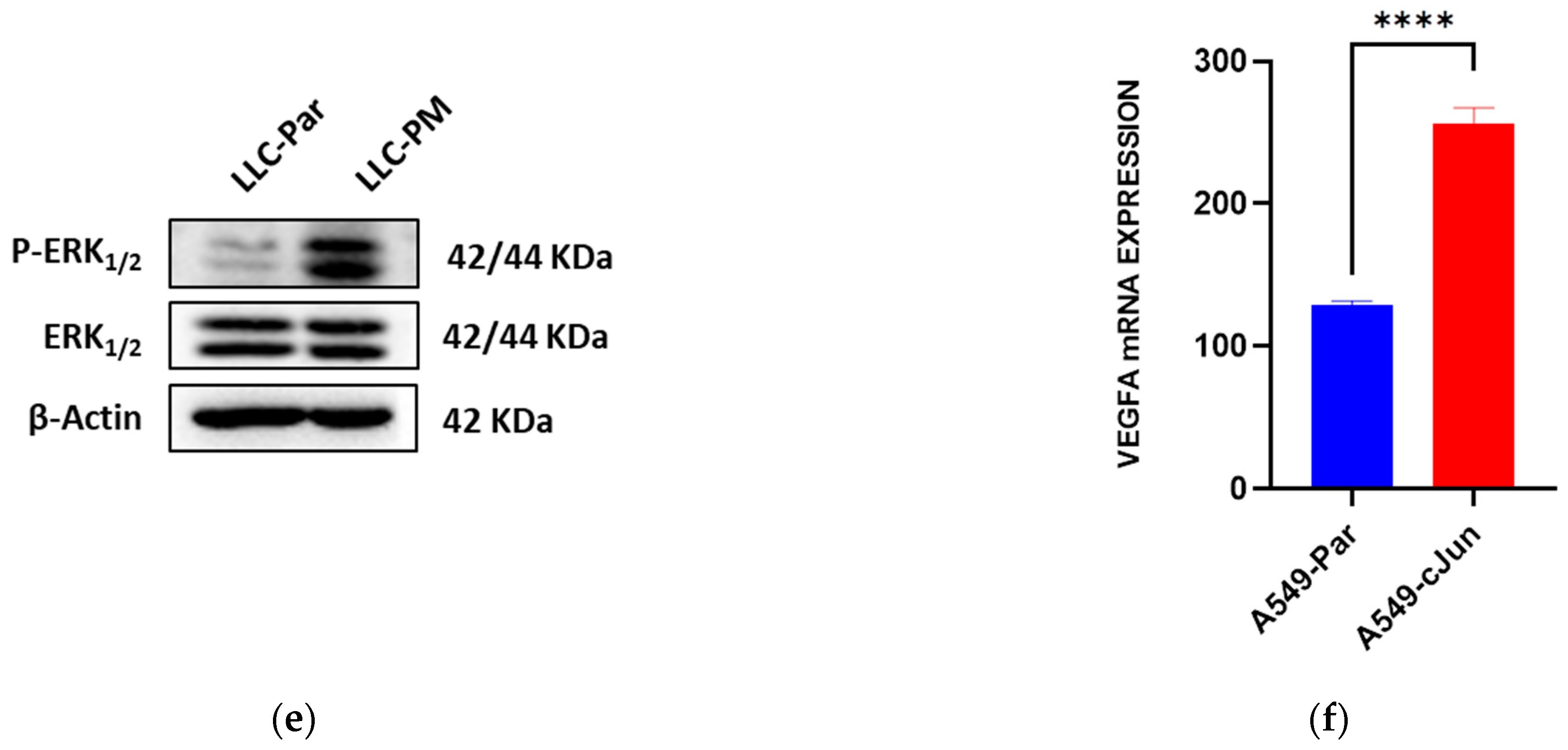
3.5. PM Promotes VEGFA Overexpression via the MAPK Pathway
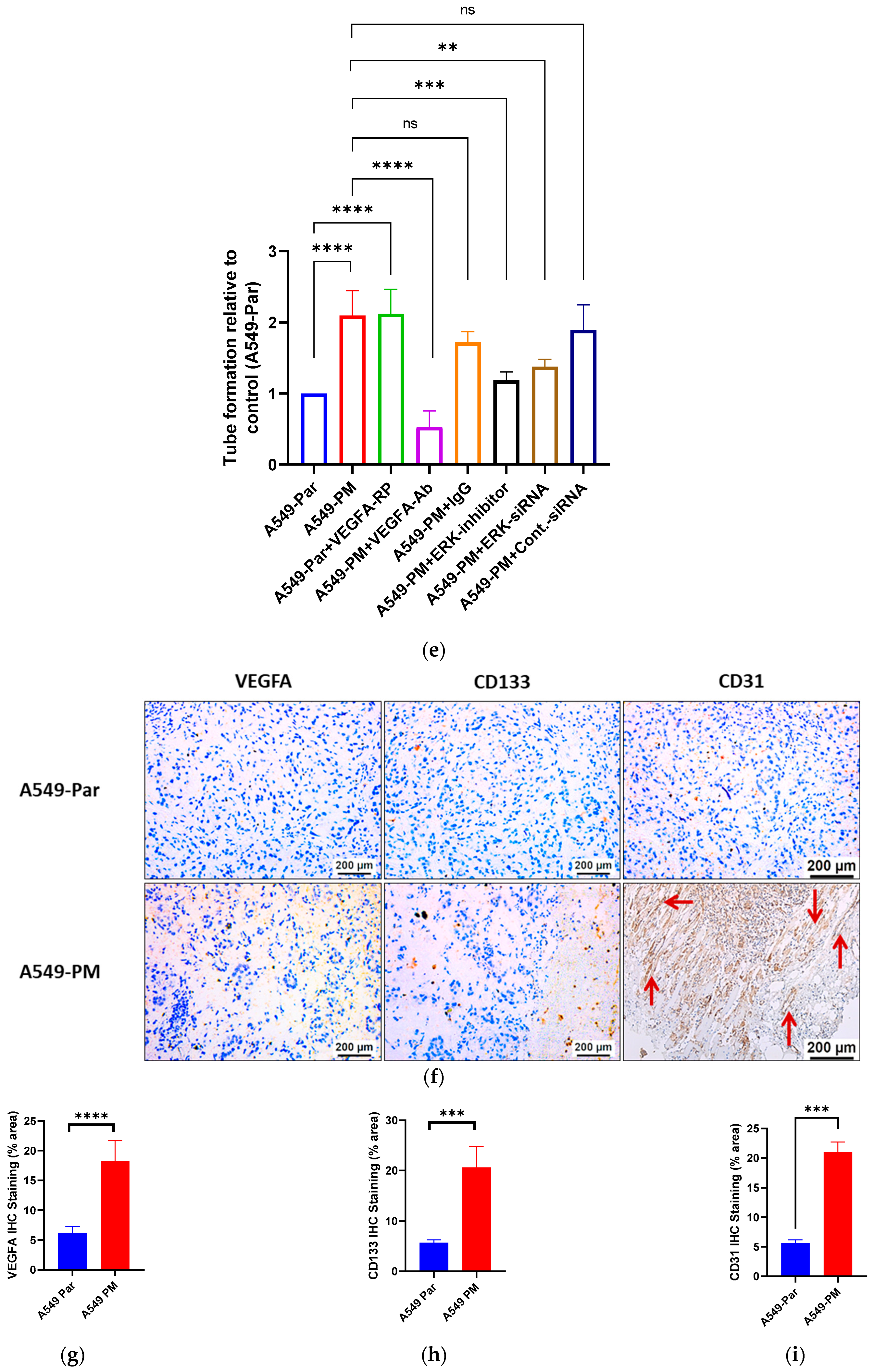
3.6. Inhibition of the MAPK Pathway Blocks PM-Enhanced VEGFA Expression and Angiogenesis Properties
3.7. PM Promotes Angiogenesis in an In Vivo Study via VEGFA Overexpression
4. Discussion
4.1. Elevated PM Levels and Lung Cancer Mortality
4.2. PM-Induced Angiogenesis in NSCLC
4.3. Molecular Mechanisms Underlying VEGFA Overexpression
4.4. In Vivo Validation of PM-Induced Angiogenesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kratzer, T.B.; Bandi, P.; Freedman, N.D.; Smith, R.A.; Travis, W.D.; Jemal, A.; Siegel, R.L. Lung cancer statistics, 2023. Cancer 2024, 130, 1330–1348. [Google Scholar] [CrossRef]
- Adjei, A.A. Lung cancer worldwide. J. Thorac. Oncol. 2019, 14, 956. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol./Współczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Zheng, M. Classification and pathology of lung cancer. Surg. Oncol. Clin. 2016, 25, 447–468. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, C.H.; Jeong, Y.J.; Chung, D.H.; Goo, J.M.; Park, C.M.; Austin, J.H. IASLC/ATS/ERS International Multidisciplinary Classification of Lung Adenocarcinoma: Novel concepts and radiologic implications. J. Thorac. Imaging 2012, 27, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Canales, J.; Parra-Cuentas, E.; Wistuba, I.I. Diagnosis and molecular classification of lung cancer. In Lung Cancer: Treatment and Research; Springer: Cham, Switzerland, 2016; pp. 25–46. [Google Scholar]
- Cheng, E.S.; Weber, M.; Steinberg, J.; Yu, X.Q. Lung cancer risk in never-smokers: An overview of environmental and genetic factors. Chin. J. Cancer Res. 2021, 33, 548. [Google Scholar] [CrossRef] [PubMed]
- Rivera, G.A.; Wakelee, H. Lung cancer in never smokers. In Lung Cancer and Personalized Medicine: Current Knowledge and Therapies; Springer: Cham, Switzerland, 2016; pp. 43–57. [Google Scholar]
- Blechter, B.; Wong, J.Y.; Chien, L.H.; Shiraishi, K.; Shu, X.O.; Cai, Q.; Zheng, W.; Ji, B.T.; Hu, W.; Rahman, M.L.; et al. Age at lung cancer diagnosis in females versus males who never smoke by race and ethnicity. Br. J. Cancer 2024, 130, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Apte, J.S.; Marshall, J.D.; Cohen, A.J.; Brauer, M. Addressing global mortality from ambient PM2.5. Environ. Sci. Technol. 2015, 49, 8057–8066. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, D.; Lin, G.; Liu, K.; Wang, Q. An ecological analysis of PM2.5 concentrations and lung cancer mortality rates in China. BMJ Open 2015, 5, e009452. [Google Scholar] [CrossRef]
- Shisong, C.; Wenji, Z.; Hongliang, G.; Deyong, H.; You, M.; Wenhui, Z.; Shanshan, L. Comparison of remotely sensed PM2.5 concentrations between developed and developing countries: Results from the US, Europe, China, and India. J. Clean. Prod. 2018, 182, 672–681. [Google Scholar] [CrossRef]
- Zhang, T.; Mao, W.; Gao, J.; Song, X.; Li, L.; Sun, X.; Ding, X.; Li, J.; Zhai, Y.; Ma, W.; et al. The effects of PM2.5 on lung cancer-related mortality in different regions and races: A systematic review and meta-analysis of cohort studies. Air Qual. Atmos. Health 2022, 15, 1523–1532. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Yan, Y.; Al-Aly, Z. Burden of cause-specific mortality associated with PM2.5 air pollution in the United States. JAMA Netw. Open 2019, 2, e1915834. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, Y.; Liu, H.L.; Lai, W.Q.; Shi, Y.; Liu, X.H.; Xi, Z.G.; Lin, B.C. Exposure to PM2.5 enhances the PI3K/AKT signaling and malignancy of ERα expression-dependent non-small cell lung carcinoma. Biomed. Environ. Sci 2021, 34, 319–323. [Google Scholar]
- Yang, L.; Wang, N.; Liu, S.; Xiao, Q.; Geng, G.; Zhang, X.; Li, H.; Zheng, Y.; Guo, F.; Li, Q.; et al. The PM2.5 concentration reduction improves survival rate of lung cancer in Beijing. Sci. Total Environ. 2023, 858, 159857. [Google Scholar] [CrossRef]
- Li, T.; Hu, R.; Chen, Z.; Li, Q.; Huang, S.; Zhu, Z.; Zhou, L.F. Fine particulate matter (PM2.5): The culprit for chronic lung diseases in China. Chronic Dis. Transl. Med. 2018, 4, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.E.; Considine, E.M.; Maestas, M.M.; Li, G. Daily PM2.5 concentration estimates by county, ZIP code, and census tract in 11 western states 2008–2018. Sci. Data 2021, 8, 112. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69. [Google Scholar]
- Wang, F.; Chen, T.; Chang, Q.; Kao, Y.W.; Li, J.; Chen, M.; Li, Y.; Shia, B.C. Respiratory diseases are positively associated with PM2.5 concentrations in different areas of Taiwan. PLoS ONE 2021, 16, e0249694. [Google Scholar] [CrossRef]
- Zeng, F.; Pang, G.; Hu, L.; Sun, Y.; Peng, W.; Chen, Y.; Xu, D.; Xia, Q.; Zhao, L.; Li, Y.; et al. Subway Fine Particles (PM2.5)-Induced Pro-Inflammatory Response Triggers Airway Epithelial Barrier Damage Through the TLRs/NF-κB-Dependent Pathway In Vitro. Environ. Toxicol. 2024, 39, 5296–5308. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Yin, H.; McDuffie, E.E.; Martin, R.V.; Brauer, M. Global health costs of ambient PM2.5 from combustion sources: A modelling study supporting air pollution control strategies. Lancet Planet. Health 2024, 8, e476–e488. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, R.; Beeraka, N.M.; Mahesh, P.A.; Xue, N.; Lu, P.; Bai, W.; Mao, Z.; Pr, H.V.; Bulygin, K.V.; et al. Correlation of time trends of air pollutants, greenspaces and tracheal, bronchus and lung cancer incidence and mortality among the adults in United States. Front. Oncol. 2024, 14, 1398679. [Google Scholar] [CrossRef]
- Kosanpipat, B.; Wongwut, T.; Norrasan, N.; Watthanawongsa, P.; Phinyo, P.; Saeteng, S.; Siwachat, S.; Chewaskulyong, B.; Tantraworasin, A. Impact of PM2.5 exposure on mortality and tumor recurrence in resectable non-small cell lung carcinoma. Sci. Rep. 2024, 14, 24660. [Google Scholar] [CrossRef]
- Ramamoorthy, T.; Nath, A.; Singh, S.; Mathew, S.; Pant, A.; Sheela, S.; Kaur, G.; Sathishkumar, K.; Mathur, P. Assessing the global impact of ambient air pollution on cancer incidence and mortality: A comprehensive meta-analysis. JCO Glob. Oncol. 2024, 10, e2300427. [Google Scholar] [CrossRef]
- Yu, P.; Xu, R.; Li, S.; Coelho, M.S.; Saldiva, P.H.; Sim, M.R.; Abramson, M.J.; Guo, Y. Associations between long-term exposure to PM2.5 and site-specific cancer mortality: A nationwide study in Brazil between 2010 and 2018. Environ. Pollut. 2022, 302, 119070. [Google Scholar] [CrossRef]
- Gualtieri, M.; Longhin, E.; Mattioli, M.; Mantecca, P.; Tinaglia, V.; Mangano, E.; Proverbio, M.C.; Bestetti, G.; Camatini, M.; Battaglia, C. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicol. Lett. 2012, 209, 136–145. [Google Scholar] [CrossRef]
- Santibáñez-Andrade, M.; Quezada-Maldonado, E.M.; Rivera-Pineda, A.; Chirino, Y.I.; García-Cuellar, C.M.; Sánchez-Pérez, Y. The road to malignant cell transformation after particulate matter exposure: From oxidative stress to genotoxicity. Int. J. Mol. Sci. 2023, 24, 1782. [Google Scholar] [CrossRef]
- Mezheyeuski, A.; Bergsland, C.H.; Backman, M.; Djureinovic, D.; Sjöblom, T.; Bruun, J.; Micke, P. Multispectral imaging for quantitative and compartment-specific immune infiltrates reveals distinct immune profiles that classify lung cancer patients. J. Pathol. 2018, 244, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.M.; Choi, J.S.; Lee, J.Y.; Kim, S.; Bae, W.Y.; Jang, Y.W.; Kim, J.E.; Lee, S.H.; Nam, S.; Jeong, J.W. Mild exposure to fine particulate matter promotes angiogenesis in non-small cell lung carcinoma. Environ. Pollut. 2023, 329, 121715. [Google Scholar] [CrossRef] [PubMed]
- Quek, Y.W.; Kang, Y.T.; Huang, H.C.; Chang, H.Y.; Huang, I.C.; Lue, K.H.; Ko, J.L. PM2.5 induces lung inflammation through ANGPTL4. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2024, 829, 111887. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis. Annu. Rev. Med. 2006, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Wang, T.H.; Huang, K.Y.; Chen, C.C.; Chang, Y.H.; Chen, H.Y.; Hsueh, C.; Liu, Y.T.; Yang, S.C.; Yang, P.C.; Chen, C.Y. PM2.5 promotes lung cancer progression through activation of the AhR-TMPRSS2-IL18 pathway. EMBO Mol. Med. 2023, 15, e17014. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Ho, T.L.; Chao, C.C.; He, X.Y.; Chen, P.C.; Cheng, F.J.; Huang, W.C.; Huang, C.L.; Liu, P.I.; Tang, C.H. Particulate matter facilitates amphiregulin-dependent lung cancer proliferation through glutamine metabolism. Int. J. Biol. Sci. 2024, 20, 3126. [Google Scholar] [CrossRef]
- Mukai, N.; Akahori, T.; Komaki, M.; Li, Q.; Kanayasu-Toyoda, T.; Ishii-Watabe, A.; Kobayashi, A.; Yamaguchi, T.; Abe, M.; Amagasa, T.; et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp. Cell Res. 2008, 314, 430–440. [Google Scholar] [CrossRef]
- Brown, N.S.; Bicknell, R. Cell migration and the boyden chamber. In Metastasis Research Protocols: Volume II: Analysis of Cell Behavior In Vitro and In Vivo; Humana Press: Totowa, NJ, USA, 2001; pp. 47–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yang, J.; He, R.; Wang, D.; Huang, Y.; Zhao, G.; Ning, M.; Zeng, T.; Li, G. Integrated multi-omics analysis for lung adenocarcinoma in Xuanwei, China. Aging 2023, 15, 14263. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Q.; Pan, G.; Meng, Q.H.; Tuo, X.; Cai, X.; Chen, Z.; Li, Y.; Huang, T.; Duan, X.; et al. Integrated analysis of genome-wide copy number alterations and gene expression profiling of lung cancer in Xuanwei, China. PLoS ONE 2017, 12, e0169098. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Li, J.; Wang, N.; Yu, H.; Han, S.; Xiang, M.; Zhang, H.; Zeng, D.; Jiang, J.; Ma, H. SIRPG promotes lung squamous cell carcinoma pathogenesis via M1 macrophages: A multi-omics study integrating data and Mendelian randomization. Front. Oncol. 2024, 14. [Google Scholar] [CrossRef]
- Huang, F.; Pan, B.; Wu, J.; Chen, E.; Chen, L. Relationship between exposure to PM2.5 and lung cancer incidence and mortality: A meta-analysis. Oncotarget 2017, 8, 43322. [Google Scholar] [CrossRef] [PubMed]
- Vinikoor-Imler, L.C.; Davis, J.A.; Luben, T.J. An ecologic analysis of county-level PM2.5 concentrations and lung cancer incidence and mortality. Int. J. Environ. Res. Public Health 2011, 8, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Mengersen, K.; Tong, S.; Kimlin, M.; Zhou, M.; Liu, Y.; Hu, W. County-level variation in the long-term association between PM2.5 and lung cancer mortality in China. Sci. Total Environ. 2020, 738, 140195. [Google Scholar] [CrossRef]
- Badyda, A.J.; Grellier, J.; Dąbrowiecki, P. Ambient PM2.5 exposure and mortality due to lung cancer and cardiopulmonary diseases in Polish cities. In Respiratory Treatment and Prevention; Springer: Cham, Switzerland, 2017; pp. 9–17. [Google Scholar]
- Yang, B.; Chen, D.; Zhao, H.; Xiao, C. The effects for PM2.5 exposure on non-small-cell lung cancer induced motility and proliferation. Springerplus 2016, 5, 2059. [Google Scholar] [CrossRef]
- Fan, R.; Xu, L.; Cui, B.; Li, D.; Sun, X.; Qi, Y.; Rao, J.; Wang, K.; Wang, C.; Zhao, K.; et al. Genomic characterization revealed PM2.5-associated mutational signatures in lung cancer including activation of APOBEC3B. Environ. Sci. Technol. 2023, 57, 6854–6864. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, F.; Rui, W.; Long, F.; Wang, L.; Feng, Z.; Chen, D.; Ding, W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. Vitr. 2013, 27, 1762–1770. [Google Scholar] [CrossRef]
- Lee, C.W.; Vo, T.T.; Wu, C.Z.; Chi, M.C.; Lin, C.M.; Fang, M.L.; Lee, I.T. The inducible role of ambient particulate matter in cancer progression via oxidative stress-mediated reactive oxygen species pathways: A recent perception. Cancers 2020, 12, 2505. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Zhang, M.; Sun, Y.; Fang, Z.; Wang, H.; Li, S.; Peng, Y.; Li, J.; Li, J.; Tian, J.; et al. Mechanism of PM2.5-induced human bronchial epithelial cell toxicity in central China. J. Hazard. Mater. 2020, 396, 122747. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, T.; Yang, X.; Sun, B.; Li, Y.; Duan, J.; Sun, Z. PM2.5-induced alteration of DNA methylation and RNA-transcription are associated with inflammatory response and lung injury. Sci. Total Environ. 2019, 650, 908–921. [Google Scholar] [CrossRef]
- ASHRAE. Standards 62.1 & 62.2. American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2022. Available online: https://www.ashrae.org/technical-resources/bookstore/standards-62-1-62-2 (accessed on 17 June 2025).
- Hu, X.; Li, Q.; Shao, S.; Zeng, Q.; Jiang, S.; Wu, Q.; Jiang, C. Potential lung carcinogenicity induced by chronic exposure to PM 2.5 in the rat. Environ. Sci. Pollut. Res. 2017, 24, 18991–19000. [Google Scholar] [CrossRef]
- Whitmarsh, A.J.; Davis, R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, B.W.; Spence, H.J.; McGarry, L.C.; Hennigan, R.F. Transcription factors control invasion: AP-1 the first among equals. Oncogene 2007, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Feng, N.; Zheng, M.; Ye, X.; Lin, H.; Yu, X.; Gan, Z.; Fang, Z.; Zhang, H.; Gao, M.; et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim. Biophys. Acta BBA-Gen. Subj. 2017, 1861, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Chang-Chien, J.; Kuo, M.L.; Tseng, Y.L.; Huang, H.Y.; Tsai, H.J.; Yao, T.C. Differential effects of long-and short-term exposure to PM2.5 on accelerating telomere shortening: From in vitro to epidemiological studies. Ecotoxicol. Environ. Saf. 2024, 281, 116650. [Google Scholar] [CrossRef]
- Colín-Val, Z.; Flores-Navarro, G.; Rocha-Zavaleta, L.; Robledo-Cadena, D.X.; Quintana-Belmares, R.O.; López-Marure, R. Fine particulate matter (PM2.5) promotes chemoresistance and aggressive phenotype of A549 lung cancer cells. Toxicol. Appl. Pharmacol. 2024, 487, 116955. [Google Scholar] [CrossRef]
- Wei, H.; Liang, F.; Cheng, W.; Zhou, R.; Wu, X.; Feng, Y.; Wang, Y. The mechanisms for lung cancer risk of PM2.5: Induction of epithelial-mesenchymal transition and cancer stem cell properties in human non-small cell lung cancer cells. Environ. Toxicol. 2017, 32, 2341–2351. [Google Scholar] [CrossRef] [PubMed]



| Forward (5′–3′) | Reverse (5′–3′) | |
|---|---|---|
| VEGFA | GCAGA-ATCAT-CACGA-AGTGG | GCATG-GTGAT-GTTGG-ACTCC |
| VEGFB | GAGATGTCCCTGGAAGAACACA | GAGTGGGATGGGTGATGTCAG |
| ANGPIT1 | CAAGGCCATCTGTGAAAACAAG | CAGGGGGAACCTCCATGTAG |
| FGF1 | GCCCTGACCGAGAAGTTTAATC | CCCCGTTGCTACAGTAGAGG |
| FGF13 | AGGCCGAGGGTGGTATCTG | AGATCGGGAGAACTCCGTGAG |
| FGF22 | GGGAGCGCATCGAAGAGAAC | CTGTGAGGCGTAGGTGTTGTG |
| PDGFB | CTCGATCCGCTCCTTTGATGA | CGTTGGTGCGGTCTATGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omran, K.; Jiang, Y.-J.; Ho, T.-L.; Kousar, I.; Tang, C.-H.; Tan, M. Long-Term Particulate Matter (PM) Exposure Promotes Non-Small-Cell Lung Cancer (NSCLC) Angiogenesis Through Up-Regulation of VEGFA. Cancers 2025, 17, 2868. https://doi.org/10.3390/cancers17172868
Omran K, Jiang Y-J, Ho T-L, Kousar I, Tang C-H, Tan M. Long-Term Particulate Matter (PM) Exposure Promotes Non-Small-Cell Lung Cancer (NSCLC) Angiogenesis Through Up-Regulation of VEGFA. Cancers. 2025; 17(17):2868. https://doi.org/10.3390/cancers17172868
Chicago/Turabian StyleOmran, Khaled, Ya-Jing Jiang, Trung-Loc Ho, Iqra Kousar, Chih-Hsin Tang, and Ming Tan. 2025. "Long-Term Particulate Matter (PM) Exposure Promotes Non-Small-Cell Lung Cancer (NSCLC) Angiogenesis Through Up-Regulation of VEGFA" Cancers 17, no. 17: 2868. https://doi.org/10.3390/cancers17172868
APA StyleOmran, K., Jiang, Y.-J., Ho, T.-L., Kousar, I., Tang, C.-H., & Tan, M. (2025). Long-Term Particulate Matter (PM) Exposure Promotes Non-Small-Cell Lung Cancer (NSCLC) Angiogenesis Through Up-Regulation of VEGFA. Cancers, 17(17), 2868. https://doi.org/10.3390/cancers17172868








