Clinical Outcomes and Safety of Ultra-Low-Dose Radiotherapy for Ocular Adnexal Lymphoma: A Systematic Review
Simple Summary
Abstract
1. Introduction
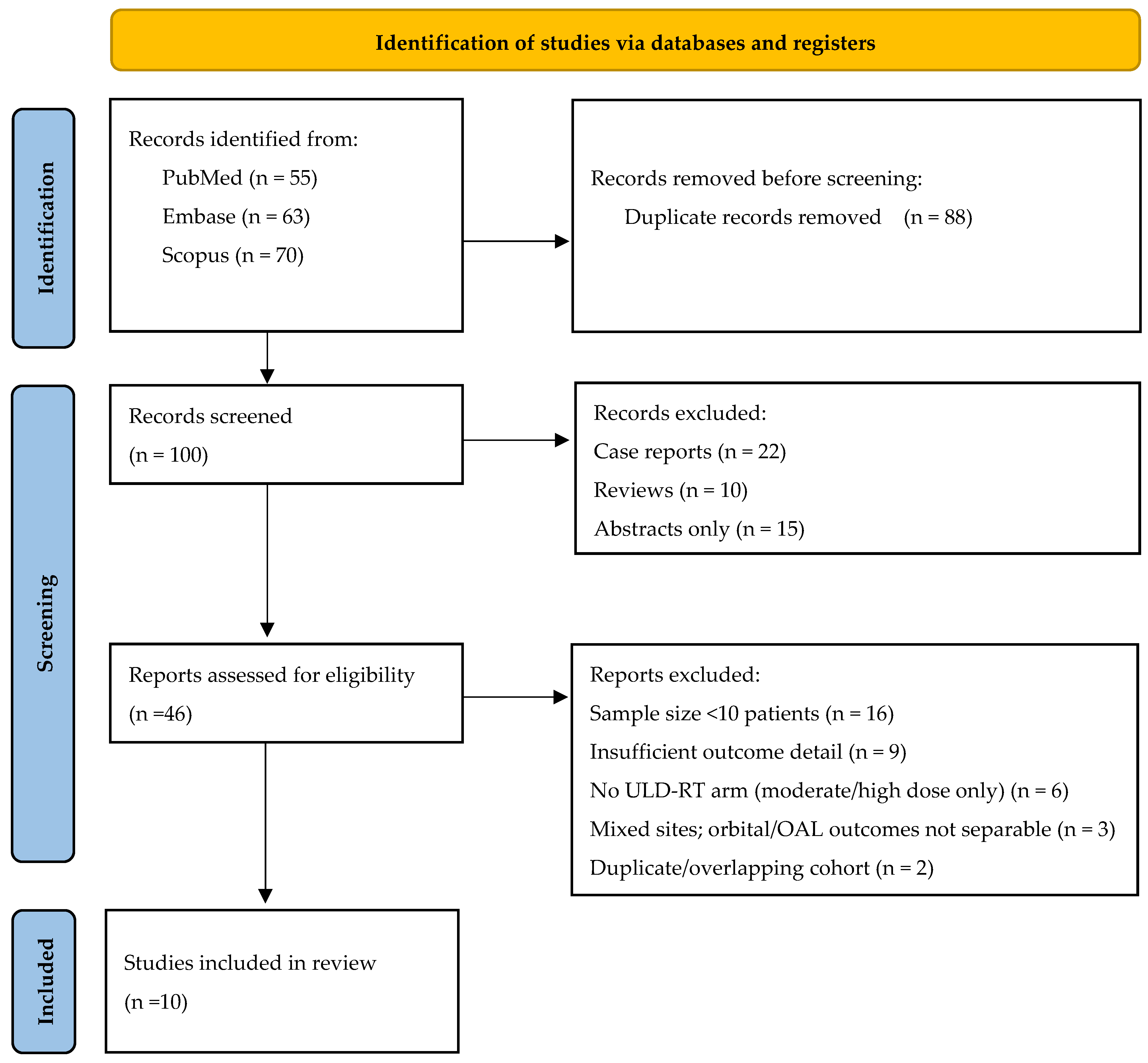
2. Materials and Methods
2.1. Criteria for Inclusion and Exclusion
2.2. Sources of Information
2.3. Strategy for Literature Search
“radiotherapy” AND (“boom boom” OR “low dose” OR “ultra-low” OR “4 Gy” OR “4 Gray” OR “two fraction”) AND lymphoma AND (ocul* OR adnex* OR orbit* OR choroid* OR eye)”
2.4. Process for Selecting Studies
2.5. Extraction of Data and Variables
2.6. Data Synthesis and Grouping of Study Outcomes
2.7. Assessment of Study Quality
2.8. Dose Notation and Conventions
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NHL | non-Hodgkin lymphoma |
| OAL | ocular adnexal lymphoma |
| MALT | mucosa-associated lymphoid tissue |
| 3D-CRT | three-dimensional conformal radiation therapy |
| ULD-RT | ultra-low-dose radiotherapy |
| BBRT | boom-boom radiotherapy |
| PBT | proton beam therapy |
| DLBCL | diffuse large B-cell lymphoma |
| OS | overall survival |
| PFS | progression-free survival |
| RCTs | randomized controlled trials |
| CTCAE | Common Terminology Criteria for Adverse Events |
| MeSH | medical subject heading |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ORR | overall response rate |
| CR | complete response |
| LCR | local control rate |
| SWiM | Synthesis Without Meta-analysis |
| NOS | Newcastle-Ottawa scale |
| MZL | marginal zone lymphoma |
| FL | follicular lymphoma |
| CLL | chronic lymphocytic leukemia |
| MDRT | medium-dose radiotherapy |
| IMRT | intensity-modulated radiotherapy |
| CFR | conventionally fractionated radiotherapy |
| PS | performance status |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Stefanovic, A.; Lossos, I.S. Extranodal marginal zone lymphoma of the ocular adnexa. Blood 2009, 114, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Dolcetti, R.; Du, M.-Q.; Doglioni, C.; Resti, A.G.; Politi, L.S.; De Conciliis, C.; Radford, J.; Bertoni, F.; Zucca, E.; et al. Ocular adnexal MALT lymphoma: An intriguing model for antigen-driven lymphomagenesis and microbial-targeted therapy. Ann. Oncol. 2008, 19, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.W.; Gospodarowicz, M.K.; Pintilie, M.; Wells, W.; Hodgson, D.C.; Sun, A.; Crump, M.; Patterson, B.J. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J. Clin. Oncol. 2003, 21, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.; Friedrich, I.; Hadi, I.; Schmidt-Hegemann, N.-S.; Hartoyo, S.N.; Trauth, R.; Reitz, D.; Manapov, F.; Siefert, A.; Dreyling, M.; et al. Primary radiation therapy in stage I/II indolent orbital lymphoma—A comprehensive retrospective recurrence and toxicity analysis. Eur. J. Haematol. 2022, 109, 21–30. [Google Scholar] [CrossRef]
- Franklin, C.I. Primary lymphoreticular tumours in the orbit. Clin. Radiol. 1975, 26, 137–140. [Google Scholar] [CrossRef]
- Gao, L.-R.; Wang, X.; Xia, C.; Song, Y.-W.; Wang, L.; Li, X.; Yang, Y.; Cao, J.-Z.; Chen, K.; Zhong, Q.-Z.; et al. Multicenter phase II study of moderate low-dose radiotherapy in indolent non-Hodgkin lymphoma: CLCG-iNHL-01 protocol. Future Oncol. 2024, 20, 71–81. [Google Scholar] [CrossRef]
- Sin, I.; Ong, W.; Hussein, A.; Master, Z.; Poh, S.; Ho, B.; Looi, W.; Yeoh, K. Orbital MALT Lymphoma Treated with Low Dose Radiotherapy Yields Excellent Outcomes. Radiother. Oncol. 2023, 182, S930–S931. [Google Scholar] [CrossRef]
- Olsen, T.G.; Heegaard, S. Orbital lymphoma. Surv. Ophthalmol. 2019, 64, 45–66. [Google Scholar] [CrossRef]
- Pinnix, C.C.; Dabaja, B.S.; Gunther, J.R.; Fang, P.Q.; Wu, S.Y.; Nastoupil, L.J.; Strati, P.; Nair, R.; Ahmed, S.; Steiner, R.; et al. Response-Adapted Ultralow-Dose Radiation Therapy for Orbital Indolent B-Cell Lymphoma: A Phase 2 Nonrandomized Controlled Trial. JAMA Oncol. 2024, 10, 1195–1203. [Google Scholar] [CrossRef]
- Meng, K.; Lim, M.C.; Poon, M.L.M.; Sundar, G.; Vellayappan, B. Low-dose “boom-boom” radiotherapy for ocular lymphoma arising from IgG4-related ophthalmic disease: Case report and literature review. Eur. J. Ophthalmol. 2022, 32, NP78–NP84. [Google Scholar] [CrossRef] [PubMed]
- König, L.; Hörner-Rieber, J.; Bernhardt, D.; Hommertgen, A.; Rieken, S.; Debus, J.; Herfarth, K. Response rates and recurrence patterns after low-dose radiotherapy with 4 Gy in patients with low-grade lymphomas. Strahlenther. Onkol. 2018, 194, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Lee, M.Y.; Choe, J.-Y.; Choi, S.H.; Kim, H.J. Ultra-low-dose radiation treatment for early-stage ocular adnexal MALT lymphoma. Eur. J. Ophthalmol. 2022, 32, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dalvin, L.A.; Lim, L.-A.S.; Mashayekhi, A.; Shields, J.A.; Shields, C.L. Ultra-low-dose (boom-boom) radiotherapy for choroidal lymphoma in three consecutive cases. Eur. J. Ophthalmol. 2021, 31, NP91–NP96. [Google Scholar] [CrossRef]
- Astafurov, K.V.; Bothun, E.D.; Laack, N.N.; Deisher, A.J.; Patel, S.V.; Dalvin, L.A. Ultra-low-dose (boom-boom) radiotherapy for management of recurrent ocular post-transplant lymphoproliferative disorder. Am. J. Ophthalmol. Case Rep. 2021, 23, 101118. [Google Scholar] [CrossRef]
- La Rocca, M.; Leonardi, B.F.; Lo Greco, M.C.; Marano, G.; Finocchiaro, I.; Iudica, A.; Milazzotto, R.; Liardo, R.L.E.; La Monaca, V.A.; Salamone, V.; et al. Radiotherapy of Orbital and Ocular Adnexa Lymphoma: Literature Review and University of Catania Experience. Cancers 2023, 15, 5782. [Google Scholar] [CrossRef]
- Pereira-Da Silva, M.V.; Di Nicola, M.L.; Altomare, F.; Xu, W.; Tsang, R.; Laperriere, N.; Krema, H. Radiation therapy for primary orbital and ocular adnexal lymphoma. Clin. Transl. Radiat. Oncol. 2023, 38, 15–20. [Google Scholar] [CrossRef]
- Rehn, S.; Elsayad, K.; Oertel, M.; Baehr, A.; Eter, N.; Haverkamp, U.; Lenz, G.; Eich, H.T. Radiotherapy Dose and Volume De-escalation in Ocular Adnexal Lymphoma. Anticancer. Res. 2020, 40, 4041–4046. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Chelius, M.; Chau, K.; Yang, J.; Hajj, C.; Imber, B.; Yahalom, J. Low grade, indolent lymphomas of the head and neck: Comparative toxicity of standard versus very low dose radiation therapy. Hematol. Oncol. 2021, 39, 304–312. [Google Scholar] [CrossRef]
- Baron, J.; Wright, C.M.; Lee, D.Y.; Carpenter, M.; Manjunath, S.H.; Briceño, C.A.; Chong, E.; Maity, A.; Plastaras, J.P.; Paydar, I. Low-Dose Radiotherapy Versus Moderate-Dose Radiotherapy for the Treatment of Indolent Orbital Adnexal Lymphomas. Front. Oncol. 2021, 11, 716002. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 March 2025).
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, R.; Yuan, X.; Yao, S.; Wang, C.; Cheng, J. Ultra-low-dose radiotherapy in the treatment of ocular adnexal lymphoma: A prospective study. Radiat. Oncol. 2022, 17, 208. [Google Scholar] [CrossRef]
- Shelukar, S.; Fernandez, C.; Bas, Z.; Komarnicky, L.; Lally, S.E.; Shields, C.L.; Binder, A.; Porcu, P.; Alpdogan, O.; Martinez-Outschoorn, U.; et al. High local control and low ocular toxicity using ultra-low-dose “boom-boom” radiotherapy for indolent orbital lymphoma. Chin. Clin. Oncol. 2022, 11, 44. [Google Scholar] [CrossRef]
- Pinnix, C.C.; Dabaja, B.S.; Milgrom, S.A.; Smith, G.L.; Abou, Z.; Nastoupil, L.; Romaguera, J.; Turturro, F.; Fowler, N.; Fayad, L.; et al. Ultra-low-dose radiotherapy for definitive management of ocular adnexal B-cell lymphoma. Head Neck 2017, 39, 1095–1100. [Google Scholar] [CrossRef]
- Park, J.; Yea, J.W.; Oh, S.A.; Kim, M.K.; Son, J.H.; Park, J.W. Prospective Study of 4 Gy Radiotherapy for Orbital Mucosa-Associated Lymphoid Tissue Lymphoma (FORMAL). Cancers 2022, 14, 4274. [Google Scholar] [CrossRef]
- Manta, A.I.; Schlect, D.J.; Wang, D.D.; Sullivan, T.J. Two-year Outcomes of Ultra-low-dose Radiotherapy in the Treatment of Ocular Adnexal B-cell Lymphomas. Ophthalmic Plast. Reconstr. Surg. 2025, 41, 94–100. [Google Scholar] [CrossRef]
- Fasola, C.E.; Jones, J.C.; Huang, D.D.; Le, Q.-T.; Hoppe, R.T.; Donaldson, S.S. Low-dose radiation therapy (2 Gy × 2) in the treatment of orbital lymphomant. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 930–935. [Google Scholar] [CrossRef]
- Imber, B.S.; Chau, K.W.; Lee, J.; Lee, J.; Casey, D.L.; Yang, J.C.; Wijentunga, N.A.; Shepherd, A.; Hajj, C.; Qi, S.; et al. Excellent response to very-low-dose radiation (4 Gy) for indolent B-cell lymphomas: Is 4 Gy suitable for curable patients? Blood Adv. 2021, 5, 4185–4197. [Google Scholar] [CrossRef]
- Knoops, L.; Haas, R.; de Kemp, S.; Majoor, D.; Broeks, A.; Eldering, E.; de Boer, J.P.; Verheij, M.; van Ostrom, C.; de Vries, A.; et al. In vivo p53 response and immune reaction underlie highly effective low-dose radiotherapy in follicular lymphoma. Blood 2007, 110, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Fang, P.Q.; Fetooh, A.; Manzar, G.S.; Corrigan, K.L.; Schrank, B.R.; Nasr, L.; Chihara, D.; Castillo, L.E.M.; Nair, R.; et al. Ultra-Low-Dose Radiation for Extranodal Marginal Zone Lymphoma of the Lung. Adv. Radiat. Oncol. 2024, 9, 101648. [Google Scholar] [CrossRef] [PubMed]
- Knop, N.; Knop, E. Conjunctiva-associated lymphoid tissue in the human eye. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1270–1279. [Google Scholar]
- Mayo, C.; Martel, M.K.; Marks, L.B.; Flickinger, J.; Nam, J.; Kirkpatrick, J. Radiation dose-volume effects of optic nerves and chiasm. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S28–S35. [Google Scholar] [CrossRef]
- Flanagan, J.P.M.; Ng, M.; Kibrom, A.Z.; Filshie, R.J.A.; Stawell, R.J.; O’Day, R.F. Ultra-low dose external beam radiotherapy for presumed choroidal lymphoma: A case report. J. Ophthalmic Inflamm. Infect. 2022, 12, 10. [Google Scholar] [CrossRef]
- Woo, Y.J.; Ko, J.; Ji, Y.W.; Kim, T.-I.; Yoon, J.S. Meibomian Gland Dysfunction Associated With Periocular Radiotherapy. Cornea 2017, 36, 1486–1491. [Google Scholar] [CrossRef]
- Venkatesh, R.; Bavaharan, B.; Mahendradas, P.; Yadav, N.K. Primary vitreoretinal lymphoma: Prevalence, impact, and management challenges. Clin. Ophthalmol. 2019, 13, 353–364. [Google Scholar] [CrossRef]
- Lee, G.-I.; Oh, D.; Kim, W.S.; Kim, S.J.; Ko, Y.H.; Woo, K.I.; Kim, Y.-D.; Ahn, Y.C. Low-Dose Radiation Therapy for Primary Conjunctival Marginal Zone B-Cell Lymphoma. Cancer Res. Treat. 2018, 50, 575–581. [Google Scholar] [CrossRef]
- Holloway, C.L.; Pickles, T.; Croteau, N.S.; Wai, E.S. Treatment Outcomes of Low-grade Lymphoma of the Orbit. Clin. Oncol. (R. Coll. Radiol.) 2022, 34, e298–e304. [Google Scholar] [CrossRef]
- Goda, J.S.; Le, L.W.; Lapperriere, N.J.; Millar, B.-A.; Payne, D.; Gospodarowicz, M.K.; Wells, W.; Hodgson, D.C.; Sun, A.; Simpson, R.; et al. Localized orbital mucosa-associated lymphoma tissue lymphoma managed with primary radiation therapy: Efficacy and toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e659–e666. [Google Scholar] [CrossRef]
| Author (Citation) | Year | Country | Study Design | Sample Size a |
|---|---|---|---|---|
| Pinnix [10] | 2024 | United States | Clinical trial (phase 2, single-arm) and retrospective study | 105 b |
| Yang [25] | 2022 | China | Prospective exploratory study | 16 |
| Shelukar [26] | 2022 | United States | Retrospective case series | 17 |
| Pinnix [27] | 2017 | United States | Retrospective study | 22 |
| Park [28] | 2022 | Korea | Clinical trial (phase 2, single-arm) | 14 |
| Manta [29] | 2024 | Australia | Retrospective study | 21 |
| König [12] | 2018 | Germany | Retrospective study | 47 |
| Fasola [30] | 2013 | United States | Retrospective study | 20 |
| Chelius [20] | 2021 | United States | Retrospective study | 266 c |
| Baron [21] | 2021 | United States | Retrospective study | 36 |
| Author (Citation) | Age, Median (Range) | Gender (Female/Male) | Histology | Disease Location | Laterality | RT Dose | Prior Therapy |
|---|---|---|---|---|---|---|---|
| Number of Patients (%) | |||||||
| Pinnix–prospective cohort [10] | 63 (29–88) | 31 (62%)/19 (38%) | MALT a: 32 (64%) FL b: 12 (24%) Low-grade unspecified: 6 (12%) | Conjunctiva only: 16 (32%) Lacrimal gland only: 6 (12%) Soft tissues/muscles: 23 (46%) Multiple sites: 5 (10%) | Uni c: 42 (84%) Bi d: 8 (16%) | 4 Gy/2 fr. | No systemic therapy within 4 weeks before radiotherapy; newly diagnosed: 36 (72%) Systemic treatment after radiotherapy (0.1–53.6 months): 10 (20%) |
| Pinnix–retrospective cohort [10] | 63 (25–87) | 26 (47%)/29 (53%) | MALT a: 38 (69%) FL b: 13 (24%) Low-grade unspecified: 4 (7%) | Conjunctiva only: 17 (31%) Lacrimal gland only: 7 (13%) Soft tissues/muscles: 16 (29%) Multiple sites: 15 (27%) | Uni c: 39 (71%) Bi d: 16 (29%) | 4 Gy/2 fr. | No systemic therapy within 4 weeks before radiotherapy; newly diagnosed: 44 (80%) |
| Yang [25] | 63 (23–86) | 7 (44%)/9 (56%) | MALT a: 11 (69%) FL b: 2 (12%) Lymphoid hyperplasia: 3 (19%) | Conjunctiva: 5 (19%) Lacrimal gland: 2 (7%) Soft tissue: 11 (41%) Rectus: 4 (14%) Eyelid: 5 (19%) | Uni c:11 (69%) Bi d: 5 (31%) | 4 Gy/2 fr. | No other therapy concomitantly with radiotherapy until evaluation of the response |
| Shelukar [26] | 67 (24–80) | 12 (71%)/5 (29%) | MALT a: 5 (29%) FL b: 4 (24%) MZL e: 3 (18%) MCL f: 1 (6%) Other low-grade: 4 (24%) | Conjunctiva: 4 (21%) Lacrimal gland: 4 (21%) Orbit: 8 (42%) Choroid: 2 (11%) Eyelid: 5 (5%) | Uni c: 15 (88%) Bi d: 2 (12%) | 4 Gy/2 fr. | No previous treatment |
| Pinnix [27] | 64.5 (25–88) | 12 (55%)/10 (45%) | MALT a: 14 (64%) FL b: 5 (23%) MCL f: 2 (9%) Low-grade unspecified: 1 (4%) | Conjunctiva: 6 (27%) Lacrimal gland: 6 (27%) Soft tissue: 9 (41%) Mixed: 1 (5%) | Uni c: 16 (73%) Bi d: 6 (27%) | 4 Gy/2 fr. | Prior systemic treatment: 10 (45%) Prior orbital radiotherapy: 1 (5%) |
| Park [28] | 60.5 (NA g) | 7 (50%)/7 (50%) | NA g | Conjunctiva: 14 (82.3%) Retrobulbar: 1 (5.9%) Mixed: 2 (11.8%) | Uni c:11 (78.6%) Bi d: 3 (21.4%) | 4 Gy/2 fr. | No previous radiotherapy in the orbit |
| Manta [29] | 62 (33–85) | 6 (29%)/15 (71%) | MZL e: 14 (67%) FL b: 5 (24%) MCL f: 2 (10%) | Conjunctiva: 9 (43%) Orbit: 14 (67%) Lacrimal gland: 7 (33%) Extraocular muscles: 4 (19%) Eyelid/preseptal tissue: 4 (19%) | Uni c: 18 (85.7%) Bi d: 3 (14.3%) | 4 Gy/2 fr. | No concomitantly chemotherapy; 5 (24%) had a history of prior treatment for ocular adnexal and/or systemic NHL i |
| König [12] | 64 (33–89) | 29 (61.7%)/18 (38.3%) | FL b: 27 (57.4%) MZL e: 20 (42.6%) | Nodal disease: 8 lesions (16%) Extranodal disease: 42 lesions (84%): Orbit: 16 lesions (32%) Salivary glands: 7 lesions (14%) Skin: 15 lesions (30%) Others: 4 lesions (8%) | NA g | 4 Gy/2 fr. | Rituximab simultaneously: 13 (26%) Primary treatment: 30 (60%) |
| Fasola [30] | 70 (38–88) | 10 (50%)/10 (50%) | FL b: 11 (55%) MZL e: 8 (40%) MCL f: 1 (5%) | Conjunctiva: 9 (33%) Lacrimal gland: 11 (41%) Retrobulbar: 2 (7%) Eyelid: 5 (19%) | Uni c: 14 (70%) Bi d: 6 (30%) | 4 Gy/2 fr. | Prior chemotherapy: 6 (30%) Prior radiotherapy: 8 (40%) |
| Chelius [20] | 61 (19–92) | 126 (47.4%)/140 (52.6%) | FL b: 117 (44.1%) MZL e/MCL f: 110 (41.4%) Cutaneous B-cell: 22 (8.3%) Small lymphocytic lymphoma/CLL h: 17 (6.4%) | Orbit: 81 (30.5%) | NA g | 4 Gy/2 fr. (12/62 pts orbit) | Relapse or refractory disease: 29 (46.8%) |
| >4 Gy (69/204 pts orbit) | Relapse or refractory disease: 32 (15.7%) | ||||||
| Baron [21] | 64.5 (16–92) | 23 (64%)/13 (36%) | MZL e: 20 (56%) FL b: 11 (30%) MCL f: 5 (14%) | Conjunctiva: 7 (19%) Lacrimal gland: 13 (36%) Eyelid: 15 (42%) Other: 1 (3%) | Uni c: 21 (58%) Bi d: 15 (42%) | 4 Gy/2 fr. (12 pts) | Concurrent/sequential Rituximab: 8 (22%) |
| Median RT dose 24 Gy (21–36 Gy) (24 pts) | |||||||
| Author (Citation) | Overall Response Rate (ORR) b | Complete Response (CR d) | Local Control Rate (LCR) | Progression-Free Survival (PFS) | Overall Survival (OS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Patients (%) | 1-Year | 2-Year | 3-Year | 4-Year | 5-Year | 1-Year | 2-Year | 5-Year | 1-Year | 2-Year | 5-Year | ||
| Pinnix—prospective cohort [10] | NA a | 44 (88%) | NA a | 89% | NA a | NA a | NA a | NA a | NA a | NA a | NA a | 98% | NA a |
| Pinnix—retrospective cohort [10] | NA a | 51 (93%) | NA a | 96% | NA a | NA a | NA a | NA a | NA a | NA a | NA a | 98% | NA a |
| Yang [25] | 14 (88%) | 12 (75%) | 85% | NA a | NA a | NA a | NA a | LPFS c 85% DPFS g 100% | NA a | NA a | 100% | NA a | NA a |
| Shelukar [26] | 15 (89%) | 11 (65%) | NA a | NA a | 100% | NA a | 94% | NA a | NA a | NA a | NA a | NA a | NA a |
| Pinnix [27] | 22 (100%) | 19 (86%) | NA a | NA a | NA a | NA a | NA a | LPFS c 100% (for 19 patients who achieved CR d) | LPFS c 75% (for 19 patients who achieved CR d) | NA a | NA a | NA a | NA a |
| Park [28] | 17/17 Ls e (100%) | 11/17 Ls e (64%) | NA a | 91% f | NA a | NA a | NA a | NA a | 90% f | NA a | NA a | 100% f | NA a |
| Manta [29] | 20 (95%) | 20 (95%) | NA a | 100% | NA a | NA a | NA a | NA a | 100% | NA a | NA a | 100% | NA a |
| König [12] | 45/50 Ls e (90%) | 43/50 Ls e (82%) | NA a | 91% c | NA a | NA a | NA a | NA a | DPFS g 83% | DPFS g 83% | NA a | 96.6% | 88.6% |
| Fasola [30] | 26/27 Ls e (96%) | 23/27 Ls e (85%) | NA a | NA a | NA a | NA a | NA a | NA a | LPFS c 100% RPFS h 96% i DPFS g 75% | NA a | NA a | NA a | NA a |
| Chelius—4 Gy cohort [20] | NA a | 45/62 (73%) | 40/45 (89%) j,k | NA a | NA a | NA a | NA a | NA a | NA a | NA a | NA a | NA a | NA a |
| Chelius—>4 Gy cohort [20] | NA a | 92/104 (89%) | 84/92 (96%) j | NA a | NA a | NA a | NA a | NA a | NA a | NA a | NA a | NA a | NA a |
| Baron—ULD-RT l [21] | 100% | 50% | NA a | 100% | NA a | 100% | NA a | NA a | NA a | NA a | NA a | 100% | NA a |
| Baron—MDRT m [21] | 88% | 58% | NA a | 100% | NA a | 89% | NA a | NA a | NA a | NA a | NA a | 95% | NA a |
| Author (Citation) | Acute Toxicity | Most Common Type Number of Patients (%) | ||
|---|---|---|---|---|
| Number of Patients (%) | ||||
| Grade 1 | Grade 2 | Grade 3 | ||
| Pinnix—prospective cohort [10] | 3 (6%) | 1 (2%) | No toxicity | Dry eye 4 (8%) |
| Pinnix—retrospective cohort [10] | 1 (1.8%) | No toxicity | No toxicity | HSV a keratitis 1 (1.8%) |
| Yang [25] | No toxicity | No toxicity | No toxicity | / |
| Shelukar [26] | Patients followed by oncologist: | NA | ||
| 1 (6%) | No toxicity | No toxicity | ||
| Patients followed by ophthalmologist: | ||||
| 5 (33.3%) | No toxicity | No toxicity | Dry eye 3 (20%), cataract 1 (6.7%), chorioretinal atrophy 1 (6.7%) | |
| Pinnix [27] | 1 (4.5%) | No toxicity | No toxicity | Dry eye 1 (4.5%) |
| Park [28] | 1 (7%) | No toxicity | No toxicity | Dry eye 1 (7%) |
| Manta [29] | No toxicity | No toxicity | No toxicity | / |
| König [12] | No toxicity | No toxicity | No toxicity | / |
| Fasola [30] | 6 (23%) | No toxicity | No toxicity | Dry eye 1 (4%), acute conjunctivitis 1 (4%), transient periorbital edema 4 (15%) |
| Chelius—ULD-RT b [20] | Orbit: 5/12 (41.7%) | No toxicity | Xerophthalmia 3 (25%) Visual changes 1 (8%) Dermatitis 1 (8%) | |
| Chelius—MDRT c [20] | Orbit: 66/69 (95.7%) | No toxicity | Xerophthalmia 34 (49%) Conjunctivitis 20 (29%) Visual changes 17 (25%) Watering eye 21 (30%) | |
| Baron—ULD-RT b [21] | ULD-RT b: 6 (50%) | No toxicity | NA | |
| Baron—MDRT c [21] | MDRT c: 20 (83.3%) | No toxicity | NA | |
| Author (Citation) | Late Toxicity | Most Common Type Number of Patients (%) | ||
|---|---|---|---|---|
| Number of Patients (%) | ||||
| Grade 1 | Grade 2 | Grade 3 | ||
| Pinnix—prospective cohort [10] | No toxicity | No toxicity | No toxicity | / |
| Pinnix—retrospective cohort [10] | No toxicity | No toxicity * | No toxicity | / |
| Yang [25] | No toxicity | No toxicity | No toxicity | / |
| Shelukar [26] | No toxicity | No toxicity | No toxicity | / |
| Pinnix [27] | No toxicity | No toxicity | No toxicity | / |
| Park [28] | No toxicity | No toxicity | No toxicity | / |
| Manta [29] | 3 (14%) | No toxicity | Mild dry eye 3 (14%) | |
| König [12] | No toxicity | No toxicity | No toxicity | / |
| Fasola [30] | No toxicity | No toxicity | No toxicity | / |
| Chelius—ULD-RT a [20] | ULD-RT a—orbit: 2/10 (20%) | No toxicity | Xerophthalmia 1 (10%) Conjunctivitis 1 (10%) Dermatitis 1 (10%) | |
| Chelius—MDRT b [20] | MDRT borbit: 48/68 (70.6%) | No toxicity | Xerophthalmia 28 (41%) Cataracts 19 (28%) Visual changes 18 (27%) Watering eye 11 (16%) Conjunctivitis 3 (4%) | |
| Baron—ULD-RT a [21] | 2 (16%) | No toxicity | No toxicity | Dry eye 2 (16%) |
| Baron—MDRT b [21] | 7 (29%) | 3 (13%) | No toxicity | Dry eye 9 (38%) Cataract 1 (4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grujić, M.; Volchenkov, S.; Akhmetali, A.; Živković Radojević, M.; Milosavljević, N.; Janković, K.; Krasić, K.; Mihajlović, M.; Shelan, M.; Nicosia, L.; et al. Clinical Outcomes and Safety of Ultra-Low-Dose Radiotherapy for Ocular Adnexal Lymphoma: A Systematic Review. Cancers 2025, 17, 2845. https://doi.org/10.3390/cancers17172845
Grujić M, Volchenkov S, Akhmetali A, Živković Radojević M, Milosavljević N, Janković K, Krasić K, Mihajlović M, Shelan M, Nicosia L, et al. Clinical Outcomes and Safety of Ultra-Low-Dose Radiotherapy for Ocular Adnexal Lymphoma: A Systematic Review. Cancers. 2025; 17(17):2845. https://doi.org/10.3390/cancers17172845
Chicago/Turabian StyleGrujić, Miloš, Stanislav Volchenkov, Aidos Akhmetali, Marija Živković Radojević, Neda Milosavljević, Katarina Janković, Katarina Krasić, Milica Mihajlović, Mohamed Shelan, Luca Nicosia, and et al. 2025. "Clinical Outcomes and Safety of Ultra-Low-Dose Radiotherapy for Ocular Adnexal Lymphoma: A Systematic Review" Cancers 17, no. 17: 2845. https://doi.org/10.3390/cancers17172845
APA StyleGrujić, M., Volchenkov, S., Akhmetali, A., Živković Radojević, M., Milosavljević, N., Janković, K., Krasić, K., Mihajlović, M., Shelan, M., Nicosia, L., & Marinković, M. (2025). Clinical Outcomes and Safety of Ultra-Low-Dose Radiotherapy for Ocular Adnexal Lymphoma: A Systematic Review. Cancers, 17(17), 2845. https://doi.org/10.3390/cancers17172845









