Endoscopic Ablation in Cholangiocarcinoma
Simple Summary
Abstract
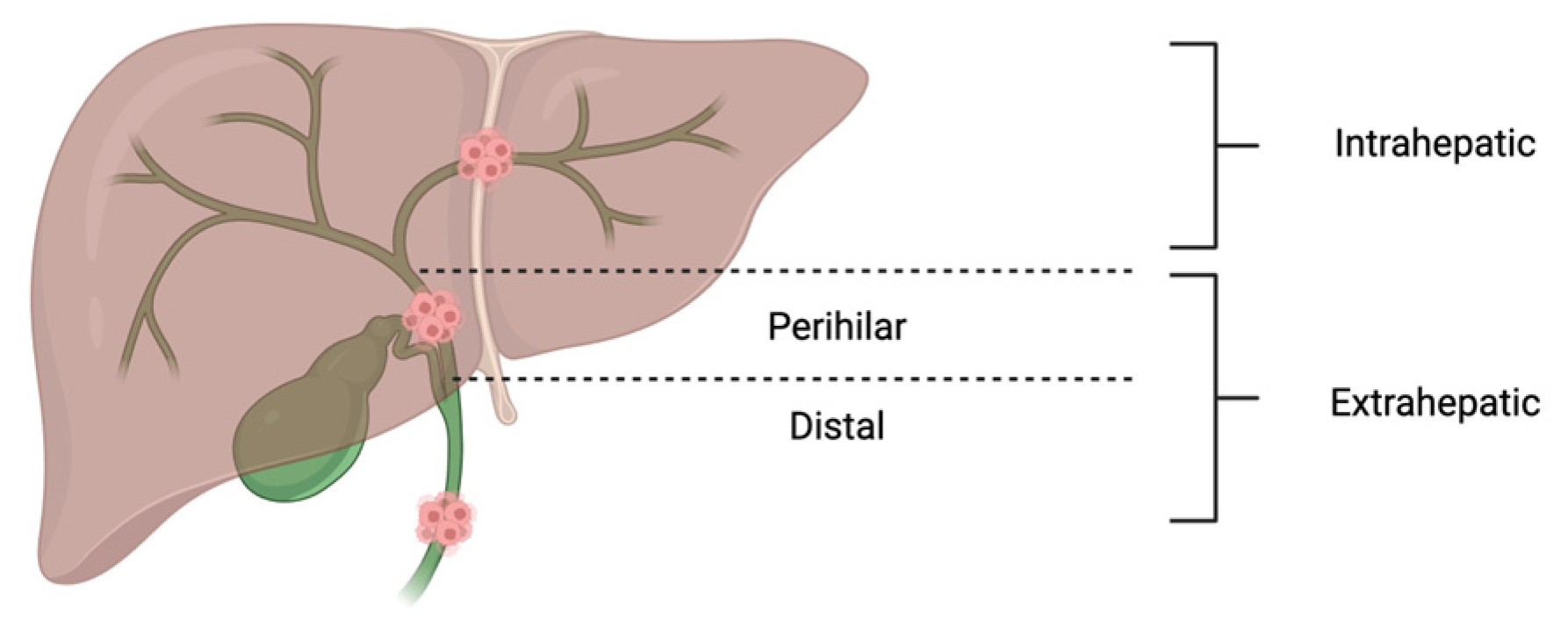
1. Introduction
2. Endoscopic Technologies
2.1. Radiofrequency Ablation
2.1.1. Clinical Application of RFA
2.1.2. Outcomes of RFA for CCA
2.1.3. Complications and Limitations of RFA
2.2. Photodynamic Therapy
2.2.1. Clinical Application of PDT
2.2.2. Outcomes of PDT for CCA
2.2.3. Complication and Limitations of PDT
2.3. Comparison Between RFA and PDT
3. Other Ablation Therapies for CCA
4. Innovation in CCA Treatment
5. Conclusions/Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Qurashi, M.; Vithayathil, M.; Khan, S.A. Epidemiology of cholangiocarcinoma. Eur. J. Surg. Oncol. 2025, 51, 107064. [Google Scholar] [CrossRef]
- Turati, F.; Bertuccio, P.; Negri, E.; Vecchia, C.L. Epidemiology of cholangiocarcinoma. Hepatoma Res. 2022, 8, 19. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer. Available online: https://seer.cancer.gov/statfacts/html/livibd.html (accessed on 1 August 2025).
- Khan, A.S.; Dageforde, L.A. Cholangiocarcinoma. Surg. Clin. N. Am. 2019, 99, 315–335. [Google Scholar] [CrossRef]
- Rebhun, J.; Shin, C.M.; Siddiqui, U.D.; Villa, E. Endoscopic biliary treatment of unresectable cholangiocarcinoma: A meta-analysis of survival outcomes and systematic review. World J. Gastrointest. Endosc. 2023, 15, 177–190. [Google Scholar] [CrossRef]
- What Is Cholangiocarcinoma? Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/diseases/21524-cholangiocarcinoma (accessed on 21 August 2025).
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Khosla, D.; Misra, S.; Chu, P.L.; Guan, P.; Nada, R.; Gupta, R.; Kaewnarin, K.; Ko, T.K.; Heng, H.L.; Srinivasalu, V.K.; et al. Cholangiocarcinoma: Recent advances in molecular pathobiology and therapeutic approaches. Cancers 2024, 16, 801. [Google Scholar] [CrossRef] [PubMed]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Van Beers, B.E. Diagnosis of cholangiocarcinoma. HPB 2008, 10, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Moon, S.H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023, 13, 233. [Google Scholar] [CrossRef]
- Hormati, A.; Jafari, S.; Jabbari, A.; Pezeshki Modares, M.; Afifian, M.; Abasi, A.; Ahmadpour, S.; Sharifi, A.R. Comparison between brush cytology and forceps biopsy under fluoroscopic guidance for the diagnosis of proximal cholangiocarcinoma. Middle East J. Dig. Dis. 2020, 12, 246–251. Available online: https://pubmed.ncbi.nlm.nih.gov/33564381/ (accessed on 1 August 2025).
- Chandrasekar, V.T.; Faigel, D. Diagnosis and treatment of biliary malignancies: Biopsy, cytology, cholangioscopy and stenting. Mini-Invasive Surg. 2021, 5, 33. [Google Scholar] [CrossRef]
- Moris, D.; Palta, M.; Kim, C.; Allen, P.J.; Morse, M.A.; Lidsky, M.E. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J. Clin. 2023, 73, 198–222. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.; Coelen, R.J.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J.; et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef]
- Kawashima, J.; Sahara, K.; Shen, F.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S.; Poultsides, G.A.; Maithel, S.K.; et al. Predicting risk of recurrence after resection of stage I intrahepatic cholangiocarcinoma. J. Gastrointest. Surg. 2024, 28, 18–25. [Google Scholar] [CrossRef]
- Sota, Y.; Einama, T.; Kobayashibayashi, K.; Fujinuma, I.; Tsunenari, T.; Takihata, Y.; Iwasaki, T.; Miyata, Y.; Okamoto, K.; Kajiwara, Y.; et al. Recurrent cholangiocarcinoma with long-term survival by multimodal treatment: A case report. Mol. Clin. Oncol. 2021, 14, 72. [Google Scholar] [CrossRef]
- Chansitthichok, S.; Chamnan, P.; Sarkhampee, P.; Lertsawatvicha, N.; Voravisutthikul, P.; Wattanarath, P. Survival of Patients with Cholangiocarcinoma Receiving Surgical Treatment in an O. viverrini Endemic Area in Thailand: A Retrospective Cohort Study. Asian Pac. J. Cancer Prev. 2020, 21, 903–909. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (Bilcap): A randomised, controlled, multicentre, phase 3 study. Lancet. Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Beri, N. Immune checkpoint inhibitors in cholangiocarcinoma. Immunotherapy 2023, 15, 541–551. [Google Scholar] [CrossRef]
- Gujarathi, R.; Peshin, S.; Zhang, X.; Bachini, M.; Meeks, M.N.; Shroff, R.T.; Pillai, A. Intrahepatic cholangiocarcinoma: Insights on molecular testing, targeted therapies, and future directions from a multidisciplinary panel. Hepatol. Commun. 2025, 9, e0743. [Google Scholar] [CrossRef]
- Makki, M.; Bentaleb, M.; Abdulrahman, M.; Suhool, A.A.; Al Harthi, S.; Ribeiro, M.A., Jr. Current interventional options for palliative care for patients with advanced-stage cholangiocarcinoma. World J. Clin. Oncol. 2024, 15, 381–390. [Google Scholar] [CrossRef]
- Hendriquez, R.; Keihanian, T.; Goyal, J.; Abraham, R.R.; Mishra, R.; Girotra, M. Radiofrequency ablation in the management of primary hepatic and biliary tumors. World J. Gastrointest. Oncol. 2022, 14, 203–215. [Google Scholar] [CrossRef]
- Baron, T.H. Endoscopic retrograde cholangiopancreatography for cholangiocarcinoma. Clin. Liver Dis. 2014, 18, 891–897. [Google Scholar] [CrossRef]
- Urban, O.; Vanek, P.; Zoundjiekpon, V.; Falt, P. Endoscopic Perspective in Cholangiocarcinoma Diagnostic Process. Gastroenterol. Res. Pract. 2019, 2019, 9704870. [Google Scholar] [CrossRef] [PubMed]
- Ponchon, T.; Gagnon, P.; Berger, F.; Labadie, M.; Liaras, A.; Chavaillon, A.; Bory, R. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: Results of a prospective study. Gastrointest. Endosc. 1995, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kurzawinski, T.; Deery, A.; Dooley, J.; Dick, R.; Hobbs, K.; Davidson, B. A prospective controlled study comparing brush and bile exfoliative cytology for diagnosing bile duct strictures. Gut 1992, 33, 1675–1677. [Google Scholar] [CrossRef]
- Desa, L.A.; Akosa, A.B.; Lazzara, S.; Domizio, P.; Krausz, T.; Benjamin, I.S. Cytodiagnosis in the management of extrahepatic biliary stricture. Gut 1991, 32, 1188–1191. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chen, C.-C.; Kuo, Y.-T.; Liao, W.-C. Endoscopic and novel approaches for evaluation of indeterminate biliary strictures. J. Formos. Med. Assoc. 2025. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78. [Google Scholar] [CrossRef]
- Jarosova, J.; Macinga, P.; Hujova, A.; Kral, J.; Urban, O.; Spicak, J.; Hucl, T. Endoscopic radiofrequency ablation for malignant biliary obstruction. World J. Gastrointest. Oncol. 2021, 13, 1383–1396. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Z.; Huang, Y.; Deng, X.; Zheng, S.; He, S.; Huang, G.; Hu, B.; Shi, M.; Liao, W.; et al. Radiofrequency ablation: Mechanisms and clinical applications. MedComm 2024, 5, e746. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Zhou, H.; Zhou, Y.; Wang, Y.; Jin, H.; Lou, Q.; Zhang, X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: A randomized trial. Endoscopy 2018, 50, 751–760. [Google Scholar] [CrossRef]
- Testoni, S.G.; Healey, A.; Dietrich, C.; Arcidiacono, P. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc. Ultrasound 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Song, Y.; Liu, S. Advances in research and application of photodynamic therapy in cholangiocarcinoma (Review). Oncol. Rep. 2024, 51, 53. [Google Scholar] [CrossRef] [PubMed]
- Zoepf, T. Photodynamic therapy of cholangiocarcinoma. HPB 2008, 10, 161–163. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sun, S.Y. Endoscopic ultrasound-guided radiofrequency ablation in gastroenterology: New horizons in search. World J. Gastroenterol. 2017, 23, 4892–4896. [Google Scholar] [CrossRef]
- Hu, B.; Sun, B.; Gao, D.-J.; Wu, J.; Ye, X.; Xia, M.-X.; Wang, T. Initial experience of ercp-guided radiofrequency ablation as the primary therapy for inoperable ampullary carcinomas. Dig. Dis. Sci. 2020, 65, 1453–1459. [Google Scholar] [CrossRef]
- Thosani, N.; Cen, P.; Rowe, J.; Guha, S.; Bailey-Lundberg, J.M.; Bhakta, D.; Patil, P.; Wray, C.J. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) for advanced pancreatic and periampullary adenocarcinoma. Sci. Rep. 2022, 12, 16516. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Huang, G.H.; Li, Z.; Hao, C.F.; Ren, J.Z.; Duan, X.H.; Zhang, K.; Chen, C.; Han, X.W.; Jiao, D.C.; et al. Percutaneous transhepatic cholangiography and intraductal radiofrequency ablation combined with biliary stent placement for malignant biliary obstruction. J. Vasc. Interv. Radiol. 2015, 26, 715–721. [Google Scholar] [CrossRef]
- Sofi, A.A.; Khan, M.A.; Das, A.; Sachdev, M.; Khuder, S.; Nawras, A.; Lee, W. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2018, 87, 944–951. [Google Scholar] [CrossRef]
- Wu, T.T.; Li, W.M.; Li, H.C.; Ao, G.K.; Zheng, F.; Lin, H. Percutaneous Intraductal Radiofrequency Ablation for Extrahepatic Distal Cholangiocarcinoma: A Method for Prolonging Stent Patency and Achieving Better Functional Status and Quality of Life. Cardiovasc. Interv. Radiol. 2017, 40, 260–269. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yoon, C.J.; Lee, J.H.; Lee, C.-H.; Hwang, J.-H.; Kim, J. Percutaneous endobiliary radiofrequency ablation with stent placement in type IV hilar cholangiocarcinoma: A prospective comparison with stent placement alone. Eur. J. Radiol. 2024, 176, 111516. [Google Scholar] [CrossRef]
- Yu, Q.; Mahbubani, A.; Kwak, D.; Liao, C.-Y.; Pillai, A.; Patel, M.; Navuluri, R.; Funaki, B.; Ahmed, O. Survival outcomes of radiofrequency ablation for intrahepatic cholangiocarcinoma from the surveillance, epidemiology, and end results (Seer) database: Comparison with radiotherapy and resection. J. Vasc. Interv. Radiol. 2025, 36, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Khizar, H.; Hu, Y.; Gu, W.; Yang, J.; Jin, H.; He, X.; Zhang, X.; Yang, J. Assessment of safety and adverse events in endoscopic radiofrequency ablation for malignant biliary obstruction. Ther. Adv. Gastroenterol. 2024, 17, 17562848241294002. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Santos, A.L.; Morais, R.; Vilas-Boas, F.; Rodrigues-Pinto, E.; Santos-Antunes, J.; Macedo, G. Endoscopic radiofrequency ablation for palliative treatment of hilar cholangiocarcinoma. VideoGIE 2021, 6, 195–198. [Google Scholar] [CrossRef]
- Cha, B.H.; Jang, M.-J.; Lee, S.H. Survival benefit of intraductal radiofrequency ablation for malignant biliary obstruction: A systematic review with meta-analysis. Clin. Endosc. 2021, 54, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Wang, Z.X.; Deng, Y.N.; Yang, Y.; Wang, G.Y.; Chen, G.H. Efficacy of Hepatic Resection vs. Radiofrequency Ablation for Patients with Very-Early-Stage or Early-Stage Hepatocellular Carcinoma: A Population-Based Study with Stratification by Age and Tumor Size. Front. Oncol. 2019, 9, 113. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy—Current limitations and novel approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular mechanisms of singlet oxygen in photodynamic therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef]
- The Physics, Biophysics and Technology of Photodynamic Therapy. Available online: https://bme.unc.edu/wp-content/uploads/sites/917/2020/07/The-physics-biophysics-and-technology-of-PDT-Phys-Med-Bio-2008.pdf (accessed on 1 August 2025).
- Nkune, N.W.; Abrahamse, H. Anti-hypoxia nanoplatforms for enhanced photosensitizer uptake and photodynamic therapy effects in cancer cells. Int. J. Mol. Sci. 2023, 24, 2656. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Cheon, Y.K.; Shim, C.S.; Cho, Y.D. Photodynamic therapy prolongs metal stent patency in patients with unresectable hilar cholangiocarcinoma. World J. Gastroenterol. 2012, 18, 5589–5594. [Google Scholar] [CrossRef]
- Moole, H.; Tathireddy, H.; Dharmapuri, S.; Moole, V.; Boddireddy, R.; Yedama, P.; Dharmapuri, S.; Uppu, A.; Bondalapati, N.; Duvvuri, A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 1278–1288. [Google Scholar] [CrossRef]
- Zoepf, T.; Jakobs, R.; Arnold, J.C.; Apel, D.; Riemann, J.F. Palliation of nonresectable bile duct cancer: Improved survival after photodynamic therapy. Am. J. Gastroenterol. 2005, 100, 2426–2430. [Google Scholar] [CrossRef]
- Ortner, M.E.; Caca, K.; Berr, F.; Liebetruth, J.; Mansmann, U.; Huster, D.; Voderholzer, W.; Schachschal, G.; Mössner, J.; Lochs, H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology 2003, 125, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Huang, M.; Zeng, S.; Zheng, J.; Peng, S.; Wang, Y.; Cheng, H.; Li, S. Innovative strategies for photodynamic therapy against hypoxic tumor. Asian J. Pharm. Sci. 2023, 18, 100775. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in combination with organic photosensitizers: Beyond the light penetration depth limit of photodynamic therapy. Int. J. Mol. Sci. 2022, 23, 12556. [Google Scholar] [CrossRef]
- Mohammad, T.; Kahaleh, M. Comparing palliative treatment options for cholangiocarcinoma: Photodynamic therapy vs. radiofrequency ablation. Clin. Endosc. 2022, 55, 347–354. [Google Scholar] [CrossRef]
- Mohan, B.P.; Chandan, S.; Khan, S.R.; Kassab, L.L.; Ponnada, S.; Artifon, E.L.A.; Otoch, J.P.; McDonough, S.; Adler, D.G. Photodynamic therapy (Pdt), radiofrequency ablation (Rfa) with biliary stents in palliative treatment of unresectable extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2022, 56, e153–e160. Available online: https://pubmed.ncbi.nlm.nih.gov/33780214/ (accessed on 1 August 2025). [CrossRef]
- Strand, D.S.; Cosgrove, N.D.; Patrie, J.T.; Cox, D.G.; Bauer, T.W.; Adams, R.B.; Mann, J.A.; Sauer, B.G.; Shami, V.M.; Wang, A.Y. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest. Endosc. 2014, 80, 794–804. [Google Scholar] [CrossRef]
- Shin, C.M.; Villa, E. Su1546 endoscopic radiofrequency ablation versus photodynamic therapy for the treatment of unresectable cholangiocarcinoma: Comparison of survival and safety profiles. Gastrointest. Endosc. 2020, 91, AB374. [Google Scholar] [CrossRef]
- Nanashima, A.; Nakashima, K.; Kawakami, H.; Ashizuka, S.; Kubota, Y. Nursing care management of photodynamic therapy in digestive tract carcinomas at a single cancer center. Photodiagnosis Photodyn. Ther. 2017, 17, 221–225. [Google Scholar] [CrossRef]
- Satiya, J.; Schwartz, I.; Tabibian, J.H.; Kumar, V.; Girotra, M. Ablative therapies for hepatic and biliary tumors: Endohepatology coming of age. Transl. Gastroenterol. Hepatol. 2020, 5, 15. [Google Scholar] [CrossRef]
- Takenaka, M.; Lee, T.H. Role of radiofrequency ablation in advanced malignant hilar biliary obstruction. Clin. Endosc. 2023, 56, 155–163. [Google Scholar] [CrossRef]
- Taggar, A.S.; Mann, P.; Folkert, M.R.; Aliakbari, S.; Myrehaug, S.D.; Dawson, L.A. A systematic review of intraluminal high dose rate brachytherapy in the management of malignant biliary tract obstruction and cholangiocarcinoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 165, 60–74. [Google Scholar] [CrossRef]
- Humphrey, S.; Newcomer, J.B.; Raissi, D.; Gabriel, G. Percutaneous microwave ablation for early-stage intrahepatic cholangiocarcinoma: A single-institutional cohort. J. Clin. Imaging Sci. 2024, 14, 4. [Google Scholar] [CrossRef]
- Song, M.; Li, J.; Li, Y.; Zhang, C.; Sigdel, M.; Hou, R.; Jiao, D.; Zhou, X. Efficacy of microwave ablation for intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Quant. Imaging Med. Surg. 2025, 15, 76069–76769. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Li, J.; Dou, J.; Li, Z.; Li, L.; Li, K.; Chen, Q.; An, C.; Zhou, Z.; He, G.; et al. Microwave ablation versus liver resection for primary intrahepatic cholangiocarcinoma within Milan criteria: A long-term multicenter cohort study. eClinicalMedicine 2024, 67, 102336. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, J. Application of immune checkpoint inhibitors in the treatment of cholangiocarcinoma. Technol. Cancer Res. Treat. 2021, 20, 15330338211039952. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Bouattour, M.; Okusaka, T.; Qin, S.; Chen, L.-T.; Kitano, M.; Lee, C.; Kim, J.W.; Chen, M.-H.; et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): Updated overall survival from a randomised phase 3 study. Lancet Gastroenterol. Hepatol. 2024, 9, 694–704. [Google Scholar] [CrossRef]
- Casak, S.J.; Kumar, V.; Song, C.; Yuan, M.; Amatya, A.K.; Cheng, J.; Mishra-Kalyani, P.S.; Tang, S.; Lemery, S.J.; Auth, D.; et al. Fda approval summary: Durvalumab and pembrolizumab, immune checkpoint inhibitors for the treatment of biliary tract cancer. Clin. Cancer Res. 2024, 30, 3371–3377. [Google Scholar] [CrossRef]
- Appleton, E.; Hassan, J.; Chan Wah Hak, C.; Sivamanoharan, N.; Wilkins, A.; Samson, A.; Ono, M.; Harrington, K.J.; Melcher, A.; Wennerberg, E. Kickstarting immunity in cold tumours: Localised tumour therapy combinations with immune checkpoint blockade. Front. Immunol. 2021, 12, 754436. [Google Scholar] [CrossRef] [PubMed]
- Moond, V.; Maniyar, B.; Harne, P.S.; Bailey-Lundberg, J.M.; Thosani, N.C. Harnessing endoscopic ultrasound-guided radiofrequency ablation to reshape the pancreatic ductal adenocarcinoma microenvironment and elicit systemic immunomodulation. Explor. Target. Anti-Tumor Ther. 2024, 5, 1056–1073. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, J.S.S.; Ray, P.; Hayashi, T.; Whisenant, T.C.; Vicente, D.; Carson, D.A.; Miller, A.M.; Schoenberger, S.P.; White, R.R. Irreversible electroporation combined with checkpoint blockade and tlr7 stimulation induces antitumor immunity in a murine pancreatic cancer model. Cancer Immunol. Res. 2019, 7, 1714–1726. [Google Scholar] [CrossRef]


| Feature | Photodynamic Therapy (PDT) | Radiofrequency Ablation (RFA) |
|---|---|---|
| Mechanism | Light-activated photosensitizer generates reactive oxygen species and induces tumor necrosis | High-frequency electrical current produces localized thermal coagulative necrosis |
| Invasiveness | Minimally invasive (endoscopic delivery via ERCP) | Minimally invasive (endoscopic via ERCP or percutaneous) |
| Tumor targeting | Selective photosensitizer uptake in tumor tissue, spares healthy tissues | Requires direct probe contact |
| Efficacy (stent patency) | Often superior in prolonging stent patency | Prolongs stent patency but generally less than PDT |
| Efficacy (survival benefit) | Often with greater survival benefit | Survival benefit observed but generally less than PDT |
| Complications | Photosensitivity reactions (must avoid sunlight for 4–6 weeks), cholangitis | Post-procedure pain, cholangitis, pancreatitis or bleeding |
| Limitations | Limited depth of penetration, photosensitivity precautions needed | Risk of thermal injury to adjacent structures, less effective for larger tumors |
| Availability | Requires special drugs and light source, limited to experienced centers | More widely available and easer to implement |
| Cost | High (porfimer sodium ~$37,000 per dose + equipment) | Lower cost (catheter ~$1295); more cost-effective in most settings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natha, C.; Vemulapalli, V.; Thosani, N. Endoscopic Ablation in Cholangiocarcinoma. Cancers 2025, 17, 2843. https://doi.org/10.3390/cancers17172843
Natha C, Vemulapalli V, Thosani N. Endoscopic Ablation in Cholangiocarcinoma. Cancers. 2025; 17(17):2843. https://doi.org/10.3390/cancers17172843
Chicago/Turabian StyleNatha, Cristina, Varun Vemulapalli, and Nirav Thosani. 2025. "Endoscopic Ablation in Cholangiocarcinoma" Cancers 17, no. 17: 2843. https://doi.org/10.3390/cancers17172843
APA StyleNatha, C., Vemulapalli, V., & Thosani, N. (2025). Endoscopic Ablation in Cholangiocarcinoma. Cancers, 17(17), 2843. https://doi.org/10.3390/cancers17172843





