Stereotactic Body Radiotherapy of Colorectal Cancer Oligometastases to the Liver: Three Years Follow-Up
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Methodology of SBRT
2.3. Patient Characteristics
2.4. Endpoints and Assessment Methods
3. Results
3.1. Toxicity
3.2. Long-Term Results
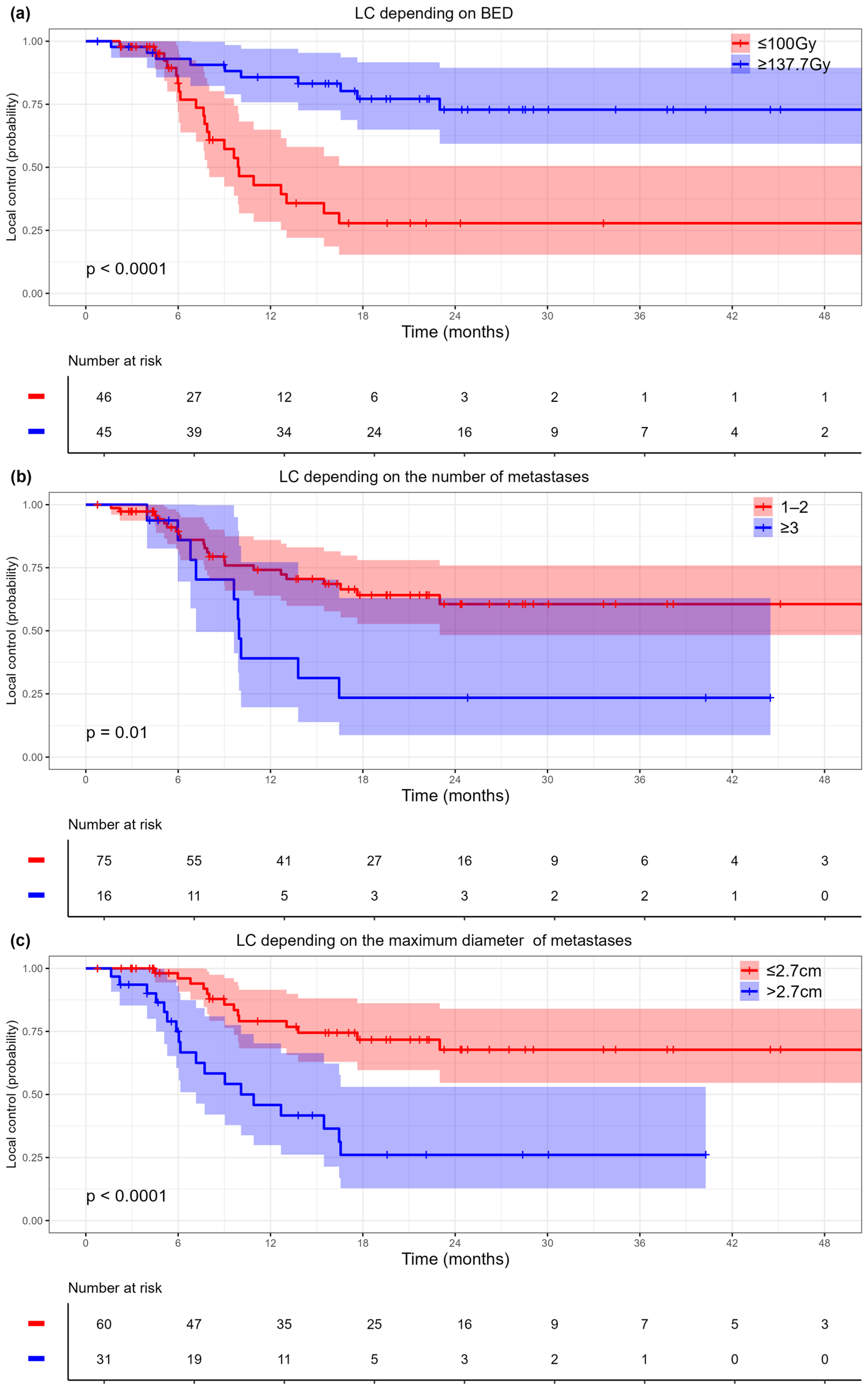
3.2.1. Local Control
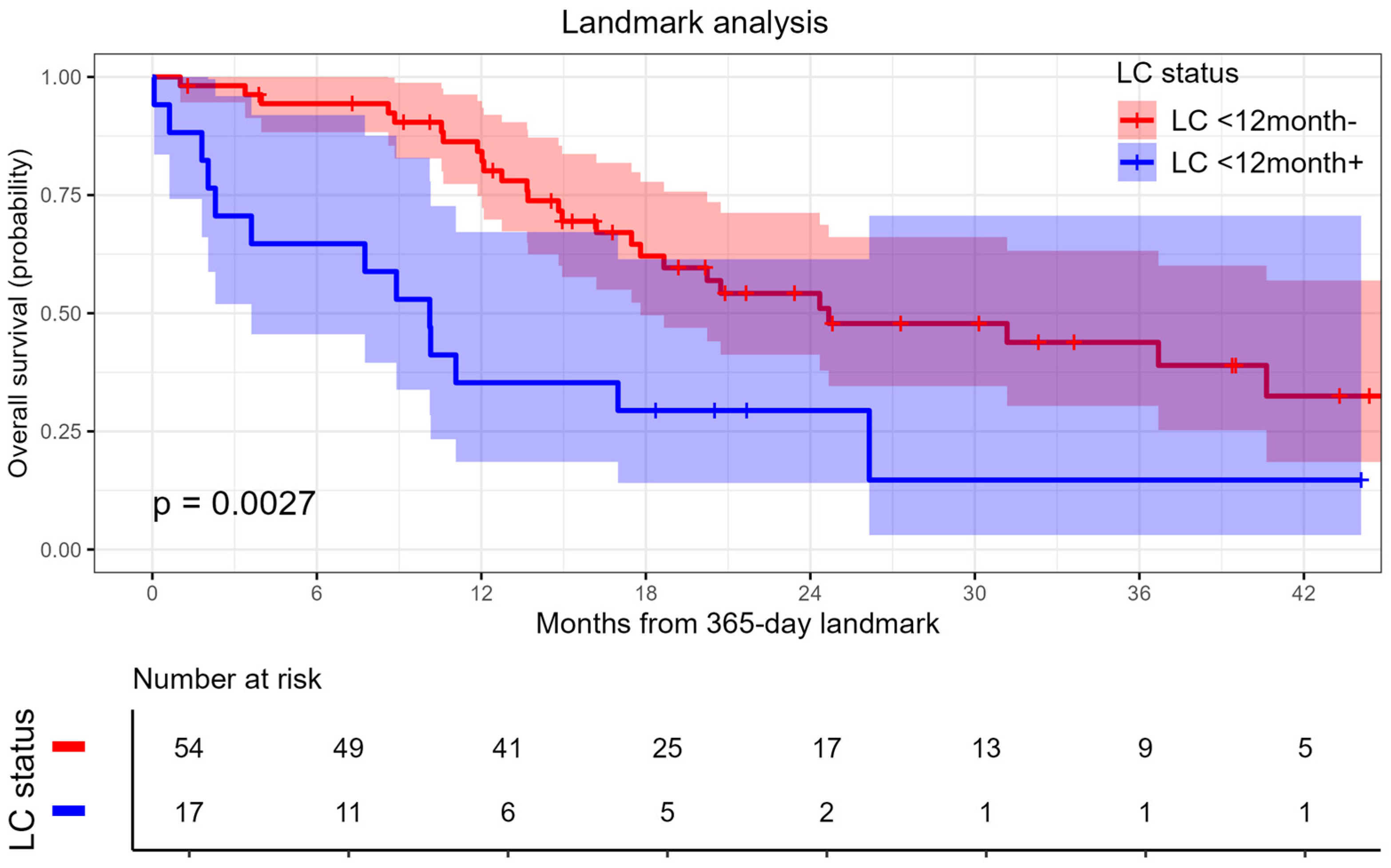
3.2.2. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef]
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A multidisciplinary international consensus. Oncologist 2012, 17, 1225–1239. [Google Scholar] [CrossRef]
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Gold, J.S.; Are, C.; Kornprat, P.; Jarnagin, W.R.; Gönen, M.; Fong, Y.; DeMatteo, R.P.; Blumgart, L.H.; D’Angelica, M. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: Trends in treatment over time in 440 patients. Ann. Surg. 2008, 247, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Van Cutsem, E.; Rougier, P.; Köhne, C.H.; Ychou, M.; Sobrero, A.; Adam, R.; Arvidsson, D.; Carrato, A.; Georgoulias, V.; et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur. J. Cancer 2007, 43, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Aloia, T.A.; Vauthey, J.N.; Loyer, E.M.; Ribero, D.; Pawlik, T.M.; Wei, S.H.; Curley, S.A.; Zorzi, D.; Abdalla, E.K. Solitary colorectal liver metastasis: Resection determines outcome. Arch. Surg. 2006, 141, 460–466. [Google Scholar] [CrossRef]
- Petrelli, F.; Coinu, A.; Zaniboni, A.; Pietrantonio, F.; Barni, S. Prognostic factors after R0 resection of colorectal cancer liver metastases: A systematic review and pooled-analysis. Rev. Recent Clin. Trials 2016, 11, 56–62. [Google Scholar] [CrossRef]
- Vaugier, L.; Mirabel, X.; Martel-Lafay, I.; Racadot, S.; Carrie, C.; Vendrely, V.; Mahé, M.-A.; Senellart, H.; Raoul, J.-L.; Campion, L.; et al. Radiosensitizing chemotherapy (Irinotecan) with stereotactic body radiation therapy for the treatment of inoperable liver and/or lung metastases of colorectal cancer. Cancers 2021, 13, 248. [Google Scholar] [CrossRef]
- Franzese, C.; Comito, T.; Franceschini, D.; Clerici, E.; Navarria, P.; Tomatis, S.; Reggiori, G.; Fogliata, A.; Tozzi, A.; Iftode, C.; et al. Recursive partitioning model-based analysis for survival of colorectal cancer patients with lung and liver oligometastases treated with stereotactic body radiation therapy. J. Cancer Res. Clin. Oncol. 2020, 146, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.D.; Mannina, E.M.; Althouse, S.K.; Maluccio, M.A.; Cárdenes, H.R. Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Pract. Radiat. Oncol. 2016, 6, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Scorsetti, M.; Comito, T.; Tozzi, A.; Navarria, P.; Fogliata, A.; Clerici, E.; Mancosu, P.; Reggiori, G.; Rimassa, L.; Torzilli, G.; et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Voglhuber, T.; Eitz, K.A.; Oechsner, M.; Vogel, M.M.E.; Combs, S.E. Analysis of using high-precision radiotherapy in the treatment of liver metastases regarding toxicity and survival. BMC Cancer 2021, 21, 780. [Google Scholar] [CrossRef]
- McDermott, R.L.; Dunne, E.M.; Zhao, Y.; Chan, E.K.; Swaminath, A.; Warner, A.; Baine, M.J.; Ahmed, K.A. Stereotactic ablative radiation therapy for colorectal liver metastases. Clin. Color. Cancer 2023, 22, 120–128. [Google Scholar] [CrossRef]
- McPartlin, A.; Swaminath, A.; Wang, R.; Pintilie, M.; Brierley, J.; Kim, J.; Ringash, J.; Wong, R.; Dinniwell, R.; Craig, T.; et al. Long-term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 388–395. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Guenther, L.M.; Rowe, R.G.; Acharya, P.T.; Swenson, D.W.; Meyer, S.C.; Clinton, C.M.; Guo, D.; Sridharan, M.; London, W.B.; DuBois, S.G. Response Evaluation Criteria in Solid Tumors (RECIST) following neoadjuvant chemotherapy in osteosarcoma. Pediatr. Blood Cancer 2018, 65, e26896. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermo-Sifiliográficas 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Stera, S.; Miebach, G.; Buergy, D.; Fleckenstein, J.; Lohr, F.; Wenz, F.; Eichhorn, M.E.; Winter, H.; Krempien, R.; Boda-Heggemann, J.; et al. Liver SBRT with active motion-compensation results in excellent local control for liver oligometastases: An outcome analysis of a pooled multi-platform patient cohort. Radiother. Oncol. 2021, 158, 230–236. [Google Scholar] [CrossRef]
- Rodríguez, M.R.; Chen-Zhao, X.; Hernando, O.; Montero, A.; López-Guerra, J.L.; Matute, R.; Eraso, A.; Gaztañaga, M.; Couñago, F.; Luna, J. SBRT-SG-01: Final results of a prospective multicenter study on stereotactic body radiotherapy for liver metastases. Clin. Transl. Oncol. 2024, 26, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.; Erler, D.; Dagan, R.; Redmond, K.; Lo, S.; Foote, M.C.; Yu, E.; Cho, C.B.; Ahn, P.H.; Lee, J.Y.; et al. Evaluation of definitive stereotactic body radiotherapy and outcomes in adults with extracranial oligometastasis. JAMA Netw. Open 2020, 3, e2026312. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Nicosia, L.; Ricardi, U.; Franzese, C.; Cuccia, F.; Figlia, V.; Ruggieri, R.; Salgarello, M.; Mazzola, R.; Pasinetti, N.; et al. Acute toxicity in patients with oligometastatic cancer following metastasis-directed stereotactic body radiotherapy: An interim analysis of the E2-RADIatE OligoCare cohort. Radiother. Oncol. 2024, 199, 110466. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Cheung, P.; Chu, W.; Myrehaug, S.; Poon, I.; Sahgal, A.; Soliman, H. Outcomes of extracranial stereotactic body radiotherapy for metastatic colorectal cancer: Dose and site of metastases matter. Radiother. Oncol. 2020, 142, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Guler, O.C.; Hurmuz, P.; Atalar, B.; Onal, C.; Igdem, S.; Alco, G.; Sager, O.; Dincoglan, F.; Demiral, S.; Uysal, B.; et al. Multi-institutional analysis of extracranial oligometastatic colorectal cancer patients treated with stereotactic body radiation therapy: TROD 02-008 study. Strahlenther. Und Onkol. 2024, 200, 958–966. [Google Scholar] [CrossRef]
- Leite, P.D.; Gaya, A.M.; Lanciano, R.M.; Yang, J.J.; Blanck, O.; Urwin, R.; Davis, J.N.; Mahadevan, A. Stereotactic body radiotherapy (SBRT) for colorectal liver metastasis: Clinical outcomes from the international multi-institutional RSSearch Patient Registry. J. Clin. Oncol. 2019, 37 (Suppl. 15), e15040. [Google Scholar] [CrossRef]
- Kok, E.N.D.; Jansen, E.P.M.; Heeres, B.C.; Aarts, M.J.; Aerts, J.G.J.V.; Arends, M.P.; van den Berkmortel, F.W.P.J.; Boers-Sonderen, M.J.; de Groot, J.W.B.; de Hingh, I.H.J.T.; et al. High versus low dose stereotactic body radiation therapy for hepatic metastases. Clin. Transl. Radiat. Oncol. 2019, 20, 45–50. [Google Scholar] [CrossRef]
- Joo, J.H.; Park, J.H.; Kim, J.C.; Yu, C.S.; Lim, S.B.; Park, I.J.; Kim, T.W.; Hong, Y.S.; Kim, K.P.; Yoon, S.M. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 876–883. [Google Scholar] [CrossRef]
- Sheikh, S.; Chen, H.; Sahgal, A.; Erler, D.; Cheung, P.; Chu, W.; Dagan, R.; Foote, M.; Lo, S.; Redmond, K.; et al. An analysis of a large multi-institutional database reveals important associations between treatment parameters and clinical outcomes for stereotactic body radiotherapy (SBRT) of oligometastatic colorectal cancer. Radiother. Oncol. 2022, 167, 187–194. [Google Scholar] [CrossRef]
- Klement, R.J.; Guckenberger, M.; Alheid, H.; Allgäuer, M.; Becker, G.; Blanck, O.; Boda-Heggemann, J.; Brunner, T.; Duma, M.; Gerum, S.; et al. Stereotactic body radiotherapy for oligo-metastatic liver disease—Influence of pre-treatment chemotherapy and histology on local tumor control. Radiother. Oncol. 2017, 123, 227–233. [Google Scholar] [CrossRef]
- Scorsetti, M.; Comito, T.; Clerici, E.; Franzese, C.; Tozzi, A.; Iftode, C.; Di Brina, L.; Navarria, P.; Mancosu, P.; Reggiori, G.; et al. Phase II trial on SBRT for unresectable liver metastases: Long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat. Oncol. 2018, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All Patients (n = 91) | BED ≤ 100 Gy (n = 46) | BED ≥ 137.7 Gy (n = 45) | p–Value |
|---|---|---|---|---|
| Sex (female), n (%) | 45 (49.5%) | 22 (47.8%) | 23 (51.1%) | 0.834 |
| Age (years), median (min–max) | 62 (27–85) | 62 (31–85) | 61 (27–83) | 0.249 |
| ECOG, n (%): | ||||
| 0–1 | 65 (71.4%) | 30 (65.2%) | 35 (77.8%) | 0.246 |
| 2 | 26 (28.6%) | 16 (34.8%) | 10 (22.2%) | |
| pT, n (%): | ||||
| 2 | 5 (5.5%) | 1 (2.2%) | 4 (8.9%) | 0.477 |
| 3 | 53 (58.2%) | 28 (60.9%) | 25 (55.6%) | |
| 4 | 33 (36.3%) | 17 (37%) | 16 (35.6%) | |
| pN, n (%): | ||||
| 0 | 27 (29.7%) | 13 (28.3%) | 14 (31.1%) | 0.830 |
| 1 | 41 (45.1%) | 20 (43.5%) | 21 (46.7%) | |
| 2 | 23 (25.3%) | 13 (28.3%) | 10 (22.2%) | |
| RAS mutation 1, n (%) | 62 (69.7%) | 32 (72.7%) | 30 (66.7%) | 0.645 |
| Synchronous metastases, n (%) | 38 (41.8) | 21 (45.7) | 17 (37.8) | 0.525 |
| Number of metastases, n (%): | ||||
| 1 | 45 (49.5%) | 20 (43.5%) | 25 (55.6%) | 0.524 |
| 2 | 30 (33%) | 17 (37%) | 13 (28.9%) | |
| ≥3 | 16 (17.6%) | 9 (19.6%) | 7 (15.6%) | |
| Diameter of metastasis (cm), median (min–max) | 2.5 (0.8–6.5) | 2.5 (0.8–7) | 1.9 (0.8–6.5) | 0.075 |
| Previous liver resection, n (%) | 67 (73.6) | 32 (69.6) | 35 (77.8) | 0.477 |
| Prior chemotherapy (lines), median (min–max) | 2 (0–4) | 2 (0–3) | 2 (0–4) | 0.813 |
| Extrahepatic metastases, n (%) | 24 (26.4) | 11 (23.9) | 13 (28.9) | 0.639 |
| Type of oligometastases 2, n (%): | ||||
| De novo | 24 (26.7%) | 14 (31.1%) | 10 (22.2%) | 0.467 |
| Induced | 20 (22.2%) | 11 (24.4%) | 9 (20%) | |
| Repeated | 46 (51.1%) | 20 (44.4%) | 26 (57.8%) |
| Univariate Analysis | Multivariate Analysis * | |||
|---|---|---|---|---|
| Predictor | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Sex (male vs. female) | 1.78 (0.89–3.57) | 0.104 | ||
| Age, years | 1 (0.97–1.02) | 0.799 | ||
| Prior chemotherapy, lines | 1.17 (0.83–1.67) | 0.37 | ||
| ECOG (2 vs. 0–1) | 1.02 (0.46–2.28) | 0.961 | ||
| RAS mutation | 1.82 (0.9–3.69) | 0.096 | ||
| Microsatellite instability | 0.62 (0.08–4.51) | 0.633 | ||
| pT | 1.23 (0.68–2.25) | 0.495 | ||
| pN (1–2 vs. 0) | 1.18 (0.55–2.53) | 0.68 | ||
| Extrahepatic metastases | 0.69 (0.3–1.59) | 0.387 | ||
| Previous liver resection | 1 (0.45–2.22) | 0.999 | ||
| Type (synchronous vs. metachronous) | 1.18 (0.58–2.42) | 0.642 | ||
| Diameter of metastasis, cm | 1.38 (1.13–1.68) | 0.001 | ||
| Diameter of metastasis (>2.7 cm vs. ≤2.7 cm) | 3.77 (1.86–7.63) | <0.001 | 2.73 (1.32–5.59) | <0.001 |
| The number of metastases | 1.51 (1.07–2.12) | 0.018 | ||
| The number of metastases (≥3 vs. 1–2) | 2.59 (1.22–5.49) | 0.013 | 2.24 (1.05–4.77) | 0.037 |
| BED (≥137.7 Gy vs. ≤100 Gy) | 0.21 (0.1–0.45) | <0.001 | 0.25 (0.12–0.55) | <0.001 |
| Univariate Analysis | Multivariate Analysis * | |||
|---|---|---|---|---|
| Predictor | HR (95% CI) | p–Value | HR (95% CI) | p–Value |
| Sex (male vs. female) | 1.03 (0.56–1.87) | 0.93 | ||
| Age, years | 1.01 (0.99–1.03) | 0.33 | ||
| Prior chemotherapy, lines | 1.2 (0.89–1.61) | 0.23 | ||
| ECOG (2 vs. 0–1) | 1.56 (0.79–3.07) | 0.2 | ||
| RAS mutation | 2.09 (1.12–3.88) | 0.02 | 2.27 (1.21–4.25) | 0.01 |
| Microsatellite instability | 1.04 (0.25–4.34) | 0.953 | ||
| pT | 1.04 (0.62–1.74) | 0.887 | ||
| pN (1–2 vs. 0) | 0.85 (0.46–1.57) | 0.597 | ||
| Extrahepatic metastases | 1.07 (0.55–2.08) | 0.843 | ||
| Previous liver resection | 1.79 (0.86–3.73) | 0.12 | ||
| Type (synchronous vs. metachronous) | 2.31 (1.19–4.49) | 0.013 | 2.11 (1.04–4.26) | 0.037 |
| Diameter of metastasis, cm | 1.25 (1.03–1.52) | 0.026 | ||
| Diameter of metastasis (>2.6 cm vs. ≤2.6 cm) | 2.2 (1.21–3.98) | 0.009 | 2.03 (1.07–3.83) | 0.03 |
| The number of metastases | 1.36 (1.03–1.81) | 0.032 | ||
| The number of metastases (≥3 vs. 1–2) | 2.2 (1.12–4.26) | 0.022 | 1.85 (0.93–3.63) | 0.076 |
| BED (≥137.7 Gy vs. ≤100 Gy) | 1.13 (0.61–2.1) | 0.688 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskalenko, A.; Chernykh, M.; Ichshanov, D.; Malinina, K.; Ikonnikova, A.; Lyadov, V. Stereotactic Body Radiotherapy of Colorectal Cancer Oligometastases to the Liver: Three Years Follow-Up. Cancers 2025, 17, 2823. https://doi.org/10.3390/cancers17172823
Moskalenko A, Chernykh M, Ichshanov D, Malinina K, Ikonnikova A, Lyadov V. Stereotactic Body Radiotherapy of Colorectal Cancer Oligometastases to the Liver: Three Years Follow-Up. Cancers. 2025; 17(17):2823. https://doi.org/10.3390/cancers17172823
Chicago/Turabian StyleMoskalenko, Alexey, Marina Chernykh, Damir Ichshanov, Ksenia Malinina, Anna Ikonnikova, and Vladimir Lyadov. 2025. "Stereotactic Body Radiotherapy of Colorectal Cancer Oligometastases to the Liver: Three Years Follow-Up" Cancers 17, no. 17: 2823. https://doi.org/10.3390/cancers17172823
APA StyleMoskalenko, A., Chernykh, M., Ichshanov, D., Malinina, K., Ikonnikova, A., & Lyadov, V. (2025). Stereotactic Body Radiotherapy of Colorectal Cancer Oligometastases to the Liver: Three Years Follow-Up. Cancers, 17(17), 2823. https://doi.org/10.3390/cancers17172823






