Simple Summary
The human microbiome plays a crucial role in the development and treatment of head and neck squamous cell carcinoma (HNSCC). Recently, a strong association between specific microbial alterations, such as increased levels of Fusobacterium nucleatum and Porphyromonas gingivalis, and the incidence of HNSCC was described. Our narrative review aims to explain how the oral microbiome influences the efficacy of various treatments, including chemotherapy and radiotherapy, noting that specific bacterial profiles may predict treatment responses. Additionally, the potential of microbiome modulation, through probiotics, to improve patient outcomes and reduce treatment-related toxicities like oral mucositis was described. Despite the promising links between microbiome composition and cancer treatment, further research to establish definitive causal relationships and therapeutic applications in HNSCC management is needed.
Abstract
The oral microbiome is the largest and most diverse microbiome in the human body, second only to the gut microbiome. Mounting evidence supports its role in the genesis, promotion, and aggressiveness of oral cancer as well as certain cancers of distant sites. In this review, we focus on describing specific microbial alterations, which were proven to be associated either with head and neck squamous cell carcinoma (HNSCC) development or with its treatment efficacy, especially with radiotherapy, chemotherapy, targeted treatment, and immunotherapy. Purpose: To discuss associations of oral and gut microbiome with the development of HNSCC and with its anti-cancer treatment efficacy. Methods: A literature search was conducted in PubMed/Medline. Due to the nature of this narrative review, we prioritized the most recent studies. Regarding the type of studies, umbrella reviews, meta-analyses, and systematic reviews were prioritized, in that order, over individual studies. Results: The microbiome plays an important role in cancer development, progression, and treatment response across solid tumors, including HNSCC. Better treatment response and/or reduction in treatment toxicities can be achieved through microbiome alteration; e.g., the use of specific probiotics can result in a reduction in acute radiotherapy-induced mucositis in HNSCC radiotherapy, and fecal microbiota transplantation from immune-checkpoint inhibitor (ICI) responders to non-responders can overcome primary resistance to ICI. Conclusions: We highlighted specific research areas on how to utilize microbiome modification to enhance anti-cancer treatment response and/or decrease the incidence and severity of anti-cancer treatment toxicities.
Keywords:
microbiome; head and neck; HNSCC; radiotherapy; chemotherapy; immunotherapy; tumor microenvironment 1. Introduction
Despite decades of research, cancer remains a major global health problem. In recent years, the role of microorganisms in the initiation and development of cancer has been increasingly investigated. Head and neck squamous cell carcinomas (HNSCC) currently account for ca. 4% of all malignancies, making it the sixth most common cancer worldwide. The incidence of HNSCC has been on the rise worldwide [1]. HNSCC covers a group of heterogeneous cancers of distinguished anatomical locations (oral cavity, oropharynx, pharynx, and larynx), pathoetiology, and molecular characteristics [2].
HNSCC has been associated with the carriage of several oral bacteria (e.g., Porphyromonas gingivalis, Fusobacterium nucleatum, Streptococcus spp.), some viruses (e.g., HPV, HHV-8, HSV, or EBV), and yeasts (Candida albicans). The specificity of the oral microbiome lies in the fact that a significant correlation has been demonstrated between oral dysbiosis, which can be caused by chronic alcoholism, smoking, malhygiene, microbial infections, and tumors of the oral cavity, as well as tumors of distant sites such as the oropharynx, esophagus, pancreas, colorectum, breast, prostate, or lungs [3,4,5,6].
Meta-analyses have shown that dysbiosis significantly increases the risk of oral cancer [7,8]. Among the identified microbial agents whose abundance is significantly higher in oral SCC tissue, or in other areas of the oral cavity, are, e.g., F. nucleatum, Pseudomonas aeruginosa, and P. gingivalis [9,10,11].
Treatment of HNSCC is complex and should be decided within the multidisciplinary team (otorhinolaryngologist, clinical oncologist, radiation oncologist, etc.). For the localized and locally advanced disease, surgery and/or (chemo)radiotherapy are the methods of choice. For recurrent and/or metastatic disease (R/M HNSCC), systemic therapy is implemented, often with the palliative goal [12].
Although little is known about oral microbial changes after surgery for HNSCC, there is information regarding gut microbiome alterations in patients after surgical treatment for colorectal cancer. A unique microenvironment resembling the microbiome of neither healthy controls nor the colorectal patients was reported [13]. Large variations exist among patients in tumor radio-responsiveness and in the incidence and severity of radiotherapy-induced side effects like dermatitis or mucositis. Exposure to ionizing radiation leads to changes in the microbiome that can contribute to more severe side effects [14,15,16,17]. The shift in microbial representation has been shown to affect the treatment response to radiotherapy and could potentially be associated with early recurrence and worse prognosis. Some studies have already proposed that the microbiome can be modified to maximize treatment response and minimize adverse effects using probiotics or prebiotics in head and neck cancer [18]. However, the oral microbiome, in terms of predictive and prognostic biomarkers, has yet to be investigated in detail.
Growing evidence supports not only the role of the microbiome in the development and progression of cancer but also the hypothesis that the host microbiome can affect anti-cancer chemotherapy efficacy and toxicity [19]. Chemotherapy (CHT) was for a long time the cornerstone of R/M HNSCC treatment; however, with the arrival of immunotherapy (immune-checkpoint inhibitors, ICIs), the treatment was revolutionized, and the therapeutic paradigm has shifted toward a preference for ICIs. Because of their structural and molecular heterogeneity, it has been challenging to develop targeted therapeutics. Cetuximab (monoclonal antibody against epidermal growth factor receptor, EGFR) remains the only targeted treatment for HNSCC [12].
Despite advances in our knowledge of the risk factors associated with HNSCC, survival rates for these cancers have not improved significantly. This only highlights the urgency of finding new means of early primary and/or tertiary prevention of these diseases. Understanding the oral microbiome and identifying risk agents; e.g., identifying protective organisms for prophylactic use or associated pathogens for targeted eradication may not only have an impact on primary prevention but could also open new possibilities for adjuvant treatment in terms of probiotic administration to reduce the risk of recurrence or metachronous cancer, or to reduce the incidence and severity of radiation-induced oral mucositis (RIOM). Ultimately, fully comprehending how the human microbiome, the oral as well as the gut microbiome, affects the response to systemic treatment in cancer patients will open new possibilities of personalized medicine enabling us to choose the most effective treatment strategy, and/or to modify a patient’s microbiome to receive the best treatment efficacy.
2. Methods
A literature search was conducted in PubMed/Medline (prior to 29 June 2024) to retrieve relevant articles on this topic. The keywords used for database search were “microbiome”, “head and neck cancer”, “head and neck squamous cell carcinoma”, “radiotherapy”, “chemotherapy”, “cetuximab”, “immunotherapy”, and “surgery”. Only English language articles were considered. Due to the nature of this narrative review, we prioritized the most recent studies. Regarding the type of studies, umbrella reviews, meta-analyses, and systematic reviews were prioritized, in that order, over individual studies. We excluded letters to the editor, editorials, and conference reports.
3. Results
3.1. Microbial Risk Factors of Developing Head and Neck Cancer
Although viral oncogenesis has been extensively studied since the 20th century, scientific knowledge of bacterial oncogenesis has not been well elucidated [20]. The first reports about oncogenic microbiome date back to the 19th century, when Virchow described pro-cancerogenic effects of Schistosoma on the urinary bladder [21]. His hypothesis of chronic inflammation remains one of the widely supported mechanisms of bacterial carcinogenesis, the other ones being antigen-driven proliferation and metabolite secretion (hormones, oncoproteins, and/or toxic metabolites) [20,22].
Emerging evidence promotes oral microbes as potential biomarkers for various malignancies, e.g., of the oral cavity as well as of distant sites, and even premalignant lesions [23]. The most common premalignant lesions in the oral cavity are leukoplakia, erythroplakia, and erythroleukoplakia, sometimes labeled as oral potentially malignant disorders (OPMD) [24]. Identifying specific oral microbiota associated with OPMD and/or early-stage HNSCC could initiate research efforts to develop screening programs. Such an initiative could reduce morbidity and mortality as the proper treatment could be started in earlier stages. Lee et al. reported significant differences in the microbiome of healthy controls, patients with OPMD, and patients with oral squamous cell carcinoma (OSCC). They demonstrated the significantly higher abundance of Fusobacterium spp., Prevotella spp., Porphyromonas spp., Veillonella spp., Actinomyces spp., Clostridium spp., Haemophilus spp., Streptococcus spp., and Enterobacteriaceae in patients with OPMD and OSCC compared to the healthy controls. Furthermore, they reported significant differences in the abundance of Bacillus spp., Enterococcus spp., Parvimonas spp., Peptostreptococcus spp., and Slackia spp. between OPMD and OSCC [25]. This can imply that the microbiome plays a crucial role in the carcinogenesis of OSCC. If we could identify the causative mechanism behind OPMD development or its transformation into OSCC, we could design a proper intervention and thus modify the oral microbiome to reduce the chances of HNSCC development [26,27,28,29].
HNSCC represents a heterogeneous group of diseases of various anatomical sites, cellular origin, and different pathoetiology [30]. Poor oral health and oral dysbiosis are associated with tumors of the oral cavity, as well as tumors of distant sites [3,4,5,6]. Such dysbiosis leads to immune dysregulation, which contributes to the development of various diseases, including systemic infections and solid and hematological malignancies [31,32]. The oncogenic microbiome has synergistic effects with other carcinogens. Several studies have described that certain oral microbiome alterations caused by smoking, hygiene, diet, and alcohol consumption enhance tumor development; however, no consensus data exist at this time [7,8,24,33,34,35,36,37]. Frank et al. reported a significantly higher diversity of oral microbiome in HNSCC compared to healthy controls; moreover, in HNSCC patients, significant interindividual variability was observed across bacterial profiles [38]. Some concordance has been found in the varied data; however, no clear pattern has yet been revealed. The reasons behind such high variety in results can be many, e.g., sample type (oral swabs, saliva, tumor tissue samples, etc.), tumor stage, comorbidities, variations in gut microbiome, etc. Among the identified microbial agents significantly associated with the development of oral cancer are P. gingivalis, F. nucleatum, and Ps. aeruginosa [9,10,11]. Wang et al. observed significant differences in the abundance of Actinomyces spp. and Parvimonas spp. in HNSCC patients and healthy controls. In the HNSCC patients’ microbiome, Actinomyces spp. were rather depleted, and the representation of Parvimonas spp. was higher compared to the healthy controls. Moreover, these results correlated with the T stage of tumors [39].
As it seems now, many distinctive pro-cancerous mechanisms are applied in microbial oncogenesis, ultimately leading to a proinflammatory state, e.g., production of lipopolysaccharides, enzymes, bacterial toxins, etc. [40]. Streptococcus salivarius, Corynebacterium spp., and Stomatococcus spp. show strong oxidizing properties, which they utilize to convert alcohol into acetaldehyde, which can then interfere with DNA synthesis and repair mechanisms [41,42]. P. gingivalis stimulates the production of dendritic precursor cells, which inhibit cytotoxic CD8+ lymphocytes; it also stimulates the transcription of proteins facilitating cell division, subsequently promoting cell proliferation [43,44].
Incongruent with the above-mentioned studies, Kumpitsch et al. reported no significant difference in the diversity of microbiota between HNSCC patients and controls; however, significant differences in the abundance of microbes were reported—the abundance of the genera Veillonella, Rothia, and Haemophilus from the phylum Firmicutes was significantly higher in HNSCC patients [45]. Similarly, Mäkinen et al. observed a direct association of OSCC and the oral microbiome. Microbial diversity was not significantly affected, but the relative abundance of S. anginosus, Abiotrophia defectiva, and F. nucleatum was significantly higher in HNSCC patients. On the contrary, Prevotella histolitica, Haemophilus parainfluenzae, and F. periodonticum were dominantly represented in the healthy cohort [46]. Huang et al. observed a specific microbiome in the HNSCC tumor tissue. On the surface of oral SCCs, both anaerobes, e.g., Actinomyces spp., Clostridium spp., Fusobacterium spp., Porphyromonas spp., and Bacteroides spp., and aerobes, e.g., Klebsiella spp., Citrobacter spp., Streptococcus spp., Enterobacter spp., and Serratia spp., were identified. S. anginosus colonizes dental plaques and produces nitric oxide and cyclooxygenase-2, facilitating DNA damage [47].
Across most of the studies, a similar pattern of increased abundance of Lactobacillus spp. and decreased abundance of Neisseria spp. was observed in HNSCC patients compared to healthy controls [48,49,50,51,52,53,54,55]. These two genera are consistently found differentially abundant in oral, oropharyngeal, and laryngeal cancers, suggesting potential cancer-protective properties of Neisseria spp. and cancer-promoting properties of Lactobacillus spp. Frank et al. established an OSCC model in mice by chemically induced carcinogenesis (4-nitroquinoline-1-oxide, 4NQO), in which a direct link between oral dysbiosis and OSCC dynamics was demonstrated. As they proposed, a myriad of mechanisms are involved in this bacterial-induced cancerogenesis. They hypothesized that Lactobacillus spp. promotes cancer development via production of tryptophan and its metabolites, which act as a cancer-promoting aryl-hydrocarbon receptor (AhR) pathway activator. AhR activation is a critical pathway in many cancers, including HNSCC, which enhances cancer stem cell migration [38].
Healthy oral microbiome composition is also crucial for preventing periodontitis, which is strongly associated with HNSCC development [56]. Dental plaque biofilms cause chronic inflammation, which leads to the irreversible destruction of tooth-supporting tissues. This process is facilitated via many bacteria; however, F. nucleatum appears to play the crucial role. F. nucleatum is essential in establishing polymicrobial plaques. It helps to navigate other bacteria, such as streptococcal species, S. anginosus, or P. gingivalis, into the plaque [57]. Currently, only one meta-analysis investigated the plausible association between F. nucleatum and HNSCC. Bronzatoa et al. reported its higher abundance in HNSCC patients compared to controls. However, as the authors discussed, its presence can be only a result of a hypoxic environment within the dental plaque, and no direct evidence supporting the hypothesis of F. nucleatum-mediated tumor transformation exists [58]. On the other hand, F. nucleatum directly interacts with epithelial cells, promoting cancerogenesis, faster growth, and spread of HNSCC. F. nucleatum also produces FadA adhesins, which bind to E-cadherin receptors on endothelial cells, enabling them to hematogenously transfer to distant niches [59].
The prognostic effects of F. nucleatum on disease/relapse-free survival in HNSCC were investigated by two independent trials. They reported that F. nucleatum was significantly associated with longer overall survival and a lower relapse rate [60,61]. These findings are quite unexpected, especially considering its association with higher aggressiveness and worse prognosis in other cancer types, e.g., esophageal, colorectal, and pancreatic carcinomas [62,63,64,65,66]. The reason why is currently unknown; however, F. nucleatum was observed to be significantly more abundant in HPV+ HNSCC, which represents a distinctive entity. HPV+ HNSCCs usually affect younger people, non-smokers, non- or just slight alcohol consumers, and are associated with better prognosis [60,61]. Data on bacterial oncogenesis in these patients are limited and require further scientific research.
3.2. Pathologic Mechanisms of F. nucleatum-Facilitated Impact on HNSCC
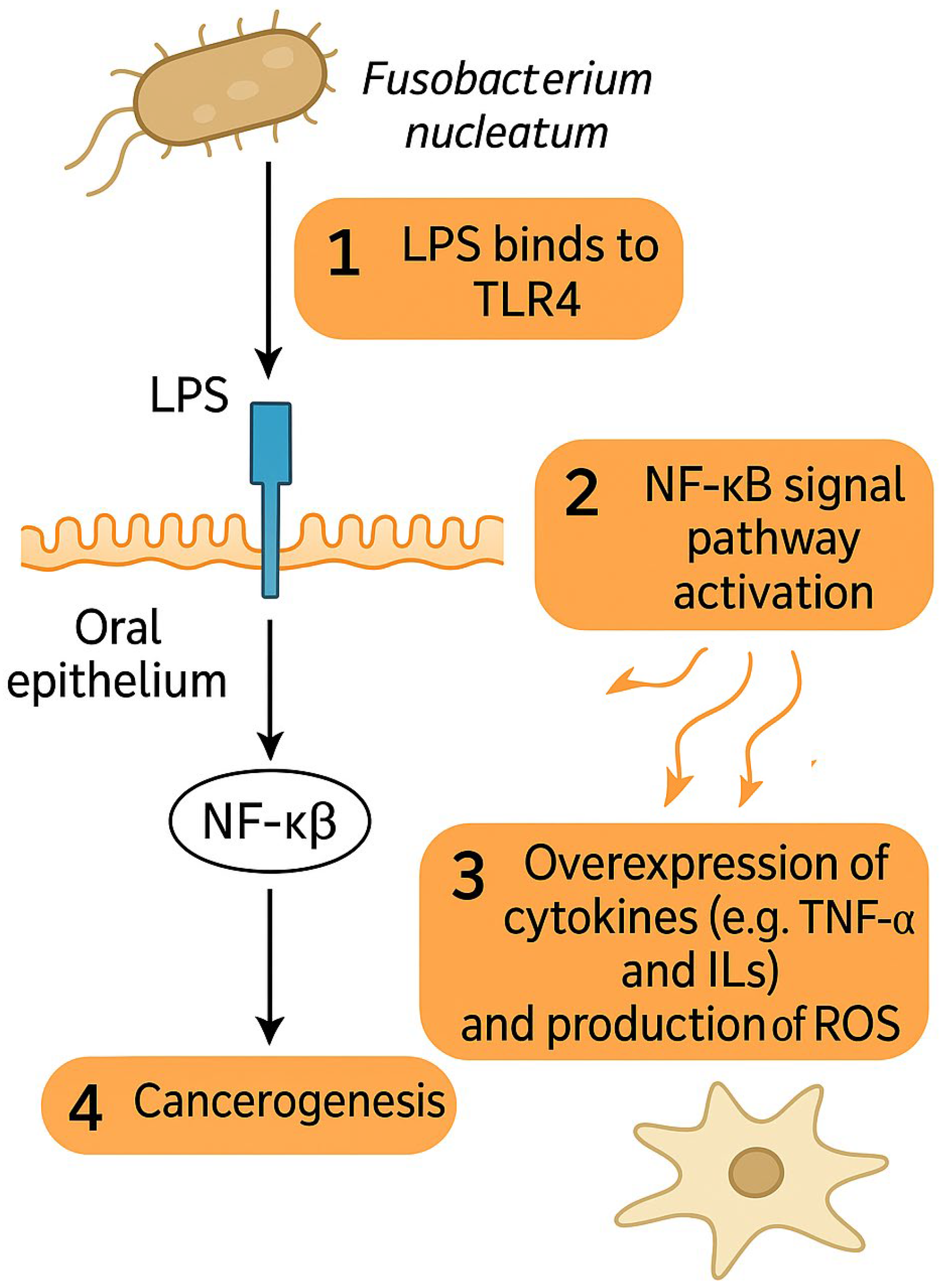
The impact of F. nucleatum on HNSCC development has recently become an object of scientific interest and research. Carcinogenic properties of F. nucleatum have mostly been attributed to its interaction with Toll-like receptors (TLRs) of oral epithelium and to its pro-inflammatory effects and are depicted in Figure 1 [67]. Lipopolysaccharides of the cell membrane of F. nucleatum can directly bind to the TLR4 of epithelial cells and thus activate the NF-κB signaling pathway, leading to the over-expression of various cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-8, IL-10, IL-12, and reactive oxygen species (ROS) production, similar to that observed in colonic cancer [67,68,69,70]. OSCC infiltrated with F. nucleatum have also been observed to exhibit increased invasiveness, migration, and proliferation of cancer cells [71,72,73]. F. nucleatum was observed to facilitate functional loss of E-cadherin (CDH1), increased cell migration, and the upregulation of Snail family transcriptional repressor 1 (SNAI1) in cancerous as well as non-cancerous oral epithelial cells, indicating epithelial–mesenchymal transition (EMT) [74].
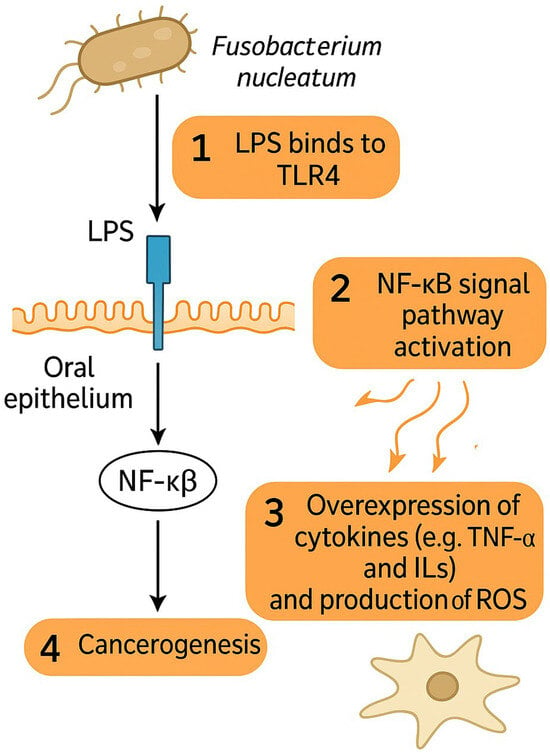
Figure 1.
F. nucleatum promotes cancerogenesis via interaction of LPS with TLR4 on epithelial cells, which leads to overexpression of cytokines and ROS via NF-κB signal pathway activation. Abbreviations: LPS—lipopolysaccharide, TLR4—Toll-like receptor 4, NF-κB—Nuclear factor kappa-light-chain-enhancer of activated B cells, TNF-α—tumor necrosis factor alpha, ROS—reactive oxygen species.
How and when F. nucleatum colonizes HNSCC tissue is still unclear. Recent evidence supports that F. nucleatum uses its Fap2 adhesin to bind to a Gal-GalNAc oligosaccharide on cancer cells; however, these findings come from breast and colon cancer models [75,76]. No studies have yet investigated whether OSCC colonization with F. nucleatum is facilitated via the Fap2/Gal-GalNAc mechanism, although indirect evidence of Gal-GalNAc overexpression in OSCC cells coinciding with increased abundance of F. nucleatum supports this theory [77,78].
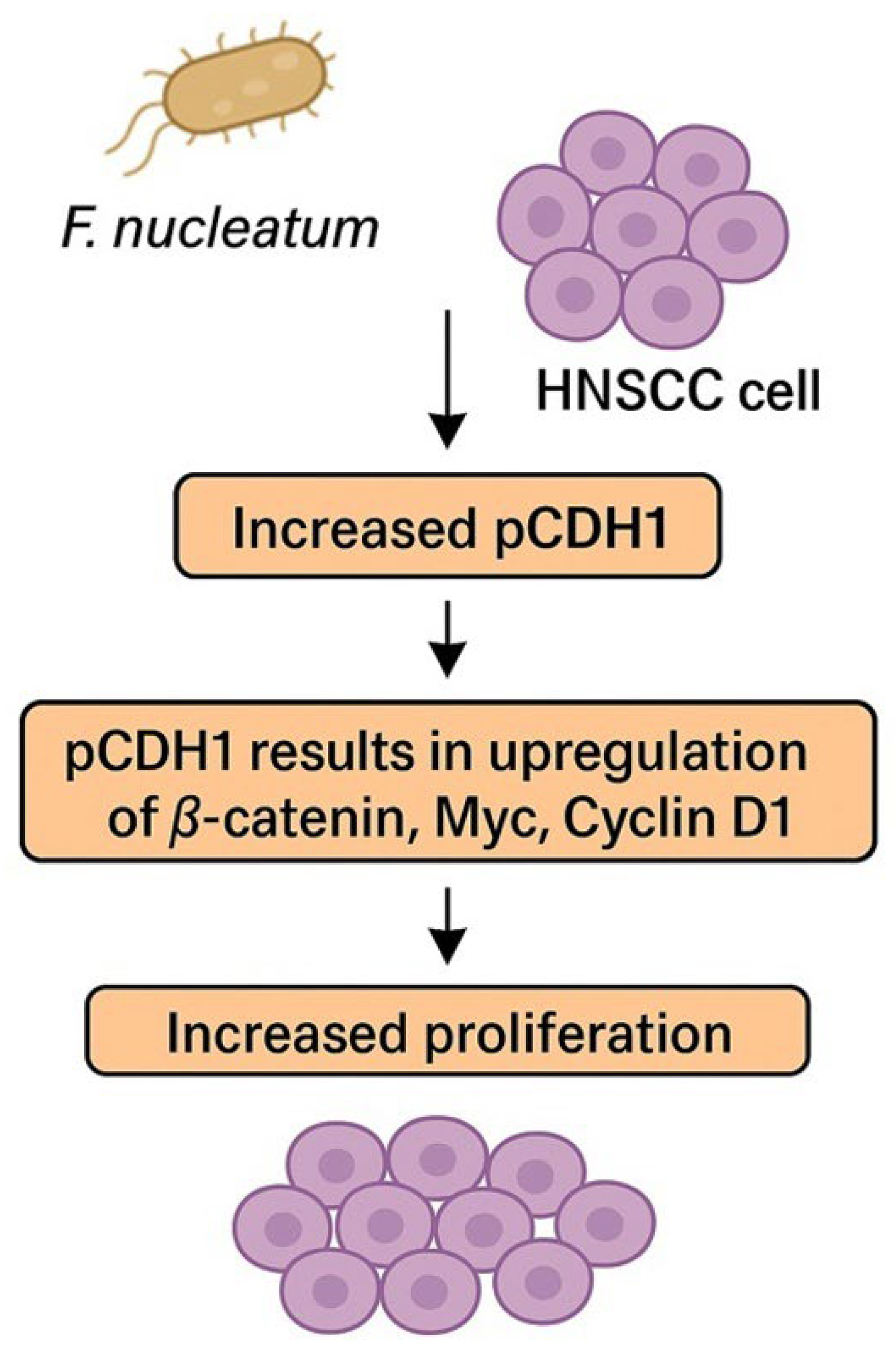
The CDH1 pathway seems to play a pivotal role in accelerating the OSCC cell cycle and stimulating cell proliferation [79]. In preclinical models, higher representation of phosphorylated CDH1 (pCDH1) was found in OSCC cells infiltrated with F. nucleatum. pCDH1 results in the upregulation of β-catenin, Myc, and Cyclin D1, subsequently inducing cell proliferation. The pathway is summarized in Figure 2. Cyklin D1 is often overexpressed in OSCC and is associated with tumor progression, higher grade, and poor prognosis [79,80,81]. Interestingly, no effect of F. nucleatum on CDH1/β-catenin in noncancerous cells was observed, which suggests that F. nucleatum is not responsible for cancerogenic transformation of the oral epithelium [79]. This discovery could imply that F. nucleatum is not responsible for the cancerogenesis of OSCC, even though a higher representation of F. nucleatum was observed in OSCC samples compared to healthy individuals [58]. However, enriched representation of F. nucleatum was also observed in OPMDs, which proves that colonization of OSCC/HNSCC with F. nucleatum begins much earlier in the process of cancerogenesis and potentially supports the role of F. nucleatum in this process [77,78].
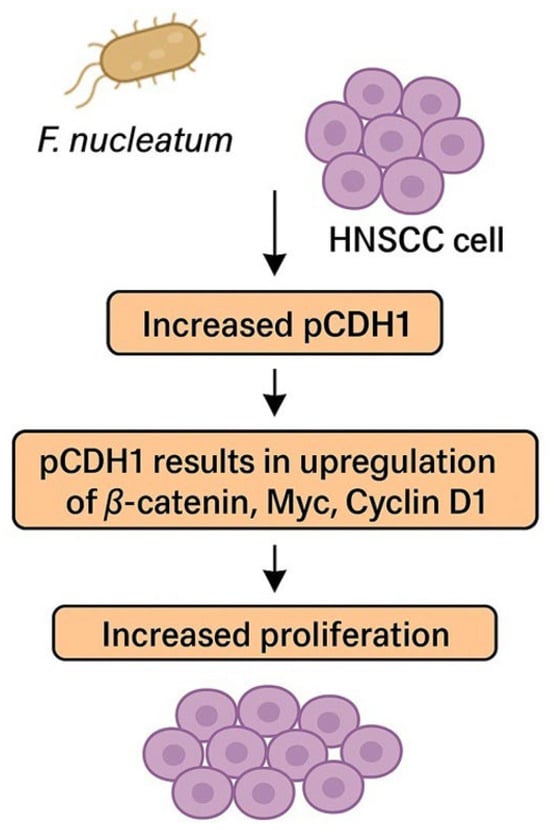
Figure 2.
F. nucleatum promotes cancer cell proliferation via phosphorylation of CDH1. Abbreviations: HNSCC—head and neck squamous cell carcinoma, pCDH1—phosphorylated cadherin 1.
F. nucleatum has been observed to play a crucial role in enhancing the invasiveness of OSCC. Via direct interaction of Fap2 with epithelial cells‘ TLRs, the IL-6-STAT3 signal pathway is augmented, leading to the upregulation of matrix metalloproteinases such as MMP1 and MMP9 [67]. Overexpression of MMPs leads to excessive degradation of the extracellular matrix, which facilitates cancer cells’ invasiveness and dissemination [82,83,84,85].
Moreover, F. nucleatum has a unique capability of suppressing the immune system in the tumor microenvironment (TME) via the inhibition of immune cells’ activity [86]. An inverse association between F. nucleatum representation and abundance of CD3+ and CD4+ T-lymphocytes was observed in breast and colon tissue. Fap2 adhesin binds to the T-cell immunoreceptor with Ig and ITIM domain (TIGIT), which is an important immunomodulatory receptor of T-cells and NK-cells, and inhibits it [87]. This Fap2-TIGIT interaction protects F. nucleatum from the immune system response, and furthermore, it protects the surrounding cancer cells via a bystander effect [67,87,88,89,90]. Fap2 is also linked to the cell death of lymphocytes, further promoting its local immunosuppressive effects [90].
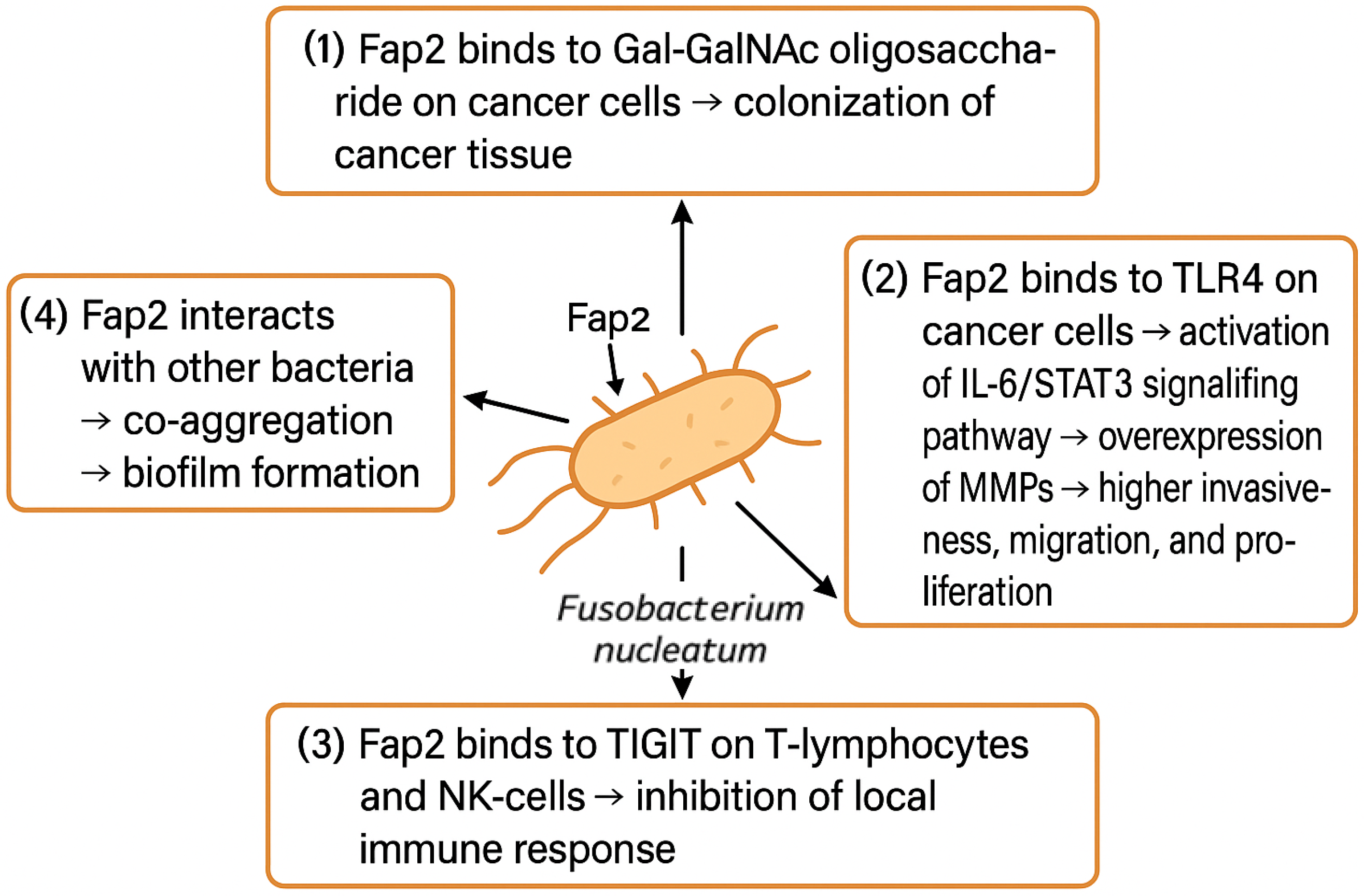
Fap2 adhesin is one of the most significant virulence factors of F. nucleatum. As depicted in Figure 3, it facilitates the colonization of cancer tissues, promotes its invasiveness, migration, and proliferation, and suppresses the local immune response, but it also enables the F. nucleatum to form biofilms through aggregation of other bacterial species [91]. Certain bacteria, e.g., P. gingivalis, are not able to survive in the OCSS environment alone. F. nucleatum can produce ammonia to neutralize the local pH, which enables acid-sensitive bacteria to co-exist in F. nucleatum biofilm. Whether such co-aggregations have a direct impact on cancerogenesis is unclear, as studies report incongruent results. Binder Gallimindi’s group observed significantly higher proliferation of OSCC cells co-cultivated with the mixture of P. gingivalis and F. nucleatum; however, Harrandah and colleagues reported that OSCC cells infected with F. nucleatum alone exhibited a higher proliferation rate compared to the combination of periodontal pathogens [67,92].
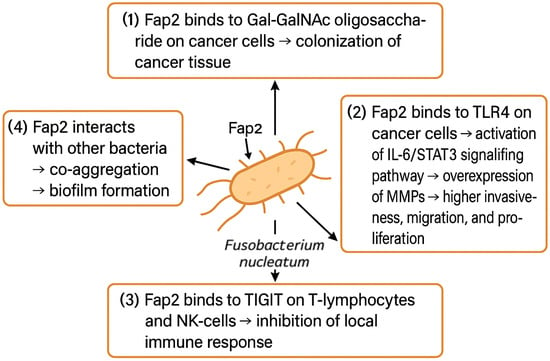
Figure 3.
F. nucleatum promotes OSCC tissue colonization, proliferation, invasiveness, and migration and suppresses local immune response. Abbreviations: Gal-GalNAc—galactose- and N-acetyl-D-galactosamine, TLR4—Toll-like receptor 4, IL-6—interleukin 6, STAT3—signal transducer and activator of transcription 3, MMPs—metalloproteinases, TIGIT—T-cell immunoreceptor with Ig and ITIM domain, NK-cell—natural killer cell.
3.3. Microbiome and Radical Treatment—Surgery and (Chemo)Radiotherapy
Treatment of localized and locally advanced HNSCCs is complex and should be debated within a multidisciplinary board. Treatment modality is chosen based on the stage, localization, and histopathological characteristics of the tumor. In early stages, surgery alone can be sufficient, and in more aggressive or extensive cancer, adjuvant radiotherapy (RT) is applied. Sometimes, radical chemoradiotherapy (CRT) can offer comparable results with surgery and adjuvant treatment, however, saving patients from possibly mutilating surgery. Each of these modalities can impact the oral microbiome [12].
A very limited number of studies linked to the perioperative changes in the oral microbiome have been published. One study described a higher abundance of Haemophilus spp., Neisseria spp., Aggregatibacter spp., and Leptotrichia spp. in the post-resection microbiome of HNSCC patients [49]. In case of successful resection, these bacterial alterations reverted to normal within three months since the curative surgery [93].
RT of HNSCC is associated with a high incidence of RT-induced toxicity, acute as well as late, e.g., xerostomia, radiation-induced oral mucositis (RIOM), dermatitis, dysphagia, etc., which can significantly affect patients’ Quality of Life (QoL) [94,95,96]. The intensity and/or severity of these adverse events is enhanced with CRT [97]. It remains unclear whether oral dysbiosis is associated with (C)RT-related complications and/or treatment efficiency. In this chapter, we will discuss the impact of the oral microbiome on (C)RT efficacy and acute toxicity. Its role in the genesis of late toxicity (neuromuscular damage, fibrogenesis, etc.) has not been published so far [98].
The most common RT-induced adverse event in HNSCC patients is xerostomia. Xerostomia is caused by high radiation doses to salivary glands, which leads to significantly decreased salivary flow [97]. Xerostomia thus often leads to impaired speech and taste, and malnutrition, overall diminishing the QoL of HNSCC patients. Salivary flow is insufficient in delivering alkaline salivary solutions, thus lowering intraoral pH, which predisposes HNSCC patients to develop oral mucositis, dental caries, mucosal ulcerations, and oral infections [99]. Interrupted salivary flow leads to microbial alterations in the oral cavity, resulting in higher relative abundances of S. mutans, Lactobacillus spp., Candida spp., and Staphylococcus spp.; in contrast, the relative abundance of S. sanguinis, Neisseria spp., and Fusobacterium spp. decreases [74]. Mojdami et al. later reported results in agreement with previous studies. They observed an increased relative abundance of the genera Streptococcus, Lactobacillus, Treponema, and Prevotella [14]. Similarly, Chen et al. observed a significant reduction in the relative abundance of Haemophilus spp., Veillonella spp., and Granulicatella spp., and an increase in the relative abundance of genera Lactobacillus, Scardovia, Acinetobacter, and Enterococcus after CRT [45].
RIOM represents a common debilitating toxicity associated with RT/CRT of the head and neck region, affecting ca. 80–90% patients, among whom up to 60% may develop severe RIOM (Grade 3 or 4) [100,101,102]. In recent years, growing evidence has shown that oral dysbiosis contributes to the incidence and severity of RIOM [16,103,104,105]. Hu et al. demonstrated a dose-dependent oral microbiome alteration after RT. Higher administered doses were associated with an increased abundance of Pseudomonas spp., Treponema spp., and Granulicatella spp., and a decreased abundance of Prevotella spp., Fusobacterium spp., Leptotrichia spp., Campylobacter spp., Peptostreptococcus spp., and Atopobium spp. Pre-existing dysbiosis can predispose patients to developing more severe RIOM. Patients who during RT developed RIOM Gr 2 and higher had an increased abundance of several Gram-negative bacteria (e.g., Fusobacterium spp., Haemophilus spp., Tannerella spp., Porphyromonas spp., and Eikenella spp.) before RT [15]. Such microbial alterations subsequently lead to microbial imbalance, which results in deterred immunomodulatory functions and promotes chronic inflammation. CRT-induced cell apoptosis results in oral mucosal damage, which is then exacerbated by the amplified inflammatory cascade [102,105].
Regardless of the acute toxicity, certain microbial alterations after RT can be observed in all HNSCC patients. Gaetti-Jardim et al. reported a reduced abundance of Gram-negative obligate anaerobes and an enhanced abundance of S. mutans [106]. Studies investigating microbial changes between the pre- and post-RT microbiome concordantly described a dose-dependent reduction in bacterial diversity after RT. The reconstitution of microbial diversity to the pre-RT composition has been observed with the prolonging time since the end of RT [107].
RT for HNSCC affects oral cavity epithelia directly through ionizing radiation and indirectly through reduced salivary flow, both contributing to cariogenesis [11,14,16,17]. RT-induced dental issues can significantly deteriorate the QoL of HNSCC patients and can lead to osteoradionecrosis of the jaw. Regular dental follow-ups are necessary, and specialized dental care should be provided to all HNSCC patients who underwent RT/CRT [108]. Vesty et al. suggested oral probiotics containing Streptococcus salivarius M18 to modulate host immune response and inter-bacterial cross-talk to improve oral health; unfortunately, they failed [109].
So far, five studies have been conducted on probiotics and prebiotics administration to prevent or alleviate symptoms of RIOM [110,111,112,113,114]. Probiotics can competitively inhibit pathogens by the production of bacteriocins, and they can also modulate cell proliferation and apoptosis. Prebiotics stimulate the growth of beneficial microbiota and can also inhibit the activity of some bacterial pathogens. The probiotic strain Bifidobacterium animalis subsp. lactis HN019 has been shown to play a role in modulating the immuno-inflammatory response of the human body, benefiting oral health, alleviating the symptoms of periodontitis, and shortening the duration of its treatment [115]. In a recent study, the administration of probiotics based on Bifidobacterium spp. and Lactobacillus spp. was shown to significantly reduce the grade of radiotherapy-induced mucositis (RIOM) [116]. Also, the exopolysaccharide produced by Bifidobacterium breve was shown to have antitumor effects against HNSCC, which could lead to the future inclusion of probiotics with B. breve in the adjuvant therapy of HNSCC [116,117]. Sharma et al. observed a significant decrease in severe RIOM incidence in the probiotics group (administered Lactobacillus brevis CD2) compared to the placebo group (52 vs. 77%) [111]. Limaye et al. observed that the group of HNSCC patients, to whom the prebiotic Lactococcus lactis was given, had a 35% reduction in ulcerative RIOM prevalence throughout the RT (15.5 vs. 45.7%) [112].
As oral dysbiosis can significantly affect the morbidity of HNSCC patients undergoing RT, some authors were interested in whether pretherapeutic antibiotics administration could affect the incidence of RIOM and/or treatment results [118]. RT-induced mucositis is associated with the local microbiome. It was demonstrated that germ-free mice exhibited a higher radioresistance of their intestinal mucosa compared to regular mice, whose radiotherapy-related toxicity was much higher [111,119]. One single-center analysis reported peri-therapeutic antibiotics administration as an independent prognostic factor of reduced disease-free survival [120]. Just recently, Rühle et al. published an analysis on 220 HNSCC cancer patients undergoing definitive CRT. They reported that patients pretreated or treated concurrently with RT with antibiotics had significantly lower disease-free and even overall survival (OS)—the median of OS was 10 months shorter (36 vs. 26 months) [118]. Altogether with the Nenclares group, data on 492 HNSCC patients agreeably demonstrated a worse prognosis of HNSCC patients (pre)-treated with antibiotics. We could discuss how the overall survival could be lower because of the co-morbidities requiring the antibiotics therapy (e.g., pneumonia, port infection, etc.); however, in both cohorts, an independent significant association was found between antibiotics use and decreased progression-free survival [118,120].
Alterations of bacterial representation in the oral microbiome have been shown to affect the treatment response to RT. F. nucleatum has been suggested as a predictor of the therapeutic response of HNSCC to RT [121]. Mechanisms underlying its impact have not been elucidated; however, the ability of F. nucleatum to aggregate and to form biofilms has been speculated to increase radioresistance by attracting neutrophils and cytokine release [122,123].
Plausible microbiome modifications to maximize the treatment response and minimize adverse effects with probiotics in HNSCC have already been proposed; however, the proper identification and characterization of protective and/or prognostic factors have yet to be investigated in detail [18,124,125].
3.4. Microbiome and Systemic Therapy for HNSCC
Treatment of HNSCC requires a multimodal approach. For the localized and locally advanced stage of HNSCC, either definitive CRT, especially for locally advanced disease (LA), or primary surgical approach followed by an adjuvant RT is the standard of care [12]. However, despite the technological progress that RT has made in the last 20 years (IMRT, IGRT, VMAT techniques), the recurrence rates are still quite high. Recurrence rates for primary locally advanced HNSCC can vary depending on many factors, including the specific tumor location, extent of disease, response to treatment, and other clinical factors. Nevertheless, the following overview can be provided [126].
Patients with locally advanced HNSCC treated with radical CRT have a recurrence rate of around 30–50%. Outcomes may vary depending on the exact treatment protocol and the individual patient’s response to therapy [127]. Patients treated with surgery followed by adjuvant RT (with or without chemotherapy) have a recurrence rate of approximately 30–40%. Again, outcomes may vary depending on the specific treatment conditions and other factors [128]. Patients at high risk of recurrence, i.e., especially patients with observed lymph node extracapsular extension, may benefit from the addition of concurrent CHT to adjuvant RT [129].
It is important to emphasize that individual prognosis and recurrence rates may vary considerably depending on the specific clinical characteristics of each patient. The multidisciplinary team takes many factors into account, especially the performance status of the patients, comorbidities—renal insufficiency, chronic cardiac failure, autoimmune diseases, etc., molecular characteristics of the tumor, e.g., expression of programmed-death ligand 1 (PD-L1) either on tumor cells (tumor positive score, TPS) or on both tumor and immune cells in the tumor (combine positive score, CPS), or next-generation sequencing (NGS) results, when deciding the most appropriate treatment approach and assessing the risk of recurrence. As for R/M HNSCC, this stage is usually incurable. Currently, for R/M HNSCC, the most used regimens include monotherapy or a combination of chemotherapeutics such as cisplatin (cDDP), carboplatin (CBDCA), 5-fluorouracil (5-FU), docetaxel, paclitaxel, immune-checkpoint inhibitors as pembrolizumab and nivolumab, and targeted treatment agents such as cetuximab (anti-epidermal growth factor receptor monoclonal antibody). Currently, the immunotherapy strategies are preferred to the targeted therapy in HNSCC [12].
3.4.1. Chemotherapy
Among the most often used chemotherapeutics in the treatment of R/M HNSCC belong alkylating agents (cDDP, CBDCA), nucleic acid inhibitors (5-FU), and microtubule depolymerization inhibitors (docetaxel and paclitaxel) [130,131,132,133,134,135,136].
The human microbiome plays a significant role in the development and progression of cancer; moreover, it was proven that the host microbiome can also affect anti-cancer CHT efficacy and toxicity. Alexander et al. proposed three possible outcomes of microbial impact on chemotherapeutics: direct influence on drug efficacy through pharmacokinetic alterations; abrogation and compromise of anticancer effects; and mediation of toxicity [19].
Animal studies have shown that manipulation of the microbiome can affect the efficacy and toxicity of CHT [137,138]. In animal models, cisplatin efficacy and toxicity severity and/or incidence have been found to be associated with specific microbial alterations [137,139,140]. In the Lewis lung carcinoma mouse model, Gui et al. observed significant survival reduction in mice treated with cisplatin and concurrent antibiotics (vancomycin, ampicillin, and neomycin) compared to cisplatin alone. In contrast, mice that were given cisplatin concurrently with Lactobacillus spp. exhibited better a cisplatin response rate, more rapid shrinkage of the tumor, and longer survival rate compared to the mice treated with cisplatin alone [141]. Chen et al. similarly investigated the effect of different microbial profiles on cisplatin efficacy in the Lewis lung carcinoma mouse model, reporting that Akkermansia muciniphila enhances the anti-cancer effects of cDDP. Compared to cDDP alone, combined treatment with A. muciniphila led to slowed-down tumor growth, downregulation of Ki67 and p53, and upregulation of pro-apoptotic proteins [142]. Hsiao et al. observed attenuated nephrotoxicity of cisplatin in Wistar rats, which were given cisplatin concurrently with Limosilactobacillus reuteri and Clostridium butyricum [143]. cDPP is usually preferred to CBDCA in HNSCC treatment, and it does not appear to be an attractive target for (pre-)clinical microbial research. However, one Chinese study described higher intestinal toxicity in mice treated with CBDCA, which had a higher abundance of Prevotella capri in their gut microbiome [144].
5-FU is usually used in combination with platinum-based CHT, usually cDDP, in the first or second line of R/M HNSCC [12]. Yuan et al. performed similar experiments to Gui and collaborators, as described above. They investigated the treatment efficacy of 5-FU in a mouse model. Mice treated concurrently with 5-FU and a combination of antibiotics (vancomycin, ampicillin, and neomycin) had a much worse treatment response compared to mice treated only with 5-FU; the use of probiotics had no improvement in the response rate [145]. Yu et al. investigated the impact of the gut microbiome on CHT efficacy in colorectal cancer. They reported that F. nucleatum increases chemoresistance to 5-FU in colorectal cancer by modulating autophagy [146]. Certain probiotics exhibited protective properties against intestinal mucositis, suggesting their potential use in the alleviation of toxicity. Use of the strain Limosilactobacillus fermentum BR11, probiotic product VSL#3 (containing S. thermophilus, B. breve, B. longum subsp. longum and B. longum subsp. infantis, L. delbrueckii subsp. bulgaricus, L. acidophilus, Lacticaseibacillus paracasei, and Lactiplantibacillus plantarum), and species Lacticas. casei and Lacticas. rhamnosus are associated with reduced chemotoxicity, reduced diarrhea, and weight loss in rats [147,148,149,150]. Similar effects were observed with L. acidophilus, Lacticas. rhamnosus, and Lacticas. paracasei, and with B. animalis subsp. lactis, B. bifidum, and B. longum subsp. infantis when used concurrently with 5-FU [151,152,153].
Clinical studies on the relationship between CHT and the host microbiome in head–neck cancer are either lacking or focus on CRT. However, one small Chinese study on 44 OSCC patients investigated how the microbiota affects the efficacy of induction CHT with TPF (docetaxel, cisplatin, and 5-fluorouracil). Rui et al. observed different microbial profiles in responders and non-responders. In the responder group, Slackia spp. abundance was enriched; among the non-responders, Mycoplasma spp. and F. nucleatum were enriched [154]. In accordance with the study by Yu and collaborators, these findings suggest that F. nucleatum could promote chemoresistance even in head and neck cancers [144].
The microbiome plays an important role in modulating the effects of CHT through modulation of the immune system, drug metabolism, intestinal barrier protection, and the production of toxic metabolites [155,156,157]. Limited data are available on the impact of CHT on the oral microbiome of HNSCC patients. CHT alone affects the oral microbiome, decreases the abundance of Streptococcus spp., and causes a relative increase in the abundance of Gram-negative anaerobic bacteria [158]. It can also lead to dysbiosis, resulting in metabolic changes, e.g., dysregulation of the enterosalivary nitrate-nitrite-nitric oxide pathway, leading to biochemical changes in the mouth cavity. Suggesting that these changes may alter HNSCC patients’ risk of recurrence [159]. Manipulation of the microbiome represents a potential therapeutic strategy to improve the efficacy of CHT and reduce its side effects.
A literary review regarding the influence of the gut microbiome on systemic anti-cancer therapy is summarized in Supplementary Table S1.
3.4.2. Targeted Therapy
Cetuximab is a monoclonal antibody targeting epidermal growth factor receptor (EGFR) and is approved for HNSCC treatment [160]. As far as authors are concerned, no studies investigating how the microbiome alters the toxicity or treatment results of cetuximab monotherapy have been published. The lack of evidence might be caused by the declining usage of cetuximab in HNSCC treatment. CRT with cDDP has been found superior in terms of treatment response, as well as tolerability; moreover, immune checkpoint inhibitors also surpassed cetuximab-based regimens in palliative settings [161]. The efficacy of targeted treatment after ICIs is being investigated by a phase II trial (NCT04375384) [162].
Only two single-arm phase II clinical trials, CAVE-mCRC and CAVE-LUNG, investigated cetuximab in combination with ICI avelumab in metastatic colorectal carcinoma (mCRC) and chemo-refractory non-small cell lung carcinoma (NSCLC) patients, respectively. Specific taxa, Agathobacter rectalis M104/1 and Blautia sp. SR1/5, were identified in long-term responding patients in both trials [163], as to whether these microbiotas can affect the treatment response in HNSCCs requires further evaluation.
3.4.3. Immunotherapy
Since the landmark trials, KEYNOTE-048 and CheckMate141, immunotherapy with ICIs has become the cornerstone of R/M HNSCC palliative treatment in the first and second line [12,164,165]. Currently, two ICIs are routinely used in HNSCC treatment, i.e., nivolumab and pembrolizumab. Both IgG4 monoclonal antibodies target programmed death receptor-1 (PD-1) on the cytotoxic CD8+ T-lymphocytes, diminishing inhibitory signals from the programmed death ligand-1 (PD-L1) exhibited on the cancer cell surface [166,167]. Therefore, CD8+ T-lymphocytes can be subsequently activated and thus eliminate cancer cells [168]. Even though immunotherapy revolutionized treatment across most of the solid tumors, its objective response rate is usually around 40% [169,170]. Many studies have already demonstrated how closely the human microbiome and immunotherapy are linked, suggesting it could be connected to the primary and/or secondary (acquired) resistance to the ICIs [171,172,173,174,175,176,177,178,179]. Common effort is currently aimed at identifying “cold” and “hot” microbial constitutions that could predict the ICI treatment response and could eventually lead to attempts on how to modify the human microbiome to enhance treatment efficacy. Some studies have already tried to manipulate the gut microbiome for therapeutic purposes through, e.g., fecal transplantation, probiotics use, antibiotics administration, etc. [162].
The association of ICI efficacy and microbial compositions stems from pre-clinical research on a mouse model. Vetizou et al. reported germ-free mice as non-responders to ICIs. Once these mice received either oral gavage or fecal transplantation containing Bacteroides fragilis, they became ICI-responsive [180]. Similar results were reported in other studies, regardless of the target of ICIs (PD-(L)1 or CTLA-4, i.e., cytotoxic T-lymphocyte-associated protein [171,181,182]. Most current clinical studies investigated the effect of gut microbiome on ICI efficacy; however, their results were, for a long time, incongruent. Broad-spectrum antibiotics were shown to disrupt gut microbiota and thus reduce the efficacy of ICIs; however, non-targeted commercial prebiotics also did not improve ICI efficacy [183,184,185]. Also, the exact mechanism of how the gut microbiome influences ICI efficacy is still unknown.
Currently, the most attention regarding the impact of the gut microbiome on ICI treatment efficacy is paid to metastatic melanoma, especially to enhance the rate and depth of the response [162,183,186]. Only limited data are available on the role of the gut microbiome and its metabolome on ICI efficacy in R/M HNSCC. Bari et al. reported significantly higher bacterial diversity in their gut microbiome, a lower Firmicutes-to-Bacteroidetes phylum ratio, and a higher abundance of the genus Bacteroides and family Lachnospiraceae; uniformly, the responders had a higher abundance of Eubacterium oxidoreductans and Bact. uniformis in the baseline and post-treatment samples, and Ruminococcus spp. was enriched in the durable responders. Interestingly, they also found a different metabolomic signature. Significantly lower levels of adenosine, inosine, and xanthine were reported in baseline samples of responders; in this group, adenosine, inosine, and xanthine levels continued to decrease, and vice versa, the levels rose in non-responders throughout the therapy, suggesting that inosine is consumed by the activated T-cytotoxic cells [187]. Despite promising results from other solid tumors, a recent pilot study failed to find an association between the oral microbiome and nivolumab treatment response in HNSCC patients. Farris and colleagues observed no associations in bacterial diversity (baseline and week 7) and clinical response, PD-L1 expression, HPV status, and treatment duration [188].
The key issue currently is the difference and diversity in the data obtained. Many studies identified the differences in microbial composition affecting ICI efficacy; however, all the studies failed to agree on specific universal bacterial agents [171,172,173,174,175]. Moreover, even a meta-analysis of these studies failed to do so [189].
4. Discussion
4.1. Bacterial Alterations in HNSCC
Traditionally, HNSCC, especially arising in the oropharynx, can be stratified according to the evidence of HPV-infected cells, HPV-positivity being the harbinger of radiosensitivity, and of better prognosis [190]. Recently, bacterial oncogenesis has been profoundly studied; however, no convincing data have yet been reported. Several obstacles were reported: (a) HNSCC is a highly heterogeneous group of diseases that may differ in anatomical sites, its pathoetiology, and histopathology; (b) high interindividual variability is typical even for the healthy oral microbiome; and (c) often inconsistent results were reported. As we already addressed, different investigators reported either higher or unchanged bacterial diversity in HNSCC patients and healthy controls. Surprisingly, investigators often reported even contradictory findings of increased/decreased relative abundance in the HNSCC patients compared to controls [7,8,34,35,36,45,46,47]. Across many studies, an increased abundance of F. nucleatum, P. gingivalis, Ps. aeruginosa, Lactobacillus spp., and a decreased representation of Neisseria spp. and Actinomyces spp. were observed [9,10,11,25,38,45,46,47,48,49,50,51,54,55].
Microbial alterations discovered in HNSCC patients are summarized in Supplementary Table S2.
Particularly, a large amount of evidence has proved that F. nucleatum is closely related to tumor development [191]. F. nucleatum usually inhabits the oral cavity and causes periodontal disease [64,146,192]. A significant correlation has been demonstrated between oral dysbiosis, especially the increased abundance of F. nucleatum, and tumors of the oral cavity, as well as tumors of distant sites such as the oropharynx, esophagus, pancreas, gut, breast, prostate, or lungs [3,4,5,7]. F. nucleatum-associated tumors are generally linked with worse treatment response, higher aggressiveness, and worse prognosis [62,193]. Surprisingly, it appears to be the opposite in HNSCC. HNSCC tested positive for F. nucleatum, had a lower recurrence rate, and a prolonged overall survival [61]. The reasons why are currently unknown, although microbial inhibition might be involved.
The genus Bifidobacterium occurs naturally in the oral and intestinal lumen [194]. Bifidobacteria have combined local and systemic effects, including competitive inhibition of pathogenic bacteria, production of organic acids and bacteriocin-like compounds, and immunomodulation [118,195,196]. A probiotic formula containing the species B. adolescentis, B. longum, and B. bifidum exhibited inhibitory effects on the growth of F. nucleatum in vitro [197]. This was confirmed in another independent study, where a direct effect on CRT efficacy and toxicity was observed [198]. Details of how bifidobacteria interact with F. nucleatum remain unclear; however, one of the possible anti-cancer mechanisms includes the production of exopolysaccharides, which stimulate the Bax gene pathway, resulting in the overexpression of Caspase 3 and 8, resulting in the induction of apoptosis [109,110]. Whether cross-talk between bifidobacteria and F. nucleatum in the oral cavity is responsible for the better treatment response in F. nucleatum-associated HNSCC still lacks any evidence.
4.2. Human Microbiome Has a Direct Influence on Anti-Cancer Therapy in HNSCC
Recently, the human microbiome has been vigorously researched as its impact on anti-cancer treatment efficacy appears to be eminent [155]. Mounting evidence supports that specific gut microbiome composition affects treatment response to various chemotherapeutics, e.g., gemcitabine, cyclophosphamide, oxaliplatin, etc. [146,159,199,200,201,202]. In the case of HNSCC, almost all data came from animal models. The only published clinical study investigated the treatment response to the induction of TPF chemotherapy. Interestingly, F. nucleatum was significantly more abundant in the non-responder group [154]. Based on the literature review, F. nucleatum interferes with the metabolism of 5-FU and thus induces chemoresistance in CRC [146]. Hypothetically, this can be a similar case. An interesting concept of inflammation-induced chemoresistance was proposed based on a mouse model. Mice with OSCC had orally inoculated P. gingivalis, which resulted in chronic inflammation. These mice were then treated with paclitaxel or docetaxel, with or without anti-inflammation drugs. Mice who were infected with P. ginigvalis were more chemoresistant compared to the uninfected mice; however, the infected mice treated concurrently with antiinflammation medication became chemosensitive to paclitaxel/docetaxel again [203,204]. An interesting finding of the ability to alleviate toxicity and enhance CHT efficacy was made with butyrate-producing bacteria, i.e., mainly the genera Ruminococcus, Clostridium, Eubacterium, and Coprococcus [205]. C. butyricum in combination with Lim. reuteri effectively attenuated cDDP-associated renal damage; moreover, when given concomitantly with cDDP, it enhanced chemosensitivity in cellular models as a potential histone deacetylase inhibitor [143,206].
In terms of RT, the microbiome is an important player in local control, as well as a systemic therapy response, regardless of the site of RT [62,146,192,193,207,208,209,210,211,212]. Clinical studies on rectal cancer convincingly report that the specific gut microbiome is associated with CRT response in relation to reaching complete remission or not [146,192,193,207]. Microbial alterations were also linked to radiation-induced adverse events in patients with prostate, gynecological, rectal, and other cancers [147,148,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237]. Focusing on the oral microbiome and HNSCC, the depth of knowledge is far shallower. RT of the head and neck region results in oral microbiome alterations; however, its pre-RT constitution is associated with the incidence and severity of RIOM [16,106,107,108]. Interestingly, so far, no specific microbial taxa have been linked to either radiosensitivity or radioresistance in HNSCC. The only clinical studies that demonstrated a significant effect on patients’ prognosis and treatment response reported that broad-spectrum antibiotics administered before or during CRT were linked with shorter time to recurrence and shorter survival [108,110]. These findings correlate with pre-clinical data investigating the relationship between dysbiosis, butyrate acid, and RT. Uribe-Herranz and colleagues investigated the impact of antibiotics on RT efficacy in a melanoma and lung/cervical model in C57/BL6 mice. Mice were stratified into three groups: (a) vancomycin-treated (covers butyrate-producing bacteria), (b) neomycin/metronidazole-treated (does not affect butyrate-producing bacteria), and (c) vancomycin-treated mice who had per oral intake of butyrate. Vancomycin-treated mice had a better response to RT; however, this effect was abrogated in the group with a dietary intake of butyrate, and neomycin/metronidazole-treated mice exhibited no change in response to RT [223]. Whether these findings can be extrapolated to the HNSCC is a subject of discussion, as no direct evidence exists; however, it would be highly interesting to investigate, especially considering that butyrate was observed to have a chemosensitizing effect with cDDP on cellular lines [143,180].
Only limited data are available regarding the role of the human microbiome and its metabolites on ICI efficacy in R/M HNSCC. Bari et al. reported a significantly higher abundance of Eu. oxidoreductans, Bact. uniformis, and Ruminococcus spp. in the ICI responders [187]. Interestingly, these bacteria are closely associated with butyrate production [205,237], which is consistent with preclinical findings that butyrate enhances ICI-treatment efficacy via activation of CD8+ [172,180,238,239,240]. Across the immunotherapy of solid tumors, it is becoming a generally accepted fact in the scientific community that the host microbiome can detrimentally affect ICI treatment efficacy. Experiments with fecal transplantation (FMT) showed that the response to ICI treatment can be modified via a change in gut microbiome. Routy et al. [171] hypothesized that abnormal gut microbiome composition is associated with primary resistance to the ICIs. Moreover, they described how mice react to ICI treatment in several situations: (a) germ-free mice did not respond to ICIs; (b) germ-free mice who received FMT from a human responder to ICIs responded to the therapy; (c) germ-free mice who received FMT from a human non-responder to ICIs did not respond to the therapy; and (d) germ-free mice who received FMT from a human non-responder to ICIs, but had per orally administered A. muciniphila, responded to the ICIs [171]. Bernal et al. observed that A. muciniphila was significantly less abundant in the stool of oropharyngeal SCC patients [217]. Other authors suggested that the higher abundance of A. muciniphila can be related to better treatment outcomes with ICIs [172,173,180]. Unfortunately, the situation is not that simple. Based on the decreased representation of A. muciniphila in HPV+ HNSCCs, we could expect worse treatment efficacy of ICIs in HPV-associated HNSCCs; however, the situation is reversed, i.e., the efficacy of ICIs is in HPV-associated rather than in HPV-negative HNSCCs (1.29× higher response rate, median OS 11.5 vs. 6.3 months) [241]. So far, the only clinical trial investigating the association between the oral microbiome and ICI treatment outcomes (specifically with nivolumab) was conducted by Farris and colleagues, and they failed to report any significant association [188].
The effects of the oral microbiota on treatment outcomes in HNSCC are summarized in Supplementary Table S3.
4.3. Probiotics Are Potent Agents in Anti-Cancer Therapy and Toxicity Modulation in HNSCC
Probiotic administration is intensively studied in efforts to enhance anti-cancer treatment outcomes and/or to reduce the severity of treatment toxicities. Lactobacilli, Bifidobacterium spp., Lactococcus spp., and Enterococcus spp. appear to be promising probiotic taxa with such activities [242,243]. The effect of probiotics on the alleviation of RT-induced adverse toxicity was mostly studied in patients undergoing pelvic RT; however, the evidence from clinical trials is still limited [147,148,231,232]. Several authors reported positive effects of Lactobacillus-based probiotics on RT-induced diarrhea [215,226,228,234].
So far, limited evidence regarding the probiotic effect in HNSCC has been published; however, similar results have been reported. Probiotic strains of Lactobacillus spp., Lactococcus spp., and Bifidobacterium spp. were all observed to reduce RT- and/or CHT-induced mucositis in HNSCC treatment [110,111,112,113,114]. Interestingly, a decrease in bifidobacterial representation was observed after RT [222]. It was hypothesized that, thanks to bifidobacterial anti-cancer effects and their beneficial effect on the oral microbiome, they could be an interesting agent for concomitant and/or adjuvant administration to HNSCC patients [195,196,197,198]. Similar results were observed in HNO97 cellular cultures with L. acidophilus and S. mutans, promoting further clinical research [244].
Based on the results of patients who had been pre-treated with probiotics and then received surgery for CRC, it was theorized that proper nutritional preparations and probiotics administration can improve results and/or decrease surgery-related toxicity in HNSCC patients undergoing radical surgical treatment; however, no prospective trials have been completed [245,246]. Limited data on immunonutrition in HNSCC are available, mostly regarding nutritional support [247]. Nevertheless, oral and/or enteral administration of probiotics is safe and helpful in patients who have undergone radical surgery for HNSCC. Gunduz et al. observed lower complications in patients after the HNSCC surgery, including a decreased rate of post-operative sepsis [248].
The effect of probiotics on HNSCC treatment is summarized in Supplementary Table S4.
5. Conclusions
The microbiome plays an important role in cancer development, progression, and treatment response across solid tumors, including HNSCC. Recent studies highlighted significant discrepancies in microbial profiles of healthy individuals and of those with oral potentially malignant disorders (e.g., OPMD) and/or HNSCC, suggesting the microbiome’s potential as a biomarker and therapeutic target. The association between oral dysbiosis and various malignancies initiated efforts to identify specific microbial agents tied to cancer development and/or progression.
Due to the significant heterogeneity of HNSCCs and the vast diversity of the oral microbiome, inconsistent associations of increased/decreased microbial diversity and/or relative/absolute abundance of specific taxa in the salivary microbiome with HNSCC were observed. In HNSCC, cancer-protective properties are commonly attributed to Neisseria spp.; conversely, F. nucleatum, Lactobacillus spp., S. anginosus, and P. gingivalis exhibit cancerogenic effects. Interestingly, a high abundance of F. nucleatum in the oral microbiome appears to be associated with better prognosis, which is inconsistent with its negative prognostic role in other cancers.
The high inconsistency among reported data can be possibly explained by several theories; however, conclusive evidence is still missing. Microbial diversity and ranging microbial representation in HNSCC patients’ samples can be attributed to the interindividual diversity, which can be observed in healthy controls, as well as to incoherent study designs, including different samples, collection methods, and patients’ characteristics (e.g., diet, smoking habits, alcohol consumption, etc.). Further controlled studies focusing on the covariant patients’ characteristics are necessary to establish causal associations.
Almost no research data suggest that the oral microbiome would be of a similar importance compared to the gut microbiome in terms of affecting the systemic therapy response (meaning CHT and ICIs therapy) and/or its toxicity.
Surgical treatment and (C)RT led to notable changes in the oral microbiome. (C)RT of HNSCCs may lead to interrupted salivary flow via collateral radiation damage to salivary glands. Decreased salivation and xerostomia results in significant microbial changes, e.g., a higher relative abundance of the genera Streptococcus, Staphylococcus, and Lactobacillus, and a lower relative abundance of the genera Haemophilus, Neisseria, and Fusobacterium, which ultimately affect patients’ QoL and their oral cavity health. Moreover, pre-existing dysbiosis, especially the representation of some Gram-negative bacteria (e.g., Fusobacterium spp., Haemophilus spp., Porphyromonas spp., and Eikenella spp.), was observed to impact both the incidence and severity of adverse events such as RIOM and xerostomia. Interventions with Bifidobacterium- and/or Lactobacillus-based probiotics and some prebiotics have shown potential in alleviating the severity and incidence of RIOM.
Specific microbiota can impact the pharmacodynamics of chemotherapeutics like cDDP and 5-FU, which are routinely used in HNSCC treatment regimens, thus affecting the treatment efficacy and toxicity. Lactobacillus spp. and A. muciniphila were observed to enhance cDDP-treatment response in animal models, whereas others, like F. nucleatum, may contribute to chemoresistance and treatment failure. Lactobacillus-based probiotics showed decreased 5-FU-related toxicity in rats. So far, no research has evaluated the impact of the oral microbiome on the ICI response rate and efficacy in HNSCC treatment. The gut microbiome appears to play a pivotal role in predicting the ICI treatment efficacy and even toxicity, whereas the oral microbiome has not shown any significant impact on ICI efficacy.
Microbiome alterations through pre-/pro-/synbiotics to achieve a better treatment response and/or to reduce the treatment toxicities have been demonstrated on several levels, e.g., a reduction in acute RT-induced mucositis in HNSCC RT, FMT from ICI responders to non-responders to overcome primary resistance to ICIs, etc. So far in HNSCCs, Lactobacillus- and/or Bifidobacterium-based probiotics have been shown to decrease RIOM incidence and severity. It also appears that probiotics could serve as potential adjuncts to conventional therapies in improving treatment outcomes and/or reducing treatment toxicity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17132238/s1, Table S1: Influence of gut microbiome on systemic anti-cancer therapy; Table S2: Microbial alterations discovered in HNSCC patients; Table S3: The effect of oral microbiota on treatment outcomes in HNSCC; Table S4: Effect of probiotics and antibiotics on HNSCC treatment efficacy.
Author Contributions
Conceptualization, M.P. and R.S.; methodology, M.P. and M.L.; validation, R.S., N.M. and V.N.-B.; resources, M.P. and A.H.; data curation, M.P. and M.L.; writing—original draft preparation, M.P.; writing—review and editing, N.M., V.N.-B. and M.P.; supervision, R.S., N.M. and V.N.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 4NQO | 4-nitroquinoline-1-oxide |
| 5-FU | 5-fluorouracil |
| AhR | Aryl-hydrocarbon receptor |
| ATB | antibiotics |
| CAL-27 | Centre Antoine Lacassagne-27 (tongue squamous cell carcinoma cell line) |
| CBDCA | Carboplatin |
| CD8+ | Cluster of differentiation, cytotoxic T-lymphocytes |
| cDDP | Cisplatin |
| CHT | Chemotherapy |
| CR | Complete remission |
| CRC | Colorectal carcinoma |
| CRT | Chemoradiotherapy |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein |
| EBV | Epstein–Barr virus |
| EXTREME | Cetuximab, cisplatin |
| FadA | Adhesins unique to oral Fusobacterium nucleatum |
| FMT | Fecal microbiota transplantation |
| ICIs | Immune-checkpoint inhibitors |
| IMRT | Intensity Modulated Radiotherapy |
| IGRT | Image Guided Radiotherapy |
| HNSCC | Head and neck squamous cell carcinoma |
| HNO97 | Tongue squamous cell carcinoma cell line |
| HHV-8 | Human herpesvirus-8 |
| HPV | Human papillomavirus |
| HSV | Herpes simplex virus |
| LA | Locally advanced |
| mCRC | Metastatic colorectal carcinoma |
| nCRT | Neoadjuvant chemoradiotherapy |
| OPMD | Oral potentially malignant disorders |
| OS | Overall survival |
| OSCC | Oral squamous cell carcinoma |
| PFS | Progression-free survival |
| PD-1 | Programmed cell death receptor 1 |
| PD-L1 | Programmed cell death receptor ligand 1 |
| SCC15 | Tongue squamous cell carcinoma cell line |
| R/M HNSCC | Recurrent or metastatic head and neck squamous cell carcinoma |
| RIOM | Radiation-induced oral mucositis |
| RT | Radiotherapy |
| TPF | Paclitaxel, cisplatin, 5-fluorouracil |
| VMAT | Volumetric Modulated Arc Therapy |
| WSU-HN6 | Wayne State University-Head and Neck 6 (tongue squamous cell carcinoma cell line) |
| QoL | Quality of life |
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral. Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.L.; Xia, L.Y.; He, B.Y.; Guo, J.M.; Huang, C.; Zeng, X.T. Cyclooxygenase-2-1195G>A Polymorphism and Head and Neck Squamous Cell Carcinoma Susceptibility: A Meta-Analysis of 1564 Cases and 2346 Controls. Med. Sci. Monit. 2015, 21, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Veronesi, P.; Galimberti, V.; Weinstein, R.; Del Fabbro, M.; Francetti, L. Is periodontitis a risk indicator for cancer? A meta-analysis. PloS ONE 2018, 13, e0195683. [Google Scholar] [CrossRef] [PubMed]
- Bracci, P.M. Oral Health and the Oral Microbiome in Pancreatic Cancer: An Overview of Epidemiological Studies. Cancer J. 2017, 23, 310–314. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, X.; Li, Y.; Shi, X.; Gu, Y.; Qian, Q.; Hong, Y. Hematopoietic and lymphatic cancers in patients with periodontitis: A systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e21–e28. [Google Scholar] [CrossRef]
- Fitzpatrick, S.G.; Katz, J. The association between periodontal disease and cancer: A review of the literature. J. Dent. 2010, 38, 83–95. [Google Scholar] [CrossRef]
- Javed, F.; Warnakulasuriya, S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Crit. Rev. Oncol. Hematol. 2016, 97, 197–205. [Google Scholar] [CrossRef]
- Su, S.C.; Chang, L.C.; Huang, H.D.; Peng, C.Y.; Chuang, C.Y.; Chen, Y.T.; Lu, M.Y.; Chiu, Y.W.; Chen, P.Y.; Yang, S.F. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 2021, 42, 127–135. [Google Scholar] [CrossRef]
- Kakabadze, M.Z.; Paresishvili, T.; Karalashvili, L.; Chakhunashvili, D.; Kakabadze, Z. Oral microbiota and oral cancer: Review. Oncol. Rev. 2020, 14, 476. [Google Scholar] [CrossRef]
- Lafuente Ibáñez de Mendoza, I.; Maritxalar Mendia, X.; García de la Fuente, A.M.; Quindós Andrés, G.; Aguirre Urizar, J.M. Role of Porphyromonas gingivalis in oral squamous cell carcinoma development: A systematic review. J. Periodontal Res. 2020, 55, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Keam, B.; Machiels, J.P.; Kim, H.R.; Licitra, L.; Golusinski, W.; Gregoire, V.; Lee, Y.G.; Belka, C.; Guo, Y.; Rajappa, S.J.; et al. Pan-Asian adaptation of the EHNS-ESMO-ESTRO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with squamous cell carcinoma of the head and neck. ESMO Open 2021, 6, 100309. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhu, H.; Liu, D.; Li, T.; Zhang, C.; Zhu, J.; Lv, H.; Liu, K.; Hao, C.; Tian, Z.; et al. A Pilot Study: Changes of Gut Microbiota in Post-surgery Colorectal Cancer Patients. Front. Microbiol. 2018, 9, 2777. [Google Scholar] [CrossRef]
- Mojdami, Z.D.; Barbour, A.; Oveisi, M.; Sun, C.; Fine, N.; Saha, S.; Marks, C.; Elebyary, O.; Watson, E.; Tenenbaum, H.; et al. The Effect of Intensity-Modulated Radiotherapy to the Head and Neck Region on the Oral Innate Immune Response and Oral Microbiome: A Prospective Cohort Study of Head and Neck Tumour Patients. Int. J. Mol. Sci. 2022, 23, 9594. [Google Scholar] [CrossRef]
- Hu, Y.J.; Shao, Z.Y.; Wang, Q.; Jiang, Y.T.; Ma, R.; Tang, Z.S.; Liu, Z.; Liang, J.P.; Huang, Z.W. Exploring the dynamic core microbiome of plaque microbiota during head-and-neck radiotherapy using pyrosequencing. PLoS ONE 2013, 8, e56343. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Biswas, K.; Mackenzie, B.W.; Taylor, M.W.; Douglas, R.G. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support Care Cancer 2020, 28, 2683–2691. [Google Scholar] [CrossRef]
- Sami, A.; Elimairi, I.; Stanton, C.; Ross, R.P.; Ryan, C.A. The Role of the Microbiome in Oral Squamous Cell Carcinoma with Insight into the Microbiome-Treatment Axis. Int. J. Mol. Sci. 2020, 21, 8061. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Badnjević, A.; Cervino, G.; Cicciù, M. Probiotics in the Treatment of Radiotherapy-Induced Oral Mucositis: Systematic Review with Meta-Analysis. Pharmaceuticals 2023, 16, 654. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Chang, A.H.; Parsonnet, J. Role of bacteria in oncogenesis. Clin. Microbiol. Rev. 2010, 23, 837–857. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Burcher, K.M.; Burcher, J.T.; Inscore, L.; Bloomer, C.H.; Furdui, C.M.; Porosnicu, M. A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers. Cancers 2022, 14, 4116. [Google Scholar] [CrossRef]
- Lee, W.H.; Chen, H.M.; Yang, S.F.; Liang, C.; Peng, C.Y.; Lin, F.M.; Tsai, L.L.; Wu, B.C.; Hsin, C.H.; Chuang, C.Y.; et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef]
- Snider, E.J.; Freedberg, D.E.; Abrams, J.A. Potential Role of the Microbiome in Barrett’s Esophagus and Esophageal Adenocarcinoma. Am. J. Dig. Dis. 2016, 61, 2217–2225. [Google Scholar] [CrossRef]
- Galvao-Moreira, L.V.; da Cruz, M.C.F.N. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016, 53, 17–19. [Google Scholar] [CrossRef]
- Chen, J.; Domingue, J.C.; Sears, C.L. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin. Immunol. 2017, 32, 25–34. [Google Scholar] [CrossRef]
- Hu, J.; Han, S.; Chen, Y.; Ji, Z. Variations of Tongue Coating Microbiota in Patients with Gastric Cancer. BioMed Res. Int. 2015, 2015, 173729. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92, Erratum in Nat. Rev. Dis. Primers 2023, 9, 4. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yang, X.J.; Chao, Y.L.; Zheng, H.M.; Sheng, H.F.; Liu, H.Y.; He, Y.; Zhou, H.W. The Potential Effect of Oral Microbiota in the Prediction of Mucositis During Radiotherapy for Nasopharyngeal Carcinoma. eBioMedicine 2017, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, J.; Zhou, X.; You, M.; Du, Q.; Yang, X.; He, J.; Zou, J.; Cheng, L.; Li, M.; et al. Oral microbiota distinguishes acute lymphoblastic leukemia pediatric hosts from healthy populations. PLoS ONE 2014, 9, e102116. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Freedman, N.D.; Alekseyenko, A.V.; Wu, J.; Yang, L.; Pei, Z.; et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018, 6, 59. [Google Scholar] [CrossRef]
- Hsiao, J.R.; Chang, C.C.; Lee, W.T.; Huang, C.C.; Ou, C.Y.; Tsai, S.T.; Chen, K.C.; Huang, J.S.; Wong, T.Y.; Lai, Y.H.; et al. The interplay between oral microbiome, lifestyle factors and genetic polymorphisms in the risk of oral squamous cell carcinoma. Carcinogenesis 2018, 39, 778–787. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wang, H.; Retuerto, M.; Zhang, H.; Burkey, B.; Ghannoum, M.A.; Eng, C. Bacteriome and mycobiome associations in oral tongue cancer. Oncotarget 2017, 8, 97273–97289. [Google Scholar] [CrossRef]
- Yost, S.; Stashenko, P.; Choi, Y.; Kukuruzinska, M.; Genco, C.A.; Salama, A.; Weinberg, E.O.; Kramer, C.D.; Frias-Lopez, J. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int. J. Oral Sci. 2018, 10, 32. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yeh, Y.M.; Yu, H.Y.; Chin, C.Y.; Hsu, C.W.; Liu, H.; Huang, P.J.; Hu, S.N.; Liao, C.T.; Chang, K.P.; et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front. Microbiol. 2018, 3, 862. [Google Scholar] [CrossRef]
- Frank, D.N.; Qiu, Y.; Cao, Y.; Zhang, S.; Lu, L.; Kofonow, J.M.; Robertson, C.E.; Liu, Y.; Wang, H.; Levens, C.L.; et al. A dysbiotic microbiome promotes head and neck squamous cell carcinoma. Oncogene 2022, 41, 1269–1280. [Google Scholar] [CrossRef]
- Wang, H.; Funchain, P.; Bebek, G.; Altemus, J.; Zhang, H.; Niazi, F.; Peterson, C.; Lee, W.T.; Burkey, B.B.; Eng, C. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017, 9, 14. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Salaspuro, V.; Salaspuro, M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. Int. J. Cancer 2004, 111, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Comparison of the microbiological features of chronic and aggressive periodontitis. Periodontology 2000 2010, 53, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Kumpitsch, C.; Moissl-Eichinger, C.; Pock, J.; Thurnher, D.; Wolf, A. Preliminary insights into the impact of primary radiochemotherapy on the salivary microbiome in head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 16582. [Google Scholar] [CrossRef]
- Mäkinen AIet, a.l. Salivary microbiome profiles of oral cancer patients analyzed before and after treatment. Microbiome 2023, 11, 171. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef]
- Pushalkar, S.; Mane, S.P.; Ji, X.; Li, Y.; Evans, C.; Crasta, O.R.; Morse, D.; Meagher, R.; Singh, A.; Saxena, D. Microbial diversity in saliva of oral squamous cell carcinoma. FEMS Immunol. Med. Microbiol. 2011, 61, 269–277. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget. 2016, 7, 51320–51334. [Google Scholar] [CrossRef]
- Wolf, A.; Moissl-Eichinger, C.; Perras, A.; Koskinen, K.; Tomazic, P.V.; Thurnher, D. The salivary microbiome as an indicator of carcinogenesis in patients with oropharyngeal squamous cell carcinoma: A pilot study. Sci. Rep. 2017, 7, 5867. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; White, J.R.; Godoy-Vitorino, F.; Rodríguez-Hilario, A.; Navarro, K.; González, H.; Michailidi, C.; Jedlicka, A.; Canapp, S.; Bondy, J.; et al. High-resolution microbiome profiling uncovers Fusobacterium nucleatum, Lactobacillus gasseri/johnsonii, and Lactobacillus vaginalis associated to oral and oropharyngeal cancer in saliva from HPV positive and HPV negative patients treated with surgery and chemo-radiation. Oncotarget 2017, 8, 110931–110948. [Google Scholar] [PubMed]
- Shay, E.; Sangwan, N.; Padmanabhan, R.; Lundy, S.; Burkey, B.; Eng, C. Bacteriome and mycobiome and bacteriome-mycobiome interactions in head and neck squamous cell carcinoma. Oncotarget 2020, 11, 2375–2386. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.J.; Crean, S.J.; Lewis, M.A.; Spratt, D.A.; Wade, W.G.; Wilson, M.J. Viable bacteria present within oral squamous cell carcinoma tissue. J. Clin. Microbiol. 2006, 44, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome with Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef]
- Katarkar, A.; Saha, A.; Mukherjee, S.; Kundu, D.; Bandyopadhyay, P.; Chaudhuri, K. Telomerase expression in individuals with chronic and aggressive periodontitis. J. Periodontol. 2015, 86, 656–665. [Google Scholar] [CrossRef]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and oral cancer: A critical review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef]
- Bronzatoa, J.D.; Bomfim, R.A.; Edwards, D.H.; Crouch, D.; Hector, M.P.; Gomes, B.P.F.A. Detection of Fusobacterium in oral and head and neck cancer samples: A systematic review and meta-analysis. Arch. Oral Biol. 2020, 112, 104669. [Google Scholar] [CrossRef]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef]
- Chen, Z.; Wong, P.Y.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Wong, S.H.; et al. The Intersection between Oral Microbiota, Host Gene Methylation and Patient Outcomes in Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 3425. [Google Scholar] [CrossRef]
- Neuzillet, C.; Marchais, M.; Vacher, S.; Hilmi, M.; Schnitzler, A.; Meseure, D.; Leclere, R.; Lecerf, C.; Dubot, C.; Jeannot, E.; et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci. Rep. 2021, 11, 7870. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Li, F.; Wang, L.P.; Chen, X.F.; Wang, D.; Cao, L.; Ping, Y.; Zhao, S.; Li, B.; Thorne, S.H.; et al. CTL- vs Treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma. Br. J. Cancer 2015, 113, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Iwatsuki, M.; Yoshida, N.; Watanabe, M.; Baba, H. Review of the gut microbiome and esophageal cancer: Pathogenesis and potential clinical implications. Ann. Gastroenterol. Surg. 2017, 1, 99–104. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human Microbiome Fusobacterium nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef]
- Hayashi, M.; Ikenaga, N.; Nakata, K.; Luo, H.; Zhong, P.; Date, S.; Oyama, K.; Higashijima, N.; Kubo, A.; Iwamoto, C.; et al. Intratumor Fusobacterium nucleatum promotes the progression of pancreatic cancer via the CXCL1-CXCR2 axis. Cancer Sci. 2023, 114, 3666–3678. [Google Scholar] [CrossRef]
- Udayasuryan, B.; Ahmad, R.N.; Nguyen, T.T.D.; Umaña, A.; Monét Roberts, L.; Sobol, P.; Jones, S.D.; Munson, J.M.; Slade, D.J.; Verbridge, S.S. Fusobacterium nucleatum induces proliferation and migration in pancreatic cancer cells through host autocrine and paracrine signaling. Sci. Signal. 2022, 15, eabn4948. [Google Scholar] [CrossRef]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef]
- Atanasova KR, Yilmaz, Ö. Prelude to oral microbes and chronic diseases: Past, present and future. Microbes Infect. 2015, 17, 473–483. [Google Scholar] [CrossRef]
- Geng, F.; Zhang, Y.; Lu, Z.; Zhang, S.; Pan, Y. Fusobacterium nucleatum Caused DNA Damage and Promoted Cell Proliferation by the Ku70/p53 Pathway in Oral Cancer Cells. DNA Cell Biol. 2020, 39, 144–151. [Google Scholar] [CrossRef]
- Kang, W.; Jia, Z.; Tang, D.; Zhang, Z.; Gao, H.; He, K.; Feng, Q. Fusobacterium nucleatum Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-κB Signaling Pathways in Human Gingival Fibroblasts. Oxid. Med. Cell. Longev. 2019, 2019, 1681972. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, A.A.; Shelton, R.M.; Landini, G.; Cooper, P.R.; Milward, M.R. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adhes. Migr. 2018, 12, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef]
- Abed, J.; Emgård, J.E.M.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef]
- Amer, A.; Galvin, S.; Healy, C.M.; Moran, G.P. The microbiome of potentially malignant oral leukoplakia exhibits enrichment for Fusobacterium, Leptotrichia, Campylobacter, and Rothia species. Front. Microbiol. 2017, 8, 2391. [Google Scholar] [CrossRef]
- Decsi, G.; Soki, J.; Pap, B.; Dobra, G.; Harmati, M.; Kormondi, S.; Pankotai, T.; Braunitzer, G.; Minarovits, J.; Sonkodi, I.; et al. Chicken or the egg: Microbial alterations in biopsy samples of patients with oral potentially malignant disorders. Pathol. Oncol. Res. 2019, 25, 1023–1033. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Huang, X.; Wang, Q.; Fu, R.; Wen, X.; Liu, J.A.; Zhang, L.F. Nucleatum enhances oral squamous cell carcinoma proliferation via E-cadherin/β-Catenin pathway. BMC Oral Health 2024, 24, 518. [Google Scholar] [CrossRef]
- Kujan, O.; Huang, G.; Ravindran, A.; Vijayan, M.; Farah, C.S. CDK4, CDK6, cyclin D1 and Notch1 immunocytochemical expression of oral brush liquid-based cytology for the diagnosis of oral leukoplakia and oral cancer. J. Oral Pathol. Med. 2019, 48, 566–573. [Google Scholar] [CrossRef]
- Siril, Y.J.; Kouketsu, A.; Saito, H.; Takahashi, T.; Kumamoto, H. Immunohistochemical expression levels of cyclin D1 and CREPT reflect the course and prognosis in oral precancerous lesions and squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2022, 51, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Uitto, V.-J.; Baillie, D.; Wu, Q.; Gendron, R.; Grenier, D.; Putnins, E.E.; Kanervo, A.; Firth, J.D. Fusobacterium nucleatum increases collagenase 3 production and migration of epithelial cells. Infect. Immun. 2005, 73, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Amano, A.; Lamont, R.J.; Murakami, Y. Involvement of protease-activated receptor 4 in over-expression of matrix metalloproteinase 9 induced by Porphyromonas gingivalis. Med. Microbiol. Immunol. 2015, 204, 605–612. [Google Scholar] [CrossRef]
- Ha, N.H.; Woo, B.H.; Kim, D.J.; Ha, E.S.; Choi, J.I.; Kim, S.J.; Park, B.S.; Lee, J.H.; Park, H.R. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015, 36, 9947–9960. [Google Scholar] [CrossRef]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef]
- Mima, K.; Sukawa, Y.; Nishihara, R.; Qian, Z.R.; Yamauchi, M.; Inamura, K.; Kim, S.A.; Masuda, A.; Nowak, J.A.; Nosho, K.; et al. Fusobacterium nucleatum and T-cells in colorectal carcinoma. JAMA Oncol. 2015, 1, 653–661. [Google Scholar] [CrossRef]
- Chen, T.; Li, Q.; Zhang, X.; Long, R.; Wu, Y.; Wu, J.; Fu, X. TOX expression decreases with progression of colorectal cancers and is associated with CD4 T-cell density and Fusobacterium nucleatum infection. Hum. Pathol. 2018, 79, 93–101. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Kaplan, C.W.; Ma, X.; Paranjpe, A.; Jewett, A.; Lux, R.; Kinder-Haake, S.; Shi, W. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect. Immun. 2010, 78, 4773–4778. [Google Scholar] [CrossRef]
- Coppenhagen-Glazer, S.; Sol, A.; Abed, J.; Naor, R.; Zhang, X.; Han, Y.W.; Bachrach, G. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect. Immun. 2015, 83, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Harrandah, A.; Chukkapalli, S.; Bhattacharyya, I.; Progulske-Fox, A.; Chan, E. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J. Oral Microbiol. 2020, 31, 1849493. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.K.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Meehan, K.; Lau, E.H.L.; et al. Restoration of the Oral Microbiota After Surgery for Head and Neck Squamous Cell Carcinoma Is Associated with Patient Outcomes. Front. Oncol. 2021, 11, 737843. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Dijkema, T.; Raaijmakers, C.P.; Ten Haken, R.K.; Roesink, J.M.; Braam, P.M.; Houweling, A.C.; Moerland, M.A.; Eisbruch, A.; Terhaard, C.H. Parotid gland function after radiotherapy: The combined michigan and utrecht experience. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 449–453. [Google Scholar] [CrossRef]
- Ling, D.C.; Kabolizadeh, P.; Heron, D.E.; Ohr, J.P.; Wang, H.; Johnson, J.; Kubicek, G.J. Incidence of hospitalization in patients with head and neck cancer treated with intensity-modulated radiation therapy. Head Neck 2015, 37, 1750–1755. [Google Scholar] [CrossRef]
- Trotti, A. Toxicity in head and neck cancer: A review of trends and issues. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1–12. [Google Scholar] [CrossRef]
- Pandey, D.; Szczesniak, M.; Maclean, J.; Yim, H.C.H.; Zhang, F.; Graham, P.; El-Omar, E.M.; Wu, P. Dysbiosis in Head and Neck Cancer: Determining Optimal Sampling Site for Oral Microbiome Collection. Pathogens 2022, 11, 1550. [Google Scholar] [CrossRef]
- Cheng, S.C.; Wu, V.W.; Kwong, D.L.; Ying, M.T. Assessment of post-radiotherapy salivary glands. Br. J. Radiol. 2011, 84, 393–402. [Google Scholar] [CrossRef]
- Nishimura, Y.; Nakamatsu, K.; Shibata, T.; Kanamori, S.; Koike, R.; Okumura, M.; Suzuki, M. Importance of the initial volume of parotid glands in xerostomia for patients with head and neck cancers treated with IMRT. Jpn. J. Clin. Oncol. 2005, 35, 375–379. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-Induced Oral Mucositis. Front. Oncol. 2017, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Q.; Yu, W.; Chang, B.; Le, A.D. Oral Mucositis: An Update on Innate Immunity and New Interventional Targets. J. Dent. Res. 2020, 99, 1122–1130. [Google Scholar] [CrossRef]
- Vasconcelos, R.M.; Sanfilippo, N.; Paster, B.J.; Kerr, A.R.; Li, Y.; Ramalho, L.; Queiroz, E.L.; Smith, B.; Sonis, S.T.; Corby, P.M. Host-Microbiome Cross-talk in Oral Mucositis. J. Dent. Res. 2016, 95, 725–733. [Google Scholar] [CrossRef]
- Hong, B.Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef]
- Subramaniam, N.; Muthukrishnan, A. Oral mucositis and microbial colonization in oral cancer patients undergoing radiotherapy and chemotherapy: A prospective analysis in a tertiary care dental hospital. J. Investig. Clin. Dent. 2019, 10, e12454. [Google Scholar] [CrossRef]
- Gaetti-Jardim EJr Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; Dos Santos, C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral Biol. 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Gao, L.; Hu, Y.; Wang, Y.; Jiang, W.; He, Z.; Zhu, C.; Ma, R.; Huang, Z. Exploring the variation of oral microbiota in supragingival plaque during and after head-and-neck radiotherapy using pyrosequencing. Arch. Oral Biol. 2015, 60, 1222–1230. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Boutell, S.; Taylor, M.W.; Douglas, R.G.; Biswas, K. Randomised, double-blind, placebo-controlled trial of oral probiotic Streptococcus salivarius M18 on head and neck cancer patients post-radiotherapy: A pilot study. Sci. Rep. 2020, 10, 13201. [Google Scholar] [CrossRef]
- Galeana-Patiño, C.E.; Ortiz, M.I.; Cariño-Cortés, R.; López-Santillán, I.C.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Muñoz-Pérez, V.M. Probiotics, as Adjuvant Therapy and Preventive Measure on Progression, and Complications of Head and Neck Cancer. Curr. Pharm. Biotechnol. 2023, 24, 1504–1514. [Google Scholar] [CrossRef]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Limaye, S.A.; Haddad, R.I.; Cilli, F.; Sonis, S.T.; Colevas, A.D.; Brennan, M.T.; Hu, K.S.; Murphy, B.A. Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer 2013, 119, 4268–4276. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Belgioia, L.; Cante, D.; LAPorta, M.R.; Caspiani, O.; Guarnaccia, R.; Argenone, A.; Muto, P.; Musio, D.; De Felice, F.; et al. Lactobacillus brevis CD2 for Prevention of Oral Mucositis in Patients with Head and Neck Tumors: A Multicentric Randomized Study. Anticancer Res. 2019, 39, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; Su, Y.; Xie, H.; Zeng, L.; Kuang, J.; et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef]
- Araújo, M.M.; Botelho, P.B. Probiotics, prebiotics, and synbiotics in chronic constipation: Outstanding aspects to be considered for the current evidence. Front. Nutr. 2022, 9, 935830. [Google Scholar] [CrossRef]
- Wang, L.; Vuletic, I.; Deng, D.; Crielaard, W.; Xie, Z.; Zhou, K.; Zhang, J.; Sun, H.; Ren, Q.; Guo, C. Bifidobacterium breve as a delivery vector of IL-24 gene therapy for head and neck squamous cell carcinoma in vivo. Gene Ther. 2017, 24, 699–705. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Li, Q.; Tian, K.; Xu, L.; Liu, G.; Guo, C. Exopolysaccharide, Isolated from a Novel Strain Bifidobacterium breve lw01 Possess an Anticancer Effect on Head and Neck Cancer—Genetic and Biochemical Evidences. Front. Microbiol. 2019, 10, 1044. [Google Scholar] [CrossRef]
- Rühle, A.; Zou, J.; Glaser, M.; Halle, L.; Gkika, E.; Schäfer, H.; Knopf, A.; Becker, C.; Grosu, A.L.; Popp, I.; et al. The influence of antibiotic administration on the outcomes of head-and-neck squamous cell carcinoma patients undergoing definitive (chemo)radiation. Eur. Arch. Otorhinolaryngol. 2023, 280, 2605–2616. [Google Scholar] [CrossRef]
- Crawford, P.A.; Gordon, J.I. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA 2005, 102, 13254–13259. [Google Scholar] [CrossRef]
- Nenclares, P.; Bhide, S.A.; Sandoval-Insausti, H.; Pialat, P.; Gunn, L.; Melcher, A.; Newbold, K.; Nutting, C.M.; Harrington, K.J. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur. J. Cancer 2020, 131, 9–15. [Google Scholar] [CrossRef]
- Ohsawa, M.; Nishi, H.; Emi, M.; Yoshikawa, T.; Hamai, Y.; Ibuki, Y.; Kurokawa, T.; Hirohata, R.; Kitasaki, N.; Kawada-Matsuo, M.; et al. Impact of Fusobacterium nucleatum in the treatment of cancer, including radiotherapy and its future potential in esophageal cancer. J. Radiat. Res. 2024, 65 (Suppl. S1), i126–i134. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, A.J.; Hong, C.S.; Lin, A.J.; Xiang, Y.; Cooper, D.E.; Zhang, J.; Xu, E.S.; Kuo, H.C.; Mowery, Y.M.; Carpenter, D.J.; et al. Neutrophils promote tumor resistance to radiation therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 18584–18589. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, J.W.; Yoon, H.I.; Lee, C.G.; Keum, K.C.; Lee, I.J. The Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Head and Neck Cancer Patients Treated with Radiotherapy. J. Clin. Med. 2018, 7, 512. [Google Scholar] [CrossRef]
- Almståhl, A.; Wikström, M.; Fagerberg-Mohlin, B. Microflora in oral ecosystems in subjects with radiation-induced hyposalivation. Oral Dis. 2008, 14, 541–549. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, C.R.; Huang, T.W. Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 13268. [Google Scholar] [CrossRef]
- Noble, A.R.; Greskovich, J.F.; Han, J.; Reddy, C.A.; Nwizu, T.I.; Khan, M.F.; Scharpf, J.; Adelstein, D.J.; Burkey, B.B.; Koyfman, S.A. Risk Factors Associated with Disease Recurrence in Patients with Stage III/IV Squamous Cell Carcinoma of the Oral Cavity Treated with Surgery and Postoperative Radiotherapy. Anticancer Res. 2016, 36, 785–792. [Google Scholar]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner HJr Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed. Pharmacol. J. 2019, 12, 07–15. [Google Scholar] [CrossRef]
- Montgomery, B.; Lin, D.W. Chapter 10—Toxicities of chemotherapy for genitourinary malignancies. In Complications of Urologic Surgery, 4th ed.; W.B. Saunders: Philadelphia, PA, USA, 2010; pp. 117–123. [Google Scholar]
- Hartinger, J.; Veselý, P.; Šíma, M.; Netíková, I.; Matoušková, E.; Petruželka, L. 5-fluorouracil Toxicity Mechanism Determination in Human Keratinocytes: In vitro Study on HaCaT Cell Line. Prague Med. Rep. 2017, 118, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; de Souza, T.; Coyle, L.; Di Piazza, M.; Herpers, B.; Ferreira, S.; Zhang, M.; Vappiani, J.; Sévin, D.C.; Gabor, A.; et al. New insights into the mechanisms underlying 5-fluorouracil-induced intestinal toxicity based on transcriptomic and metabolomic responses in human intestinal organoids. Arch. Toxicol. 2021, 95, 2691–2718. [Google Scholar] [CrossRef] [PubMed]
- Pazdur, R.; Kudelka, A.P.; Kavanagh, J.J.; Cohen, P.R.; Raber, M.N. The taxoids: Paclitaxel (Taxol) and docetaxel (Taxotere). Cancer Treat. Rev. 1993, 19, 351–386. [Google Scholar] [CrossRef] [PubMed]
- Ismail, U.; Killeen, R.B. Taxane Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Verweij, J.; Clavel, M.; Chevalier, B. Paclitaxel (Taxol) and docetaxel (Taxotere): Not simply two of a kind. Ann. Oncol. 1994, 5, 495–505. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Kovács, T.; Mikó, E.; Ujlaki, G.; Sári, Z.; Bai, P. The Microbiome as a Component of the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 137–153. [Google Scholar]
- Chambers, L.M.; Esakov Rhoades, E.L.; Bharti, R.; Braley, C.; Tewari, S.; Trestan, L.; Alali, Z.; Bayik, D.; Lathia, J.D.; Sangwan, N.; et al. Disruption of the Gut Microbiota Confers Cisplatin Resistance in Epithelial Ovarian Cancer. Cancer Res. 2022, 82, 4654–4669. [Google Scholar] [CrossRef]
- Cheung, M.K.; Yue, G.G.L.; Tsui, K.Y.; Gomes, A.J.; Kwan, H.S.; Chiu, P.W.Y.; Lau, C.B.S. Discovery of an interplay between the gut microbiota and esophageal squamous cell carcinoma in mice. Am. J. Cancer Res. 2020, 10, 2409–2427. [Google Scholar]
- Gui, Q.F.; Lu, H.F.; Zhang, C.X.; Xu, Z.R.; Yang, Y.H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 2015, 14, 5642–5651. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, X.; Chen, S.; Fu, X.; Ma, G.; Zhang, A. Akkermansia muciniphila Enhances the Antitumor Effect of Cisplatin in Lewis Lung Cancer Mice. J. Immunol. Res. 2020, 2020, 2969287. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Chen, H.L.; Tsai, J.N.; Lin, M.Y.; Liao, J.W.; Wei, M.S.; Ko, J.L.; Ou, C.C. Administration of Lactobacillus reuteriCombined with Clostridium butyricum Attenuates Cisplatin-Induced Renal Damage by Gut Microbiota Reconstitution, Increasing Butyric Acid Production, and Suppressing Renal Inflammation. Nutrients 2021, 13, 2792. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhou, B.; Xia, X.; Chen, S.; Deng, Y.; Wang, Y.; Wu, L.; Tian, Y.; Zhao, B.; Xu, H.; et al. Prevotella copri is associated with carboplatin-induced gut toxicity. Cell Death Dis. 2019, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed. Pharmacother. 2018, 108, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Smith, C.L.; Geier, M.S.; Yazbeck, R.; Torres, D.M.; Butler, R.N.; Howarth, G.S. Lactobacillus fermentum BR11 and fructo-oligosaccharide partially reduce jejunal inflammation in a model of intestinal mucositis in rats. Nutr. Cancer 2008, 60, 757–767. [Google Scholar] [CrossRef]
- Bowen, J.M.; Stringer, A.M.; Gibson, R.J.; Yeoh, A.S.; Hannam, S.; Keefe, D.M. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol. Ther. 2007, 6, 1449–1454. [Google Scholar]
- Osterlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef]
- Yeung, C.Y.; Chan, W.T.; Jiang, C.B.; Cheng, M.L.; Liu, C.Y.; Chang, S.W.; Chiang Chiau, J.S.; Lee, H.C. Amelioration of Chemotherapy-Induced Intestinal Mucositis by Orally Administered Probiotics in a Mouse Model. PLoS ONE 2015, 10, e0138746. [Google Scholar] [CrossRef]
- Quaresma, M.; Damasceno, S.; Monteiro, C.; Lima, F.; Mendes, T.; Lima, M.; Justino, P.; Barbosa, A.; Souza, M.; Souza, E.; et al. Probiotic mixture containing Lactobacillus spp. and Bifidobacterium spp. attenuates 5-fluorouracil-induced intestinal mucositis in mice. Nutr. Cancer. 2020, 72, 1355–1365. [Google Scholar] [CrossRef]
- Mi, H.; Dong, Y.; Zhang, B.; Wang, H.; Peter, C.C.K.; Gao, P.; Fu, H.; Gao, Y. Bifidobacterium Infantis Ameliorates Chemotherapy-Induced Intestinal Mucositis Via Regulating T Cell Immunity in Colorectal Cancer Rats. Cell Physiol. Biochem. 2017, 42, 2330–2341. [Google Scholar] [CrossRef]
- Kato, S.; Hamouda, N.; Kano, Y.; Oikawa, Y.; Tanaka, Y.; Matsumoto, K.; Amagase, K.; Shimakawa, M. Probiotic Bifidobacterium bifidum G9-1 attenuates 5-fluorouracil-induced intestinal mucositis in mice via suppression of dysbiosis-related secondary inflammatory responses. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Zhang, X.; Huang, J.; Wei, D.; Li, Z.; Shao, Z.; Ju, H.; Ren, G. The baseline oral microbiota predicts the response of locally advanced oral squamous cell carcinoma patients to induction chemotherapy: A prospective longitudinal study. Radiother. Oncol. 2021, 164, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Deng, Y.; Hou, X.; Wang, H.; Du, H.; Liu, Y. Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals 2024, 17, 604. [Google Scholar] [CrossRef]
- Singh, N.K.; Beckett, J.M.; Kalpurath, K.; Ishaq, M.; Ahmad, T.; Eri, R.D. Synbiotics as Supplemental Therapy for the Alleviation of Chemotherapy-Associated Symptoms in Patients with Solid Tumours. Nutrients 2023, 15, 1759. [Google Scholar] [CrossRef]
- Vanhoecke, B.; De Ryck, T.; Stringer, A.; Van De Wiele, T.; Keefe, D. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis. 2015, 21, 17–30. [Google Scholar] [CrossRef]
- Lim, Y.; Tang, K.D.; Karpe, A.V.; Beale, D.J.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. Chemoradiation therapy changes oral microbiome and metabolomic profiles in patients with oral cavity cancer and oropharyngeal cancer. Head Neck 2021, 43, 1521–1534. [Google Scholar] [CrossRef]
- Chidharla, A.; Parsi, M.; Kasi, A. Cetuximab; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Tang, W.H.; Sun, W.; Long, G.X. Concurrent cisplatin or cetuximab with radiotherapy in patients with locally advanced head and neck squamous cell carcinoma: A meta-analysis. Medicine 2020, 99, e21785. [Google Scholar] [CrossRef]
- Burcher, K.M.; Bloomer, C.H.; Gavrila, E.; Kalada, J.M.; Chang, M.J.; Gebeyehu, R.R.; Song, A.H.; Khoury, L.M.; Lycan, T.W.; Kinney, R.; et al. Study protocol: Phase II study to evaluate the effect of cetuximab monotherapy after immunotherapy with PD-1 inhibitors in patients with head and neck squamous cell cancer. Ther. Adv. Med. Oncol. 2024, 16, 17588359231217959. [Google Scholar] [CrossRef]
- Martini, G.; Ciardiello, D.; Dallio, M.; Famiglietti, V.; Esposito, L.; Corte, C.M.D.; Napolitano, S.; Fasano, M.; Gravina, A.G.; Romano, M.; et al. Gut microbiota correlates with antitumor activity in patients with mCRC and NSCLC treated with cetuximab plus avelumab. Int. J. Cancer 2022, 151, 473–480. [Google Scholar] [CrossRef]
- Harrington, K.J.; Burtness, B.; Greil, R.; Soulières, D.; Tahara, M.; de Castro GJr Psyrri, A.; Brana, I.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab with or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. J. Clin. Oncol. 2023, 41, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Blumenschein GJr Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; Worden, F.; et al. CheckMate 141: 1-Year Update and Subgroup Analysis of Nivolumab as First-Line Therapy in Patients with Recurrent/Metastatic Head and Neck Cancer. Oncologist 2018, 23, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Gunturi, A.; McDermott, D.F. Nivolumab for the treatment of cancer. Expert Opin. Investig. Drugs 2015, 24, 253–260. [Google Scholar] [CrossRef]
- Ribas, A. Tumor immunotherapy directed at PD-1. N. Engl. J. Med. 2012, 366, 2517–2519. [Google Scholar] [CrossRef]
- Dohlman, A.B.; Arguijo Mendoza, D.; Ding, S.; Gao, M.; Dressman, H.; Iliev, I.D.; Lipkin, S.M.; Shen, X. The cancer microbiome atlas: A pan-cancer comparative analysis to distinguish tissue-resident microbiota from contaminants. Cell Host Microbe 2021, 29, 281–298.e5. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Peters, B.A.; Wilson, M.; Moran, U.; Pavlick, A.; Izsak, A.; Wechter, T.; Weber, J.S.; Osman, I.; Ahn, J. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 2019, 11, 61. [Google Scholar] [CrossRef]
- Lee, K.A.; Thomas, A.M.; Bolte, L.A.; Björk, J.R.; de Ruijter, L.K.; Armanini, F.; Asnicar, F.; Blanco-Miguez, A.; Board, R.; Calbet-Llopart, N.; et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 2022, 28, 535–544. [Google Scholar] [CrossRef]
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R.; et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Zegarra-Ruiz, D.F.; El Beidaq, A.; Iñiguez, A.J.; Di Ricco, M.; Manfredo Vieira, S.; Ruff, W.E.; Mubiru, D.; Fine, R.L.; Sterpka, J.; Greiling, T.M.; et al. A diet-sensitive commensal lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe 2019, 25, 113–127. [Google Scholar] [CrossRef]
- Pandey, S.P.; Bender, M.J.; McPherson, A.C.; Phelps, C.M.; Sanchez, L.M.; Rana, M.; Hedden, L.; Sangani, K.A.; Chen, L.; Shapira, J.H.; et al. Tet2 deficiency drives liver microbiome dysbiosis triggering Tc1 cell autoimmune hepatitis. Cell Host Microbe 2022, 30, 1003–1019. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Bari, S.; Jain, S.; Yadav, H.; Liu, M.; Hodge, E.; Kirtane, K.; Chung, C.H.; Conejo-Garcia, J.; Muzaffar, J. Gut microbiome/metabolome predicts response to immune checkpoint blockers (ICB) in patients with recurrent metastatic head and neck squamous cell cancer (RM HNSCC). J. Clin. Oncol. 2022, 40, 6055. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Harrington, K.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Vokes, E.; Gillison, M.; Even, C.; et al. Abstract CT022: Evaluation of oral microbiome profiling as a response biomarker in squamous cell carcinoma of the head and neck: Analyses from CheckMate 141. Cancer Res. 2017, 77 (Suppl. S13), CT022. [Google Scholar] [CrossRef]
- Gharaibeh, R.Z.; Jobin, C. Microbiota and cancer immunotherapy: In search of microbial signals. Gut 2019, 68, 385–388. [Google Scholar] [CrossRef]
- Goon, P.; Schürmann, M.; Oppel, F.; Shao, S.; Schleyer, S.; Pfeiffer, C.J.; Todt, I.; Brasch, F.; Scholtz, L.U.; Göerner, M.; et al. Viral and Clinical Oncology of Head and Neck Cancers. Curr. Oncol. Rep. 2022, 24, 929–942. [Google Scholar] [CrossRef]
- Brooks, J.P.; Edwards, D.J.; Harwich, M.D.; Rivera, M.C.; Fettweis, J.M.; Serrano, M.G.; A Reris, R.; Sheth, N.U.; Huang, B. The truth about metagenomics: Quantifying and counteracting bias in 16S rRNA studies. BMC Microbiol. 2015, 15, 66. [Google Scholar] [CrossRef]
- Shi, W.; Shen, L.; Zou, W.; Wang, J.; Yang, J.; Wang, Y.; Liu, B.; Xie, L.; Zhu, J.; Zhang, Z. The Gut Microbiome Is Associated with Therapeutic Responses and Toxicities of Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients-A Pilot Study. Front. Cell. Infect. Microbiol. 2020, 10, 562463. [Google Scholar] [CrossRef]
- Mann, E.H.; Maughan, T.S. Fusobacterium nucleatum, rectal cancer and radiotherapy. Ann. Oncol. 2020, 31, 1277–1278. [Google Scholar] [CrossRef]
- Teughels, W.; Van Essche, M.; Sliepen, I.; Quirynen, M. Probiotics and oral healthcare. Periodontology 2000 2008, 48, 111–147. [Google Scholar] [CrossRef]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef]
- Araújo, L.D.C.; Furlaneto, F.A.C.; Da Silva, L.A.B.; Kapila, Y.L. Use of the Probiotic Bifidobacterium animalis subsp. lactis HN019 in Oral Diseases. Int. J. Mol. Sci. 2022, 23, 9334. [Google Scholar] [CrossRef]
- Liang, J.Q.; Zeng, Y.; Lau Eyt Sun, Y.; Huang, Y.; Zhou, T.; Xu, Z.; Yu, J.; Ng, S.C.; Chan, F.K.L. A Probiotic Formula for Modulation of Colorectal Cancer Risk via Reducing CRC-Associated Bacteria. Cells 2023, 12, 1244. [Google Scholar] [CrossRef]
- Badgeley, A.; Anwar, H.; Modi, K.; Murphy, P.; Lakshmikuttyamma, A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188494. [Google Scholar] [CrossRef]
- Liu, Y.; Baba, Y.; Ishimoto, T.; Tsutsuki, H.; Zhang, T.; Nomoto, D.; Okadome, K.; Yamamura, K.; Harada, K.; Eto, K.; et al. Fusobacterium nucleatum confers chemoresistance by modulating autophagy in oesophageal squamous cell carcinoma. Br. J. Cancer 2021, 124, 963–974. [Google Scholar] [CrossRef]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Inamura, K. Gut microbiota contributes towards immunomodulation against cancer: New frontiers in precision cancer therapeutics. Semin. Cancer Biol. 2021, 70, 11–23. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Song, J.M.; Woo, B.H.; Lee, J.H.; Yoon, S.; Cho, Y.; Kim, Y.D.; Park, H.R. Oral Administration of Porphyromonas gingivalis, a Major Pathogen of Chronic Periodontitis, Promotes Resistance to Paclitaxel in Mouse Xenografts of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2494. [Google Scholar] [CrossRef]
- Woo, B.H.; Kim, D.J.; Choi, J.I.; Kim, S.J.; Park, B.S.; Song, J.M.; Lee, J.H.; Park, H.R. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potential. Oncotarget. 2017, 8, 46981–46992. [Google Scholar] [CrossRef]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef]
- Koprinarova, M.; Markovska, P.; Iliev, I.; Anachkova, B.; Russev, G. Sodium butyrate enhances the cytotoxic effect of cisplatin by abrogating the cisplatin imposed cell cycle arrest. BMC Mol. Biol. 2010, 11, 49. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Jin, C.; Yue, K.; Sheng, D.; Zhang, T.; Dou, X.; Liu, J.; Jing, H.; Zhang, L.; et al. Prospective, longitudinal analysis of the gut microbiome in patients with locally advanced rectal cancer predicts response to neoadjuvant concurrent chemoradiotherapy. J. Transl. Med. 2023, 21, 221. [Google Scholar] [CrossRef]
- Jang, B.S.; Chang, J.H.; Chie, E.K.; Kim, K.; Park, J.W.; Kim, M.J.; Song, E.J.; Nam, Y.D.; Kang, S.W.; Jeong, S.Y.; et al. Gut Microbiome Composition Is Associated with a Pathologic Response After Preoperative Chemoradiation in Patients with Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 736–746. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, X.; Yang, D.; Li, Y.; Gong, T.; Li, B.; Cheng, J.; Chen, R.; Guo, X.; Yuan, W. Effects of probiotic supplementation on related side effects after chemoradiotherapy in cancer patients. Front. Oncol. 2022, 12, 1032145. [Google Scholar] [CrossRef]
- Qiu, B.; Xi, Y.; Liu, F.; Li, Y.; Xie, X.; Guo, J.; Guo, S.; Wu, Y.; Wu, L.; Liang, T.; et al. Gut Microbiome Is Associated with the Response to Chemoradiotherapy in Patients with Non-small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 407–418. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, F.; Qiu, B.; Li, Y.; Xie, X.; Guo, J.; Wu, L.; Liang, T.; Wang, D.; Wang, J.; et al. Analysis of Gut Microbiota Signature and Microbe-Disease Progression Associations in Locally Advanced Non-Small Cell Lung Cancer Patients Treated with Concurrent Chemoradiotherapy. Front. Cell. Infect. Microbiol. 2022, 12, 892401. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsumoto, Y.; Murakami, K.; Endo, S.; Toyozumi, T.; Otsuka, R.; Kinoshita, K.; Hu, J.; Iida, S.; Morishita, H.; et al. Gut microbiome can predict chemoradiotherapy efficacy in patients with esophageal squamous cell carcinoma. Esophagus 2023, 20, 691–703. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, W.; Zhang, Z.; Sha, G.; Wang, D.; Tang, D. Intratumoral microbiota: A new force in diagnosing and treating pancreatic cancer. Cancer Lett. 2023, 554, 216031. [Google Scholar] [CrossRef]
- Benkhaled, S.; Peters, C.; Jullian, N.; Arsenijevic, T.; Navez, J.; Van Gestel, D.; Moretti, L.; Van Laethem, J.L.; Bouchart, C. Combination, Modulation and Interplay of Modern Radiotherapy with the Tumor Microenvironment and Targeted Therapies in Pancreatic Cancer: Which Candidates to Boost Radiotherapy? Cancers 2023, 15, 768. [Google Scholar] [CrossRef] [PubMed]
- Gerassy-Vainberg, S.; Blatt, A.; Danin-Poleg, Y.; Gershovich, K.; Sabo, E.; Nevelsky, A.; Daniel, S.; Dahan, A.; Ziv, O.; Dheer, R.; et al. Radiation induces proinflammatory dysbiosis: Transmission of inflammatory susceptibility by host cytokine induction. Gut 2018, 67, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Z.; Li, X.; Cao, H.; Jiang, H.; Niu, Q.; Hu, B. Influence of tumor mycobiome on cancer pathogenesis (Review). Oncol. Lett. 2023, 26, 541. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Hetta, H.F.; Saleh, M.M.; Ali, M.E.; Ahmed, A.A.; Salah, M. Alterations in skin microbiome mediated by radiotherapy and their potential roles in the prognosis of radiotherapy-induced dermatitis: A pilot study. Sci. Rep. 2021, 11, 5179. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhang, G.; Ma, Y.; Zhang, Q.; Li, Z.; Ran, J.; Hou, X.; Geng, Y.; Yang, Z.; et al. The impact of pelvic radiotherapy on the gut microbiome and its role in radiation-induced diarrhoea: A systematic review. Radiat. Oncol. 2021, 16, 187. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Wheeler, K.M.; Liss, M.A. The Microbiome and Prostate Cancer Risk. Curr. Urol. Rep. 2019, 20, 66. [Google Scholar] [CrossRef]
- Scott, A.J.; Merrifield, C.A.; Younes, J.A.; Pekelharing, E.P. Pre-, pro- and synbiotics in cancer prevention and treatment—A review of basic and clinical research. Ecancermedicalscience 2018, 12, 869. [Google Scholar] [CrossRef]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley des Varannes, S.; Le Vacon, F.; de La Cochetière, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef]
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-de-Gómez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2020, 130, 466–479. [Google Scholar] [CrossRef]
- Manichanh, C.; Varela, E.; Martinez, C.; Antolin, M.; Llopis, M.; Doré, J.; Giralt, J.; Guarner, F.; Malagelada, J.R. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am. J. Gastroenterol. 2008, 103, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Eade, T.; Lamoury, G.; Carroll, S.; Morgia, M.; Kneebone, A.; Hruby, G.; Stevens, M.; Boyle, F.; Clarke, S.; et al. The Gut Microbiome and Gastrointestinal Toxicities in Pelvic Radiation Therapy: A Clinical Review. Cancers 2021, 13, 2353. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.D.; Kim, H.J.; Seo, J.G.; Kang, S.W.; Bae, J.W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE 2013, 8, e82659. [Google Scholar] [CrossRef]
- Mitra, A.; Grossman Biegert, G.W.; Delgado, A.Y.; Karpinets, T.V.; Solley, T.N.; Mezzari, M.P.; Yoshida-Court, K.; Petrosino, J.F.; Mikkelson, M.D.; Lin, L.; et al. Microbial Diversity and Composition Is Associated with Patient-Reported Toxicity during Chemoradiation Therapy for Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 163–171. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mak, T.D.; Jacobs, J.P.; Moon, B.H.; Strawn, S.J.; Braun, J.; Brenner, D.J.; Fornace AJJr Li, H.H. An Integrated Multi-Omic Approach to Assess Radiation Injury on the Host-Microbiome Axis. Radiat. Res. 2016, 186, 219–234. [Google Scholar] [CrossRef]
- Montassier, E.; Batard, E.; Massart, S.; Gastinne, T.; Carton, T.; Caillon, J.; Le Fresne, S.; Caroff, N.; Hardouin, J.B.; Moreau, P.; et al. 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb. Ecol. 2014, 67, 690–699. [Google Scholar] [CrossRef]
- Wang, A.; Ling, Z.; Yang, Z.; Kiela, P.R.; Wang, T.; Wang, C.; Cao, L.; Geng, F.; Shen, M.; Ran, X.; et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: A pilot study. PLoS ONE 2015, 10, e0126312. [Google Scholar] [CrossRef]
- Tooley, K.L.; Howarth, G.S.; Lymn, K.A.; Lawrence, A.; Butler, R.N. Oral ingestion of streptococcus thermophilus diminishes severity of small intestinal mucositis in methotrexate treated rats. Cancer Biol. Ther. 2006, 5, 593–600. [Google Scholar] [CrossRef]
- Tooley, K.L.; Howarth, G.S.; Lymn, K.A.; Lawrence, A.; Butler, R.N. Oral ingestion of Streptococcus thermophilus does not affect mucositis severity or tumor progression in the tumor-bearing rat. Cancer Biol. Ther. 2011, 12, 131–138. [Google Scholar] [CrossRef]
- Urbancsek, H.; Kazar, T.; Mezes, I.; Neumann, K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation-induced diarrhoea. Eur. J. Gastroenterol. Hepatol. 2001, 13, 391–396. [Google Scholar] [CrossRef]
- Salminen, E.; Elomaa, I.; Minkkinen, J.; Vapaatalo, H.; Salminen, S. Preservation of intestinal integrity during radiotherapy using live Lactobacillus acidophilus cultures. Clin. Radiol. 1988, 39, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Delia, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; De Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Chitapanarux, I.; Chitapanarux, T.; Traisathit, P.; Kudumpee, S.; Tharavichitkul, E.; Lorvidhaya, V. Randomized controlled trial of live Lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 2010, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Julia, P.J.; Munoz-Munoz, J.; van Sinderen, D. A comprehensive review on the impact of β-glucan metabolism by Bacteroides and Bifidobacterium species as members of the gut microbiota. Int. J. Biol. Macromol. 2021, 181, 877–889. [Google Scholar] [CrossRef]
- Santoni, M.; Piva, F.; Conti, A.; Santoni, A.; Cimadamore, A.; Scarpelli, M.; Battelli, N.; Montironi, R. Re: Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Eur. Urol. 2018, 74, 521–522. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Bernal, M.O.; Schneeberger, P.H.; Taylor, R.; Rey, V.; Hansen, A.R.; Taylor, K.; Bayley, A.; Hope, A.; Hosni, A.; Bratman, S.V.; et al. Role of the oral and gut microbiota as a biomarker in locoregionally advanced oropharyngeal squamous cell carcinoma (ROMA LA-OPSCC). J. Clin. Oncol. 2019, 37, 6045. [Google Scholar]
- Galvis, M.M.; Borges, G.A.; Oliveira, T.B.; Toledo, I.P.; Castilho, R.M.; Guerra, E.N.S.; Kowalski, L.P.; Squarize, C.H. Immunotherapy improves efficacy and safety of patients with HPV positive and negative head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 150, 102966. [Google Scholar] [CrossRef]
- Žuntar, I.; Petric, Z.; Bursać Kovačević, D.; Putnik, P. Safety of Probiotics: Functional Fruit Beverages and Nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef]
- Naeem, H.; Hassan, H.U.; Shahbaz, M.; Imran, M.; Memon, A.G.; Hasnain, A.; Murtaza, S.; Alsagaby, S.A.; Al Abdulmonem, W.; Hussain, M.; et al. Role of Probiotics against Human Cancers, Inflammatory Diseases, and Other Complex Malignancies. J. Food Biochem. 2024, 2024, 6632209. [Google Scholar] [CrossRef]
- Al-Asfour, A.; Bhardwaj, R.G.; Karched, M. Growth Suppression of Oral Squamous Cell Carcinoma Cells by Lactobacillus acidophilus. Int. Dent. J. 2024, 74, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Y.; Guo, T.; Li, Y.; Cai, H. Perioperative immunonutrition for gastrointestinal cancer: A systematic review of randomized controlled trials. Surg. Oncol. 2012, 21, e87–e95. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, K.; Varadhan, K.K.; Ljungqvist, O.; Lobo, D.N. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann. Surg. 2012, 255, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.A.; Mayer, C.; Bojaxhiu, B.; Aeberhard, C.; Schuetz, P.; Stanga, Z.; Giger, R. Effect of preoperative immunonutrition on complications after salvage surgery in head and neck cancer. J. Otolaryngol. Head Neck Surg. 2019, 48, 25. [Google Scholar] [CrossRef]
- Gunduz, M.; Murakami, D.; Gunduz, I.; Tamagawa, S.; Hiraoka, M.; Sugita, G.; Hotomi, M. Recurrent bacterial translocation from gut and sepsis in Head and neck cancer patients and its prevention by probiotics. Med. Hypotheses 2018, 120, 124–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).