A Comprehensive Molecular and Clinical Study of Patients with Young-Onset Colorectal Cancer
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population and Data Collection
2.2. Statistical Analysis
2.3. Molecular Testing
3. Results
3.1. Patient Characteristics
3.2. Molecular Findings
3.3. Survival Analysis
| Characteristic | HR 1 | 95% CI 1 | p-Value |
|---|---|---|---|
| Age at analysis/date at diagnosis | 1.00 | 0.92, 1.08 | >0.9 |
| Gender | |||
| Female | — | — | |
| Male | 1.53 | 0.62, 3.78 | 0.4 |
| Location | |||
| DC/sigmoid | — | — | |
| AC | 1.05 | 0.33, 3.32 | >0.9 |
| Rectum | 0.74 | 0.29, 1.88 | 0.5 |
| TC | 0.99 | 0.21, 4.69 | >0.9 |
| KRAS | |||
| Absent | — | — | |
| Present | 4.53 | 1.63, 12.6 | 0.004 |
| MS | |||
| Stable | — | — | |
| Equivical | 3.18 | 0.62, 16.2 | 0.2 |
| High | 0.46 | 0.06, 3.61 | 0.5 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal cancer incidence patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidence of colon and rectal cancer in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Bardot, A.; Ferlay, J.; Cabasag, C.J.; Morrison, D.S.; De, P.; Tervonen, H.; Walsh, P.M.; Bucher, O.; et al. Changes in colorectal cancer incidence in seven high-income countries: A population-based study. Lancet Gastroenterol. Hepatol. 2019, 4, 511–518. [Google Scholar] [CrossRef]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R.; Chung, D.C. The rising tide of early-onset colorectal cancer: A comprehensive review. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Goubran, R.A.; Gill, S.; Steel, M.; Shaw, A.; Demers, A.; Brenner, D.R. Risk factors for early-onset colorectal cancer: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1229–1240.e5. [Google Scholar] [CrossRef] [PubMed]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; Ferrucci, L.M. Risk factors associated with early-onset colorectal cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759.e2. [Google Scholar] [CrossRef]
- Low, E.E.; Demb, J.; Liu, L.; Earles, A.; Bustamante, R.; Williams, C.D.; Sosa, E.V.; Martinez, M.E.; Murphy, C.C.; Melkonian, S.C. Risk factors for early-onset colorectal cancer. Gastroenterology 2020, 159, 492–501.e7. [Google Scholar] [CrossRef]
- Dai, R.; Kelly, B.N.; Ike, A.; Xu, J.; Huang, Y.; Shah, S.C.; Shah, Y.; O’Neil, D.S.; Yu, J.; Abrams, J.A.; et al. The impact of the gut microbiome, environment, and diet in early-onset colorectal cancer development. Cancers 2024, 16, 676. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.O.; Murphy, E.A.; Singh, K.P.; Hsu, L.-L.; Berger, F.G.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Willauer, A.N.; Liu, Y.; Pereira, A.A.L.; Lam, M.; Morris, J.S.; Raghav, K.P.S.; Menter, D.G.; Broaddus, R.; Napolitano, S.; Eng, C.; et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019, 125, 2002–2010. [Google Scholar] [CrossRef]
- You, Y.N.; Xing, Y.; Feig, B.W.; Chang, G.J.; Cormier, J.N. Young-onset colorectal cancer: Is it time to pay attention? Arch. Intern. Med. 2012, 172, 287–289. [Google Scholar] [CrossRef]
- Pearlman, R.; Frankel, W.L.; Swanson, B.J.; Zhao, W.; Yilmaz, A.; Miller, K.; Lee, L.A.; Bacher, J.; Huelsman, K.M.; Arnold, M.; et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Kim, R.D.; Marshall, J.L.; Shields, A.F.; Lenz, H.-J.; et al. Comparative molecular analyses of early-onset and average-onset colorectal cancer. JCO Precis. Oncol. 2020, 4, PO.20.00032. [Google Scholar]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Kemeny, N.E.; et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 2018, 33, 125–136.e3. [Google Scholar] [CrossRef]
- Cohen, R.; Hain, E.; Buhard, O.; Guilloux, A.; Bachet, J.-B.; Schulmann, K.; Bork, P.; Zaanan, A.; Duval, A.; Laurent-Puig, P.; et al. Association of polymerase ε mutations with immunotherapy benefit in mismatch repair-deficient colorectal cancer. J. Clin. Oncol. 2021, 39, 686–697. [Google Scholar]
- Watson, R.; Liu, T.-C.; Ruzinova, M.B. High frequency of KRAS mutation in early-onset colorectal adenocarcinoma. Hum. Pathol. 2016, 56, 163–170. [Google Scholar] [CrossRef]
- Sahin, I.H.; Saridogan, T.; Ayasun, R.; Khan, U.; Keefer, L.; Zhao, Q.; Ning, Y.; Lenz, H.-J. Targeting KRAS oncogene for patients with colorectal cancer. JCO Oncol. Pract. 2024, 20, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.H.; Golemis, E.A.; Serebriiskii, I.G.; Newberg, J.Y.; Kumar, A.; Liu, M.; Bowles, M.E.; Chung, J.H.; Do, K.; Raghav, K.P.S.; et al. Comprehensive genomic landscapes in early and later onset colorectal cancer. Clin. Cancer Res. 2019, 25, 5852–5858. [Google Scholar] [CrossRef] [PubMed]
- Yantiss, R.K.; Goodarzi, M.; Zhou, X.K.; Black, D.; Renfro, L.A.; Shia, J.; Klimstra, D.S. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am. J. Surg. Pathol. 2009, 33, 572–582. [Google Scholar] [CrossRef]
- Aljehani, M.A.; Bien, J.; Lee, J.S.; Fisher, G.A.; Lin, A.Y. KRAS sequence variation in young vs. late-onset colorectal cancer. JAMA Netw. Open 2023, 6, e2345801. [Google Scholar] [CrossRef]
- Serebriiskii, I.G.; Connelly, C.; Frampton, G.; Newberg, J.; Cooke, M.; Miller, V.; Ross, J.S.; Handorf, E.; Alavi, K.; Chao, J.; et al. Comprehensive characterization of RAS mutations in colon and rectal cancers. Nat. Commun. 2019, 10, 3722. [Google Scholar] [CrossRef]
- Hitchen, N.; Wong, H.L.; Wong, R.; Shapiro, J.D.; Burge, M.E.; Nott, L.M.; Lee, B.; Lim, S.H.; Wong, S.F.; Caird, S.; et al. Real world characteristics and outcomes of patients with BRAFV600E-mutant metastatic colorectal cancer in Australia: The COALA project. J. Clin. Oncol. 2025, 43, 70. [Google Scholar] [CrossRef]
- Sahin, I.H.; Xiu, J.; Khushman, M.D.; Palumbo, E.; Weinberg, B.A.; Goel, S.; Akce, M.; Singhi, A.D.; Gorantla, V.; Lou, E.; et al. Investigating the clinical and molecular characteristics of class II and III BRAF mutations and their response to anti-EGFR therapy in MSS CRC: A comprehensive analysis. J. Clin. Oncol. 2025, 43, 274. [Google Scholar] [CrossRef]
- Ferrell, M.; Guven, D.C.; Gomez, C.G.; Sahin, I.H.; Kwon, M.; Chae, Y.K.; Shah, N.J.; Altan, M.; Hwang, J.J.; Dasari, A.; et al. WNT and TGF-beta pathway alterations in young-onset colorectal cancer. Sci. Rep. 2024, 14, 17884. [Google Scholar] [CrossRef]
- Karan, C.; Tan, E.; Sarfraz, H.; Singh, A.; McFarland, D.W.; Thomas, S.; Lieu, C.H.; Overman, M.J.; Sahin, I.H. HER2–targeting approaches for colorectal cancer. JCO Oncol. Pract. 2022, 18, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Mosalem, O.; Coston, T.W.; Imperial, R.; Mauer, E.; Thompson, C.; Yilma, B.; Bekaii-Saab, T.S.; Stoppler, M.C.; Starr, J.S. A comprehensive analysis of POLE/POLD1 genomic alterations in colorectal cancer. Oncologist 2024, 29, e1224–e1227. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Q.; Wang, Y.-N.; Jin, Y.; He, M.-M.; Liu, Z.-X.; Wang, Z.-Q.; Luo, H.-Y.; Zhang, D.-S.; Wang, F.-H.; et al. POLE and POLD1 mutations and immunotherapy outcomes. JAMA Oncol. 2019, 5, 1504–1506. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lin, J.-K.; Chang, S.-C.; Chang, H.-L.; Cheng, Y.-Y.; Lin, C.-C.; Lan, Y.-T.; Lin, H.-H.; Yang, S.-H.; Wang, H.-S.; et al. Molecular profile of sporadic colorectal cancer in Taiwan. J. Biomed. Sci. 2011, 18, 36. [Google Scholar] [CrossRef]
- Lievre, A.; Bachet, J.-B.; Boige, V.; Cayre, A.; Le Corre, D.; Buc, E.; Ychou, M.; Bouche, O.; Landi, B.; Louvet, C.; et al. KRAS mutations as prognostic factor in colorectal cancer treated with cetuximab. J. Clin. Oncol. 2008, 26, 374–379. [Google Scholar] [CrossRef]
- Kadowaki, S.; Kakuta, M.; Takahashi, S.; Arai, Y.; Nishimura, Y.; Yamaguchi, K.; Yamada, Y.; Shimosegawa, T.; Hamada, T.; Akagi, Y.; et al. KRAS and BRAF mutations in resected colorectal cancer. World J. Gastroenterol. 2015, 21, 1275. [Google Scholar] [CrossRef]
- Singhal, A.; Li, B.T.; O’Reilly, E.M. Targeting KRAS in cancer. Nat. Med. 2024, 30, 969–983. [Google Scholar] [CrossRef]
- Benhattar, J.; Losi, L.; Chaubert, P.; Givel, J.-C.; Costa, J. Prognostic significance of K-ras mutations in colorectal carcinoma. Gastroenterology 1993, 104, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.; Barve, M.; Fakih, M.; Lenz, H.-J.; O’Neil, B.; Price, T.J.; Falchook, G.S.; et al. Adagrasib ± cetuximab in KRAS G12C CRC. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Kim, T.W.; Muro, K.; Zayac, A.; Marabelle, A.; Helwig, C.; Oliner, K.S.; Zhang, S.; et al. Sotorasib plus panitumumab in KRAS G12C CRC. N. Engl. J. Med. 2023, 389, 2125–2139. [Google Scholar] [CrossRef]
- Kim, D.; Herdeis, L.; Rudolph, D.; Stransky, N.; Leong, S.; Jensen, M.; Tran, J.; Romero, R.; Gill, A.L.; Xu, Y.; et al. Pan-KRAS inhibitor disables oncogenic signalling. Nature 2023, 619, 160–166. [Google Scholar] [CrossRef]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Peeples, M.; Li, N.; Anhorn, R.; Ryan, J.; Signorovitch, J. Real-world utilization of molecular diagnostic testing and matched drug therapies in the treatment of metastatic cancers. J. Med. Econ. 2018, 21, 543–552. [Google Scholar] [CrossRef]
- Gambardella, V.; Lombardi, P.; Carbonell-Asins, J.A.; Gallego, J.; Roselló, S.; Tarazona, N.; Martínez-Ciarpaglini, C.; Roda, D.; Cervantes, A.; Huerta, M.; et al. Molecular profiling and matched therapies: The MAST study. Br. J. Cancer 2021, 125, 1261–1269. [Google Scholar] [CrossRef]

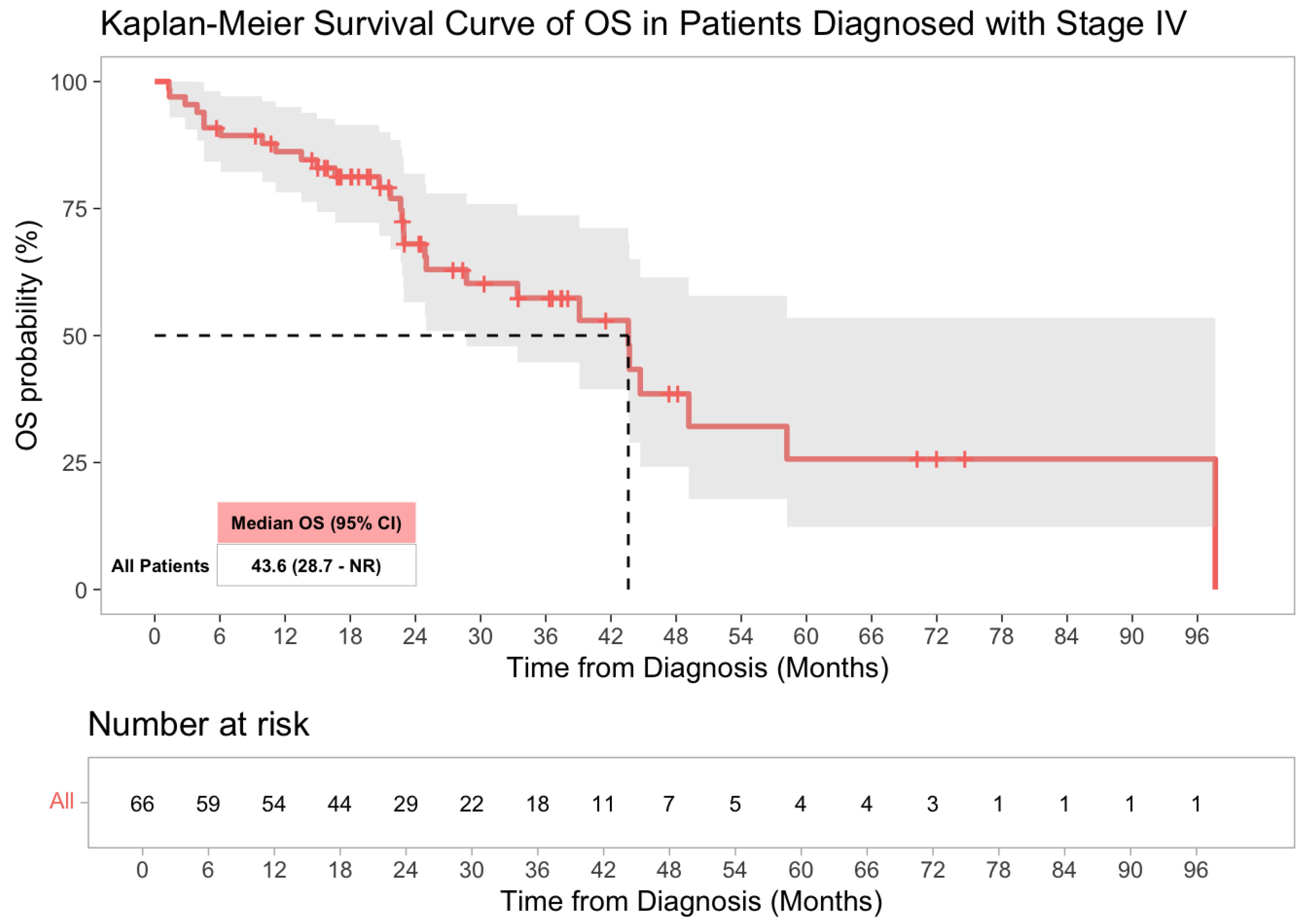
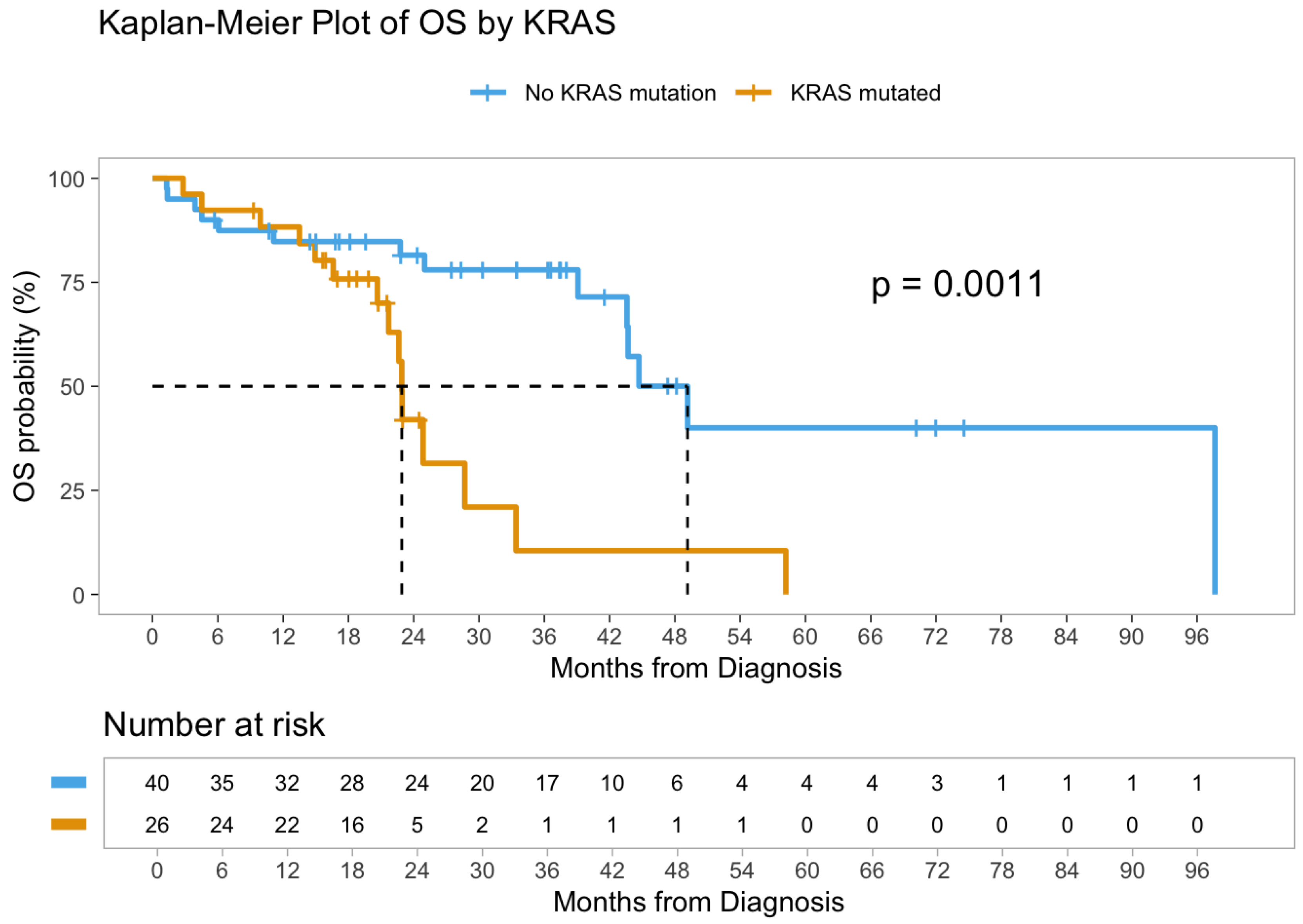
| Characteristic | Stage I–III (n = 44) | Stage IV (n = 66) | Total (n = 110) |
|---|---|---|---|
| Age at diagnosis (median, range) | 44 (25–49) | 45 (26–49) | 44.5 (25–49) |
| Gender | |||
| Female | 16 (36%) | 24 (36%) | 40 (36%) |
| Male | 28 (64%) | 42 (64%) | 70 (64%) |
| Race/Ethnicity | |||
| White | 33 (75%) | 59 (89%) | 92 (84%) |
| African American | 9 (20%) | 3 (4.5%) | 12 (11%) |
| Asian | 0 (0%) | 2 (3.0%) | 2 (1.8%) |
| American Indian/Alaska Native | 1 (2.3%) | 0 (0%) | 1 (0.9%) |
| Pacific Islander | 0 (0%) | 1 (1.5%) | 1 (0.9%) |
| Unreported/Not Disclosed | 1 (2.3%) | 1 (1.5%) | 2 (1.8%) |
| Primary Tumor Location | |||
| Descending/Sigmoid Colon | 18 (41%) | 30 (45%) | 48 (44%) |
| Rectum | 16 (36%) | 20 (30%) | 36 (33%) |
| Ascending Colon | 6 (14%) | 10 (15%) | 16 (15%) |
| Transverse Colon | 4 (9.1%) | 6 (9.1%) | 10 (9.1%) |
| Stage at Diagnosis | |||
| Stage I | 6 (14%) | 0 (0%) | 6 (5.5%) |
| Stage II | 3 (6.8%) | 0 (0%) | 3 (2.7%) |
| Stage III | 13 (30%) | 0 (0%) | 13 (12%) |
| Stage IV | 22 (50%) | 66 (100%) | 88 (80%) |
| Molecular Characteristic | Stage I–III (n = 44) | Stage IV (n = 66) | Total (n = 110) |
|---|---|---|---|
| KRAS mutation status | – | – | – |
| Wild-type (no mutation) | 30 (68%) | 40 (61%) | 70 (64%) |
| Mutant | 14 (32%) | 26 (39%) | 40 (36%) |
| KRAS mutation codon (among KRAS-mutant) | |||
| Exon 2 (codon 12/13) | 13 (93%) | 22 (84%) | 35 (88%) |
| Non-exon 2 (codon 61/146) | 1 (7.1%) | 4 (17%) | 5 (13%) |
| NRAS mutation status | |||
| Wild-type | 44 (100%) | 65 (98%) | 109 (99%) |
| Mutant | 0 (0%) | 1 (1.5%) | 1 (0.9%) |
| BRAF mutation status | |||
| V600E mutant | 2 (4.5%) | 2 (3.0%) | 4 (3.6%) |
| Non-V600E mutant | 1 (2.3%) | 1 (1.5%) | 2 (1.8%) |
| Wild-type | 41 (93%) | 63 (95%) | 104 (95%) |
| POLE/POLD1 mutation (pathogenic) | |||
| Mutant | 1 (2.3%) | 10 (15%) | 11 (10%) |
| Wild-type | 43 (97.7%) | 56 (85%) | 99 (90%) |
| ERBB2 (HER2) amplification | |||
| Positive | 2 (4.5%) | 3 (4.5%) | 5 (4.5%) |
| Negative | 42 (95%) | 63 (95%) | 105 (95%) |
| Microsatellite (MSI) Status | |||
| MSI-high | 4 (9.1%) | 3 (4.5%) | 7 (6.4%) |
| MS-stable | 39 (89%) | 59 (89%) | 98 (89%) |
| Equivocal/indeterminate | 1 (2.3%) | 4 (6.1%) | 5 (4.5%) |
| Tumor Mutational Burden | |||
| <10 mutations/Mb | 23 (52%) | 40 (61%) | 63 (57%) |
| 10–20 mutations/Mb | 12 (27%) | 20 (30%) | 32 (29%) |
| >20 mutations/Mb | 9 (20%) | 6 (9.1%) | 15 (14%) |
| CNV profile (somatic copy number) | |||
| Gain only | 7 (16%) | 8 (12%) | 15 (14%) |
| Loss only | 11 (25%) | 23 (35%) | 34 (31%) |
| Both gain and loss | 7 (16%) | 6 (9.1%) | 13 (12%) |
| No CNV (N/A) | 19 (43%) | 29 (44%) | 48 (44%) |
| Oncogenic Fusions | |||
| NTRK fusion (ETV6–NTRK3) | 0 (0%) | 2 (3.0%) | 2 (1.8%) |
| Characteristic | HR | 95% CI | p-Value |
|---|---|---|---|
| Age at analysis/date at diagnosis | 1.03 | 0.96–1.11 | 0.50 |
| Gender (male vs. female) | - | - | - |
| Female | - | -- | - |
| Male | 0.87 | 0.41–1.86 | 0.70 |
| Tumor location | |||
| DC/Sigmoid | - | - | - |
| AC | 1.38 | 0.48–3.93 | 0.50 |
| Rectum | 0.81 | 0.34–1.95 | 0.60 |
| TC | 0.94 | 0.21–4.26 | >0.90 |
| KRAS mutation | |||
| Absent | |||
| Present | 3.52 | 1.59–7.76 | 0.002 |
| MSI status | |||
| Stable | - | - | - |
| Equivocal | 3.55 | 0.77–16.3 | 0.10 |
| High | 0.52 | 0.07–3.90 | 0.50 |
| TMB | |||
| ≥10 | - | - | - |
| <10 | 1.72 | 0.77–3.83 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrollahi, E.; Wang, S.; Yanes, R.; Gonzalez Gomez, C.; Magge, T.; Overacre, A.; Hsieh, R.; Mcfarquhar, A.; Tatsuoka, C.; Singhi, A.; et al. A Comprehensive Molecular and Clinical Study of Patients with Young-Onset Colorectal Cancer. Cancers 2025, 17, 2763. https://doi.org/10.3390/cancers17172763
Nasrollahi E, Wang S, Yanes R, Gonzalez Gomez C, Magge T, Overacre A, Hsieh R, Mcfarquhar A, Tatsuoka C, Singhi A, et al. A Comprehensive Molecular and Clinical Study of Patients with Young-Onset Colorectal Cancer. Cancers. 2025; 17(17):2763. https://doi.org/10.3390/cancers17172763
Chicago/Turabian StyleNasrollahi, Elham, Shuaichao Wang, Rami Yanes, Cyndi Gonzalez Gomez, Tara Magge, Abigail Overacre, Ronan Hsieh, Ashley Mcfarquhar, Curtis Tatsuoka, Aatur Singhi, and et al. 2025. "A Comprehensive Molecular and Clinical Study of Patients with Young-Onset Colorectal Cancer" Cancers 17, no. 17: 2763. https://doi.org/10.3390/cancers17172763
APA StyleNasrollahi, E., Wang, S., Yanes, R., Gonzalez Gomez, C., Magge, T., Overacre, A., Hsieh, R., Mcfarquhar, A., Tatsuoka, C., Singhi, A., Saeed, A., & Sahin, I. H. (2025). A Comprehensive Molecular and Clinical Study of Patients with Young-Onset Colorectal Cancer. Cancers, 17(17), 2763. https://doi.org/10.3390/cancers17172763









