Contemporary Clinical Utilization of Radioembolization with Immune Checkpoint Inhibitors as First-Line Treatment in HCC: Real-World Report on Safety and Outcomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Data Collection
2.2. Immune Checkpoint Inhibitor Regimen
2.3. Yttrium-90 Radioembolization
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. 90Y and ICI Treatment Sequence
3.3. Safety and Adverse Events
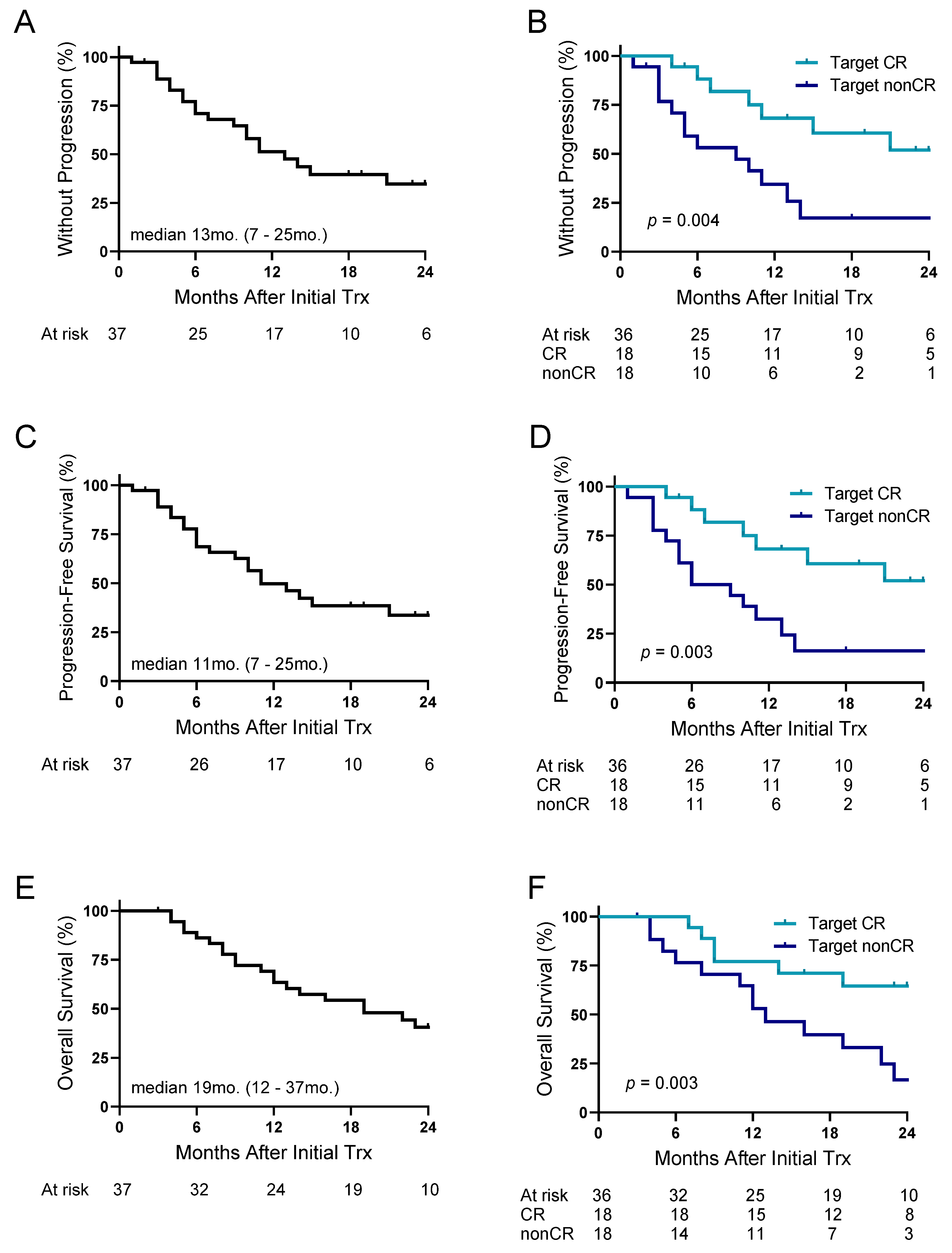
3.4. Response Rates and Outcomes Following 90Y-ICI
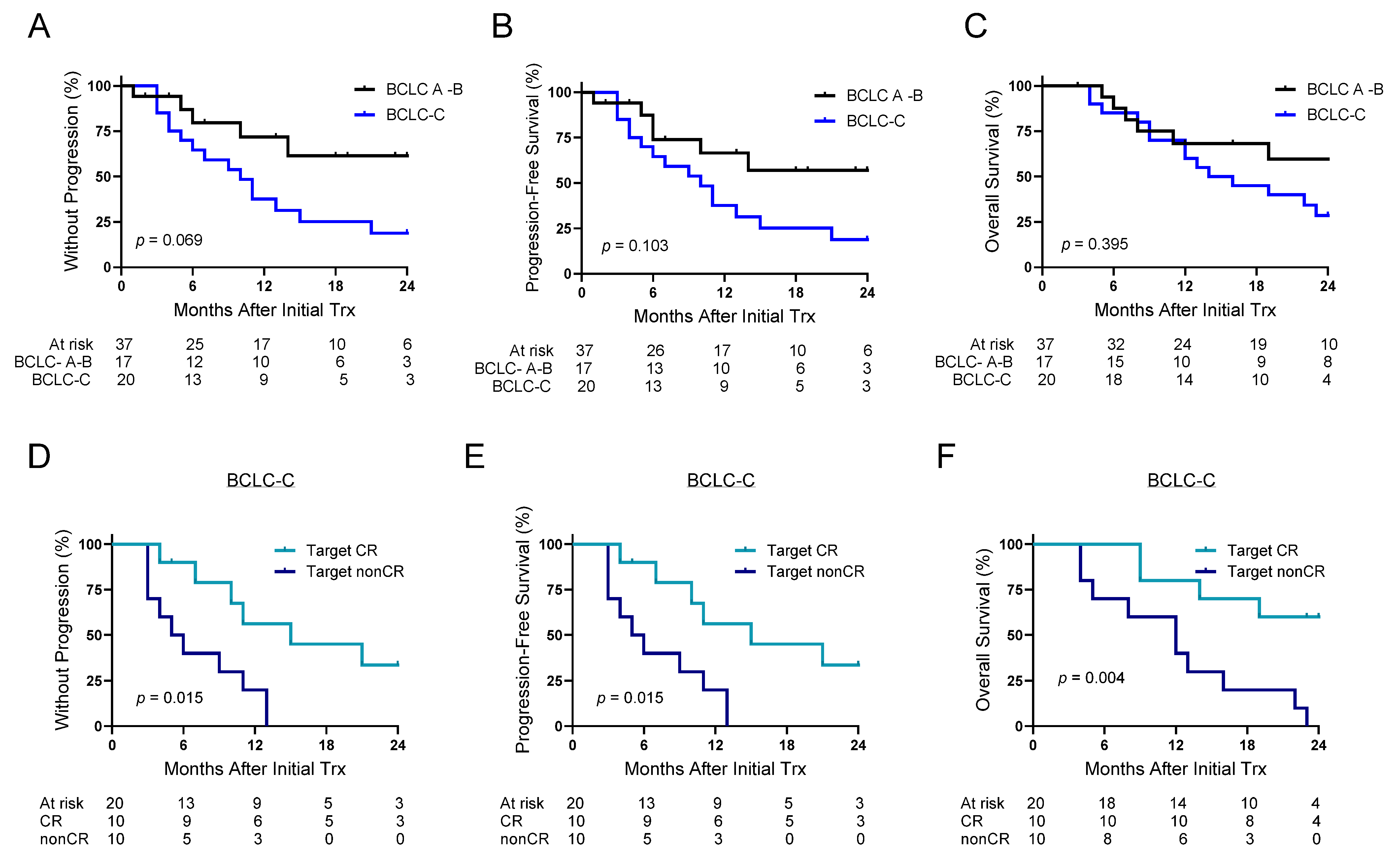
3.5. 90Y-ICI in BCLC-C Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| ICI | Immune checkpoint inhibitors |
| TKI | Tyrosine-kinase inhibitors |
| OS | Overall survival |
| ORR | Objective response rate |
| LDT | Liver-directed therapy |
| BCLC | Barcelona Clinic Liver Cancer |
| TACE | Transarterial chemoembolization |
| TARE | Transarterial radioembolization |
| 90Y | Yttrium-90 |
| Atezo | Atezolizumab |
| Bev | Bevacizumab |
| Treme | Tremelimumab |
| Durva | Durvalumab |
| PFS | Progression-free survival |
| CR | Complete response |
| OR | Objective response |
| TTP | Time to progression |
| IQR | Interquartile range |
References
- Villanueva, A.; Longo, D.L. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Évid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Galle, P.; Van Dao, T.; Reig, M.; Makowsky, M.; Paskow, M.; Gupta, C.; Kurland, J.; Negro, A.; Azevedo, S.; Braghiroli, M.I.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1399–1410. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Finn, R.S.; Sangro, B. Combination immunotherapy for hepatocellular carcinoma. J. Hepatol. 2023, 79, 506–515. [Google Scholar] [CrossRef]
- Sangro, B.; Kudo, M.; Erinjeri, J.P.; Qin, S.; Ren, Z.; Chan, S.L.; Arai, Y.; Heo, J.; Mai, A.; Escobar, J.; et al. Durvalumab with or without bevacizumab with transarterial chemoembolisation in hepatocellular carcinoma (EMERALD-1): A multiregional, randomised, double-blind, placebo-controlled, phase 3 study. Lancet 2025, 405, 216–232. [Google Scholar] [CrossRef]
- Kim, E.; Sher, A.; Abboud, G.; Schwartz, M.; Facciuto, M.; Tabrizian, P.; Knešaurek, K.; Fischman, A.; Patel, R.; Nowakowski, S.; et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): A single-centre, single-arm study. Lancet Gastroenterol. Hepatol. 2022, 7, 843–850. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; Assenat, E.; Tacher, V.; Terroir-Cassou-Mounat, M.; Mariano-Goulart, D.; Amaddeo, G.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Núñez, K.; Sandow, T.; Gimenez, J.; Hibino, M.; Cohen, A.; Thevenot, P. Yttrium-90 Induces an Effector Memory Response with Neoantigen Clonotype Expansion: Implications for Immunotherapy. Cancer Res. Commun. 2024, 4, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Núñez, K.G.; Sandow, T.; Gimenez, J.; Hibino, M.; Fort, D.; Cohen, A.J.; Thevenot, P.T. Lineage-specific regulation of PD-1 expression in early-stage hepatocellular carcinoma following 90yttrium transarterial radioembolization—Implications in treatment outcomes. Eur. J. Cancer 2023, 196, 113442. [Google Scholar] [CrossRef] [PubMed]
- Rivoltini, L.; Bhoori, S.; Camisaschi, C.; Bergamaschi, L.; Lalli, L.; Frati, P.; Citterio, D.; Castelli, C.; Mazzaferro, V. Y90-radioembolisation in hepatocellular carcinoma induces immune responses calling for early treatment with multiple checkpoint blockers. Gut 2022, 72, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Chan, S.L.; Sangro, B.; Lau, G.; Kudo, M.; Reig, M.; Breder, V.; Ryu, M.-H.; Ostapenko, Y.; Sukeepaisarnjaroen, W.; et al. Five-year overall survival update from the HIMALAYA study of tremelimumab plus durvalumab in unresectable HCC. J. Hepatol. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.D.; Bajaj, S.; He, A.; Mody, K.; Hickey, R.M.; Sarwar, A.; Krishnan, S.; Patel, T.C.; Toskich, B.B. Combining Radioembolization and Immune Checkpoint Inhibitors for the Treatment of Hepatocellular Carcinoma: The Quest for Synergy. J. Vasc. Interv. Radiol. 2024, 36, 414–424.e2. [Google Scholar] [CrossRef]
- Kudo, M.; Ren, Z.; Guo, Y.; Han, G.; Lin, H.; Zheng, J.; Ogasawara, S.; Kim, J.H.; Zhao, H.; Li, C.; et al. Transarterial chemoembolisation combined with lenvatinib plus pembrolizumab versus dual placebo for unresectable, non-metastatic hepatocellular carcinoma (LEAP-012): A multicentre, randomised, double-blind, phase 3 study. Lancet 2025, 405, 203–215. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Bhoori, S.; Romito, R.; Chiesa, C.; Morosi, C.; Maccauro, M.; Marchianò, A.; Bongini, M.; Lanocita, R.; et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: A phase 2 study. Hepatology 2012, 57, 1826–1837. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Liang, J.; Lauzon, M.; Luu, M.; Noureddin, M.; Ayoub, W.; Kuo, A.; Sankar, K.; Gong, J.; Hendifar, A.; et al. Immunotherapy and Transarterial Radioembolization Combination Treatment for Advanced Hepatocellular Carcinoma. Am. J. Gastroenterol. 2023, 118, 2201–2211. [Google Scholar] [CrossRef]
- Zhan, C.; Ruohoniemi, D.; Shanbhogue, K.P.; Wei, J.; Welling, T.H.; Gu, P.; Park, J.S.; Dagher, N.N.; Taslakian, B.; Hickey, R.M. Safety of Combined Yttrium-90 Radioembolization and Immune Checkpoint Inhibitor Immunotherapy for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2020, 31, 25–34. [Google Scholar] [CrossRef]
- Bin Lee, Y.; Nam, J.Y.; Cho, E.J.; Lee, J.-H.; Yu, S.J.; Kim, H.-C.; Paeng, J.C.; Yoon, J.-H.; Kim, Y.J. A Phase I/IIa Trial of Yttrium-90 Radioembolization in Combination with Durvalumab for Locally Advanced Unresectable Hepatocellular Carcinoma. Clin. Cancer Res. 2023, 29, 3650–3658. [Google Scholar] [CrossRef]
- Mejait, A.; Roux, C.; Soret, M.; Larrey, E.; Wagner, M.; Bijot, J.C.; Lussey-Lepoutre, C.; Thabut, D.; Goumard, C.; Maksud, P.; et al. Enhanced therapeutic outcomes with atezolizumab-bevacizumab and SIRT combination compared to SIRT alone in unresectable HCC: A promising approach for improved survival. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102282. [Google Scholar] [CrossRef]
- Tai, D.; Loke, K.; Gogna, A.; Kaya, N.A.; Tan, S.H.; Hennedige, T.; Ng, D.; Irani, F.; Lee, J.; Lim, J.Q.; et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): A single arm, single centre, phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef] [PubMed]
| General | Cohort (n = 37) |
|---|---|
| Age, median (IQR) | 64 (61–69) |
| Age, median (IQR) | 64 (61–69) |
| Gender, male, n (%) | 30 (81) |
| Declared race, n (%) | |
| Caucasian/White | 15 (40) |
| African American/Black | 14 (38) |
| Other | 8 (22) |
| Hepatology | |
| Etiology, n (%) | |
| HCV | 20 (54) |
| HCV ALD | 5 (13) |
| HBV | 4 (11) |
| Other | 8 (22) |
| Child-Pugh score, n (%) | |
| A5 | 11 (30) |
| A6 | 23 (62) |
| B7 | 1 (3) |
| B8 | 2 (5) |
| Bilirubin (mg/dL), median (IQR) | 0.7 (0.5–1.2) |
| Albumin (g/dL), median (IQR) | 3.3 (3.2–3.8) |
| Platelets (103/mL), median (IQR) | 215 (154–283) |
| Esophageal varices at baseline, n (%) | 9 (24) |
| Unable to assess varies | 5 (14) |
| HCC | |
| Diagnosis date, range | 12/28/2020–2/27/2024 |
| AFP (ng/mL), median (IQR) | 86 (9–2050) |
| AFP (ng/mL), ≥400, n (%) | 14 (38) |
| BCLC staging, n (%) | |
| A | 7 (19) |
| B | 10 (27) |
| C | 20 (54) |
| ECOG score, n (%) | |
| 0 | 23 (62) |
| 1 | 14 (38) |
| Solitary HCC, n (%) | 20 (54) |
| Index tumor size (cm), median (IQR) | 8.0 (6.0–12) |
| Cumulative tumor size (cm), median (IQR) | 10.8 (7.5–15.2) |
| Macrovascular invasion, n (%) | 14 (38) |
| Extrahepatic disease, n (%) | 9 (24) |
| Prior LDT, n (%) | 4 (11) |
| Immune Checkpoint Inhibitors | |
| ICI therapy | |
| Atezolizumab/Bevacizumab | 30 (81) |
| Tremelimumab/Durvalumab | 7 (19) |
| Treatment sequence lead | |
| ICI, n (%) | 32 (86) |
| 90Y, n (%) | 5 (14) |
| ICI Therapy | Cohort (n = 37) |
|---|---|
| Atezolizumab/Bevacizumab | |
| Start date, range | 1/22/2021–5/31/2024 |
| Patients receiving Atezo/Bev therapy, n (% of total) | 30 (81) |
| Number of cycles of Atezo, median (range) | 10 (1–49) |
| Number of cycles of Bev, median (range) | 4 (0–23) |
| Duration of Atezo/Bev, months, median (IQR) | 8 (4–16) |
| Discontinuation, n (% of Atezo/Bev) | 14 (47) |
| Time to discontinuation, months, median (IQR) | 5 (2–7) |
| Switched to different systemic therapy, n (%) | 6 (20) |
| Tremelimumab/Durvalumab | |
| Start date, range | 3/24/2023–9/13/2023 |
| Patients receiving Treme/Durva therapy, n (% of total) | 7 (18) |
| Number of cycles of Durva, median (range) | 9 (2–15) |
| Duration of Treme/Durva, months, median (IQR) | 13 (6–18) |
| Discontinuation, n (% of Treme + Durva) | 3 (43) |
| Time to discontinuation, months, median (IQR) | 6 (1–8) |
| Switch to different systemic therapy, n (%) | 2 (29) |
| First Cycle 90Y Characteristics | |
| First cycle 90Y date, range | 3/9/2021–5/20/2024 |
| Patients receiving 90Y, n (% of total) | 37 (100) |
| Target perfusion volume (mL), median (IQR) | 573 (274–978) |
| Target dose to volume (Gy), median (IQR) | 472 (313–556) |
| Lung shunt fraction (%), median (IQR) | 5.0 (3.3–8.1) |
| Multicycle 90Y, n (%) | 11 (30) |
| Number of total 90Y treatments, median (range) | 1 (1–4) |
| DEB-TACE bridge to 90Y | 3 (8) |
| Treatment Sequence Lead | |
| ICI, n (%) | 32 (86) |
| 90Y, n (%) | 5 (14) |
| Time from ICI lead -> 90Y, days, median (IQR) | 48 (21–76) |
| Time from 90Y lead -> ICI, days, median (IQR) | 18 (12–48) |
| DEB-TACE bridge to 90Y | 3 (8) |
| All AEs | Cohort (n = 37) |
|---|---|
| Any AEs, n of patients (% total) | 33 (89) |
| Any grade 3 or 4, n of patients (% total) | 6 (16) |
| AEs that led to discontinuation, n of patients (% total) | 4 (11) |
| AEs that led to delay in treatment, n of patients (% of total) | 9 (24) |
| AEs that led to death, n of patients (% of total) | 0 (0) |
| Immune-mediated AE requiring steroid use, n of patients (% of total) | 8 (22) |
| Atezolizumab/Bevacizumab AEs | Cohort (n = 30) |
| Duration of treatment, months, median (IQR) | 8 (3–16) |
| Follow-up time, months (IQR) | 15 (8–26) |
| Any, n of patients (%) | 25 (83) |
| Any grade 3 or 4, n of patients (%) | 5 (16) |
| AEs that led to discontinuation, n of patients (%) | 4 (13) |
| AEs that led to delay in treatment, n of patients (%) | 4 (13) |
| AEs that led to death, n of patients (%) | 0 (0) |
| Immune-mediated AE requiring steroid use, n of patients (%) | 5 (17) |
| Tremelimumab/Durvalumab AEs | Cohort (n = 7) |
| Duration of treatment, months, median (IQR) | 13 (6–18) |
| Follow-up time, months (IQR) | 16 (8–19) |
| Any, n of patients (%) | 5 (71) |
| Any grade 3 or 4, n of patients (%) | 0 (0) |
| AEs that led to discontinuation, n of patients (%) | 0 (0) |
| AEs that led to delay in treatment, n of patients (%) | 3 (43) |
| AEs that led to death, n of patients (%) | 0 (0) |
| Immune-mediated AE requiring steroid use, n of patients (%) | 3 (43) |
| 90Y AEs | Cohort (n = 37) |
| Number of 90Y treatments, median (range) | 1 (1–4) |
| Any, n of patients (% of total) | 20 (54) |
| Any grade 3 or 4, n of patients (% of total) | 0 (0) |
| AEs that led to discontinuation, n of patients (% of total) | 0 (0) |
| AEs that led to delay in treatment, n of patients (% of total) | 2 (5) |
| AEs that led to death, n of patients (% of total) | 0 (0) |
| Immune-mediated AE requiring steroid use, n of patients (% of total) | 0 (0) |
| Outcomes | |
|---|---|
| Time to follow-up (months), median (IQR) | 16 (8–25) |
| Overall progression, n (% total) | 21 (57) |
| Death, n (% total) | 22 (59) |
| Unable to assess response, n (% of total) | 1 (3) |
| Target Response following 90Y-ICI | |
| Target CR, n (% total) | 18 (50) |
| Target ORR (CR/PR), n (% total) | 30 (83) |
| Overall Response following 90Y-ICI | |
| Overall CR, n (% total) | 14 (39) |
| Overall ORR (CR/PR), n (% total) | 22 (61) |
| Time after ICI initiation to response rate, months, median (IQR) | 3.4 (2.3–4.2) |
| Outcomes | |
| Time to follow-up (months), median (IQR) | 16 (8–25) |
| Overall progression, n (% total) | 21 (57) |
| Death, n (% total) | 22 (59) |
| Unable to assess response, n (% of total) | 1 (3) |
| BCLC A-B | BCLC-C | p Value | |
|---|---|---|---|
| Cohort, n | 17 | 20 | |
| Hepatology | |||
| Etiology, n (%) | 0.006 | ||
| Viral | 10 (59) | 19 (95) | |
| Other | 7 (41) | 1 (5) | |
| Child-Pugh score, n (%) | 0.451 | ||
| A5/A6 | 15 (88) | 19 (95) | |
| B7/B8 | 2 (12) | 1 (5) | |
| Bilirubin (mg/dL), median (IQR) | 0.8 (0.5–1.7) | 0.7 (0.5–1.1) | 0.602 |
| Albumin (g/dL), median (IQR) | 3.3 (3.2–3.9) | 3.4 (3.3–3.8) | 0.389 |
| Platelets (103/mL), median (IQR) | 222 (134–271) | 203 (180–300) | 0.604 |
| HCC | |||
| AFP (ng/mL), median (IQR) | 41 (5–101) | 598 (55–32,066) | 0.009 |
| Solitary HCC, n (%) | 7 (41) | 13 (65) | 0.146 |
| Index tumor size (cm), median (IQR) | 8.5 (5.3–12.1) | 7.6 (6.1–10.9) | 0.692 |
| Cumulative tumor size (cm), median (IQR) | 12 (8.2–15.5) | 8.7 (7.0–15) | 0.180 |
| Macrovascular invasion, n (%) | 0 (0) | 14 (70) | |
| Extrahepatic disease, n (%) | 0 (0) | 9 (45) | |
| Immune Checkpoint Inhibitors | |||
| Patients receiving Atezo/Bev therapy, n (% of total) | 14 (82) | 16 (80) | |
| Duration of Atezo/Bev, months, median (IQR) | 10 (5–25) | 6 (3–11) | 0.073 |
| Discontinuation, n (% of Atezo/Bev) | 2 (14) | 12 (75) | <0.001 |
| Patients receiving Treme/Durva therapy, n (% of total) | 3 (18) | 4 (20) | |
| Duration of Treme/Durva, months, median (IQR) | 16 (6–18) | 11 (3–18) | 0.658 |
| Discontinuation, n (% of Treme/Durva) | 1 (33) | 2 (50) | 0.724 |
| First Cycle 90Y Characteristics | |||
| Target perfusion volume (mL), median (IQR) | 622 (384–1084) | 458 (201–922) | 0.111 |
| Target dose to volume (Gy), median (IQR) | 375 (206–537) | 501 (415–562) | 0.083 |
| Lung shunt fraction (%), median (IQR) | 6.1 (3.6–10.7) | 4.5 (2.4–6.0) | 0.070 |
| Multicycle 90Y, n (%) | 6 (35) | 5 (25) | 0.295 |
| Number of total 90Y treatments, median (range) | 2 (1–3) | 1 (1–4) | 0.599 |
| DEB-TACE bridge to 90Y | 3 (18) | 1 (5) | 0.211 |
| Treatment Sequence Lead | |||
| Sequence lead, ICI vs. 90Y lead | 12 (71) | 20 (100) | 0.003 |
| Time from ICI lead -> 90Y, days, median (IQR) | 37 (14–74) | 53 (25–80) | 0.572 |
| Time from 90Y lead -> ICI, days, median (IQR) | 18 (12–48) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez, K.G.; Sandow, T.; Grahovac, A.; Vallejo-Calzada, R.; Gimenez, J.; Bohorquez, H.; Cohen, A.; Mizrahi, J.; Du, L.; Thevenot, P. Contemporary Clinical Utilization of Radioembolization with Immune Checkpoint Inhibitors as First-Line Treatment in HCC: Real-World Report on Safety and Outcomes. Cancers 2025, 17, 2745. https://doi.org/10.3390/cancers17172745
Núñez KG, Sandow T, Grahovac A, Vallejo-Calzada R, Gimenez J, Bohorquez H, Cohen A, Mizrahi J, Du L, Thevenot P. Contemporary Clinical Utilization of Radioembolization with Immune Checkpoint Inhibitors as First-Line Treatment in HCC: Real-World Report on Safety and Outcomes. Cancers. 2025; 17(17):2745. https://doi.org/10.3390/cancers17172745
Chicago/Turabian StyleNúñez, Kelley G., Tyler Sandow, Alexandre Grahovac, Ricardo Vallejo-Calzada, Juan Gimenez, Humberto Bohorquez, Ari Cohen, Jonathan Mizrahi, Lingling Du, and Paul Thevenot. 2025. "Contemporary Clinical Utilization of Radioembolization with Immune Checkpoint Inhibitors as First-Line Treatment in HCC: Real-World Report on Safety and Outcomes" Cancers 17, no. 17: 2745. https://doi.org/10.3390/cancers17172745
APA StyleNúñez, K. G., Sandow, T., Grahovac, A., Vallejo-Calzada, R., Gimenez, J., Bohorquez, H., Cohen, A., Mizrahi, J., Du, L., & Thevenot, P. (2025). Contemporary Clinical Utilization of Radioembolization with Immune Checkpoint Inhibitors as First-Line Treatment in HCC: Real-World Report on Safety and Outcomes. Cancers, 17(17), 2745. https://doi.org/10.3390/cancers17172745







