Serum Levels of CA125 and HE4 as a Tool for Predicting Regional Lymph Node Metastatic Involvement in Endometrial Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Examination of Tumour Markers
2.3. Histological Examination
2.4. Data Analysis
3. Results
3.1. CA125 and HE4 Sensitivity and Specificity for Determining Metastatic Involvement
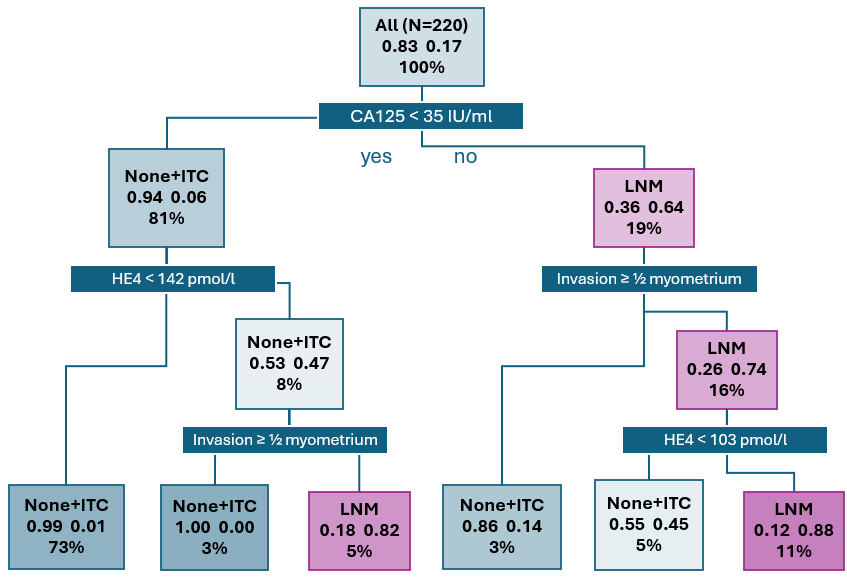
3.2. LNM Prediction Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 5 May 2025).
- World Health Organization. GLOBOCAN 2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018; World Health Organization: Geneva, Switzerland, 2018; Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/24-corpus-uteri-fact-sheet.pdf (accessed on 29 July 2020).
- Clarke, M.A.; Long, B.J.; Del Mar Morillo, A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of Endometrial Cancer Risk with Postmenopausal Bleeding in Women: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Endometrial Cancer Treatment (PDQ®)–Patient Version [Internet]; National Cancer Institute: Bethesda, MD, USA, 2019. Available online: https://www.cancer.gov/types/uterine/patient/endometrial-treatment-pdq (accessed on 19 December 2019).
- Concin, N.; Matias-Guiu, X.; Cibula, D.; Colombo, N.; Creutzberg, C.L.; Ledermann, J.; Mirza, M.R.; Vergote, I.; Abu-Rustum, N.R.; Bosse, T.; et al. ESGO-ESTRO-ESP Guidelines for the Management of Patients with Endometrial Carcinoma: Update 2025. Lancet Oncol. 2025, 26, e423–e435. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, G.S.; Paiva, L.L.; Schmidt, R.; Vieira, M.; Reis, R.; Andrade, C. Sentinel Lymph Node Mapping vs Systematic Lymphadenectomy for Endometrial Cancer: Surgical Morbidity and Lymphatic Complications. J. Minim. Invasive Gynecol. 2020, 27, 938–945.e2. [Google Scholar] [CrossRef]
- Bogani, G.; Dowdy, S.C.; Cliby, W.A.; Ghezzi, F.; Rossetti, D.; Mariani, A. Role of Pelvic and Para-Aortic Lymphadenectomy in Endometrial Cancer: Current Evidence. J. Obstet. Gynaecol. Res. 2014, 40, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Daoud, T.; Sardana, S.; Stanietzky, N.; Klekers, A.R.; Bhosale, P.; Morani, A.C. Recent Imaging Updates and Advances in Gynecologic Malignancies. Cancers 2022, 14, 5528. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Salvesen, H.B. What Is the Best Preoperative Imaging for Endometrial Cancer? Curr. Oncol. Rep. 2016, 18, 25. [Google Scholar] [CrossRef]
- Lin, Y.; Dobrotwir, A.; McNally, O.; Abu-Rustum, N.R.; Narayan, K. Role of Imaging in the Routine Management of Endometrial Cancer. Int. J. Gynecol. Obstet. 2018, 143, 109–117. [Google Scholar] [CrossRef]
- Epstein, E.; Blomqvist, L. Imaging in Endometrial Cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 721–739. [Google Scholar] [CrossRef]
- Bast, R.C.; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a Monoclonal Antibody with Human Ovarian Carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef]
- Qu, W.; Gao, Q.; Chen, H.; Tang, Z.; Zhu, X.; Jiang, S.-W. HE4-Test of Urine and Body Fluids for Diagnosis of Gynecologic Cancer. Expert. Rev. Mol. Diagn. 2017, 17, 239–244. [Google Scholar] [CrossRef]
- Galgano, M.T.; Hampton, G.M.; Frierson, H.F. Comprehensive Analysis of HE4 Expression in Normal and Malignant Human Tissues. Mod. Pathol. 2006, 19, 847–853. [Google Scholar] [CrossRef]
- Antonsen, S.L.; Høgdall, E.; Christensen, I.J.; Lydolph, M.; Tabor, A.; Loft Jakobsen, A.; Fagö-Olsen, C.L.; Andersen, E.S.; Jochumsen, K.; Høgdall, C. HE4 and CA125 Levels in the Preoperative Assessment of Endometrial Cancer Patients: A Prospective Multicenter Study (ENDOMET). Acta Obstet. Gynecol. Scand. 2013, 92, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Reijnen, C.; IntHout, J.; Massuger, L.F.A.G.; Strobbe, F.; Küsters-Vandevelde, H.V.N.; Haldorsen, I.S.; Snijders, M.P.L.M.; Pijnenborg, J.M.A. Diagnostic Accuracy of Clinical Biomarkers for Preoperative Prediction of Lymph Node Metastasis in Endometrial Carcinoma: A Systematic Review and Meta-Analysis. Oncologist 2019, 24, e880–e890. [Google Scholar] [CrossRef]
- Behrouzi, R.; Barr, C.E.; Crosbie, E.J. HE4 as a Biomarker for Endometrial Cancer. Cancers 2021, 13, 4764. [Google Scholar] [CrossRef] [PubMed]
- Lombaers, M.S.; Cornel, K.M.C.; Visser, N.C.M.; Bulten, J.; Küsters-Vandevelde, H.V.N.; Amant, F.; Boll, D.; Bronsert, P.; Colas, E.; Geomini, P.M.A.J.; et al. Preoperative CA125 Significantly Improves Risk Stratification in High-Grade Endometrial Cancer. Cancers 2023, 15, 2605. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.E.; Njoku, K.; Jones, E.R.; Crosbie, E.J. Serum CA125 and HE4 as Biomarkers for the Detection of Endometrial Cancer and Associated High-Risk Features. Diagnostics 2022, 12, 2834. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.; Teng, F.; Bai, Z.; Tian, W.; Xue, F. Predictive Value of Serum HE4 and CA125 Concentrations for Lymphatic Metastasis of Endometrial Cancer. Int. J. Gynaecol. Obstet. 2017, 136, 58–63. [Google Scholar] [CrossRef]
- O’Toole, S.A.; Huang, Y.; Norris, L.; Power Foley, M.; Shireen, R.; McDonald, S.; Kamran, W.; Ibrahim, N.; Ward, M.; Thompson, C.; et al. HE4 and CA125 as Preoperative Risk Stratifiers for Lymph Node Metastasis in Endometrioid Carcinoma of the Endometrium: A Retrospective Study in a Cohort with Histological Proof of Lymph Node Status. Gynecol. Oncol. 2021, 160, 514–519. [Google Scholar] [CrossRef]
- Chung, H.H.; Kim, J.W.; Park, N.-H.; Song, Y.-S.; Kang, S.-B.; Lee, H.-P. Use of Preoperative Serum CA-125 Levels for Prediction of Lymph Node Metastasis and Prognosis in Endometrial Cancer. Acta Obstet. Gynecol. Scand. 2006, 85, 1501–1505. [Google Scholar] [CrossRef]
- Abdalla, N.; Pazura, M.; Słomka, A.; Piórkowski, R.; Sawicki, W.; Cendrowski, K. The role of HE4 and CA125 in differentiation between malignant and non-malignant endometrial pathologies. Ginekol. Pol. 2016, 87, 781–786. [Google Scholar] [CrossRef]
- Creasman, W. Revised FIGO Staging for Carcinoma of the Endometrium. Int. J. Gynecol. Obstet. 2009, 105, 109. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Plotti, F.; Scaletta, G.; Terranova, C.; Montera, R.; De Cicco Nardone, C.; Luvero, D.; Rossini, G.; Gatti, A.; Schirò, T.; Moncelli, M.; et al. The Role of Human Epididymis Protein 4 as a Biomarker in Gynecologic Malignancies. Minerva Ginecol. 2019, 71, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.L.; Hill, K.A.; Shiro, B.C.; Diehl, S.J.; Gajewski, W.H. Preoperative Serum CA-125 Levels in Treating Endometrial Cancer. J. Reprod. Med. 2005, 50, 585–590. [Google Scholar] [PubMed]
- Ginath, S.; Menczer, J.; Fintsi, Y.; Ben-Shem, E.; Glezerman, M.; Avinoach, I. Tissue and Serum CA125 Expression in Endometrial Cancer. Int. J. Gynecol. Cancer 2002, 12, 372–375. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Sozzi, G.; Rosati, A.; Restaino, S.; Gambino, G.; Cianciolo, A.; Ceccaroni, M.; Uccella, S.; Franchi, M.; Chiantera, V.; et al. Predictive Score of Nodal Involvement in Endometrial Cancer Patients: A Large Multicentre Series. Ann. Surg. Oncol. 2022, 29, 2594–2599. [Google Scholar] [CrossRef]
- Shawn LyBarger, K.; Miller, H.A.; Frieboes, H.B. CA125 as a Predictor of Endometrial Cancer Lymphovascular Space Invasion and Lymph Node Metastasis for Risk Stratification in the Preoperative Setting. Sci. Rep. 2022, 12, 19783. [Google Scholar] [CrossRef]
- Ünsal, M.; Kimyon Comert, G.; Karalok, A.; Basaran, D.; Turkmen, O.; Boyraz, G.; Tasci, T.; Koc, S.; Boran, N.; Tulunay, G.; et al. The Preoperative Serum CA125 Can Predict the Lymph Node Metastasis in Endometrioid-Type Endometrial Cancer. Ginekol. Pol. 2018, 89, 599–606. [Google Scholar] [CrossRef]
- Frühauf, F.; Dvořák, M.; Haaková, L.; Hašlík, L.; Herboltová, P.; Chaloupková, B.; Kožnarová, J.; Kubešová, B.; Lukáčová, I.; Marek, R. Ultrasound staging of endometrial cancer—Recommended methodology of examination. Ceska Gynekol. 2014, 79, 466–476. [Google Scholar]
- Christensen, J.W.; Dueholm, M.; Hansen, E.S.; Marinovskij, E.; Lundorf, E.; Ørtoft, G. Assessment of Myometrial Invasion in Endometrial Cancer Using Three-Dimensional Ultrasound and Magnetic Resonance Imaging. Acta Obstet. Gynecol. Scand. 2016, 95, 55–64. [Google Scholar] [CrossRef]
- Jantarasaengaram, S.; Praditphol, N.; Tansathit, T.; Vipupinyo, C.; Vairojanavong, K. Three-Dimensional Ultrasound with Volume Contrast Imaging for Preoperative Assessment of Myometrial Invasion and Cervical Involvement in Women with Endometrial Cancer. Ultrasound Obstet. Gynecol. 2014, 43, 569–574. [Google Scholar] [CrossRef]
- Fischerova, D.; Smet, C.; Scovazzi, U.; Sousa, D.N.; Hundarova, K.; Haldorsen, I.S. Staging by Imaging in Gynecologic Cancer and the Role of Ultrasound: An Update of European Joint Consensus Statements. Int. J. Gynecol. Cancer 2024, 34, 363–378. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, A.; Yun, M.; Kim, Y.T.; Kang, W.J. Comparison of FDG PET/CT and MRI in Lymph Node Staging of Endometrial Cancer. Ann. Nucl. Med. 2016, 30, 104–113. [Google Scholar] [CrossRef]
- Bollineni, V.R.; Ytre-Hauge, S.; Bollineni-Balabay, O.; Salvesen, H.B.; Haldorsen, I.S. High Diagnostic Value of 18F-FDG PET/CT in Endometrial Cancer: Systematic Review and Meta-Analysis of the Literature. J. Nucl. Med. 2016, 57, 879–885. [Google Scholar] [CrossRef]
- Tanaka, T.; Terai, Y.; Yamamoto, K.; Yamada, T.; Ohmichi, M. The Diagnostic Accuracy of Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography and Sentinel Node Biopsy in the Prediction of Pelvic Lymph Node Metastasis in Patients with Endometrial Cancer: A Retrospective Observational Study. Medicine 2018, 97, e12522. [Google Scholar] [CrossRef]
| Characteristic | N = 220 |
|---|---|
| Age (years) | |
| Mean (SD) | 64 (10) |
| Median (IQR) | 65 (59–70) |
| Metastatic involvement, n (%) | |
| No | 167 (75.9%) |
| ITC | 16 (7.3%) |
| Micrometastasis | 13 (5.9%) |
| Macrometastasis | 24 (10.9%) |
| CA125 (IU/mL) | |
| Mean (SD) | 38 (88) |
| Median (IQR) | 16 (10–29) |
| HE4 (pmol/L) | |
| Mean (SD) | 110 (142) |
| Median (IQR) | 78 (59–109) |
| Histotype, n (%) | |
| Endometrioid LG | 159 (72.3%) |
| Endometrioid HG | 34 (15.5%) |
| Serous | 16 (7.3%) |
| Mixed with endometrioid component | 11 (5.0%) |
| Surgical staging, n (%) | |
| Bilateral SNB detection | 158 (71.8%) |
| Unilateral SNB + systemic PLN contralaterally | 6 (2.7%) |
| Systemic PLN + PALN | 53 (24.1%) |
| Bulky lymph node resection | 3 (1.4%) |
| Tumour invasion, n (%) | |
| Limited to endometrium | 35 (15.9%) |
| Invasion of <½ myometrium | 88 (40.0%) |
| Invasion of ≥½ myometrium | 47 (21.4%) |
| Infiltration of cervical stroma | 41 (18.6%) |
| Infiltration of uterine serosa | 9 (4.1%) |
| FIGO, n (%) | |
| IA | 119 (54.1%) |
| IB | 28 (12.7%) |
| II | 29 (13.2%) |
| IIIA | 6 (2.7%) |
| IIIB | 1 (0.5%) |
| IIIC1 | 27 (12.3%) |
| IIIC2 | 8 (3.6%) |
| IVA | 0 (0.0%) |
| IVB | 2 (0.9%) |
| FIGO stage, n (%) | |
| Local disease (stage I + II) | 176 (80.0%) |
| Advanced disease (stage III + IV) | 44 (20.0%) |
| Obesity, n (%) | |
| No | 94 (42.7%) |
| Yes | 126 (57.3%) |
| Hypertension, n (%) | |
| No | 86 (39.1%) |
| Yes | 134 (60.9%) |
| IHD, n (%) | |
| No | 205 (93.2%) |
| Yes | 15 (6.8%) |
| Diabetes mellitus, n (%) | |
| No | 171 (77.7%) |
| Yes | 49 (22.3%) |
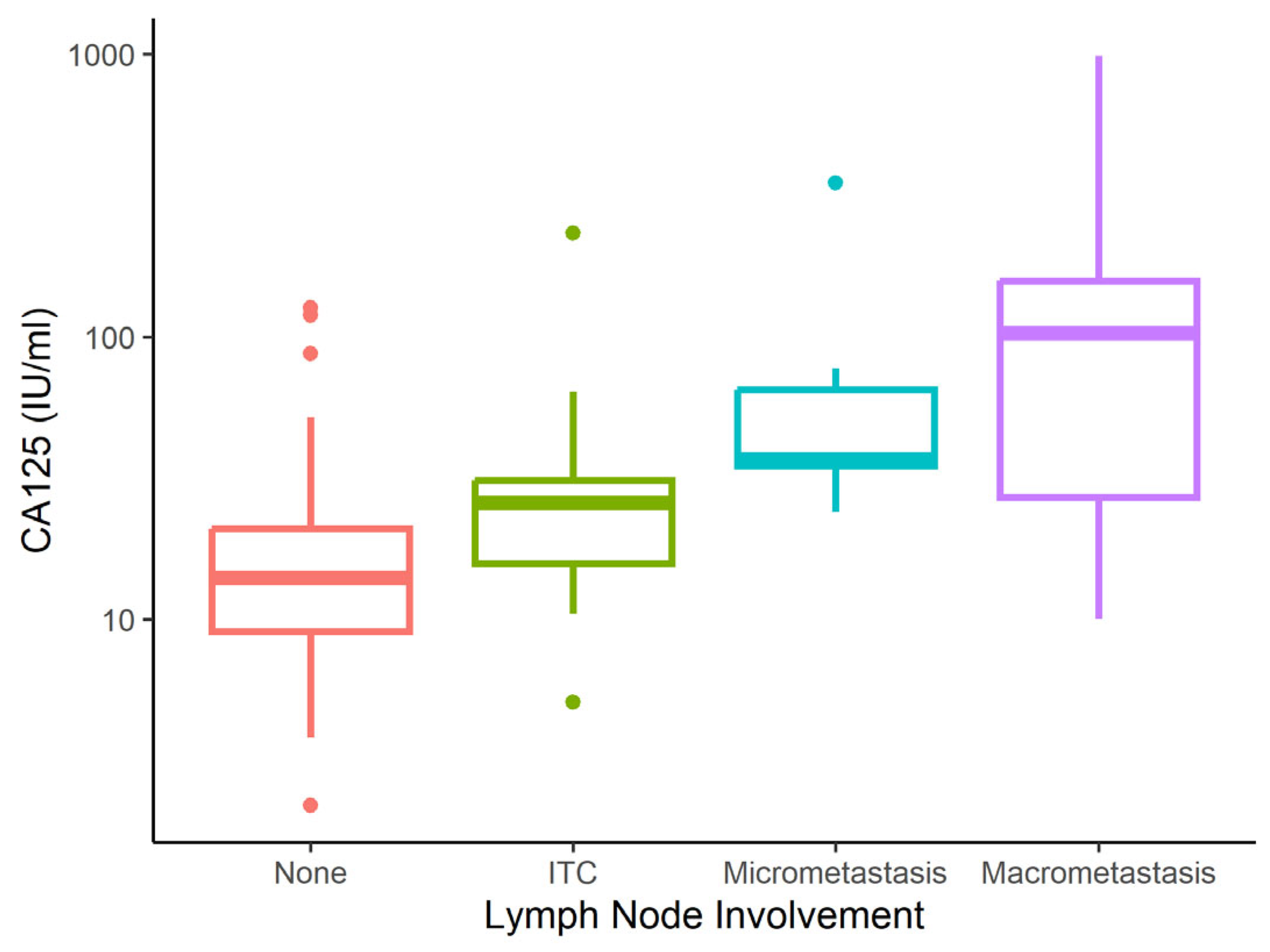
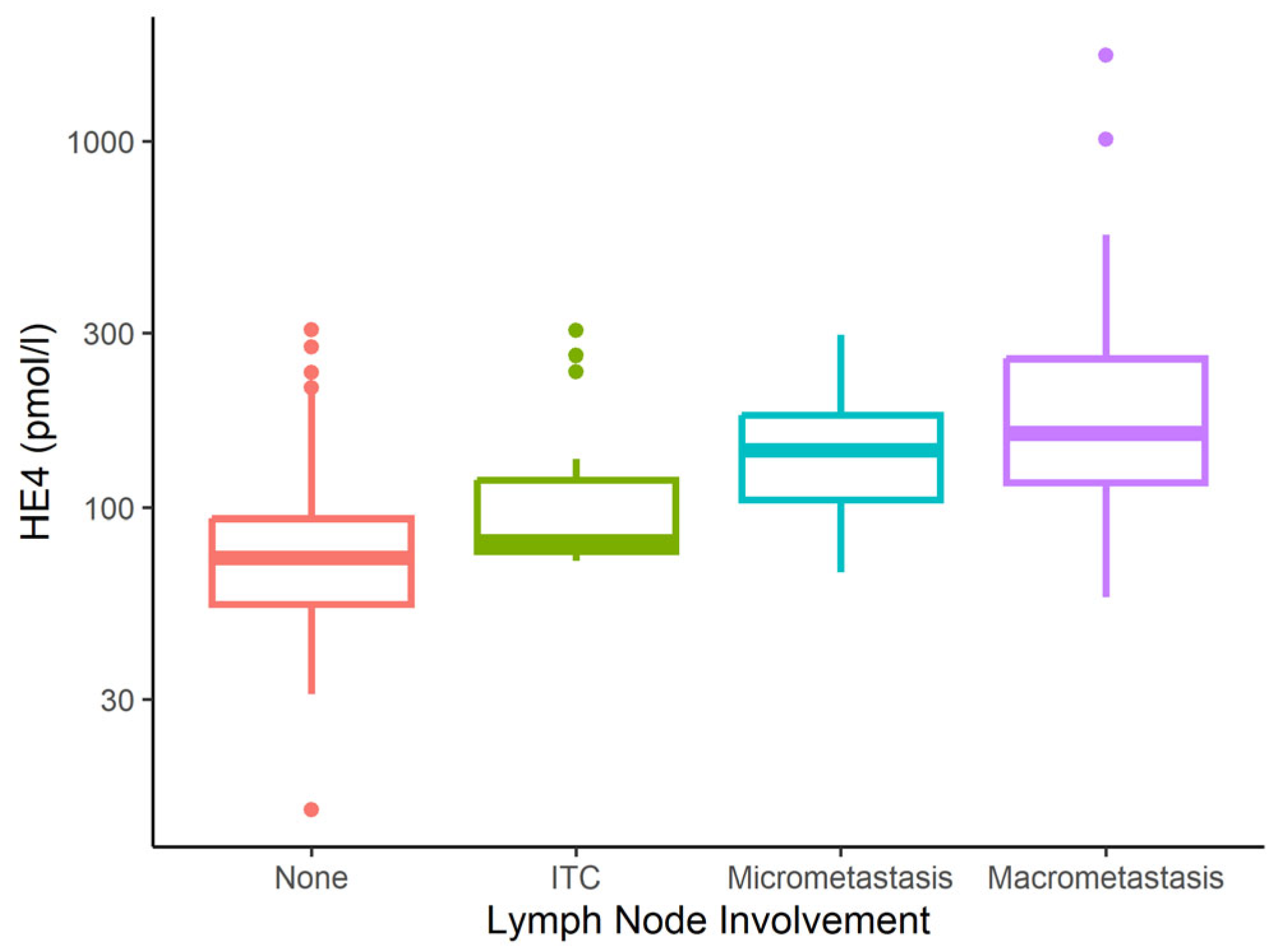
| Characteristic | None N = 167 | Micrometastasis N = 13 | Macrometastasis N = 24 | ITC N = 16 | p-Value 1 |
|---|---|---|---|---|---|
| Age (years) | 0.041 | ||||
| Mean (SD) | 63 (10) | 65 (13) | 67 (5) | 68 (8) | |
| Median (IQR) | 65 (58–69) | 62 (54–79) | 69 (64–70) | 68 (65–73) | |
| CA125 (IU/mL) | <0.001 | ||||
| Mean (SD) | 19 (19) | 70 (86) | 153 (219) | 39 (54) | |
| Median (IQR) | 14 (9–21) | 37 (35–65) | 103 (28–158) | 26 (16–31) | |
| HE4 (pmol/L) | <0.001 | ||||
| Mean (SD) | 81 (45) | 153 (75) | 276 (367) | 121 (75) | |
| Median (IQR) | 73 (55–93) | 144 (105–179) | 160 (118–255) | 81 (76–120) | |
| Histotype, n (%) | |||||
| Endometrioid LG | 126 (75.4%) | 10 (76.9%) | 9 (37.5%) | 14 (87.5%) | |
| Endometrioid HG | 22 (13.2%) | 2 (15.4%) | 9 (37.5%) | 1 (6.2%) | |
| Serous | 11 (6.6%) | 1 (7.7%) | 4 (16.7%) | 0 (0.0%) | |
| Mixed with endometroid component | 8 (4.8%) | 0 (0.0%) | 2 (8.3%) | 1 (6.2%) | |
| Surgical staging, n (%) | <0.001 | ||||
| Bilateral SNB detection | 125 (74.9%) | 13 (100.0%) | 7 (29.2%) | 13 (81.2%) | |
| Unilateral SNB + systemic PLN contralaterally | 3 (1.8%) | 0 (0.0%) | 2 (8.3%) | 1 (6.2%) | |
| Systemic PLN + PALN | 38 (22.8%) | 0 (0.0%) | 14 (58.3%) | 1 (6.2%) | |
| Bulky lymph node resection | 1 (0.6%) | 0 (0.0%) | 1 (4.2%) | 1 (6.2%) | |
| Tumour invasion, n (%) | |||||
| Limited to endometrium | 35 (21.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Invasion of <½ myometrium | 78 (46.7%) | 0 (0.0%) | 1 (4.2%) | 9 (56.2%) | |
| Invasion of ≥½ myometrium | 26 (15.6%) | 10 (76.9%) | 8 (33.3%) | 3 (18.8%) | |
| Infiltration of cervical stroma | 27 (16.2%) | 3 (23.1%) | 7 (29.2%) | 4 (25.0%) | |
| Infiltration of uterine serosa | 1 (0.6%) | 0 (0.0%) | 8 (33.3%) | 0 (0.0%) | |
| FIGO, n (%) | |||||
| IA | 110 (65.9%) | 0 (0.0%) | 0 (0.0%) | 9 (56.2%) | |
| IB | 26 (15.6%) | 0 (0.0%) | 0 (0.0%) | 2 (12.5%) | |
| II | 25 (15.0%) | 0 (0.0%) | 0 (0.0%) | 4 (25.0%) | |
| IIIA | 6 (3.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| IIIB | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | |
| IIIC1 | 0 (0.0%) | 13 (100.0%) | 14 (58.3%) | 0 (0.0%) | |
| IIIC2 | 0 (0.0%) | 0 (0.0%) | 8 (33.3%) | 0 (0.0%) | |
| IVA | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| IVB | 0 (0.0%) | 0 (0.0%) | 2 (8.3%) | 0 (0.0%) | |
| FIGO stage, n (%) | <0.001 | ||||
| Local disease | 161 (96.4%) | 0 (0.0%) | 0 (0.0%) | 15 (93.8%) | |
| Advanced disease | 6 (3.6%) | 13 (100.0%) | 24 (100.0%) | 1 (6.2%) | |
| Obesity, n (%) | 0.177 | ||||
| No | 70 (41.9%) | 8 (61.5%) | 7 (29.2%) | 9 (56.2%) | |
| Yes | 97 (58.1%) | 5 (38.5%) | 17 (70.8%) | 7 (43.8%) | |
| Hypertension, n (%) | 0.506 | ||||
| No | 68 (40.7%) | 5 (38.5%) | 6 (25.0%) | 7 (43.8%) | |
| Yes | 99 (59.3%) | 8 (61.5%) | 18 (75.0%) | 9 (56.2%) | |
| IHD, n (%) | 0.537 | ||||
| No | 157 (94.0%) | 12 (92.3%) | 22 (91.7%) | 14 (87.5%) | |
| Yes | 10 (6.0%) | 1 (7.7%) | 2 (8.3%) | 2 (12.5%) | |
| Diabetes mellitus, n (%) | 0.146 | ||||
| No | 131 (78.4%) | 10 (76.9%) | 21 (87.5%) | 9 (56.2%) | |
| Yes | 36 (21.6%) | 3 (23.1%) | 3 (12.5%) | 7 (43.8%) |
| Characteristic | None + ITC, N = 183 | Micro-/Macrometastasis, N = 37 | p-Value 1 |
|---|---|---|---|
| Age (years) | 0.086 | ||
| Mean (SD) | 64 (10) | 67 (9) | |
| Median (IQR) | 65 (58–70) | 68 (62–71) | |
| Metastatic involvement, n (%) | <0.001 | ||
| No | 167 (91.3%) | 0 (0.0%) | |
| ITC | 16 (8.7%) | 0 (0.0%) | |
| Micrometastasis | 0 (0.0%) | 13 (35.1%) | |
| Macrometastasis | 0 (0.0%) | 24 (64.9%) | |
| CA125 (IU/mL) | <0.001 | ||
| Mean (SD) | 21 (24) | 124 (187) | |
| Median (IQR) | 14 (9–23) | 64 (30–125) | |
| HE4 (pmol/L) | <0.001 | ||
| Mean (SD) | 85 (49) | 233 (303) | |
| Median (IQR) | 74 (56–96) | 154 (105–225) | |
| Histotype, n (%) | 0.010 | ||
| Endometrioid LG | 140 (76.5%) | 19 (51.4%) | |
| Endometrioid HG | 23 (12.6%) | 11 (29.7%) | |
| Serous | 11 (6.0%) | 5 (13.5%) | |
| Mixed with endometroid component | 9 (4.9%) | 2 (5.4%) | |
| Surgical staging, n (%) | 0.035 | ||
| Bilateral SNB detection | 138 (75.4%) | 20 (54.1%) | |
| Unilateral SNB + systemic PLN contralaterally | 4 (2.2%) | 2 (5.4%) | |
| Systemic PLN + PALN | 39 (21.3%) | 14 (37.8%) | |
| Bulky lymph nodes resection | 2 (1.1%) | 1 (2.7%) | |
| Tumour invasion, n (%) | <0.001 | ||
| Limited to endometrium | 35 (19.1%) | 0 (0.0%) | |
| Invasion of <½ myometrium | 87 (47.5%) | 1 (2.7%) | |
| Invasion of ≥½ myometrium | 29 (15.8%) | 18 (48.6%) | |
| Infiltration of cervical stroma | 31 (16.9%) | 10 (27.0%) | |
| Infiltration of uterine serosa | 1 (0.5%) | 8 (21.6%) | |
| FIGO, n (%) | <0.001 | ||
| IA | 119 (65.0%) | 0 (0.0%) | |
| IB | 28 (15.3%) | 0 (0.0%) | |
| II | 29 (15.8%) | 0 (0.0%) | |
| IIIA | 6 (3.3%) | 0 (0.0%) | |
| IIIB | 1 (0.5%) | 0 (0.0%) | |
| IIIC1 | 0 (0.0%) | 27 (73.0%) | |
| IIIC2 | 0 (0.0%) | 8 (21.6%) | |
| IVA | 0 (0.0%) | 0 (0.0%) | |
| IVB | 0 (0.0%) | 2 (5.4%) | |
| FIGO stage, n (%) | <0.001 | ||
| Local disease | 176 (96.2%) | 0 (0.0%) | |
| Advanced disease | 7 (3.8%) | 37 (100.0%) | |
| Obesity, n (%) | 0.768 | ||
| No | 79 (43.2%) | 15 (40.5%) | |
| Yes | 104 (56.8%) | 22 (59.5%) | |
| Hypertension, n (%) | 0.201 | ||
| No | 75 (41.0%) | 11 (29.7%) | |
| Yes | 108 (59.0%) | 26 (70.3%) | |
| IHD, n (%) | 0.722 | ||
| No | 171 (93.4%) | 34 (91.9%) | |
| Yes | 12 (6.6%) | 3 (8.1%) | |
| Diabetes mellitus, n (%) | 0.332 | ||
| No | 140 (76.5%) | 31 (83.8%) | |
| Yes | 43 (23.5%) | 6 (16.2%) |
| Study | Year | CA125 Cut-Off (IU/mL) | Sensitivity (%) | Specificity (%) | HE4 Cut-Off (pmol/L) | Sensitivity (%) | Specificity (%) | Study Design | Sample Size |
|---|---|---|---|---|---|---|---|---|---|
| Antonsen et al. [15] | 2013 | 35 | 62.1 | 78.9 | 70 | 75.8 | 48.4 | Prospective | 352 |
| Wang et al. [20] | 2016 | 13.5 | 72.2 | 51.9 | 72.9 | 82.4 | 52.3 | Retrospective | 258 |
| O’Toole et al. [21] | 2020 | 35 | 57 | 91.4 | 81 | 78.6 | 53.4 | Retrospective | 147 |
| Chung et al. [22] | 2011 | 28.5 | 61.5 | 94.9 | – | – | – | Retrospective | 92 |
| Lombaers et al. [18] | 2023 | 35 | 68.4 | 78.7 | – | – | – | Retrospective | 333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crha, T.; Ovesná, P.; Weinberger, V.; Felsinger, M.; Babjak, B.; Valík, D.; Hausnerová, J.; Minář, L. Serum Levels of CA125 and HE4 as a Tool for Predicting Regional Lymph Node Metastatic Involvement in Endometrial Carcinoma. Cancers 2025, 17, 2740. https://doi.org/10.3390/cancers17172740
Crha T, Ovesná P, Weinberger V, Felsinger M, Babjak B, Valík D, Hausnerová J, Minář L. Serum Levels of CA125 and HE4 as a Tool for Predicting Regional Lymph Node Metastatic Involvement in Endometrial Carcinoma. Cancers. 2025; 17(17):2740. https://doi.org/10.3390/cancers17172740
Chicago/Turabian StyleCrha, Tomáš, Petra Ovesná, Vít Weinberger, Michal Felsinger, Branislav Babjak, Dalibor Valík, Jitka Hausnerová, and Luboš Minář. 2025. "Serum Levels of CA125 and HE4 as a Tool for Predicting Regional Lymph Node Metastatic Involvement in Endometrial Carcinoma" Cancers 17, no. 17: 2740. https://doi.org/10.3390/cancers17172740
APA StyleCrha, T., Ovesná, P., Weinberger, V., Felsinger, M., Babjak, B., Valík, D., Hausnerová, J., & Minář, L. (2025). Serum Levels of CA125 and HE4 as a Tool for Predicting Regional Lymph Node Metastatic Involvement in Endometrial Carcinoma. Cancers, 17(17), 2740. https://doi.org/10.3390/cancers17172740






