CF10 Displayed Improved Activity Relative to 5-FU in a Mouse CRLM Model Under Conditions of Physiological Folate
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Reagents, and Clonogenic Assay
2.2. Western Blotting
2.3. Cell Viability and Rescue Assay
2.4. TS Activity Assay
2.5. Liver Tumor Cell Inoculation to Simulate CRLM Formation
2.6. Treatment and Fluorescence Imaging of Tumor Progression
2.7. PRISM Assay
2.8. Statistical Analysis
3. Results
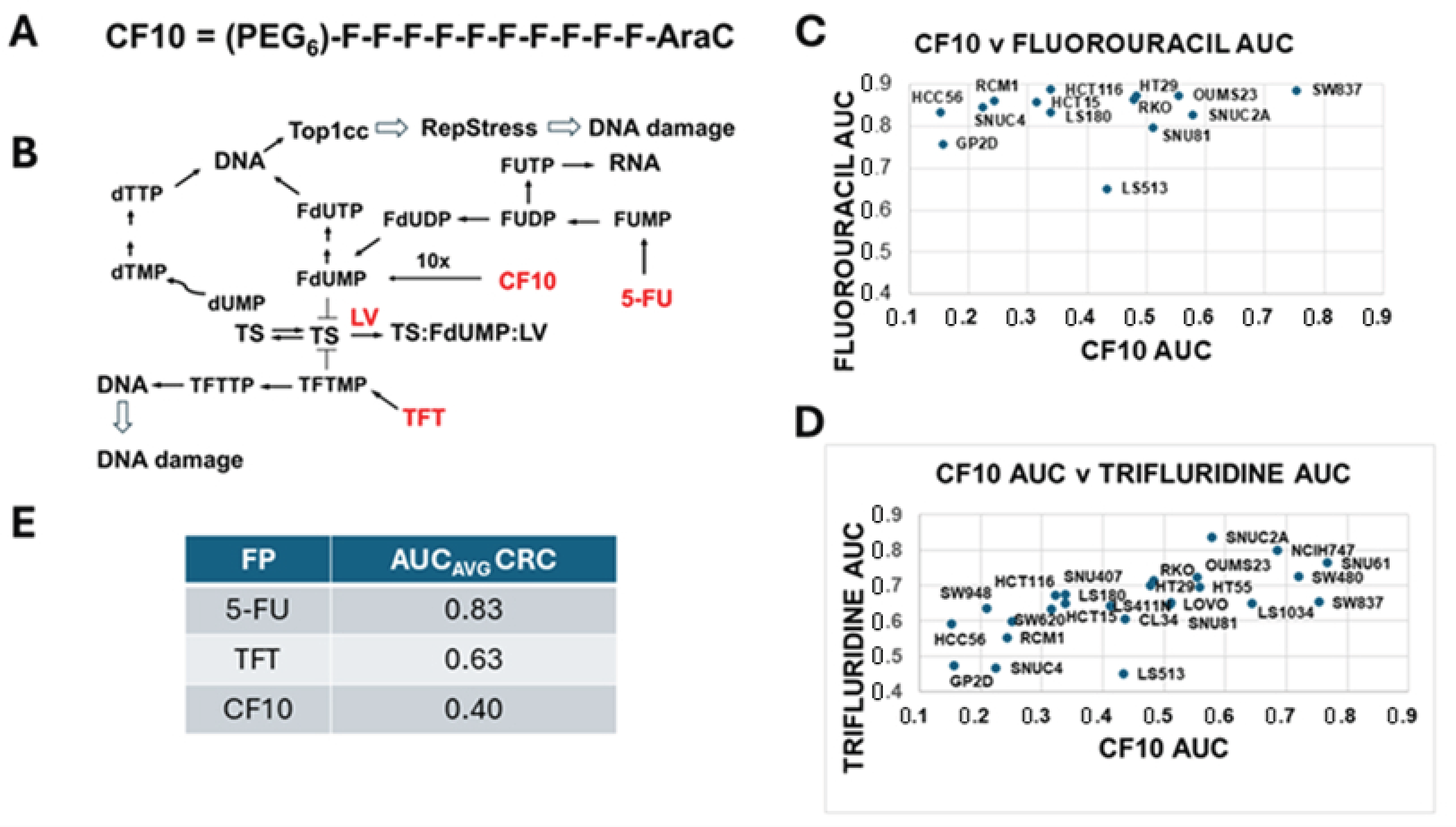
3.1. CF10 Displayed Enhanced Potency to CRC Cells Relative to 5-FU and TFT
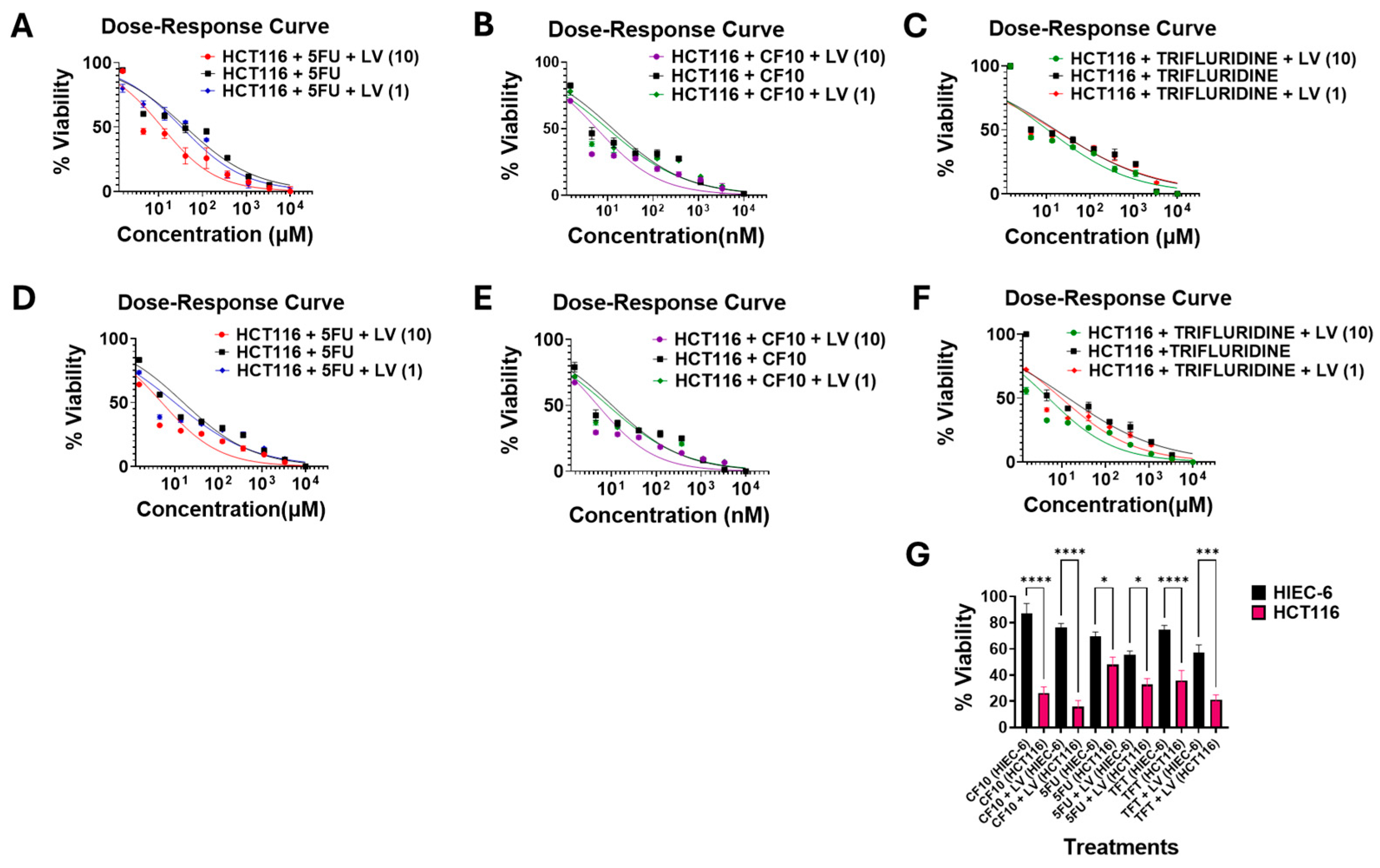
3.2. FP Potency Was Enhanced by Folate Restriction and LV Co-Treatment
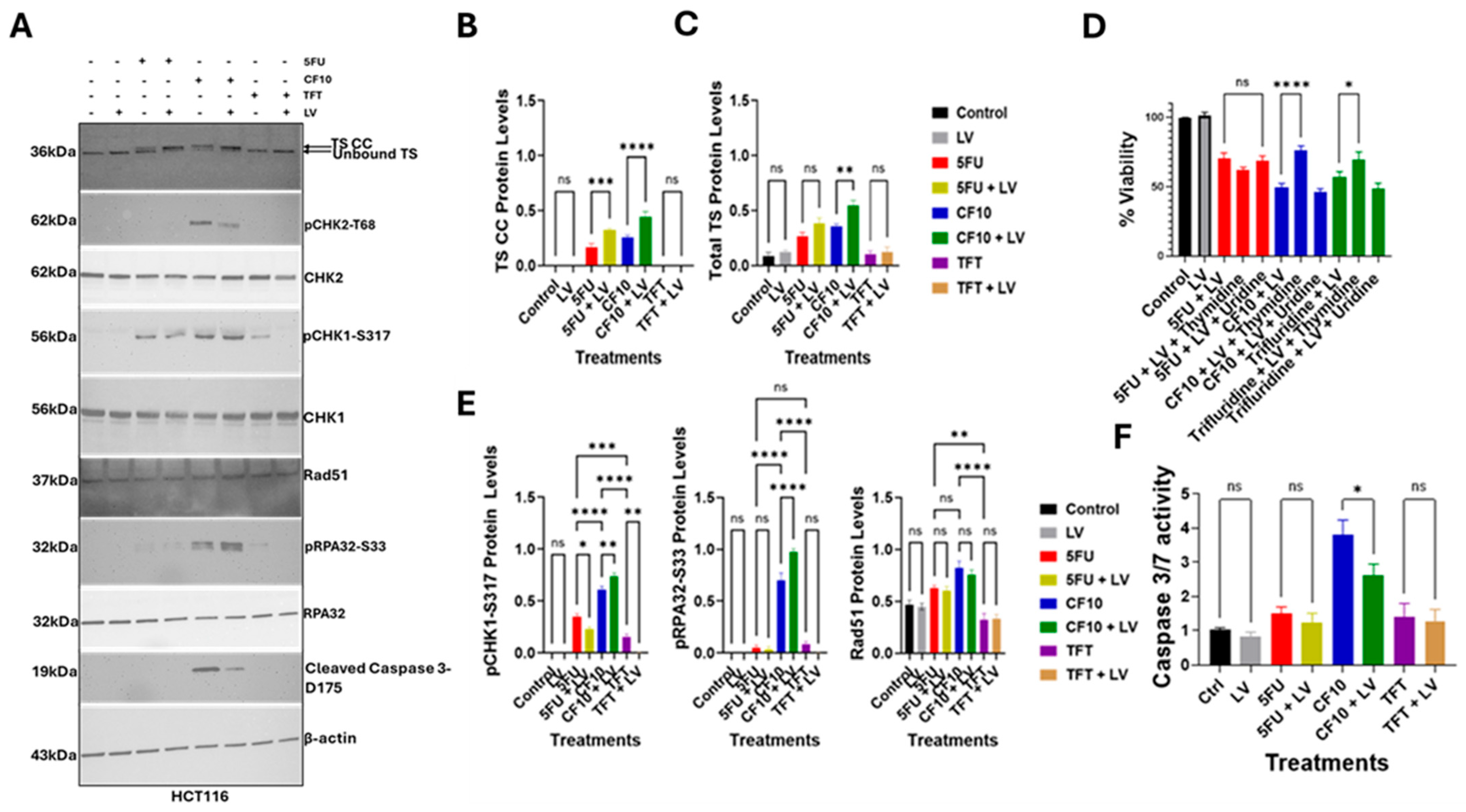
3.3. LV Stimulated TS Ternary Complex Formation for CF10, 5-FU but Not TFT
3.4. CF10 Was a Potent Inducer of Replication Stress and Apoptosis
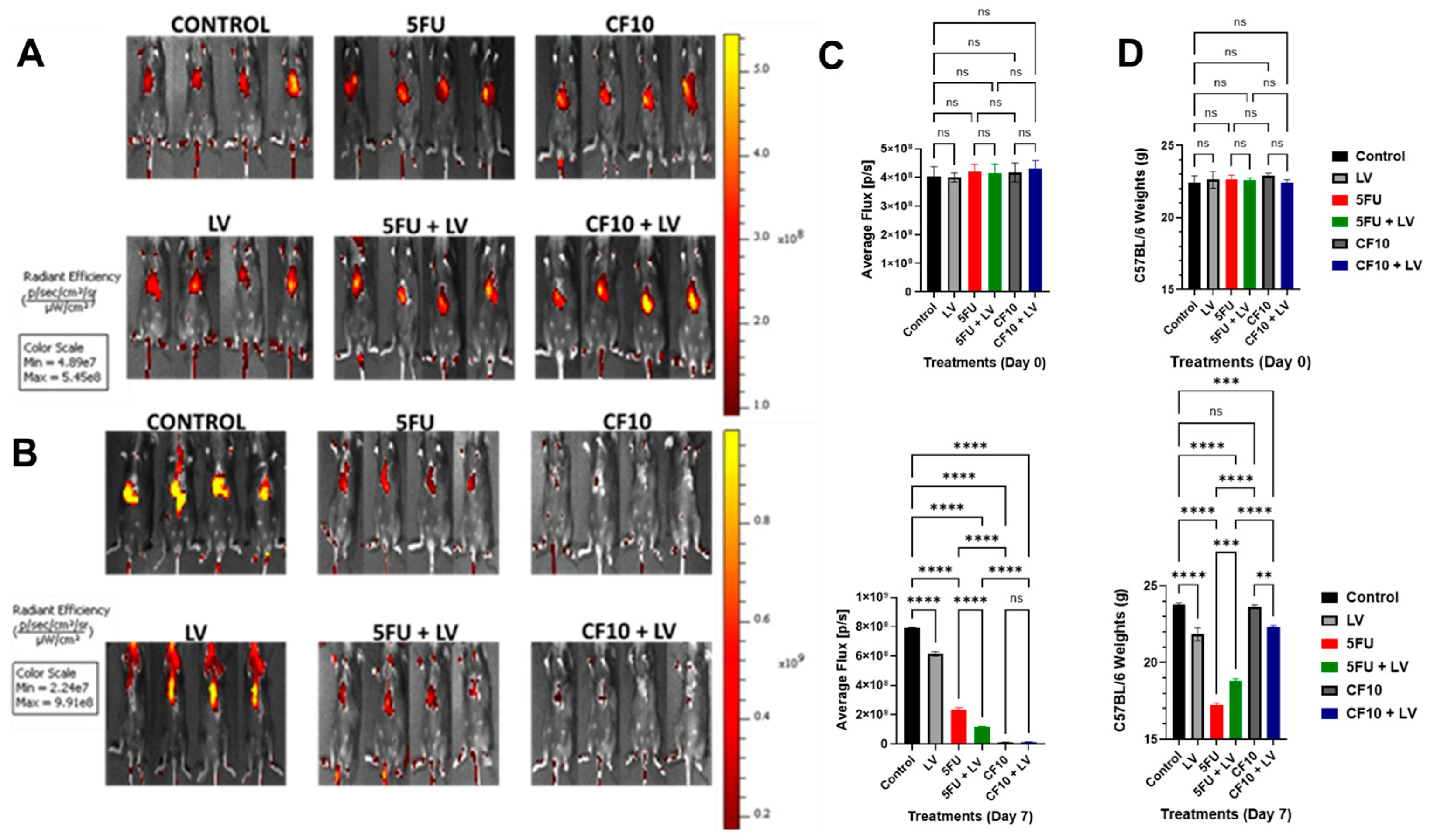
3.5. CF10 Was Highly Effective at Eradicating Liver-Metastatic Tumor Burden
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, P.M.; Danenberg, P.V.; Johnston, P.G.; Lenz, H.J.; Ladner, R.D. Standing the test of time: Targeting thymidylate biosynthesis in cancer therapy. Nat. Rev. Clin. Oncol. 2014, 11, 282–298. [Google Scholar] [CrossRef]
- Zeineddine, F.A.; Zeineddine, M.A.; Yousef, A.; Gu, Y.; Chowdhury, S.; Dasari, A.; Huey, R.W.; Johnson, B.; Kee, B.; Lee, M.S.; et al. Survival improvement for patients with metastatic colorectal cancer over twenty years. NPJ Precis. Oncol. 2023, 7, 16. [Google Scholar] [CrossRef]
- Peters, G.J. Therapeutic potential of TAS-102 in the treatment of gastrointestinal malignancies. Ther. Adv. Med. Oncol. 2015, 7, 340–356. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Sarkar, S.; Kiren, S.; Gmeiner, W.H. Review of Prodrug and Nanodelivery Strategies to Improve the Treatment of Colorectal Cancer with Fluoropyrimidine Drugs. Pharmaceutics 2024, 16, 734. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Dominijanni, A.; Haber, A.O.; Ghiraldeli, L.P.; Caudell, D.L.; D’Agostino, R., Jr.; Pasche, B.C.; Smith, T.L.; Deng, Z.; Kiren, S.; et al. Improved Antitumor Activity of the Fluoropyrimidine Polymer CF10 in Preclinical Colorectal Cancer Models through Distinct Mechanistic and Pharmacologic Properties. Mol. Cancer Ther. 2021, 20, 553–563. [Google Scholar] [CrossRef]
- Okechukwu, C.C.; Ma, X.; Sah, N.; Mani, C.; Palle, K.; Gmeiner, W.H. Enhanced Therapeutic Efficacy of the Nanoscale Fluoropyrimidine Polymer CF10 in a Rat Colorectal Cancer Liver Metastasis Model. Cancers 2024, 16, 1360. [Google Scholar] [CrossRef]
- Pritchard, D.M.; Watson, A.J.; Potten, C.S.; Jackman, A.L.; Hickman, J.A. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: Evidence for the involvement of RNA perturbation. Proc. Natl. Acad. Sci. USA 1997, 94, 1795–1799. [Google Scholar] [CrossRef]
- Ma, W.W.; Saif, M.W.; El-Rayes, B.F.; Fakih, M.G.; Cartwright, T.H.; Posey, J.A.; King, T.R.; von Borstel, R.W.; Bamat, M.K. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 2017, 123, 345–356. [Google Scholar] [CrossRef]
- Temmink, O.H.; de Bruin, M.; Comijn, E.M.; Fukushima, M.; Peters, G.J. Determinants of trifluorothymidine sensitivity and metabolism in colon and lung cancer cells. Anticancer Drugs 2005, 16, 285–292. [Google Scholar] [CrossRef]
- Fischel, J.L.; Formento, P.; Ciccolini, J.; Rostagno, P.; Etienne, M.C.; Catalin, J.; Milano, G. Impact of the oxaliplatin-5 fluorouracil-folinic acid combination on respective intracellular determinants of drug activity. Br. J. Cancer 2002, 86, 1162–1168. [Google Scholar] [CrossRef]
- Fischel, J.L.; Rostagno, P.; Formento, P.; Dubreuil, A.; Etienne, M.C.; Milano, G. Ternary combination of irinotecan, fluorouracil-folinic acid and oxaliplatin: Results on human colon cancer cell lines. Br. J. Cancer 2001, 84, 579–585. [Google Scholar] [CrossRef]
- Holbeck, S.L.; Collins, J.M.; Doroshow, J.H. Analysis of Food and Drug Administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol. Cancer Ther. 2010, 9, 1451–1460. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Sordet, O.; Zhang, H.L.; Kohlhagen, G.; Antony, S.; Gmeiner, W.H.; Pommier, Y. A novel polypyrimidine antitumor agent FdUMP[10] induces thymineless death with topoisomerase I-DNA complexes. Cancer Res. 2005, 65, 4844–4851. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Reinhold, W.C.; Pommier, Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol. Cancer Ther. 2010, 9, 3105–3114. [Google Scholar] [CrossRef]
- Gmeiner, W.H. Entrapment of DNA topoisomerase-DNA complexes by nucleotide/nucleoside analogs. Cancer Drug Resist. 2019, 2, 994–1001. [Google Scholar] [CrossRef]
- Pardee, T.S.; Gomes, E.; Jennings-Gee, J.; Caudell, D.; Gmeiner, W.H. Unique dual targeting of thymidylate synthase and topoisomerase1 by FdUMP[10] results in high efficacy against AML and low toxicity. Blood 2012, 119, 3561–3570. [Google Scholar] [CrossRef]
- Haber, A.O.; Jain, A.; Mani, C.; Nevler, A.; Agostini, L.C.; Golan, T.; Palle, K.; Yeo, C.J.; Gmeiner, W.H.; Brody, J.R. AraC-FdUMP[10] (CF10) is a next generation fluoropyrimidine with potent antitumor activity in PDAC and synergy with PARG inhibition. Mol. Cancer Res. 2021, 19, 565–572. [Google Scholar] [CrossRef]
- Finan, J.M.; Di Niro, R.; Park, S.Y.; Jeong, K.J.; Hedberg, M.D.; Smith, A.; Mccarthy, G.A.; Haber, A.O.; Muschler, J.; Sears, R.C.; et al. The polymeric fluoropyrimidine CF10 overcomes limitations of 5-FU in pancreatic ductal adenocarcinoma cells through increased replication stress. Cancer Biol. Ther. 2024, 25, 2421584. [Google Scholar] [CrossRef]
- Suzuki, N.; Emura, T.; Fukushima, M. Mode of action of trifluorothymidine (TFT) against DNA replication and repair enzymes. Int. J. Oncol. 2011, 39, 263–270. [Google Scholar] [CrossRef]
- White, S.B.; Procissi, D.; Chen, J.; Gogineni, V.R.; Tyler, P.; Yang, Y.; Omary, R.A.; Larson, A.C. Characterization of CC-531 as a Rat Model of Colorectal Liver Metastases. PLoS ONE 2016, 11, e0155334. [Google Scholar] [CrossRef]
- Machover, D. A comprehensive review of 5-fluorouracil and leucovorin in patients with metastatic colorectal carcinoma. Cancer 1997, 80, 1179–1187. [Google Scholar] [CrossRef]
- Peters, G.J.; Smitskamp-Wilms, E.; Smid, K.; Pinedo, H.M.; Jansen, G. Determinants of activity of the antifolate thymidylate synthase inhibitors Tomudex (ZD1694) and GW1843U89 against mono- and multilayered colon cancer cell lines under folate-restricted conditions. Cancer Res. 1999, 59, 5529–5535. [Google Scholar]
- Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer 2013, 13, 294. [Google Scholar] [CrossRef]
- Klubes, P.; Cerna, I.; Meldon, M.A. Effect of concurrent calcium leucovorin infusion on 5-fluorouracil cytotoxicity against murine L1210 leukemia. Cancer Chemother. Pharmacol. 1981, 6, 121–125. [Google Scholar] [CrossRef]
- Corsello, S.M.; Nagari, R.T.; Spangler, R.D.; Rossen, J.; Kocak, M.; Bryan, J.G.; Humeidi, R.; Peck, D.; Wu, X.; Tang, A.A.; et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 2020, 1, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.G.; Lau, E.; Dharmapalan, S.; Parker, M.; Chen, Y.; Alvarez, M.A.; Wang, D. Multi-output prediction of dose-response curves enables drug repositioning and biomarker discovery. NPJ Precis. Oncol. 2024, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2016, 14, 235–246. [Google Scholar] [CrossRef]
- Xia, Y.; Ji, X.; Jang, I.S.; Surka, C.; Hsu, C.; Wang, K.; Rolfe, M.; Bence, N.; Lu, G. Genetic and pharmacological interrogation of cancer vulnerability using a multiplexed cell line screening platform. Commun. Biol. 2021, 4, 834. [Google Scholar] [CrossRef]
- Brody, J.R.; Gallmeier, E.; Yoshimura, K.; Hucl, T.; Kulesza, P.; Canto, M.I.; Hruban, R.H.; Schulick, R.D.; Kern, S.E. A proposed clinical test for monitoring fluoropyrimidine therapy: Detection and stability of thymidylate synthase ternary complexes. Cancer Biol. Ther. 2006, 5, 923–927. [Google Scholar] [CrossRef]
- Very, N.; Hardiville, S.; Decourcelle, A.; Thevenet, J.; Djouina, M.; Page, A.; Vergoten, G.; Schulz, C.; Kerr-Conte, J.; Lefebvre, T.; et al. Thymidylate synthase O-GlcNAcylation: A molecular mechanism of 5-FU sensitization in colorectal cancer. Oncogene 2022, 41, 745–756. [Google Scholar] [CrossRef]
- Pommier, Y. Drugging topoisomerases: Lessons and challenges. ACS Chem. Biol. 2013, 8, 82–95. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Pommier, Y.; Nussenzweig, A.; Takeda, S.; Austin, C. Human topoisomerases and their roles in genome stability and organization. Nat. Rev. Mol. Cell Biol. 2022, 23, 407–427. [Google Scholar] [CrossRef]
- Sordet, O.; Nakamura, A.J.; Redon, C.E.; Pommier, Y. DNA double-strand breaks and ATM activation by transcription-blocking DNA lesions. Cell Cycle 2010, 9, 274–278. [Google Scholar] [CrossRef]
- Ocvirk, J.; Brodowicz, T.; Wrba, F.; Ciuleanu, T.E.; Kurteva, G.; Beslija, S.; Koza, I.; Papai, Z.; Messinger, D.; Yilmaz, U.; et al. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J. Gastroenterol. 2010, 16, 3133–3143. [Google Scholar] [CrossRef]
- van der Wilt, C.L.; Backus, H.H.; Smid, K.; Comijn, L.; Veerman, G.; Wouters, D.; Voorn, D.A.; Priest, D.G.; Bunni, M.A.; Mitchell, F.; et al. Modulation of both endogenous folates and thymidine enhance the therapeutic efficacy of thymidylate synthase inhibitors. Cancer Res. 2001, 61, 3675–3681. [Google Scholar] [PubMed]
- Liu, J.; Skradis, A.; Kolar, C.; Kolath, J.; Anderson, J.; Lawson, T.; Talmadge, J.; Gmeiner, W.H. Increased cytotoxicity and decreased in vivo toxicity of FdUMP[10] relative to 5-FU. Nucleosides Nucleotides 1999, 18, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Rahman, S.; Schwantes, I.R.; Bartlett, A.; Eil, R.; Farsad, K.; Fowler, K.; Goodyear, S.M.; Hansen, L.; Kardosh, A.; et al. Updated Management of Colorectal Cancer Liver Metastases: Scientific Advances Driving Modern Therapeutic Innovations. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Redman, J.M.; Rhea, L.P.; Brofferio, A.; Whelpley, M.; Gulley, J.L.; Gatti-Mays, M.E.; McMahon, S.; Cordes, L.M.; Strauss, J. Successful 5-fluorouracil (5-FU) infusion re-challenge in a metastatic colorectal cancer patient with coronary artery disease who experienced symptoms consistent with coronary vasospasm during first 5-FU infusion. J. Gastrointest. Oncol. 2019, 10, 1010–1014. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Holmlund, J.T.; Schiller, J.H.; Geary, R.S.; Kwoh, T.J.; Dorr, A.; Nemunaitis, J. A phase I trial of c-Raf kinase antisense oligonucleotide ISIS 5132 administered as a continuous intravenous infusion in patients with advanced cancer. Clin. Cancer Res. 2000, 6, 1626–1631. [Google Scholar]
- Lenz, H.J.; Stintzing, S.; Loupakis, F. TAS-102, a novel antitumor agent: A review of the mechanism of action. Cancer Treat. Rev. 2015, 41, 777–783. [Google Scholar] [CrossRef]
- Greenlee, J.D.; King, M.R. A syngeneic MC38 orthotopic mouse model of colorectal cancer metastasis. Biol. Methods Protoc. 2022, 7, bpac024. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, K.; Schmitz, J.C.; Priest, D.G. Disposition of leucovorin and its metabolites in dietary folic acid-deplete mice—Comparison between tumor, liver, and plasma. Cancer Chemother. Pharmacol. 1997, 40, 126–130. [Google Scholar] [CrossRef]
- Raghunathan, K.; Schmitz, J.C.; Priest, D.G. Impact of schedule on leucovorin potentiation of fluorouracil antitumor activity in dietary folic acid deplete mice. Biochem. Pharmacol. 1997, 53, 1197–1202. [Google Scholar] [CrossRef]
- Raghunathan, K.; Priest, D.G. Modulation of fluorouracil antitumor activity by folic acid in a murine model system. Biochem. Pharmacol. 1999, 58, 835–839. [Google Scholar] [CrossRef]
- Chapin, W.J.; Hwang, W.T.; Mamtani, R.; O’Hara, M.H. Low- vs High-Dose 5-FU in Triplet Chemotherapy Plus Bevacizumab for Patients With Colorectal Cancer. JAMA Netw. Open 2024, 7, e2424855. [Google Scholar] [CrossRef]
- Talukdar, A.; Kundu, B.; Sarkar, D.; Goon, S.; Mondal, M.A. Topoisomerase I inhibitors: Challenges, progress and the road ahead. Eur. J. Med. Chem. 2022, 236, 114304. [Google Scholar] [CrossRef]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef]
- Taflin, H.; Odin, E.; Carlsson, G.; Gustavsson, B.; Hemmingsson, O.; Wettergren, Y.; Urbanowicz, K.; Turyn, J.; Smolenski, R.T.; Peters, G.J. Increased potentiation of 5-fluorouracil induced thymidylate synthase inhibition by 5,10-methylenetetrahydrofolate (arfolitixorin) compared to leucovorin in patients with colorectal liver metastases; The Modelle-001 Trial. BJC Rep. 2024, 2, 89. [Google Scholar] [CrossRef]

| FR Drug | Media | IC50 (µM) ± SEM | IC50 (µM) + LV (1 µM) ± SEM | IC50 (µM) + LV (10 µM) ± SEM | LV Effect (1 µM) | LV Effect (10 µM) |
|---|---|---|---|---|---|---|
| 5FU | DMEM | 4.08 ± 0.0133 | 3.30 ± 0.0151 | 1.20 ± 0.0122 | 1.24 | 3.40 |
| 5FU | FR | 1.58 ± 0.0177 | 1.01 ± 0.0136 | 0.506 ± 0.0157 | 1.56 | 3.12 |
| CF10 | DMEM | 0.0131 ± 0.000125 | 0.0101 ± 0.000132 | 0.00583 ± 0.000138 | 1.30 | 2.25 |
| CF10 | FR | 0.0105 ± 0.000132 | 0.00842 ± 0.000145 | 0.00501 ± 0.000144 | 1.25 | 2.10 |
| TFT | DMEM | 1.66 ± 0.0105 | 1.54 ± 0.0105 | 1.09 ± 0.0112 | 1.08 | 1.52 |
| TFT | FR | 1.41 ± 0.0114 | 0.967 ± 0.0146 | 0.495 ± 0.0150 | 1.46 | 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okechukwu, C.C.; Ma, X.; Li, W.; D’Agostino, R., Jr.; Rees, M.G.; Ronan, M.M.; Roth, J.A.; Gmeiner, W.H. CF10 Displayed Improved Activity Relative to 5-FU in a Mouse CRLM Model Under Conditions of Physiological Folate. Cancers 2025, 17, 2739. https://doi.org/10.3390/cancers17172739
Okechukwu CC, Ma X, Li W, D’Agostino R Jr., Rees MG, Ronan MM, Roth JA, Gmeiner WH. CF10 Displayed Improved Activity Relative to 5-FU in a Mouse CRLM Model Under Conditions of Physiological Folate. Cancers. 2025; 17(17):2739. https://doi.org/10.3390/cancers17172739
Chicago/Turabian StyleOkechukwu, Charles Chidi, Xue Ma, Wencheng Li, Ralph D’Agostino, Jr., Matthew G. Rees, Melissa M. Ronan, Jennifer A. Roth, and William H. Gmeiner. 2025. "CF10 Displayed Improved Activity Relative to 5-FU in a Mouse CRLM Model Under Conditions of Physiological Folate" Cancers 17, no. 17: 2739. https://doi.org/10.3390/cancers17172739
APA StyleOkechukwu, C. C., Ma, X., Li, W., D’Agostino, R., Jr., Rees, M. G., Ronan, M. M., Roth, J. A., & Gmeiner, W. H. (2025). CF10 Displayed Improved Activity Relative to 5-FU in a Mouse CRLM Model Under Conditions of Physiological Folate. Cancers, 17(17), 2739. https://doi.org/10.3390/cancers17172739






