UK Real-World Evidence of Using Durvalumab Plus Cisplatin and Gemcitabine in Advanced Biliary Tract Cancer via an Early Access Scheme
Simple Summary
Abstract
1. Introduction
2. Method
2.1. Study Population
2.2. Statistical Analysis
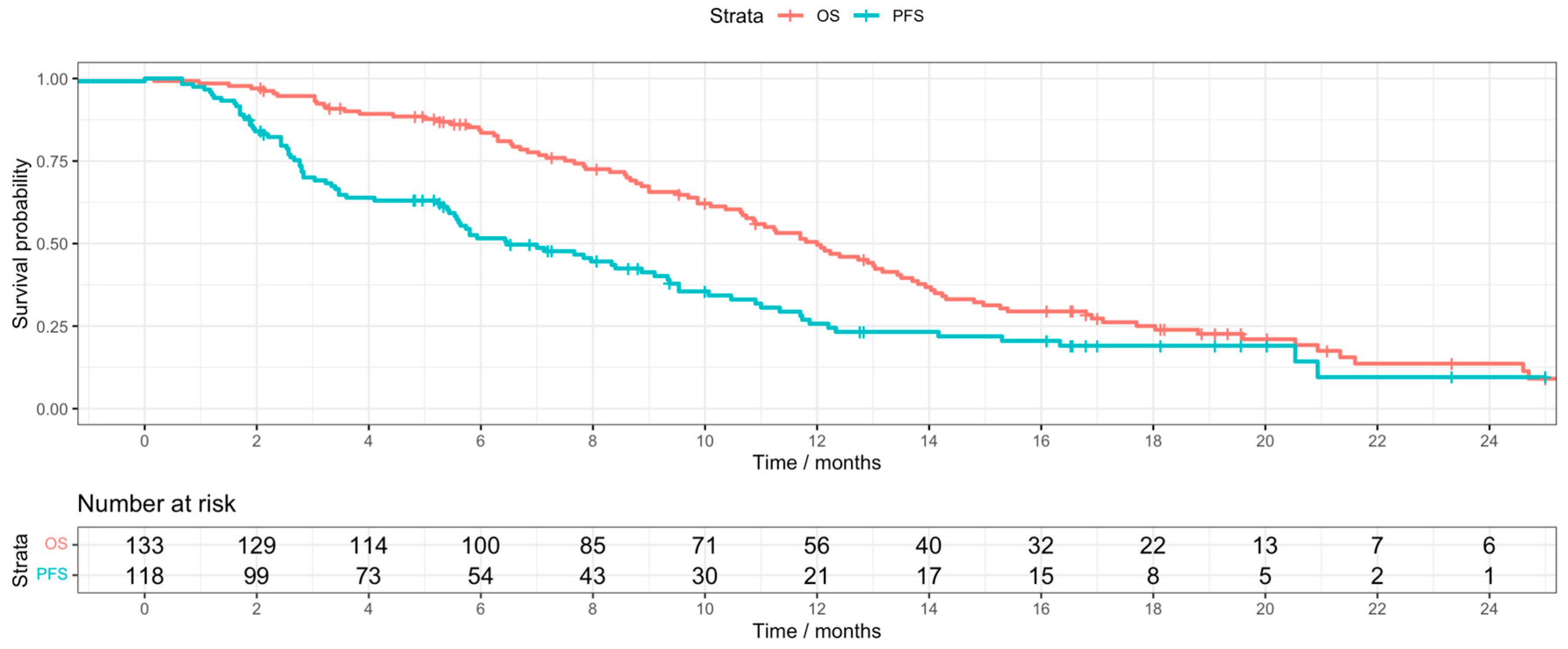
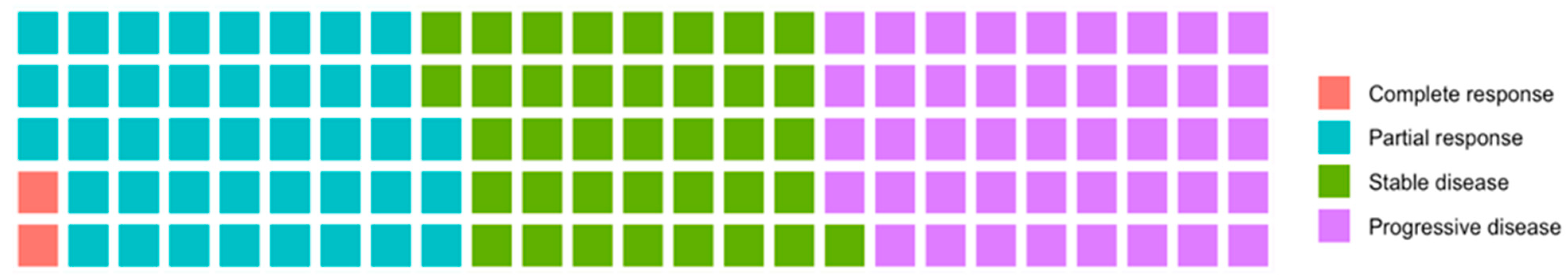
3. Results
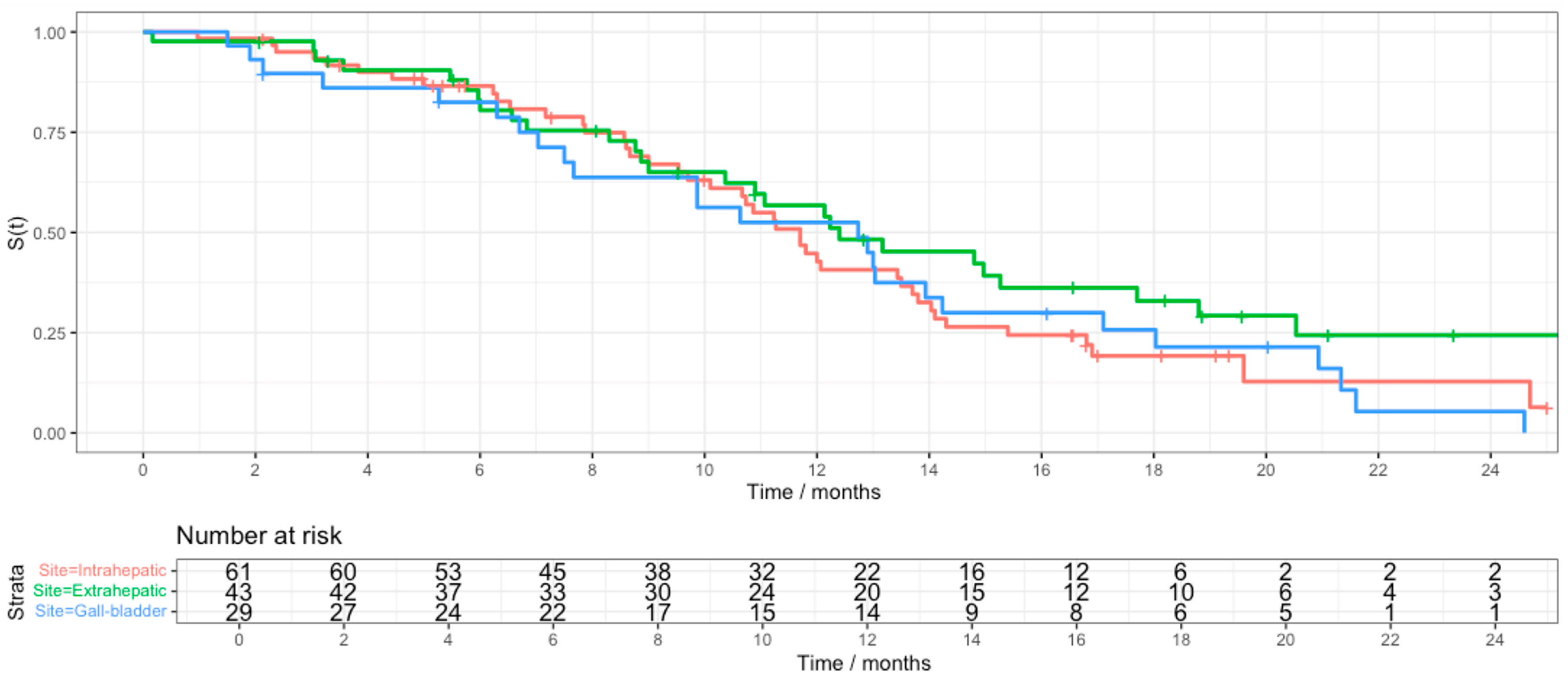
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary Tract Cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.H.; Shamseddine, A.I.; Tawil, A.N.; Berjawi, G.; Sidani, C.; Shamseddeen, W.; Barada, K.A. Prognostic Factors in Patients with Advanced Cholangiocarcinoma: Role of Surgery, Chemotherapy and Body Mass Index. World J. Gastroenterol. WJG 2008, 14, 3224. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.L.; et al. MPDL3280A (Anti-PD-L1) Treatment Leads to Clinical Activity in Metastatic Bladder Cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.; Garon, E.B.; Goldman, J.W.; Leighl, N.B.; Hellmann, M.D.; Patnaik, A.; Gandhi, L.; Eder, J.P.; Ahn, M.J.; Horn, L.; et al. Pembrolizumab as First-Line Therapy for Patients with PD-L1-Positive Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial. Ann. Oncol. 2017, 28, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Fontugne, J.; Augustin, J.; Pujals, A.; Compagnon, P.; Rousseau, B.; Luciani, A.; Tournigand, C.; Cherqui, D.; Azoulay, D.; Pawlotsky, J.M.; et al. PD-L1 Expression in Perihilar and Intrahepatic Cholangiocarcinoma. Oncotarget 2017, 8, 24644. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Boor, P.P.C.; Bruno, M.J.; Sprengers, D.; Kwekkeboom, J. Immune Suppressive Checkpoint Interactions in the Tumour Microenvironment of Primary Liver Cancers. Br. J. Cancer 2021, 126, 10. [Google Scholar] [CrossRef]
- Ioka, T.; Ueno, M.; Oh, D.-Y.; Fujiwara, Y.; Chen, J.-S.; Doki, Y.; Mizuno, N.; Park, K.; Asagi, A.; Hayama, M.; et al. Evaluation of Safety and Tolerability of Durvalumab (D) with or without Tremelimumab (T) in Patients (Pts) with Biliary Tract Cancer (BTC). J. Clin. Oncol. 2019, 37, 387. [Google Scholar] [CrossRef]
- Oh, D.Y.; He, A.R.; Bouattour, M.; Okusaka, T.; Qin, S.; Chen, L.T.; Kitano, M.; Lee, C.K.; Kim, J.W.; Chen, M.H.; et al. Durvalumab or Placebo plus Gemcitabine and Cisplatin in Participants with Advanced Biliary Tract Cancer (TOPAZ-1): Updated Overall Survival from a Randomised Phase 3 Study. Lancet Gastroenterol. Hepatol. 2024, 9, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Saesen, R.; Van Hemelrijck, M.; Bogaerts, J.; Booth, C.M.; Cornelissen, J.J.; Dekker, A.; Eisenhauer, E.A.; Freitas, A.; Gronchi, A.; Hernán, M.A.; et al. Defining the Role of Real-World Data in Cancer Clinical Research: The Position of the European Organisation for Research and Treatment of Cancer. Eur. J. Cancer 2023, 186, 52–61. [Google Scholar] [CrossRef]
- Rimini, M.; Fornaro, L.; Lonardi, S.; Niger, M.; Lavacchi, D.; Pressiani, T.; Lucchetti, J.; Giordano, G.; Pretta, A.; Tamburini, E.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer: An Early Exploratory Analysis of Real-World Data. Liver Int. 2023, 43, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Muddu, V.K.; Shah, A.; John, A.; Raj, A.; Bahl, A.; Rajappa, S.J.; Raja, T.; Ghosh, J.; Lavingia, V.; Vora, A.; et al. Gemcitabine Cisplatin and Durvalumab Experience in Advanced Biliary Tract Cancers: A Real-World, Multicentric Data From India. JCO Glob. Oncol. 2024, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-K.; Tang, Y.-J.; Wu, C.-E.; Hou, M.-M.; Hsu, H.-C.; Su, P.-J.; Chiang, N.-J.; Chen, S.-C.; Yeh, C.-N.; Chen, J.-S.; et al. Real-World Effectiveness and Prognostic Factors of Durvalumab plus Chemotherapy in a Multicentric Cohort with Advanced Biliary Tract Cancer. Oncologist 2024, oyae306. [Google Scholar] [CrossRef] [PubMed]
- Hadley Wickham Ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2011, 174, 245–246.
- Therneau, T.M. A Package for Survival Analysis in S. Available online: https://www.mayo.edu/research/documents/tr53pdf/doc-10027379 (accessed on 4 August 2025).
- Liao, P.; Cao, L.; Chen, H.; Pang, S.Z. Analysis of Metastasis and Survival between Extrahepatic and Intrahepatic Cholangiocarcinoma: A Large Population-Based Study. Medicine 2021, 100, e25635. [Google Scholar] [CrossRef] [PubMed]
- Danese, M.D.; Mody, K.; Thota, R.; Lindsey, S.C.; Bachini, M.; Abdel-Wahab, R.; Audhuy, F.; Duryea, J.; Bobiak, S. Treatment Patterns and Survival in Locally Advanced or Metastatic Biliary Tract Cancer Using SEER Medicare Data. Gastro Hep Adv. 2023, 2, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Ojerholm, E.; Smith, A.; Hwang, W.T.; Baumann, B.C.; Tucker, K.N.; Lerner, S.P.; Mamtani, R.; Boursi, B.; Christodouleas, J.P. Neutrophil-to-Lymphocyte Ratio as a Bladder Cancer Biomarker: Assessing Prognostic and Predictive Value in SWOG 8710. Cancer 2016, 123, 794. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.J.; Ronga, R.; Ambrosio, F.; Arundine, D.; Longo, V.; Galetta, D.; Gridelli, C.; Maione, P.; Palma, V.; Damiano, V.; et al. Neutrophil-to-Lymphocyte Ratio Is a Major Prognostic Factor in Non-Small Cell Lung Carcinoma Patients Undergoing First Line Immunotherapy with Pembrolizumab. Cancer Diagn. Progn. 2023, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Dulk, M.d.; Lang, S.A.; Ulmer, T.F.; Neumann, U.P.; Bednarsch, J. The Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 12691. [Google Scholar] [CrossRef] [PubMed]

| Site | Intrahepatic, N = 62 1 | Extrahepatic, N = 43 1 | Gallbladder, N = 29 1 |
|---|---|---|---|
| Age | 65 (59, 71) | 67 (60, 73) | 61 (55, 72) |
| ECOG | |||
| 0 | 36 (58%) | 15 (35%) | 13 (45%) |
| 1 | 23 (37%) | 26 (60%) | 16 (55%) |
| 2 | 3 (4.8%) | 1 (2.3%) | 0 (0%) |
| Not available | 0 (0%) | 1 (2.3%) | 0 (0%) |
| Sex | |||
| Female | 21 (34%) | 17 (40%) | 22 (76%) |
| Male | 41 (66%) | 26 (60%) | 7 (24%) |
| Metastatic | 45 (76%) | 25 (61%) | 17 (59%) |
| Unknown | 3 | 2 | 0 |
| Previous Surgery | 6 (9.8%) | 14 (36%) | 12 (44%) |
| Unknown | 1 | 4 | 2 |
| Drainage/Stent insertion | 12 (19%) | 25 (58%) | 10 (34%) |
| ALT | |||
| Elevated | 14 (23%) | 13 (30%) | 16 (34%) |
| Normal range | 48 (77%) | 30 (70%) | 13 (66%) |
| NLR | |||
| <3 | 24 (39%) | 23 (53%) | 16 (55%) |
| >3 | 38 (61%) | 20 (47%) | 13 (45%) |
| Ca 19.9 at diagnosis | |||
| Elevated | 41 (69%) | 29 (69%) | 19 (70%) |
| Normal | 18 (31%) | 13 (31%) | 8 (30%) |
| Unknown | 3 | 1 | 2 |
| CEA at diagnosis | |||
| Elevated | 25 (61%) | 21 (66%) | 15 (65%) |
| Normal | 16 (39%) | 11 (34%) | 8 (35%) |
| Unknown | 21 | 11 | 6 |
| Site | Intrahepatic, N = 16 1 | Extrahepatic, N = 17 1 | Gallbladder, N = 8 1 |
|---|---|---|---|
| Age | 66 (60, 71) | 67 (58, 72) | 62 (60, 66) |
| ECOG | |||
| 0 | 15 (94%) | 6 (35%) | 13 (63%) |
| 1 | 1 (6.3%) | 11 (65%) | 16 (38%) |
| Sex | |||
| Female | 2 (13%) | 8 (47%) | 4 (50%) |
| Male | 14 (88%) | 9 (53%) | 4 (50%) |
| BMI | |||
| Normal | 1 (6.3%) | 8 (47%) | 2 (25%) |
| Overweight | 9 (56%) | 5 (29%) | 3 (43%) |
| Obese | 6 (38%) | 4 (24%) | 3 (38%) |
| Metastatic | 10 (67%) | 8 (50%) | 4 (50%) |
| Unknown | 1 | 1 | 0 |
| Previous Surgery | 2 (13%) | 6 (40%) | 3 (43%) |
| ALT | |||
| Elevated | 3 (56%) | 5 (29%) | 4 (25%) |
| Normal range | 13 (81%) | 12 (71%) | 6 (75%) |
| NLR | |||
| <3 | 9 (56%) | 10 (59%) | 4 (50%) |
| >3 | 7 (44%) | 7 (41%) | 4 (50%) |
| Ca 19.9 at diagnosis | |||
| Elevated | 9 (56%) | 12 (71%) | 3 (38%) |
| Normal | 18 (31%) | 13 (31%) | 8 (30%) |
| Unknown | 3 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, H.; Hassan, M.; Babiker, O.; Rowley, W.; Qaisar, A.; Phillips, E.; Griffin, E.; Bell, C.; Baraka, B.; Acharige, S.; et al. UK Real-World Evidence of Using Durvalumab Plus Cisplatin and Gemcitabine in Advanced Biliary Tract Cancer via an Early Access Scheme. Cancers 2025, 17, 2732. https://doi.org/10.3390/cancers17172732
Daniels H, Hassan M, Babiker O, Rowley W, Qaisar A, Phillips E, Griffin E, Bell C, Baraka B, Acharige S, et al. UK Real-World Evidence of Using Durvalumab Plus Cisplatin and Gemcitabine in Advanced Biliary Tract Cancer via an Early Access Scheme. Cancers. 2025; 17(17):2732. https://doi.org/10.3390/cancers17172732
Chicago/Turabian StyleDaniels, Harry, Mona Hassan, Omer Babiker, William Rowley, Aitzaz Qaisar, Emma Phillips, Ellana Griffin, Catherine Bell, Bahaaeldin Baraka, Shyamika Acharige, and et al. 2025. "UK Real-World Evidence of Using Durvalumab Plus Cisplatin and Gemcitabine in Advanced Biliary Tract Cancer via an Early Access Scheme" Cancers 17, no. 17: 2732. https://doi.org/10.3390/cancers17172732
APA StyleDaniels, H., Hassan, M., Babiker, O., Rowley, W., Qaisar, A., Phillips, E., Griffin, E., Bell, C., Baraka, B., Acharige, S., Aquino, M., Plant, R., Mencel, J., Chan, S., Parslow, D., Arora, A., Scott-Brown, M., Khakoo, S., Braconi, C., ... Sivakumar, S. (2025). UK Real-World Evidence of Using Durvalumab Plus Cisplatin and Gemcitabine in Advanced Biliary Tract Cancer via an Early Access Scheme. Cancers, 17(17), 2732. https://doi.org/10.3390/cancers17172732






